Abstract
Group A β-hemolytic streptococcal (GAS) infection is associated with a spectrum of neuropsychiatric disorders. The leading hypothesis regarding this association proposes that a GAS infection induces the production of auto-antibodies, which cross-react with neuronal determinants in the brain through the process of molecular mimicry. We have recently shown that exposure of rats to GAS antigen leads to the production of anti-neuronal antibodies concomitant with the development of behavioral alterations. The present study tested the causal role of the antibodies by assessing the behavior of naïve rats following passive transfer of purified antibodies from GAS-exposed rats. Immunoglobulin G (IgG) purified from the sera of GAS-exposed rats was infused directly into the striatum of naïve rats over a 21-day period. Their behavior in the induced-grooming, marble burying, food manipulation and beam walking assays was compared to that of naïve rats infused with IgG purified from adjuvant-exposed rats as well as of naïve rats. The pattern of in vivo antibody deposition in rat brain was evaluated using immunofluorescence and colocalization. Infusion of IgG from GAS-exposed rats to naïve rats led to behavioral and motor alterations partially mimicking those seen in GAS-exposed rats. IgG from GAS-exposed rats reacted with D1 and D2 dopamine receptors and 5HT-2A and 5HT-2C serotonin receptors in vitro. In vivo, IgG deposits in the striatum of infused rats colocalized with specific brain proteins such as dopamine receptors, the serotonin transporter and other neuronal proteins. Our results demonstrate the potential pathogenic role of autoantibodies produced following exposure to GAS in the induction of behavioral and motor alterations, and support a causal role for autoantibodies in GAS-related neuropsychiatric disorders.
Keywords: streptococcus group A (GAS), Sydenham’s chorea (SC), pediatric autoimmune neuropsychiatric disorders associated with streptococcus (PANDAS), dopamine, serotonin, animal model
1. Introduction
Group A β-hemolytic streptococcal (GAS) infection is associated with a spectrum of neurological and neuropsychiatric disorders, including Sydenham’s chorea (SC), pediatric autoimmune neuropsychiatric disorders associated with streptococcus (PANDAS), obsessive-compulsive disorder (OCD), and Tourette’s syndrome (TS) (Dale, 2005; Husby et al., 1976; Peterson et al., 2000; Swedo et al., 1998; Swedo et al., 1994; Taranta and Stollerman, 1956). SC is the classical post-streptococcal neurological disorder occurring weeks to months after GAS infection, and is characterized by involuntary movements and neuropsychiatric disturbances, including obsessive-compulsive symptoms, tics, and emotional lability (Marques-Dias et al., 1997; Swedo et al., 1993).
The leading hypothesis regarding the relationship between GAS infection and neuropsychiatric disorders proposes that a GAS infection induces the production of autoantibodies, which cross-react with neuronal determinants in the brain (especially in the basal ganglia, prefrontal cortex and thalamus) through the process of molecular mimicry (Bonthius and Karacay, 2003; Bronze and Dale, 1993; Church et al., 2002; Cunningham, 2000, 2012; Husby et al., 1976; Kirvan et al., 2003; Pavone et al., 2004; Swedo et al., 1994).
Five criteria should be met to establish a disorder as an autoantibody-mediated autoimmune disorder: (A) presence of autoantibodies in the serum or cerebrospinal fluid (CSF); (B) a therapeutic effect of plasma exchange; (C) presence of antibodies at the tissue involved in the disorder pathogenesis; (D) induction of symptoms by immunizing with the antigen in an animal model; and (E) induction of symptoms by passive transfer of antibodies to animals (Archelos and Hartung, 2000). Several studies reported the presence of autoantibodies in the sera and CSF of GAS-related neuropsychiatric disorders patients (Bronze and Dale, 1993; Church et al., 2002; Gause et al., 2009; Husby et al., 1976; Kotby et al., 1998; Singer et al., 1998), and plasma exchange treatment has been reported to alleviate symptoms in these patients (Garvey et al., 2005; Perlmutter et al., 1999), fulfilling criteria A and B. We (Brimberg et al., 2012) and others (Hoffman et al., 2004) have found that exposure of rodents to GAS antigen leads to behavioral alterations, fulfilling Criterion D. Others (Ben-Pazi et al., 2012; Doyle et al., 2012; Hallett et al., 2000; Loiselle et al., 2004; Singer et al., 2005; Taylor et al., 2002; Yaddanapudi et al., 2010) have attempted to demonstrate that passive transfer of Immunoglobulin G (IgG) alters behavior (Criterion E). Some have been successful (Doyle et al., 2012; Hallett et al., 2000; Taylor et al., 2002; Yaddanapudi et al., 2010) while others have not (Ben-Pazi et al., 2012; Loiselle et al., 2004; Singer et al., 2005). The aim of the present study was to extend our previous findings (Brimberg et al., 2012) and test whether intra-striatal passive transfer of antibodies to animals would lead to the induction of a behavioral syndrome similar to those reported (Criterion E).
We have recently shown that exposure of male Lewis rats to GAS antigen led to a syndrome which resembles behavioral, pharmacological, immunological and neural characteristics of GAS-related neuropsychiatric disorders (Brimberg et al., 2012). Behaviorally, GAS-exposed rats showed increased compulsive-like behavior and motor disturbances, similar to the neuropsychiatric symptoms found in GAS-related neuropsychiatric disorders. Moreover, the abnormal behaviors in GAS-exposed rats were attenuated by pharmacological treatments used to treat the corresponding symptoms in human patients (i.e., a selective serotonin reuptake inhibitor (SSRI) and a D2 blocker, respectively). Immunologically, IgG was found in the striatum, prefrontal cortex (PFC) and thalamus of GAS-exposed rats (Brimberg et al., 2012), corresponding to the brain regions implicated in GAS-related neuropsychiatric disorders (Barsottini et al., 2002; Citak et al., 2004; Dilenge et al., 1999; Giedd et al., 2000; Huyser et al., 2009). IgG in sera obtained from GAS-exposed rats demonstrated strong immunoreactivity with D1 and D2 dopamine receptors and activated calcium/calmodulin-dependent protein kinase II signaling, as has previously been found for IgG in sera obtained from SC and PANDAS patients (Kirvan et al., 2003; Kirvan et al., 2006). In addition, we have found alterations in dopamine and glutamate levels in the medial frontal cortex and basal ganglia of GAS-exposed rats (Brimberg et al., 2012), further supporting a functional effect of the autoantibodies.
The aim of the present study was to directly test the role of the autoantibodies in inducing disease-like symptoms and to further test the involvement of the dopaminergic and the serotonergic systems in the induction of behavioral alterations. To this end, IgG purified from the sera of GAS-exposed rats and from adjuvant-exposed rats was infused directly into the striatum of naïve rats (GAS-I and Control-I rats, respectively) and the behavior of infused rats as well as of naïve rats was assessed (for more details see Fig. 1). Our results show that infusion of IgG from GAS-exposed rats leads to behavioral and motor alterations partially mimicking those seen in GAS-exposed rats. In addition, we show for the first time that IgG from GAS-exposed rats reacts with 5HT-2A and 5HT-2C serotonin receptors in vitro. In vivo, IgG deposits in the striatum of infused rats colocalized with specific brain proteins such as dopamine receptors, the serotonin transporter and other neuronal proteins.
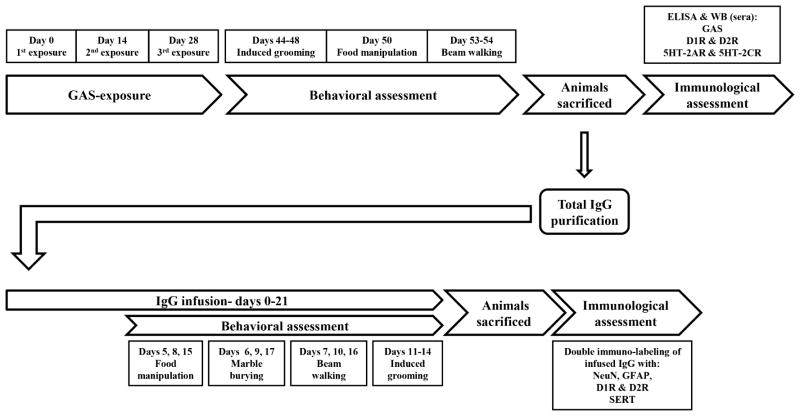
Figure 1.
Time line and behavioral and immunological procedures included in the study assessing the effects of GAS-exposure (upper half) and in the study assessing the effects of IgG infusion (lower half). 5HT-2AR=5HT-2A serotonin receptor; 5HT-2CR=5HT-2C serotonin receptor; D1R=D1 dopamine receptor; D2R=D2 dopamine receptor; GFAP=Glial fibrillary acidic protein; NeuN=neuronal nuclei; SERT=serotonin transporter; WB=western blot.
2. Materials and Methods
2.1. Animals
Male Lewis rats (Harlan, Jerusalem, Israel), 5 weeks old, were housed in groups of 2–3 per cage under a reversed 12-h light–dark cycle (lights on at 1900–0700 h) with ad libitum food and water. Rats were weighed twice a week. All experimental protocols were carried out according to the guidelines of the Institutional Animal Care and Use Committee of Tel-Aviv University.
2.2. GAS-exposure
2.2.1. Streptococcus pyogenes
M protein type 18 (Manfraedo) was obtained from Dr Allon Moses (Hadassah University Medical Center, Jerusalem, Israel), and grown as previously described (Brimberg et al., 2012). In short, streptococci were grown in 400 mL Todd-Hewitt broth (HyLab, Rehovot, Israel) overnight at 37°C with rocking at 250 rpm. The next morning, the cells were collected by centrifugation at 5000 rpm for 15 minutes at 4°C. The cell pellets (in ~1.5 gr) were stored frozen at −20°C until used.
2.2.2. Mutanolysin-Extracted GAS Antigen
A whole cell digest of M type 18 Streptococcus pyogenes was prepared as previously described (Brimberg et al., 2012). In short, cell pellets were suspended in phosphate-buffered saline (PBS) containing mutanolysin (Sigma-Aldrich, Rehovot, Israel). Following incubation at 37°C for 2 h with rocking, the digest was further disrupted by sonication (Microson ultrasonic cell disruptor, Plainveiw, NY). The insoluble material was removed by centrifugation at 12,000 × rpm (~25000 g) for 30 min at 4°C. Protein concentration in the supernatant was determined using the Coomasie-Plus Bradford reagent (PIERCE) according to the supplier recommendations. The supernatant was dialyzed extensively against water (10,000 MWCO, Sigma-Aldrich, Rehovot, Israel) then lyophilized and the powder was stored at −70°C.
2.2.3. Exposure of donor rats to GAS antigen
The exposure protocol followed Brimberg et al. (2012). Twenty eight rats were handled for 2 min daily for 4 days before the beginning of the exposure protocol. The first exposure was done at 5 weeks of age. Before each injection, rats were lightly anaesthetized with Isoflorene (VetMarket, Petach Tikva, Israel). Each rat in the GAS group was immunized subcutaneously with 200 μl of 1: 1 emulsion of PBS containing 1.2 mg of the GAS antigen and Complete Freund’s adjuvant (CFA, Sigma-Aldrich, Rehovot, Israel) supplemented with 4 mg/ml of heat-killed mycobacteria H37RA (Difco Laboratories, Detroit, MI). In order to increase the permeability of the blood brain barrier (Linthicum et al., 1982) rats have received an intraperitoneal injection of 1010 heat-killed Bordetella pertussis (Bioport, Lansing, MI, USA) as an additional adjuvant. Two boosts were introduced two and four weeks following the first exposure. Each rat was boosted with 200 μl 1: 1 emulsion of incomplete Freund’s adjuvant (IFA, Sigma-Aldrich): PBS containing 1.2 mg of the GAS antigen. Control animals were injected with PBS and adjuvants only. Behavioral testing began when the rats were 11 weeks old.
2.2.4. Preparation of pooled GAS and control donor IgG
Rats were euthanized and blood was collected. After clotting and centrifugation, serum was collected and stored at −70°C. Protein L resin (Genscript, USA) was packed in a polypropylene column (1 ml) and equilibrated with 10 ml of wash buffer (20 mM Na2HPO4, 0.15 M NaCl, pH 8.0). Sera samples were filtered by passing them through a 0.45 μm filter, and loaded onto the column. The column was washed with wash buffer. Total IgG was eluted with 10–15 ml elution buffer (0.1M glycine, pH 2.5), and neutralized to pH 7.4 with neutralization buffer (1M Tris-HCl, pH 8.5). Samples were dialyzed extensively against PBS (10,000 MWCO, Sigma-Aldrich, Rehovot, Israel). Prior to microinfusion, IgG was sterilized by filtration, pooled and brought to a concentration of 2 mg/ml. IgG concentration was determined by a NanoDrop Spectrophotometer (Thermo Scientific).
2.2.5. Intra-striatal infusion of IgG
Fourteen Lewis rats, 6 weeks old, were anesthetized with an intraperitoneal injection of ketamine and xylazine. Bilateral osmotic pump connector, 28 gauge, stainless steel cannulae (PlasticOne, USA), were implanted into the dorsolateral striatum at the following coordinates (Paxinos and Watson, 1998): 0.9 mm anterior to bregma, ±3.6 mm lateral to the midline, and 4.9 mm ventral to the skull. The cannulae were connected to an osmotic pump (Alzet Corp, Palo Alto, CA), containing 200μl of IgG (2 mg/ml) purified from the sera of either GAS-exposed rats (GAS-I group, n=8) or control rats (Control-I group, n=6), through a sterile polyethylene tube (containing sterile PBS). The choice of IgG concentration was based on previous studies in which IgG purified from PANDAS, SC or TS patients was infused into the striatum using osmotic pumps (Doyle et al., 2012; Hallett et al., 2000; Taylor et al., 2002). IgG was micro-infused bilaterally for 21 days, at a rate of 0.5 μl/hour. A third group of control rats were anesthetized but were not implanted with cannulae (naïve group, n=8). Behavioral testing began 5 days following cannula implantation.
2.3. Behavioral Assessment
A 22-h food restriction schedule with water freely available was initiated at age 9 weeks for the GAS exposure experiment, and 3 days after surgery for the intra-striatal IgG infusion experiment.
2.3.1. Food manipulation
Rats’ ability to manipulate a food pellet was assessed as previously described (Ayalon et al., 2004; Brimberg et al., 2012). In short, one day following a 10 min exposure to a 38 × 21 cm Plexiglas observation box (habituation), food-deprived rats were placed in the box, and 5 min later several Purina rat food pellets (approximately 1g each) were introduced into the box. Rats were given 10 min to consume the food pellet, and their ability to manipulate it was rated independently by two observers, according to the scale of Kolb and Holmes (1983): 0, Unable to manipulate the food pellet with the forepaws; 1, Holds the food pellet against the floor with its forepaws when it eats; 2, Eats the food pellet from the floor and sometimes hold the food pellet in its forepaws; 3, Picks the food pellet up in its forepaws and partially eats it but drops the food pellet before it is all consumed; and 4, Sits up on its hind paws and holds the food pellet in its front paws until it is finished. Half scores were given when the animal behavior was in-between scale definitions. The observer rating behavior was blind to the animal condition.
2.3.2. Beam walking
Motor coordination and balance were assessed by measuring the ability of rats to traverse a 1 m long beam with a width of 5, 2.5 or 1.5cm (Brimberg et al., 2012; Carter et al., 1999; Urakawa et al., 2007). The beam was placed 1 m above the floor, with one end mounted on a narrow support and the other end attached to the rat’s home cage. On Day 1 each rat received three training trials on the 5cm wide beam. On Day 2, after two training trials on the 5 cm beam, the time it took the rat to traverse this beam and the number of foot slips were recorded. Immediately after, rats were given 2 training trials on the 2.5 and 1.5 cm wide beams and the time it took each rat to traverse these beams on the third trial and the number of foot slips were recorded. The rater was blind to the animal condition.
2.3.3. Grooming
The assessment of induced-grooming was carried out as previously described (Brimberg et al., 2012; Greer and Capecchi, 2002). Each rat was videotaped individually for 30 min on 4 consecutive days in an empty cage. Ten min after the beginning of each session, the rat was misted with water to induce grooming. The duration of grooming behavior was assessed in the 20 min of the induced-grooming of each day. The rater was blind to the animal condition.
2.3.4. Marble burying
Marble burying was assessed because we have recently found that GAS-exposure increases the number of marbles buried in this test (Lotan et al., in preparation). Rats were placed individually in a cage measuring 37 cm long × 21 cm wide × 18 cm high, containing bedding that was 5 cm in depth, with nine marbles 2.3 cm in diameter arranged in two rows along the short wall of the cage. The number of buried marbles after 15 min was counted. Marbles were considered buried if they were at least one-half covered with bedding. The observer rating behavior was blind to the animal condition.
2.3.5. Activity
Activity was assessed as previously described (Ayalon et al., 2004). In short, rats were individually placed in an activity box (45 cm wide × 65 cm long × 40 cm high) located in a quiet room and allowed 1 h of free exploration. Images from a camera located above each box were analyzed using image analysis software. The software “grabbed” the image from each box every 1 s and compared this image, pixel by pixel, with the image obtained in the previous second. Each white rat was monitored against the darker background. The percentage of pixels that went from dark to light or from light to dark from 1 s to the next (“activity counts”) was quantified. This percentage provided a measure of the magnitude of an animal’s displacement or “activity”. One-second activity values ranged from 0% (no movement) to approximately 7.5%.
2.4. Immunological assessments
2.4.1. Reagents
The following antigens were used in this study: bovine serum albumin (BSA) was purchased from the Sigma Chemical Co. (St. Louis, MO, USA). Human D1 and D2L dopamine receptors, and 5HT-2A and 2C serotonin receptor antigens were purchased from PerkinElmer (Waltham, Massachusetts, USA) and used for both the ELISA and Western blot procedures.
2.4.2. ELISA- GAS
Immunoreactivity to the GAS mutanolysin extracted streptococcal antigen was assessed using ELISA, as previously described (Brimberg et al., 2012). Ninety six well ELISA plates (Nunc) were coated with 5 μg/ml of GAS mutanolysin extracted streptococcal antigen in PBS overnight at 4°C. Plates were blocked with 300 μl/well of 2% non-fat milk in PBS for 1 hour at RT. Diluted rat serum was applied onto the plates in a dilution series (1:500, 1:2,500, and 1:125,000) in 0.05% PBST and incubated for 1 hour at RT. Following incubation, the plates were washed X3 with PBST. HRP conjugated donkey anti-rat antibodies (Jackson Immunolaboratories, USA, 1:10,000 dilution in PBST) were added to the wells (100 μl/well) for 1 h at RT, followed by X3 washes with PBST. The Plates were developed using the chromogenic HRP substrate TMB (Sigma-Aldrich, Rehovot, Israel) (100 μl/well) and color development was terminated with 1 M H2SO4 (50 μl/well). The plates were read at 450 nm.
2.4.3. ELISA and Western blot
Immunoreactivity to D1 and D2 dopamine receptors and to the 5HT-2A and 2C serotonin receptors membrane antigens was assessed using ELISA and western blot analysis of sera obtained from GAS-exposed rats:
2.4.3.1. ELISA
Ninety six well Immunolon 4 microtiter plates (Dynatech Laboratories, Chantilly, VA) were coated with 10μg/ml of each antigen (D1, D2L, and 5HT-2A and 2C receptors membrane antigens; BSA served as a negative control protein) in 0.015M carbonate buffer (pH 9.6) overnight at 4°C. Plates were blocked with 1% BSA in PBS for 1 h. Diluted rat serum (50μl/well) was applied onto the plates in a dilution series and incubated overnight at 4°C in 1% BSA in PBS. The next day, the plates were washed X3 with PBST. Alkaline phosphatase-conjugated rabbit anti-rat secondary antibody specific to IgG (Sigma Chemical Co.) was added to the plates diluted 1:1000 in 1% BSA-PBS. Plates were further incubated for 1 h at room temperature and washed with PBST. Plates were developed with 1mg/ml p-nitrophenyl phosphate colorimetric substrate (Sigma Chemical Co.) and the optical density was determined at 405 nm in a Opsys MR microplate reader (Dynex Technologies, Chantilly, VA). For the titers of antibodies against the serotonin receptors 5HT-2A and 2C, the following dilutions were performed: 1:200, 1:400, 1:800, 1:1,600, 1:3,200, 1:6,400; D1: 1:300, 1:600; 1:1200, 1:4800, 1:9600; D2: 1:320, 1:640; 1:1280, 1:5120, 1:10240; BSA: 1:200, 1:400, 1:800, 1:1,600. Titers were determined as the last dilution resulting in an OD of 0.1.
2.4.3.2. Western blot
D1 and D2 dopamine receptors (Perkin Elmer) were loaded as 10 μg per lane, and 5HT-2A and 2C serotonin receptors (abcam) were loaded as 1 μg per lane in the SDS PAGE gel. Antigens were separated on a 7–10% SDS-PAGE gel using a 5% stacking gel and separated antigens were electro-transferred onto the nitrocellulose membrane. Membranes were blocked overnight at 4°C in 5% nonfat milk in Tris-Buffered Saline Tween-20 (TBST). To determine the immunoreactivity of the sera to blotted proteins, individual lanes were separated into strips and incubated with a pool of sera from the control or GAS-exposed rats, diluted in PBST (1:150) overnight at 4°C. Rabbit anti-D1 receptor sera were diluted 1:1000 (R&D systems); rabbit anti-D2 receptor sera were diluted 1:400 (R&D systems); rabbit anti-5HT-2A receptor sera were diluted 1:1000 (abcam); rabbit anti-5HT-2C receptor sera were diluted 1:500 (abcam); all dilutions were used based on manufacturer guidelines. The strips were washed in PBST and incubated with HRP-conjugated donkey anti-rat antibodies or with HRP-conjugated goat anti-rabbit antibodies (Jackson Immunolaboratories, USA) at dilutions of 1:5000. Blots were detected using the enhanced chemiluminescence (ECL) Plus Western detection kit (Amersham Pharmacia Biotech). The bands developed from the immunoblot were compared to the relative position of separated proteins on Coomassie blue-stained immobilized nitrocellulose strips. The relative molecular weight of proteins was determined using pre-stained molecular weight protein standards (Bio-Rad).
2.4.4. Preparation of tissue sections
Rats were overdosed with 100 mg/kg sodium pentobarbital, i.p, and perfused intracardially with cold perfusion buffer. Brains were removed and placed in 4% paraformaldehyde over night, after which they were cryoprotected in 30% sucrose solution for at least 48 hours. For immunostaining, brains were sectioned in the coronal plane at 16 μm for detecting IgG deposits, and sections were mounted on gelatin-coated slides. Slides were stored at −70°C.
2.4.5. Immunostaining for IgG deposits and double staining
To assess IgG deposits in the brain, 16μm sections were incubated in PBS until reaching room temperature. Sections were then heated in citric acid (pH 6, 10mM) for 5 min and were treated with 0.5% Triton X-100 for 3 min. The sections were blocked (2% BSA solution, 10% normal horse serum and 0.25% Triton X-100 in PBS) for 1 h in room temperature. Sections were then incubated for 1 h with fluorescent Alexa 488 goat anti rat secondary antibody (1:250, Molecular Probes, Eugene, OR, USA) diluted in blocking solution. Following Immunostaining for IgG deposits, the sections were incubated overnight at 4°C with one of the following markers labeled with mouse anti-neuron-selective NeuN (anti-neuronal nuclei, 1: 250; Millipore), rabbit anti-Glial fibrillary acidic protein (GFAP, 1:400, Sigma), rabbit anti anti-D2 dopamine receptor (1:100, Millipore), rabbit anti-D1 dopamine receptor (1:300, Millipore), and rabbit anti-serotonin transporter (1:400, Millipore). Sections were washed three times with PBS, and incubated with fluorescent Alexa 594 goat anti-mouse or anti-rabbit antibodies (1:250; molecular Probes) for 1 h at room temperature. Following immunostaining, sections were coverslipped with mounting media with DAPI (Vector Laboratories, Burlingame, USA) to counterstain the nuclei. The imaging was carried out using a fluorescence microscopy (Nikon Eclipse 80i) and a NIS imaging software (Nikon).
2.5. Statistical Analysis
We used two-sample Student’s t-tests to compare means of two independent groups. When more groups were compared, we used analysis of variance (ANOVA; the specific factors for each analysis are given in the Results section) together with post hoc LSD analysis (the specific analyses are detailed in the Results section). We considered values as significant when p<0.05. All data are presented as means± SEM.
3. Results
3.1. Effects of exposure to GAS antigen
3.1.1. Altered behavior in Lewis rats exposed to GAS antigen
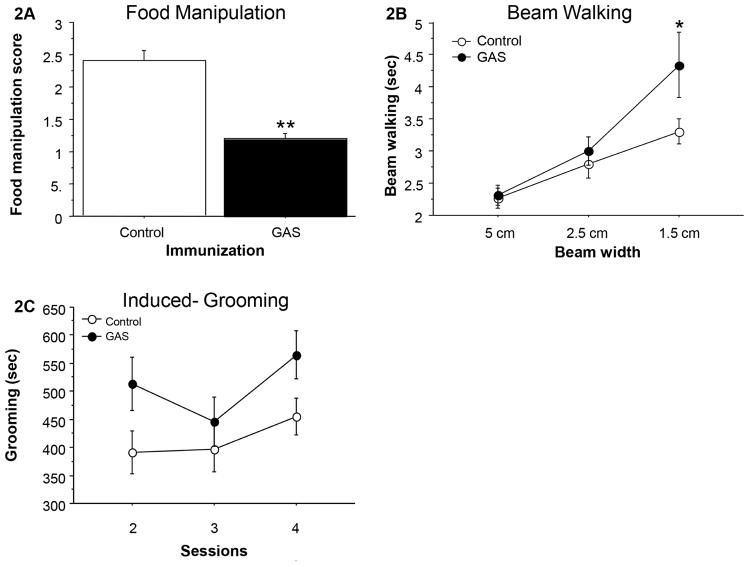
Figure 2A–C presents the behavior of GAS-exposed and control (adjuvant-exposed) rats in the food manipulation, beam walking and induced-grooming assays. GAS-exposed rats were impaired in manipulating food (Fig. 2A, t(26)=5.726, p<0.0001), and in traversing the narrow (1.5 cm) but not the wider (2.5 and 5.0 cm) beams, as evident in a longer time to traverse the beam (Fig. 2B, Exposure X Beam Width mixed ANOVA: Exposure: F(1,26)=3.484, p=0.0733; Beam Width: F(2,52)=20.814, p<0.0001; Exposure X Beam Width interaction: F (2,52) =2.479, p=0.0936; see Figure 2B for the results of the post hoc comparisons). There were no differences between the groups in the number of foot slips (data not shown, p’s>0.3). In addition, GAS rats exhibited increased grooming in the induced-grooming assay (Fig. 2C, Exposure X Session mixed ANOVA: Exposure: F(1,23)=5.145, p<0.05; Session: F(2,46)=2.818, p=0.0701; Exposure X Session interaction: F(2,46)=0.548, p=0.5821). GAS rats did not have any gross indications of skin lesions, nor did they show loss of hair, which could indicate an increase in spontaneous grooming.
Figure 2.
Effects of streptococcal exposure on (A) food manipulation, (B) beam walking, and (C) grooming. (A) The mean and standard error (SE) of food manipulation scores of GAS (n=12) and control rats (n=16). (B) The mean and SE of the time spent on the wide and narrow beams of GAS (n=12) and control rats (n=16). (C) The mean and SE of the duration of grooming in three sessions of induced-grooming of GAS (n=9) and control (n=16) rats.
*p<0.01, **p<0.0001
3.1.2. Sera from GAS-exposed rats reacted with dopamine and serotonin receptors in the ELISA and Western Immunoblot
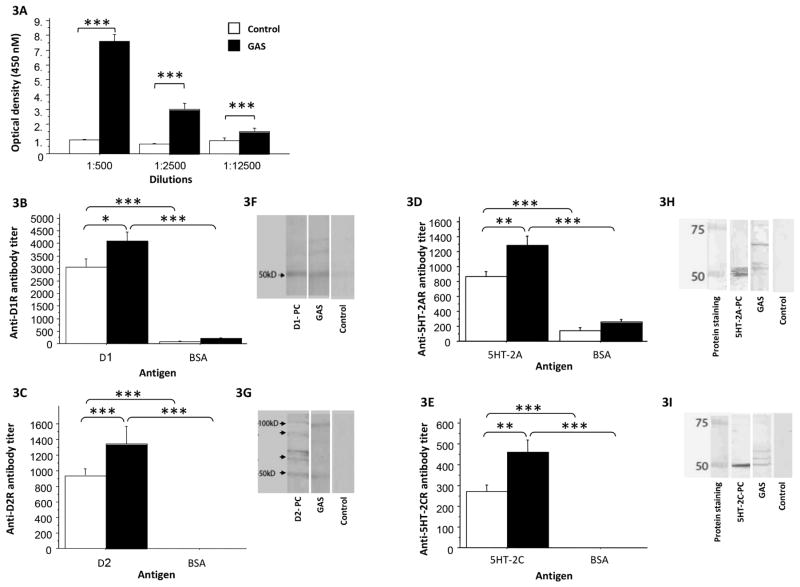
As expected, IgG antibodies in sera of GAS-exposed rats were significantly elevated against the GAS cell wall antigen, the immunogen (Fig. 3A, F(1,20)=99.53, p<0.0001). In addition, we found in the ELISA (Fig, 3B, 3C) and confirmed in the Western blot (Fig. 3F, 3G) that the streptococcal exposure led to induction of antibodies against the dopamine receptors D1 and D2, and that this reactivity was specific when compared to BSA (Fig. 3B, D1: Exposure X Protein mixed ANOVA: Exposure: F(1,38)=5.519, p<0.05; Protein: F(1,38)=187.845, p<0.0001; Exposure X Protein interaction: F(1,38)=3.095, p=0.0866; Fig. 3C, D2: Exposure X Protein mixed ANOVA: Exposure: F(1,40)=3.137, p=0.082; Protein: F(1,40)=97.616, p<0.0001; Exposure X Protein interaction: F(1,40)=3.137, p=0.082, see Fig. 3B and 3C for the results of the post hoc analysis). In addition to replicating our previous findings (Brimberg et al., 2012), we have found in the ELISA (Fig. 3D, 3E) and confirmed in the Western blot (Fig. 3H, 3I), elevated antibodies against the 5HT-2A and the 5HT-2C serotonin receptors. This reactivity was specific when compared to BSA (Fig. 3D, 5HT-2A: Exposure X Protein mixed ANOVA: Exposure: F(1,39)=12.232, p<0.005; Protein: F(1,39)=133.090, p<0.0001; Exposure X Protein interaction: F(1,39)=3.919, p=0.0548; Fig. 3E, 5HT-2C: Exposure X Protein mixed ANOVA: Exposure: F(1,38)=8.197, p<0.01; Protein: F(1,38)=125.484, p<0.0001; Exposure X Protein interaction: F(1,38)=8.197, p<0.01, see Fig. 3D and 3E for the results of the post hoc analysis).
Figure 3.
Effects of group A streptococcal (GAS) exposure on immunoreactivity of rat serum (IgG) with GAS antigen, dopamine receptors and serotonin receptors in the ELISA (A–E) and Western blot (F–I). ELISA results for anti-GAS serum IgG reactivity with: (A) GAS mutanolysin extracted antigen (GAS, n=12; control, n=10), (B) D1 dopamine receptor (GAS, n=11; control, n=10), (C) D2 dopamine receptor (GAS, n=13; control, n=10), (D) 5HT-2A serotonin receptor (GAS, n=12; control, n=10), and (E) 5HT-2C serotonin receptor (GAS, n=12; control, n=10), of sera taken from GAS and control rats, in comparison to reactivity with BSA. Western blot of pooled sera (IgG) reactivity from GAS-exposed rats compared with sera (IgG) from adjuvant-exposed control rats with: (F) D1 dopamine receptor, (G) D2 dopamine receptor, (H) 5HT-2A serotonin receptor, and (I) 5HT-2C serotonin receptor. D1-PC = anti-D1 receptor positive control; D2-PC = anti-D2 receptor positive control; 5HT-2A-PC = anti-5HT-2A receptor positive control; 5HT-2C-PC = anti-5HT-2C receptor positive control.
*p<0.01, **p<0.005, ***p<0.0001
3.2. Effects of intra-striatal passive transfer of purified total IgG from sera of GAS-exposed rats
3.2.1. Increased compulsive-like behavior and motor deficits in recipient rats
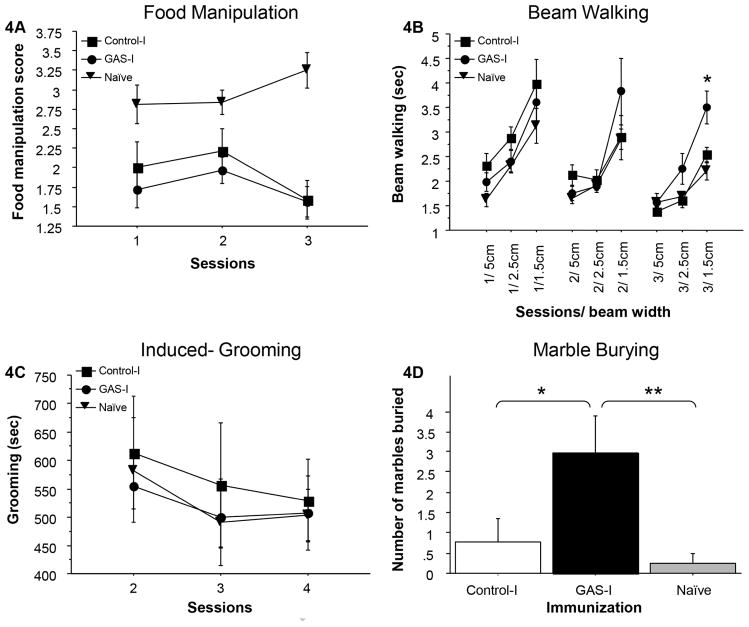
Figure 4 presents the behavior of naïve rats, of rats intra-striatally infused with total IgG purified from GAS-exposed rats (GAS-I), and of rats intra-striatally infused with total IgG purified from adjuvant-exposed rats (Control-I) in the food manipulation (Fig. 4A), beam walking (Fig. 4B), induced-grooming (Fig. 4C) and marble-burying assays (Fig. 4D). There were no significant differences between the three groups in the time spent grooming in the induced-grooming test (Fig. 4C, Condition X Session mixed ANOVA: all p’s>0.4), and both the GAS-I and Control-I groups were impaired in manipulating food compared to the naïve group (Fig. 4A, Condition X Session mixed ANOVA: Condition: F(2,19)=11.132, p<0.0005; Session: F(4,38)=2.008, p=0.1482; Condition X Session interaction: F(4,38)=4.723, p<0.005, see Fig. 4A for the results of the post hoc analysis), suggesting a non-specific effect of the intra-striatal infusion. In contrast, the GAS-I group performed significantly different from the Control-I and naïve groups in the beam walking and marble burying assays. Specifically, on the last session of beam walking, GAS-I rats required more time to traverse the narrow (1.5 cm) but not the wider (2.5 and 5 cm) beams, compared to the naïve and Control-I groups which performed similarly (Fig. 4B). This difference reflects the shortening of the time to traverse the narrow beam over sessions in the naïve and Control-I groups, which was not evident in the GAS-I group (Fig. 4B, Condition X Beam Width X Session mixed ANOVA: Condition: F(2,19)=2.64, p=0.0974; Beam Width: F(2,38)=97.423, p<0.0001; Session, F(2,38)=19.641, p<0.0001; Condition X Beam Width, F (4,38) =3.411, p<0.05; Condition X Session: F(4,38) =3.808, p<0.05; Session X Beam Width: F(4,76)=0.802, p=0.5277; Condition X Beam Width X Session: F(8,76)=0.765, p=0.6342, see Fig. 4B for the results of the post hoc analysis). A similar pattern of results was evident in the number of falls (data not shown). In addition, rats in the GAS-I group buried more marbles compared to the Control-I and naïve groups, which did not differ (Fig. 4D, F(2,18)=5.250, p<0.05, see Fig. 4D for the results of the post hoc analysis). The increased burying in the GAS-I group was not a result of a non-selective increase in behavioral output, as no significant differences were found between the three groups in activity level (in fact, the naïve group tended to be more active, ANOVA: F(2,19)=3.432, p=0.0534, data not shown).
Figure 4.
Effects of passive transfer of IgG from GAS-exposed and control rats to the striatum of naïve rats on (A) food manipulation, (B) beam walking, (C) grooming, and (D) marble burying. (A) The mean and standard error (SE) of food manipulation scores of rats infused with IgG extracted from GAS-exposed rats (GAS-I group, n=8), rats infused with IgG extracted from control rats (Control-I group, n=6) and naïve rats (n=8). (B) The mean and SE of the time spent on the wide and narrow beams of GAS-I rats (n=8), Control-I rats (n=6) and naïve rats (n=8). (C) The mean and SE of the duration of induced-grooming on three sessions of GAS-I rats (n=8), Control-I rats (n=5) and naïve rats (n=8). (d) The mean and SE of the number of marbles buried by GAS-I rats (n=8), control rats (n=5) and naïve rats (n=8).
*p<0.05, **p<0.01
3.2.2. Immunofluorescence of IgG deposits and colocalization with specific brain proteins
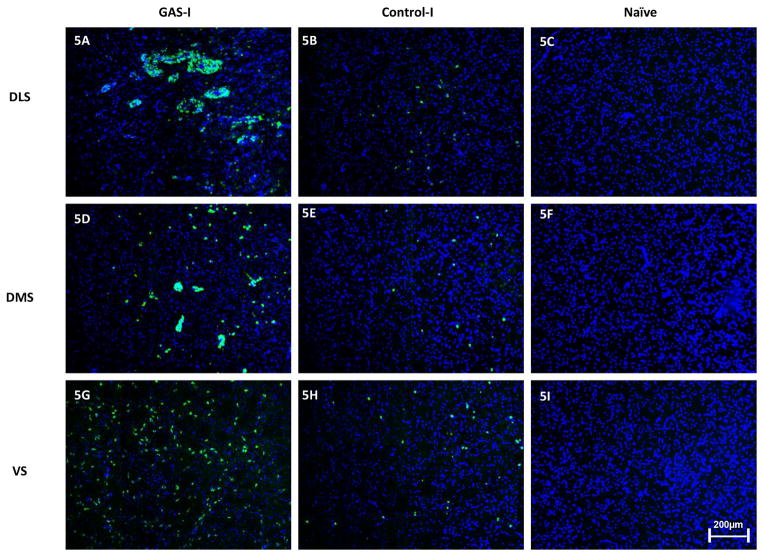
Using an immunohistochemical analysis, we assessed the pattern of in vivo IgG reactivity in the striatum. We have found IgG labeling in neurons in the striatum of GAS-I rats, in comparison to a much weaker IgG pattern in the Control-I rats. No IgG was observed in the striatum of naïve rats (Fig. 5). DAPI staining of cell nuclei overlaid the IgG reactivity in the figures.
Figure 5.
Representative immunofluorescence images showing IgG deposition in the striatum of a GAS-I rat (A, D, G), Control-I rat (B, E, H) and naïve rat (C, F, I). (A–C) Labeling in the dorso-lateral striatum (DLS, the injection site); (D–F) Labeling in the dorso-medial striatum (DMS); (G–I) Labeling in the ventral striatum (VS). Tissue sections were incubated with anti-rat alexa antibody 488 for visualization of IgG deposition; Blue signal indicates nuclear counterstaining (DAPI). 10 × microscope objective; scale bar=200μm.
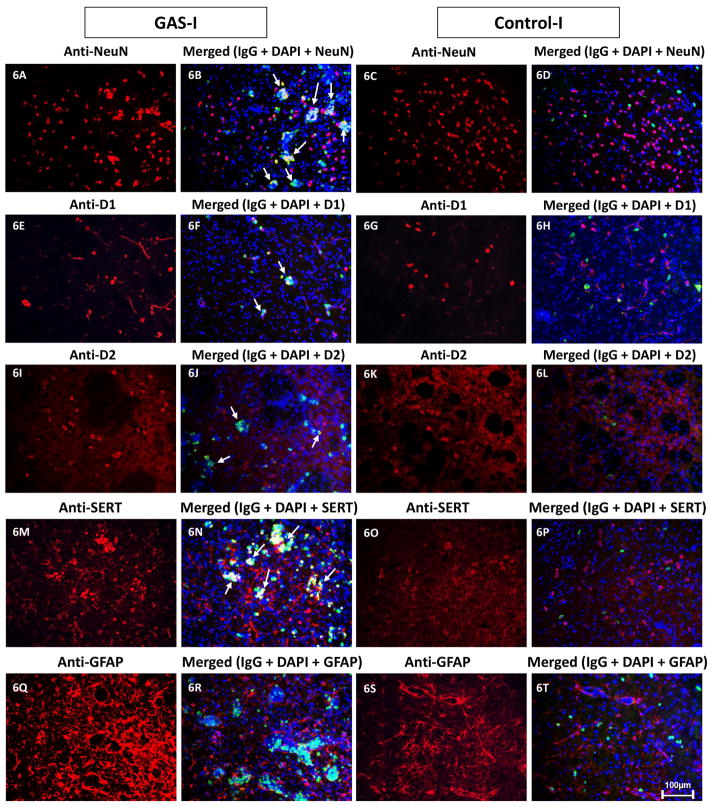
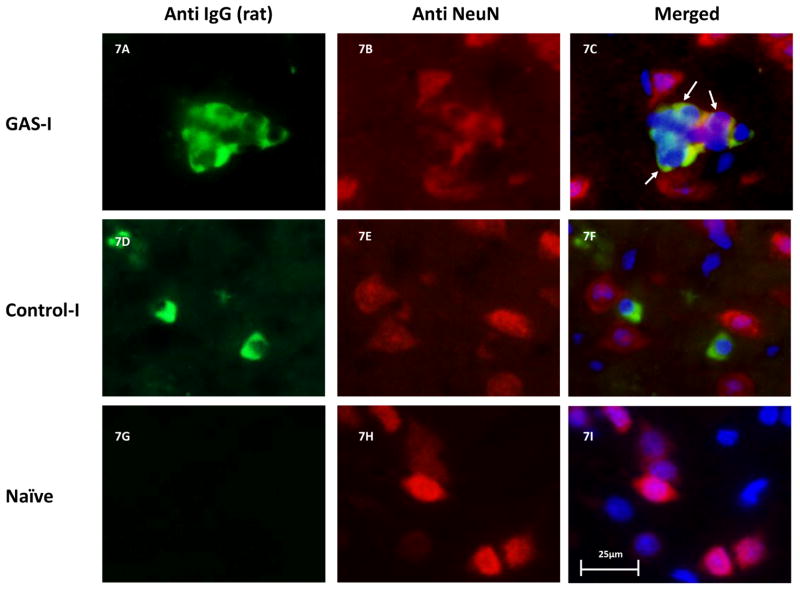
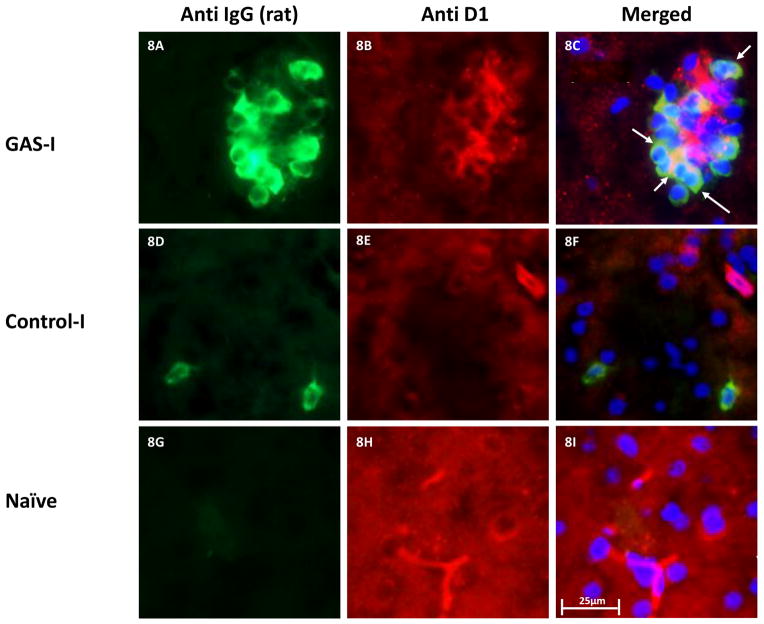
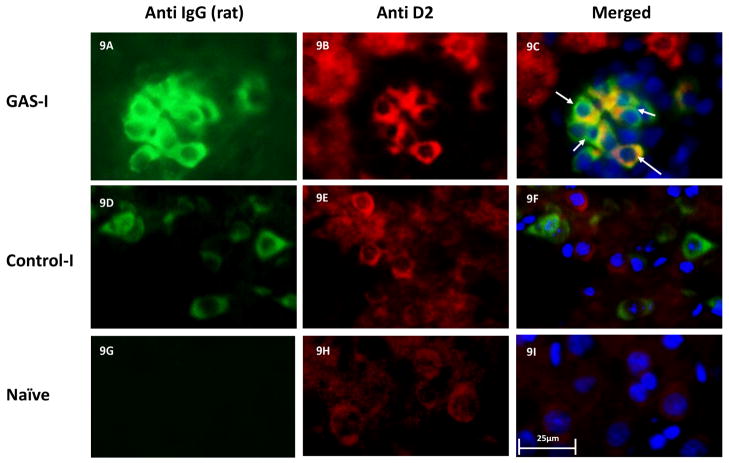
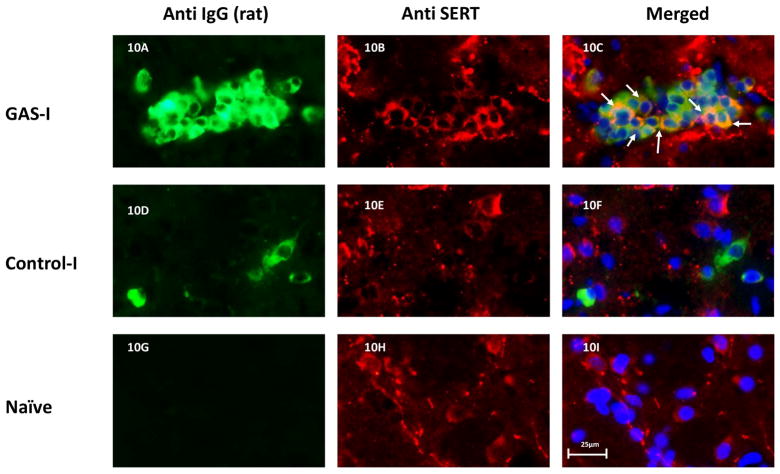
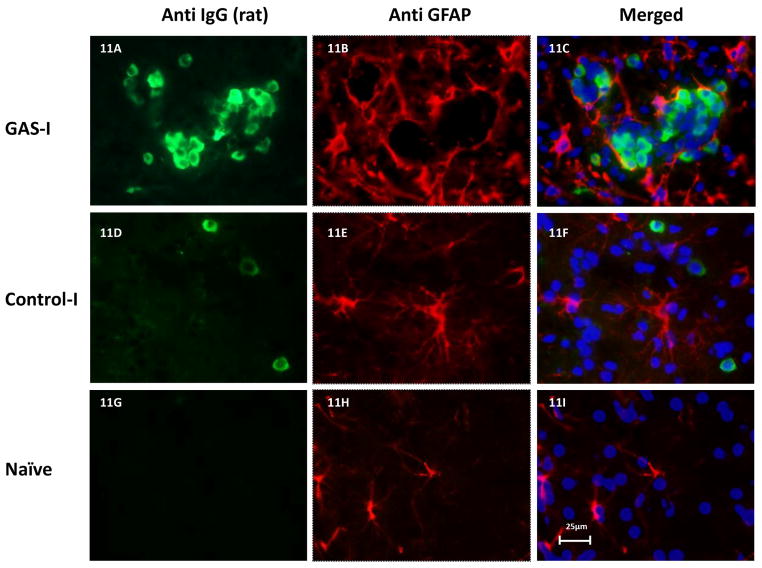
Double immunolabeling shows that IgG clusters in GAS-I rats colocalized with NeuN (labeling neurons, Fig 6B and Fig. 7C), with the D1 (Fig 6F and Fig. 8C) and D2 (Fig. 6I and Fig. 9C) dopamine receptors and with the serotonin transporter (Fig. 6N and Fig. 10C). Much less staining was observed in the Control-I rat brains (Fig. 6D, 6H, 6L, and 6P and Fig. 7F, 8F, 9F and 10F). Interestingly, double immunolabeling of GFAP (labeling astrocytes) and IgG (Fig. 6Q–T) revealed that astrocytes encircled the IgG clusters in the striatum of GAS-I rats (Fig. 6R and Fig. 11C), whereas this pattern was not observed in Control-I rats (Fig. 6T and Fig. 11F).
Figure 6.
Representative immunofluorescence images taken from the dorso-lateral striatum (DLS, the injection site) of a GAS-I rat (A–B, E–F, I–J, M–N, Q–R), and a control-I rat (C–D, G–H, K–L, O–P, S–T) showing labeling with markers for neurons (NeuN, A–D), D1 dopamine receptor (E–H), D2 dopamine receptor (I–L), serotonin transporter (SERT, M–P), and astrocytes (GFAP, Q–T), and colocalization of these markers with IgG deposition (B, D, F, H, J, LN, P, R, T). Tissue sections were incubated with anti-rat alexa antibody 488 for visualization of IgG deposition; IgG against NeuN was labeled with anti-mouse alexa antibody 594; D1 and D2 dopamine receptors, SERT and GFAP were labeled with anti-rabbit alexa antibody 594. Blue signal indicates nuclear counterstaining (DAPI). 20 × microscope objective; scale bar=100μm. The arrows point to colocalization between the infused IgG and the relevant marker.
Figure 7.
Representative immunofluorescence images showing IgG deposition in the striatum of a GAS-I rat (A–C), Control-I rat (D–F) and naïve rat (G–I) and double labeling with a marker for neurons (NeuN). Figures A, D and G display labeling of rat IgG; Figures B, E and H display NeuN labeling; Figures C, F and I display colocalization of the rats IgG and NeuN. Tissue sections were incubated with anti-rat alexa antibody 488 for visualization of IgG deposition; IgG against NeuN was labeled with anti-mouse alexa antibody 594. Blue signal indicates nuclear counterstaining (DAPI). 40 × microscope objective; scale bar=25μm. The arrows point to colocalization between the infused IgG and NeuN.
Figure 8.
Representative immunofluorescence images showing IgG deposition in the striatum of a GAS-I rat (A–C), Control-I rat (D–F) and naïve rat (G–I) and double labeling with anti-D1 dopamine receptor antibody. Figures A, D and G display labeling of rat IgG; Figures B, E and H display labeling of the D1 dopamine receptor; Figures C, F and I display colocalization of the rats IgG and the D1 dopamine receptor. Tissue sections were incubated with anti-rat alexa antibody 488 for visualization of IgG deposition; IgG against the D1 dopamine receptor was labeled with anti-rabbit alexa antibody 594. Blue signal indicates nuclear counterstaining (DAPI). 40 × microscope objective; scale bar=25μm. The arrows point to colocalization between the infused IgG and D1.
Figure 9.
Representative immunofluorescence images showing IgG deposition in the striatum of a GAS-I rat (A–C), Control-I rat (D–F) and naïve rat (G–I) and double labeling with anti-D2 dopamine receptor antibody. Figures A, D and G display labeling of rat IgG; Figures B, E and H display labeling of the D2 dopamine receptor; Figures C, F and I display colocalization of the rats IgG and the D2 dopamine receptor. Tissue sections were incubated with anti-rat alexa antibody 488 for visualization of IgG deposition; IgG against the D2 dopamine receptor was labeled with anti-rabbit alexa antibody 594. Blue signal indicates nuclear counterstaining (DAPI). 40 × microscope objective; scale bar=25μm. The arrows point to colocalization between the infused IgG and D2.
Figure 10.
Representative immunofluorescence images showing IgG deposition in the striatum of a GAS-I rat (A–C), Control-I rat (D–F) and naïve rat (G–I) and double labeling with anti-serotonin transporter (SERT) antibody. Figures A, D and G display labeling of rat IgG; Figures B, E and H display labeling of SERT; Figures C, F and I display colocalization of the rats IgG and SERT. Tissue sections were incubated with anti-rat alexa antibody 488 for visualization of IgG deposition; IgG against the SERT was labeled with anti-rabbit alexa antibody 594. Blue signal indicates nuclear counterstaining (DAPI). 40 × microscope objective; scale bar=25μm. The arrows point to colocalization between the infused IgG and SERT.
Figure 11.
Representative immunofluorescence images showing IgG deposition in the striatum of a GAS-I rat (A–C), Control-I rat (D–F) and naïve rat (G–I) and double labeling with a marker for astrocytes (GFAP). Figures A, D and G display labeling of rat IgG; Figures B, E and H display GFAP labeling; Figures C, F and I display colocalization of the rats IgG and GFAP. Figures Tissue sections were incubated with anti-rat alexa antibody 488 for visualization of IgG deposition; IgG against GFAP was labeled with anti-rabbit alexa antibody 594. Blue signal indicates nuclear counterstaining (DAPI). 40 × microscope objective; scale bar=25μm.
4. Discussion
Our study evaluated the role of antibodies produced following exposure of rats to GAS in the induction of neuropsychiatric abnormalities. The first part of the study replicated and extended our previous findings on the behavioral and biological alterations following exposure of rats to GAS extract (Brimberg et al., 2012; Cox et al., 2013). Behaviorally, GAS-exposed rats demonstrated increased grooming in the induced-grooming assay, suggestive of compulsive-like behavior, and were impaired in the food manipulation and beam walking tasks, suggesting impairments in fine motor control and gait, respectively. It should be noted, however, that the increased time it took GAS rats to traverse the narrow but not the wider beams in the beam-walking assay, may reflect increased anxiety of GAS rats rather than an impairment in gait. While we have previously found that GAS rats bury more marbles (Lotan et al., unpublished observations), a behavior which has been suggested to reflect both increased compulsive-like and anxiety-like behavior (for review see, Albelda and Joel, 2012), we did not find a significant difference between GAS and control rats in the proportion of time spent in the open arms of a plus-maze (p=0.7, Brimberg et al., 2012), a commonly used test of anxiety-like behavior. Immunologically, sera from GAS-exposed rats reacted with the D1 and D2 dopamine receptors, as we have previously reported (Brimberg et al., 2012). A novel finding of the present study is that sera from GAS-exposed rats also reacted with the 5HT-2A and 2C serotonin receptors. It should be noted that sera from adjuvant-exposed control rats exhibited background reactivity against the four receptors in the ELISA, but the higher receptor specific reactivity of sera IgG from GAS rats was confirmed by Western blots, where the adjuvant control rat sera was negative and the anti-GAS sera recognized receptor specific bands. The higher background in the control sera in the ELISA but not in the Western blot may be expected for these G protein-coupled receptors, because only in the Western blot are these receptors completely separated from the membrane (Baragli et al., 2007; Renart et al., 1979; Reynolds and Tanford, 1970; Towbin et al., 1979). The higher background in the ELISA may therefore reflect either lower avidity antibodies in the control sera that react with the receptor-membrane complex, or immune complexes from the immunization that may also create nonspecific binding. Our conclusion is supported by the lack of significant differences between reactivity of sera from adjuvant-exposed rats and naïve rats against D1 and D2 dopamine receptors in the ELISA (p’s > 0.14, Brimberg et al., unpublished observations) and by the present findings of very low binding of IgG in the striatum of rats that were infused with IgG from adjuvant-exposed rats compared with the IgG binding in the striatum of rats that were infused with IgG from GAS-exposed rats.
In the second part of our study, antibodies purified from GAS-exposed rats and infused into the striatum of naïve rats (GAS-I) were found to replicate some of the behavioral effects of exposure to GAS. Specifically, on the last session of testing, GAS-I rats were impaired in traversing a narrow, but not a wide, beam, compared to naïve rats and to rats infused with antibodies purified from adjuvant-treated control rats (Control-I). The specificity of the impairment to the narrow beam, seen also in GAS-exposed rats in the present and previous (Brimberg et al., 2012) studies, suggests that the effect of anti-GAS antibodies was specific to gait, while sparing motivation and gross motor control. However, as detailed above, the increased time to traverse the narrow beam may have reflected increased anxiety of GAS-I rats rather than a motor impairment. Because the difference between the GAS-I and the two control groups was due to the shortening of the time to traverse the narrow beam in the latter groups, it is possible that the difference reflects impaired motor learning of GAS-I rats. Alternatively, this difference may reflect the gradual development of behavioral alteration in GAS-I rats following the accumulation of antibodies, which counteracted the effects of training. A selective and specific effect of IgG infusion was also seen in the marble burying assay. GAS-I rats buried more marbles compared to Control-I and naïve rats, and this increased burying could not be attributed to a non-specific increase in activity level, because the latter was not significantly different between the three groups (in fact, the two infused groups tended to be less active than the naïve group). As we have recently found increased marble burying also in GAS-exposed rats (Lotan et al., in preparation), these results suggest that anti-GAS antibodies caused a specific increase in marble burying. GAS-I rats did not show, however, increased grooming in the induced-grooming assay, in contrast to GAS-exposed rats in the present and previous (Brimberg et al., 2012) studies. In addition, although GAS-I rats were impaired in manipulating food compared to naïve rats, so were Control-I rats, suggesting a non-specific effect of the intra-striatal infusion in this task. One of the limitations of our study is that antibodies were passively transferred intra-striatally which in itself may have led to some non-specific effects due either to mechanical disruption of striatal functioning following infusion of fluids, or an effect of the infusion of IgG, as Control-I rats were also infused with antibodies (against the adjuvant mycobacteria injected into the rats from which sera were collected). Taken together, our new evidence demonstrates that intra-striatal infusion of antibodies (IgG) purified from GAS-exposed rats mimics part of the behavioral syndrome that is induced by exposing rats to GAS antigen, and that some of these behavioral effects are specific to anti-GAS antibodies.
Our results are in line with previous studies, which reported the emergence of stereotypic behaviors and motor abnormalities following infusion of either sera or IgG purified from sera of SC, PANDAS or TS patients to the striatum of naïve rats (Doyle et al., 2012; Hallett et al., 2000; Taylor et al., 2002). Yet, for reasons currently unknown, other studies that used a similar approach failed to induce behavioral alterations (Ben-Pazi et al., 2012; Loiselle et al., 2004; Singer et al., 2005). See (Doyle et al., 2012; Singer et al., 2005) for a discussion of possible causes for the inconsistent results in these studies. Our results are also in line with the finding that systemic passive transfer of anti-streptococcal sera from GAS-exposed mice to naïve mice (concomitant with Lipopolysaccharides (LPS) administration to break the blood brain barrier) resulted in the development of motor and behavioral disturbances similar to those seen in the GAS-exposed mice (Yaddanapudi et al., 2010). Taken together with our present results, these collective studies support a role for antibodies in the induction of behavioral alterations.
Our results further suggest a role for antibodies that target proteins involved in the functioning of the dopaminergic and serotonergic systems. Specifically, we have found anti-D1 and D2 dopamine receptor antibodies, as well as anti-5HT-2A and 2C serotonin receptor antibodies in the sera of GAS-exposed rats. Several lines of evidence point to the involvement of these neurotransmitter systems in the pathophysiology of GAS-related neuropsychiatric disorders. Dysfunction of the dopaminergic system has been implicated in motor disturbances (e.g., Parkinson’s disease, Huntington’s disease, and Alzheimer’s disease (Jahanshahi et al., 2013; Michel et al., 2013; Reeves et al., 2010) as well as in OCD (Denys et al., 2004; Hesse et al., 2005; Nikolaus et al., 2010; Olver et al., 2009), and dysfunction of the serotonergic system has been implicated in OCD and anxiety disorders (Flaisher-Grinberg et al., 2008; Hesse et al., 2005; Hesse et al., 2011; Nikolaus et al., 2010; Perani et al., 2008). Dopaminergic and serotonergic drugs are used to treat motor and psychiatric symptoms, respectively, in patients affected by GAS-related neuropsychiatric disorders (Demiroren et al., 2007; Gabbay and Coffey, 2003; Moretti et al., 2008; Murphy et al., 2010; Swedo and Grant, 2005; Swedo et al., 1993). Finally, immunoreactivity to different components of the serotonergic system, such as serotonin and serotonin receptors, has been reported in sera from panic disorder and rheumatoid arthritis patients (Coplan et al., 1999; Maes et al., 2013; Schott et al., 2003; Tanaka et al., 2003), and sera from SC and PANDAS patients reacted with D1 and D2 receptors (Brimberg et al., 2012; Cox et al., 2013; Dale et al., 2012). Interestingly, Dale et al (2012) have recently found antibodies against surface D2 receptors in the sera of patients suffering from basal ganglia encephalitis, which is characterized by motor (including, parkinsonism, dystonia and chorea), and psychiatric symptoms (including, emotional lability, attention deficit and psychosis), which are also found in GAS-related neuropsychiatric disorders.
The pattern of IgG deposition in the striatum of GAS-I rats also supports the hypothesis that antibodies that target the dopaminergic and the serotonergic systems play a functional role in the induction of behavioral alterations. Specifically, IgG purified from GAS-exposed rats and infused into the striatum of naïve rats were colocalized with neurons, with cells expressing D1 dopamine receptors, with cells expressing D2 dopamine receptors and with cells expressing the serotonin transporter. Astrocytes encircled clusters of neurons containing IgG.
Colocalization of IgG with neurons in the striatum is in accordance with previous studies showing that antibodies found in sera of GAS-related neuropsychiatric disorders, such as SC, PANDAS, OCD, and TS, reacted with basal-ganglia neurons (Church et al., 2002; Cox et al., 2013; Gause et al., 2009; Husby et al., 1976; Kiessling et al., 1993; Kotby et al., 1998; Martino et al., 2011; Singer et al., 1998). There is less data regarding a possible role of astrocytes in GAS-related neuropsychiatric disorders. Kingston and Glynn (1971) found a cross-reaction of sera taken from rabbits previously exposed to GAS with astrocytes. It is possible that astrocytes encircled the antibody neuronal cell cluster in order to delimit their effect or remove them from the brain tissue.
Our results suggest a potential pathogenic role of autoantibodies produced following exposure to GAS in the induction of behavioral alterations, reminiscent of the outcomes of exposure to GAS in both patients and rats, thus establishing the fifth condition required to establish a disorder as an antibody-mediated autoimmune disorder. Our evidence illustrates induction of symptoms by intra-striatal passive transfer of IgG to animals. Additional studies are required in order to assess the exact mechanisms by which the autoantibodies produced in GAS-related neuropsychiatric disorders are pathogenic.
Acknowledgments
We thank Dr. Dan Frenkel and his laboratory members, Hilit Levy-Barazany, Dorit Trudler and Dorit Farfara (Department of Neurobiology, Tel-Aviv University, Israel) for their assistance in the immunohistochemical analyses. This research was supported by the Israel Science Foundation (grant No. 341/07) to DJ and National Institutes of Health grant HL56267, Bench to Bedside grant from the National Institutes of Mental Health, and the Oklahoma Center for the Advancement of Science and Technology grant to MWC.
Footnotes
Financial disclosures
MWC is Chief Scientific Officer with a financial interest in Moleculera Labs which provides diagnostic testing of children with autoimmune neuropsychiatric and movement disorders. Moleculera Labs resides at the University of Oklahoma Health Sciences Center and Presbyterian Health Foundation Research Park in Oklahoma City, OK. MWC has research funding from National Institutes of Health (grant No. HL56267), from the National Institutes of Mental Health (Bench to Bedside grant) and from Oklahoma Center for the Advancement of Science and Technology. DJ has research funding from the Israel Science Foundation (grant No. 341/07). All other authors assert that none has any commercial or financial involvements that might present an appearance of a conflict of interest in connection with the submitted manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Albelda N, Joel D. Current animal models of obsessive compulsive disorder: an update. Neuroscience. 2012;211:83–106. doi: 10.1016/j.neuroscience.2011.08.070. [DOI] [PubMed] [Google Scholar]
- Archelos JJ, Hartung HP. Pathogenetic role of autoantibodies in neurological diseases. Trends Neurosci. 2000;23:317–327. doi: 10.1016/s0166-2236(00)01575-7. [DOI] [PubMed] [Google Scholar]
- Ayalon L, Doron R, Weiner I, Joel D. Amelioration of behavioral deficits in a rat model of Huntington’s disease by an excitotoxic lesion to the globus pallidus. Exp Neurol. 2004;186:46–58. doi: 10.1016/S0014-4886(03)00312-1. [DOI] [PubMed] [Google Scholar]
- Baragli A, Alturaihi H, Watt HL, Abdallah A, Kumar U. Heterooligomerization of human dopamine receptor 2 and somatostatin receptor 2 - Co-immunoprecipitation and fluorescence resonance energy transfer analysis. Cellular Signalling. 2007;19:2304–2316. doi: 10.1016/j.cellsig.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Barsottini OG, Ferraz HB, Seviliano MM, Barbieri A. Brain SPECT imaging in Sydenham’s chorea. Braz J Med Biol Res. 2002;35:431–436. doi: 10.1590/s0100-879x2002000400004. [DOI] [PubMed] [Google Scholar]
- Ben-Pazi H, Sadan O, Offen D. Striatal microinjection of Sydenham chorea antibodies: using a rat model to examine the dopamine hypothesis. J Mol Neurosci. 2012;46:162–166. doi: 10.1007/s12031-011-9559-6. [DOI] [PubMed] [Google Scholar]
- Bonthius DJ, Karacay B. Sydenham’s chorea: not gone and not forgotten. Semin Pediatr Neurol. 2003;10:11–19. doi: 10.1016/s1071-9091(02)00004-9. [DOI] [PubMed] [Google Scholar]
- Brimberg L, Benhar I, Mascaro-Blanco A, Alvarez K, Lotan D, Winter C, Klein J, Moses AE, Somnier FE, Leckman JF, Swedo SE, Cunningham MW, Joel D. Behavioral, pharmacological, and immunological abnormalities after streptococcal exposure: a novel rat model of Sydenham chorea and related neuropsychiatric disorders. Neuropsychopharmacology. 2012;37:2076–2087. doi: 10.1038/npp.2012.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronze MS, Dale JB. Epitopes of streptococcal M proteins that evoke antibodies that cross-react with human brain. J Immunol. 1993;151:2820–2828. [PubMed] [Google Scholar]
- Carter RJ, Lione LA, Humby T, Mangiarini L, Mahal A, Bates GP, Dunnett SB, Morton AJ. Characterization of progressive motor deficits in mice transgenic for the human Huntington’s disease mutation. J Neurosci. 1999;19:3248–3257. doi: 10.1523/JNEUROSCI.19-08-03248.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Church AJ, Cardoso F, Dale RC, Lees AJ, Thompson EJ, Giovannoni G. Anti-basal ganglia antibodies in acute and persistent Sydenham’s chorea. Neurology. 2002;59:227–231. doi: 10.1212/wnl.59.2.227. [DOI] [PubMed] [Google Scholar]
- Citak EC, Gucuyener K, Karabacak NI, Serdaroglu A, Okuyaz C, Aydin K. Functional brain imaging in Sydenham’s chorea and streptococcal tic disorders. J Child Neurol. 2004;19:387–390. doi: 10.1177/088307380401900513. [DOI] [PubMed] [Google Scholar]
- Coplan JD, Tamir H, Calaprice D, DeJesus M, de la Nuez M, Pine D, Papp LA, Klein DF, Gorman JM. Plasma anti-serotonin and serotonin anti-idiotypic antibodies are elevated in panic disorder. Neuropsychopharmacology. 1999;20:386–391. doi: 10.1016/S0893-133X(98)00130-4. [DOI] [PubMed] [Google Scholar]
- Cox CJ, Sharma M, Leckman JF, Zuccolo J, Zuccolo A, Kovoor A, Swedo SE, Cunningham MW. Brain human monoclonal autoantibody from sydenham chorea targets dopaminergic neurons in transgenic mice and signals dopamine D2 receptor: implications in human disease. J Immunol. 2013;191:5524–5541. doi: 10.4049/jimmunol.1102592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham MW. Pathogenesis of group A streptococcal infections. Clin Microbiol Rev. 2000;13:470–511. doi: 10.1128/cmr.13.3.470-511.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham MW. Streptococcus and rheumatic fever. Curr Opin Rheumatol. 2012;24:408–416. doi: 10.1097/BOR.0b013e32835461d3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale RC. Post-streptococcal autoimmune disorders of the central nervous system. Dev Med Child Neurol. 2005;47:785–791. doi: 10.1017/S0012162205001647. [DOI] [PubMed] [Google Scholar]
- Dale RC, Merheb V, Pillai S, Wang D, Cantrill L, Murphy TK, Ben-Pazi H, Varadkar S, Aumann TD, Horne MK, Church AJ, Fath T, Brilot F. Antibodies to surface dopamine-2 receptor in autoimmune movement and psychiatric disorders. Brain. 2012;135:3453–3468. doi: 10.1093/brain/aws256. [DOI] [PubMed] [Google Scholar]
- Demiroren K, Yavuz H, Cam L, Oran B, Karaaslan S, Demiroren S. Sydenham’s chorea: a clinical follow-up of 65 patients. J Child Neurol. 2007;22:550–554. doi: 10.1177/0883073807302614. [DOI] [PubMed] [Google Scholar]
- Denys D, van der Wee N, Janssen J, De Geus F, Westenberg HG. Low level of dopaminergic D2 receptor binding in obsessive-compulsive disorder. Biol Psychiatry. 2004;55:1041–1045. doi: 10.1016/j.biopsych.2004.01.023. [DOI] [PubMed] [Google Scholar]
- Dilenge ME, Shevell MI, Dinh L. Restricted unilateral Sydenham’s chorea: reversible contralateral striatal hypermetabolism demonstrated on single photon emission computed tomographic scanning. J Child Neurol. 1999;14:509–513. doi: 10.1177/088307389901400805. [DOI] [PubMed] [Google Scholar]
- Doyle F, Cardoso F, Lopes L, Mendes M, Dias F, Cruz L, Tavares R, Camargos A, Carneiro M, Dias-Lopes C, Chavez-Olortegui C. Infusion of Sydenham’s chorea antibodies in striatum with up-regulated dopaminergic receptors: a pilot study to investigate the potential of SC antibodies to increase dopaminergic activity. Neurosci Lett. 2012;523:186–189. doi: 10.1016/j.neulet.2012.06.073. [DOI] [PubMed] [Google Scholar]
- Flaisher-Grinberg S, Klavir O, Joel D. The role of 5-HT2A and 5-HT2C receptors in the signal attenuation rat model of obsessive-compulsive disorder. Int J Neuropsychopharmacol. 2008;11:811–825. doi: 10.1017/S146114570800847X. [DOI] [PubMed] [Google Scholar]
- Gabbay V, Coffey B. Obsessive-Compulsive disorder, Tourette’s disorder, or pediatric autoimmune neuropsychiatric disorders associated with Streptococcus in an adolescent? Diagnostic and therapeutic challenges. J Child Adolesc Psychopharmacol. 2003;13:209–212. doi: 10.1089/104454603322572516. [DOI] [PubMed] [Google Scholar]
- Garvey MA, Snider LA, Leitman SF, Werden R, Swedo SE. Treatment of Sydenham’s chorea with intravenous immunoglobulin, plasma exchange, or prednisone. J Child Neurol. 2005;20:424–429. doi: 10.1177/08830738050200050601. [DOI] [PubMed] [Google Scholar]
- Gause C, Morris C, Vernekar S, Pardo-Villamizar C, Grados MA, Singer HS. Antineuronal antibodies in OCD: comparisons in children with OCD-only, OCD+chronic tics and OCD+PANDAS. J Neuroimmunol. 2009;214:118–124. doi: 10.1016/j.jneuroim.2009.06.015. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Rapoport JL, Garvey MA, Perlmutter S, Swedo SE. MRI assessment of children with obsessive-compulsive disorder or tics associated with streptococcal infection. Am J Psychiatry. 2000;157:281–283. doi: 10.1176/appi.ajp.157.2.281. [DOI] [PubMed] [Google Scholar]
- Greer JM, Capecchi MR. Hoxb8 is required for normal grooming behavior in mice. Neuron. 2002;33:23–34. doi: 10.1016/s0896-6273(01)00564-5. [DOI] [PubMed] [Google Scholar]
- Hallett JJ, Harling-Berg CJ, Knopf PM, Stopa EG, Kiessling LS. Anti-striatal antibodies in Tourette syndrome cause neuronal dysfunction. J Neuroimmunol. 2000;111:195–202. doi: 10.1016/s0165-5728(00)00320-9. [DOI] [PubMed] [Google Scholar]
- Hesse S, Muller U, Lincke T, Barthel H, Villmann T, Angermeyer MC, Sabri O, Stengler-Wenzke K. Serotonin and dopamine transporter imaging in patients with obsessive-compulsive disorder. Psychiatry Res. 2005;140:63–72. doi: 10.1016/j.pscychresns.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Hesse S, Stengler K, Regenthal R, Patt M, Becker GA, Franke A, Knupfer H, Meyer PM, Luthardt J, Jahn I, Lobsien D, Heinke W, Brust P, Hegerl U, Sabri O. The serotonin transporter availability in untreated early-onset and late-onset patients with obsessive-compulsive disorder. Int J Neuropsychopharmacol. 2011;14:606–617. doi: 10.1017/S1461145710001604. [DOI] [PubMed] [Google Scholar]
- Hoffman KL, Hornig M, Yaddanapudi K, Jabado O, Lipkin WI. A murine model for neuropsychiatric disorders associated with group A beta-hemolytic streptococcal infection. J Neurosci. 2004;24:1780–1791. doi: 10.1523/JNEUROSCI.0887-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husby G, van de Rijn I, Zabriskie JB, Abdin ZH, Williams RC., Jr Antibodies reacting with cytoplasm of subthalamic and caudate nuclei neurons in chorea and acute rheumatic fever. J Exp Med. 1976;144:1094–1110. doi: 10.1084/jem.144.4.1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huyser C, Veltman DJ, de Haan E, Boer F. Paediatric obsessive-compulsive disorder, a neurodevelopmental disorder? Evidence from neuroimaging. Neurosci Biobehav Rev. 2009;33:818–830. doi: 10.1016/j.neubiorev.2009.01.003. [DOI] [PubMed] [Google Scholar]
- Jahanshahi A, Vlamings R, van Roon-Mom WM, Faull RL, Waldvogel HJ, Janssen ML, Yakkioui Y, Zeef DH, Kocabicak E, Steinbusch HW, Temel Y. Changes in brainstem serotonergic and dopaminergic cell populations in experimental and clinical Huntington’s disease. Neuroscience. 2013;238:71–81. doi: 10.1016/j.neuroscience.2013.01.071. [DOI] [PubMed] [Google Scholar]
- Kiessling LS, Marcotte AC, Culpepper L. Antineuronal antibodies in movement disorders. Pediatrics. 1993;92:39–43. [PubMed] [Google Scholar]
- Kingston D, Glynn LE. A cross-reaction between Str. pyogenes and human fibroblasts, endothelial cells and astrocytes. Immunology. 1971;21:1003–1016. [PMC free article] [PubMed] [Google Scholar]
- Kirvan CA, Swedo SE, Heuser JS, Cunningham MW. Mimicry and autoantibody-mediated neuronal cell signaling in Sydenham chorea. Nat Med. 2003;9:914–920. doi: 10.1038/nm892. [DOI] [PubMed] [Google Scholar]
- Kirvan CA, Swedo SE, Snider LA, Cunningham MW. Antibody-mediated neuronal cell signaling in behavior and movement disorders. J Neuroimmunol. 2006;179:173–179. doi: 10.1016/j.jneuroim.2006.06.017. [DOI] [PubMed] [Google Scholar]
- Kolb B, Holmes C. Neonatal motor cortex lesions in the rat: absence of sparing of motor behaviors and impaired spatial learning concurrent with abnormal cerebral morphogenesis. Behav Neurosci. 1983;97:697–709. doi: 10.1037//0735-7044.97.5.697. [DOI] [PubMed] [Google Scholar]
- Kotby AA, El Badawy N, El Sokkary S, Moawad H, El Shawarby M. Antineuronal antibodies in rheumatic chorea. Clin Diagn Lab Immunol. 1998;5:836–839. doi: 10.1128/cdli.5.6.836-839.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linthicum DS, Munoz JJ, Blaskett A. Acute experimental autoimmune encephalomyelitis in mice. I. Adjuvant action of Bordetella pertussis is due to vasoactive amine sensitization and increased vascular permeability of the central nervous system. Cell Immunol. 1982;73:299–310. doi: 10.1016/0008-8749(82)90457-9. [DOI] [PubMed] [Google Scholar]
- Loiselle CR, Lee O, Moran TH, Singer HS. Striatal microinfusion of Tourette syndrome and PANDAS sera: failure to induce behavioral changes. Mov Disord. 2004;19:390–396. doi: 10.1002/mds.10522. [DOI] [PubMed] [Google Scholar]
- Maes M, Ringel K, Kubera M, Anderson G, Morris G, Galecki P, Geffard M. In myalgic encephalomyelitis/chronic fatigue syndrome, increased autoimmune activity against 5-HT is associated with immuno-inflammatory pathways and bacterial translocation. J Affect Disord. 2013 doi: 10.1016/j.jad.2013.03.029. [DOI] [PubMed] [Google Scholar]
- Marques-Dias MJ, Mercadante MT, Tucker D, Lombroso P. Sydenham’s chorea. Psychiatr Clin North Am. 1997;20:809–820. doi: 10.1016/s0193-953x(05)70346-4. [DOI] [PubMed] [Google Scholar]
- Martino D, Chiarotti F, Buttiglione M, Cardona F, Creti R, Nardocci N, Orefici G, Veneselli E, Rizzo R. The relationship between group A streptococcal infections and Tourette syndrome: a study on a large service-based cohort. Dev Med Child Neurol. 2011;53:951–957. doi: 10.1111/j.1469-8749.2011.04018.x. [DOI] [PubMed] [Google Scholar]
- Michel PP, Toulorge D, Guerreiro S, Hirsch EC. Specific needs of dopamine neurons for stimulation in order to survive: implication for Parkinson disease. FASEB J. 2013 doi: 10.1096/fj.12-220418. [DOI] [PubMed] [Google Scholar]
- Moretti G, Pasquini M, Mandarelli G, Tarsitani L, Biondi M. What every psychiatrist should know about PANDAS: a review. Clin Pract Epidemiol Ment Health. 2008;4:13. doi: 10.1186/1745-0179-4-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy TK, Kurlan R, Leckman J. The immunobiology of Tourette’s disorder, pediatric autoimmune neuropsychiatric disorders associated with Streptococcus, and related disorders: a way forward. J Child Adolesc Psychopharmacol. 2010;20:317–331. doi: 10.1089/cap.2010.0043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolaus S, Antke C, Beu M, Muller HW. Cortical GABA, striatal dopamine and midbrain serotonin as the key players in compulsive and anxiety disorders--results from in vivo imaging studies. Rev Neurosci. 2010;21:119–139. doi: 10.1515/revneuro.2010.21.2.119. [DOI] [PubMed] [Google Scholar]
- Olver JS, O’Keefe G, Jones GR, Burrows GD, Tochon-Danguy HJ, Ackermann U, Scott A, Norman TR. Dopamine D1 receptor binding in the striatum of patients with obsessive-compulsive disorder. J Affect Disord. 2009;114:321–326. doi: 10.1016/j.jad.2008.06.020. [DOI] [PubMed] [Google Scholar]
- Pavone P, Bianchini R, Parano E, Incorpora G, Rizzo R, Mazzone L, Trifiletti RR. Anti-brain antibodies in PANDAS versus uncomplicated streptococcal infection. Pediatr Neurol. 2004;30:107–110. doi: 10.1016/S0887-8994(03)00413-2. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press; San Diego: 1998. [Google Scholar]
- Perani D, Garibotto V, Gorini A, Moresco RM, Henin M, Panzacchi A, Matarrese M, Carpinelli A, Bellodi L, Fazio F. In vivo PET study of 5HT(2A) serotonin and D(2) dopamine dysfunction in drug-naive obsessive-compulsive disorder. Neuroimage. 2008;42:306–314. doi: 10.1016/j.neuroimage.2008.04.233. [DOI] [PubMed] [Google Scholar]
- Perlmutter SJ, Leitman SF, Garvey MA, Hamburger S, Feldman E, Leonard HL, Swedo SE. Therapeutic plasma exchange and intravenous immunoglobulin for obsessive-compulsive disorder and tic disorders in childhood. Lancet. 1999;354:1153–1158. doi: 10.1016/S0140-6736(98)12297-3. [DOI] [PubMed] [Google Scholar]
- Peterson BS, Leckman JF, Tucker D, Scahill L, Staib L, Zhang H, King R, Cohen DJ, Gore JC, Lombroso P. Preliminary findings of antistreptococcal antibody titers and basal ganglia volumes in tic, obsessive-compulsive, and attention deficit/hyperactivity disorders. Arch Gen Psychiatry. 2000;57:364–372. doi: 10.1001/archpsyc.57.4.364. [DOI] [PubMed] [Google Scholar]
- Reeves S, Mehta M, Howard R, Grasby P, Brown R. The dopaminergic basis of cognitive and motor performance in Alzheimer’s disease. Neurobiol Dis. 2010;37:477–482. doi: 10.1016/j.nbd.2009.11.005. [DOI] [PubMed] [Google Scholar]
- Renart J, Reiser J, Stark GR. Transfer of Proteins from Gels to Diazobenzyloxymethyl-Paper and Detection with Antisera - Method for Studying Antibody Specificity and Antigen Structure. Proc Natl Acad Sci U S A. 1979;76:3116–3120. doi: 10.1073/pnas.76.7.3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds JA, Tanford C. Binding of Dodecyl Sulfate to Proteins at High Binding Ratios - Possible Implications for State of Proteins in Biological Membranes. Proc Natl Acad Sci U S A. 1970;66:1002. doi: 10.1073/pnas.66.3.1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schott K, Schaefer JE, Richartz E, Batra A, Eusterschulte B, Klein R, Berg PA, Bartels M, Mann K, Buchkremer G. Autoantibodies to serotonin in serum of patients with psychiatric disorders. Psychiatry Res. 2003;121:51–57. doi: 10.1016/s0165-1781(03)00137-9. [DOI] [PubMed] [Google Scholar]
- Singer HS, Giuliano JD, Hansen BH, Hallett JJ, Laurino JP, Benson M, Kiessling LS. Antibodies against human putamen in children with Tourette syndrome. Neurology. 1998;50:1618–1624. doi: 10.1212/wnl.50.6.1618. [DOI] [PubMed] [Google Scholar]
- Singer HS, Mink JW, Loiselle CR, Burke KA, Ruchkina I, Morshed S, Parveen S, Leckman JF, Hallett JJ, Lombroso PJ. Microinfusion of antineuronal antibodies into rodent striatum: failure to differentiate between elevated and low titers. J Neuroimmunol. 2005;163:8–14. doi: 10.1016/j.jneuroim.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Swedo SE, Grant PJ. Annotation: PANDAS: a model for human autoimmune disease. J Child Psychol Psychiatry. 2005;46:227–234. doi: 10.1111/j.1469-7610.2004.00386.x. [DOI] [PubMed] [Google Scholar]
- Swedo SE, Leonard HL, Garvey M, Mittleman B, Allen AJ, Perlmutter S, Lougee L, Dow S, Zamkoff J, Dubbert BK. Pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections: clinical description of the first 50 cases. Am J Psychiatry. 1998;155:264–271. doi: 10.1176/ajp.155.2.264. [DOI] [PubMed] [Google Scholar]
- Swedo SE, Leonard HL, Kiessling LS. Speculations on antineuronal antibody-mediated neuropsychiatric disorders of childhood. Pediatrics. 1994;93:323–326. [PubMed] [Google Scholar]
- Swedo SE, Leonard HL, Schapiro MB, Casey BJ, Mannheim GB, Lenane MC, Rettew DC. Sydenham’s chorea: physical and psychological symptoms of St Vitus dance. Pediatrics. 1993;91:706–713. [PubMed] [Google Scholar]
- Tanaka S, Matsunaga H, Kimura M, Tatsumi K, Hidaka Y, Takano T, Uema T, Takeda M, Amino N. Autoantibodies against four kinds of neurotransmitter receptors in psychiatric disorders. J Neuroimmunol. 2003;141:155–164. doi: 10.1016/s0165-5728(03)00252-2. [DOI] [PubMed] [Google Scholar]
- Taranta A, Stollerman GH. The relationship of Sydenham’s chorea to infection with group A streptococci. Am J Med. 1956;20:170–175. doi: 10.1016/0002-9343(56)90186-3. [DOI] [PubMed] [Google Scholar]
- Taylor JR, Morshed SA, Parveen S, Mercadante MT, Scahill L, Peterson BS, King RA, Leckman JF, Lombroso PJ. An animal model of Tourette’s syndrome. Am J Psychiatry. 2002;159:657–660. doi: 10.1176/appi.ajp.159.4.657. [DOI] [PubMed] [Google Scholar]
- Towbin H, Staehelin T, Gordon J. Electrophoretic Transfer of Proteins from Polyacrylamide Gels to Nitrocellulose Sheets - Procedure and Some Applications. Proc Natl Acad Sci U S A. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urakawa S, Hida H, Masuda T, Misumi S, Kim TS, Nishino H. Environmental enrichment brings a beneficial effect on beam walking and enhances the migration of doublecortin-positive cells following striatal lesions in rats. Neuroscience. 2007;144:920–933. doi: 10.1016/j.neuroscience.2006.10.038. [DOI] [PubMed] [Google Scholar]
- Yaddanapudi K, Hornig M, Serge R, De Miranda J, Baghban A, Villar G, Lipkin WI. Passive transfer of streptococcus-induced antibodies reproduces behavioral disturbances in a mouse model of pediatric autoimmune neuropsychiatric disorders associated with streptococcal infection. Mol Psychiatry. 2010;15:712–726. doi: 10.1038/mp.2009.77. [DOI] [PubMed] [Google Scholar]