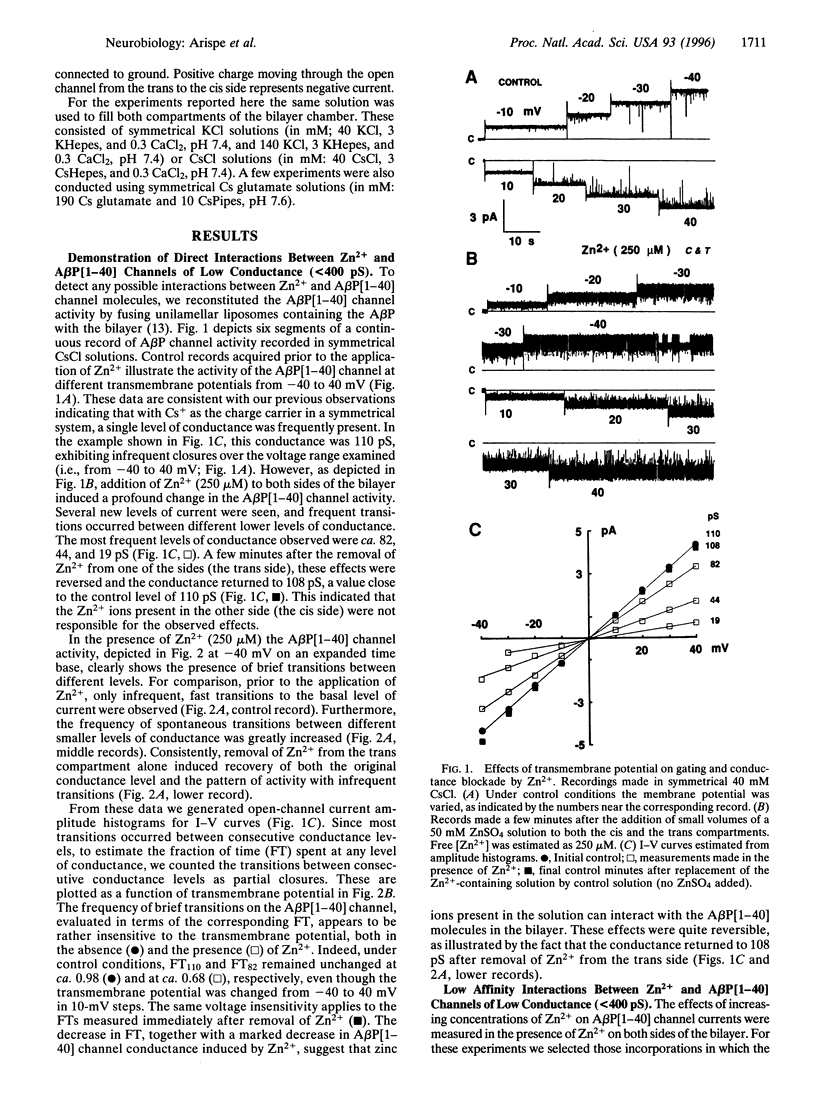
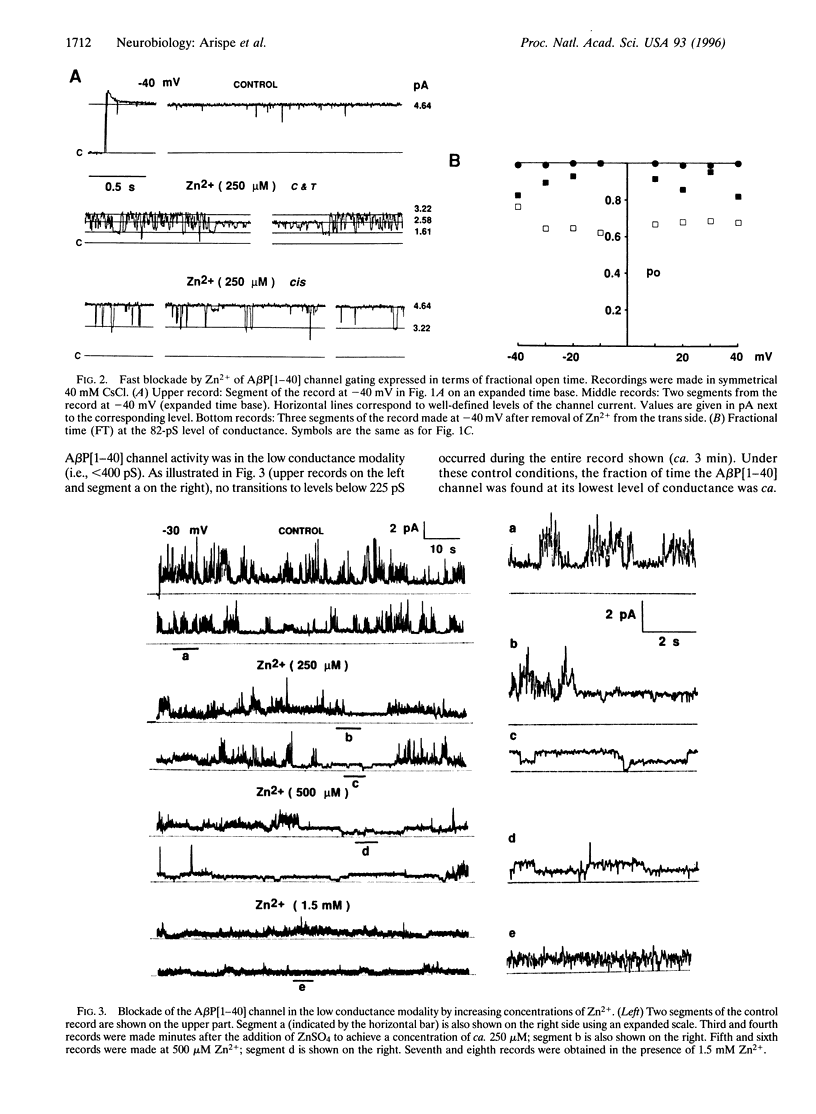
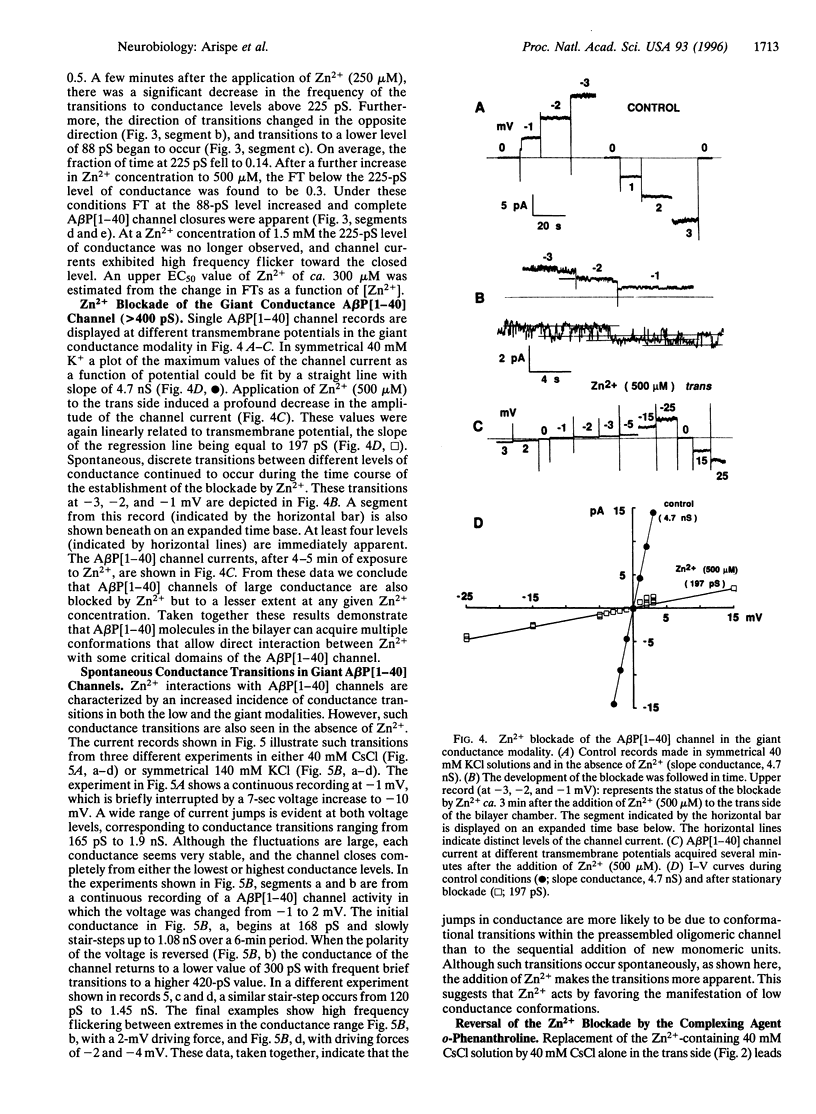
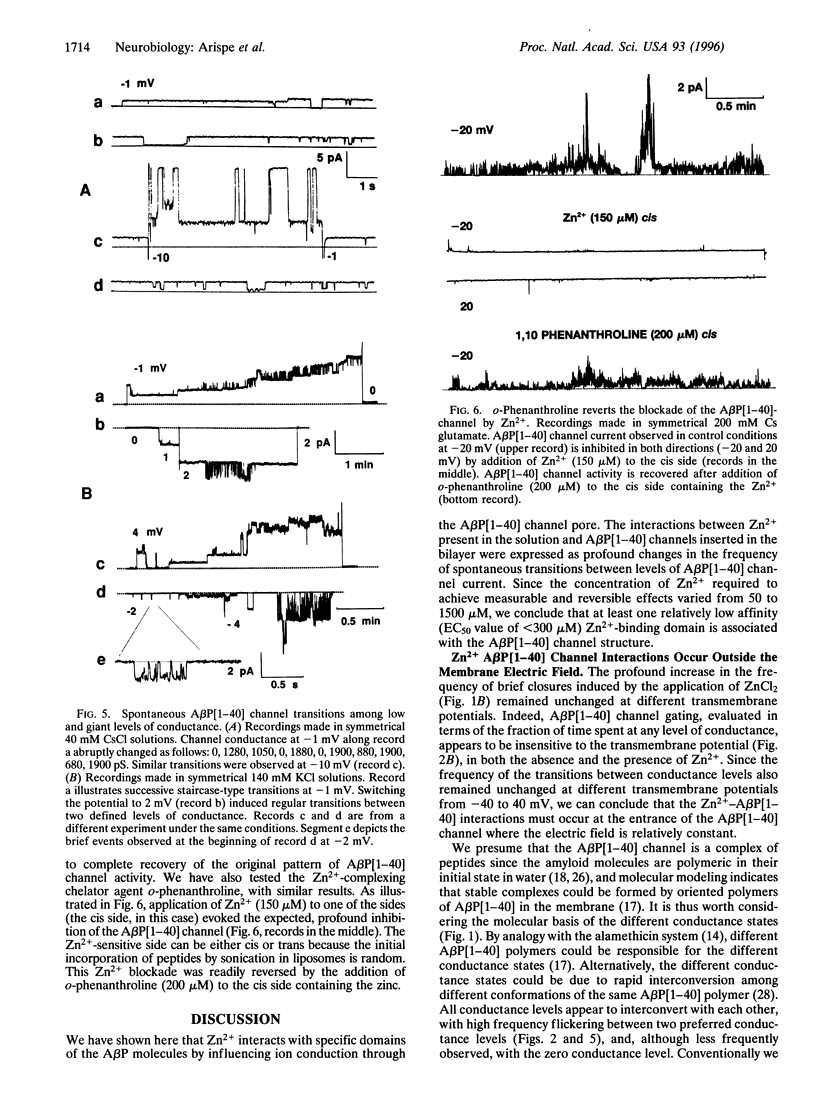
Abstract
The Alzheimer disease 40-residue amyloid beta protein (AbetaP[1-40]) forms cation-selective channels across acidic phospholipid bilayer membranes with spontaneous transitions over a wide range of conductances ranging from 40 to 4000 pS. Zn2+ has been reported to bind to AbetaP[1-40] with high affinity, and it has been implicated in the formation of amyloid plaques. We now report the functional consequences of such Zn2+ binding for the AbetaP[1-40] channel. Provided the AbetaP[1-40] channel is expressed in the low conductance (<400 pS) mode, Zn2+ blocks the open channel in a dose- dependent manner. For AbetaP[1-40] channels in the giant conductance mode (>400 pS), Zn2+ doses in the millimolar range were required to exert substantial blockade. The Zn2+ chelator o-phenanthroline reverses the blockade. We also found that Zn2+ modulates AbetaP[1-40] channel gating and conductance only from one side of the channel. These data are consistent with predictions of our recent molecular modeling studies on AbetaP[1-40] channels indicating asymmetric Zn(2+)-AbetaP[1-40] interactions at the entrance to the pore.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arispe N., Pollard H. B., Rojas E. Giant multilevel cation channels formed by Alzheimer disease amyloid beta-protein [A beta P-(1-40)] in bilayer membranes. Proc Natl Acad Sci U S A. 1993 Nov 15;90(22):10573–10577. doi: 10.1073/pnas.90.22.10573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arispe N., Pollard H. B., Rojas E. The ability of amyloid beta-protein [A beta P (1-40)] to form Ca2+ channels provides a mechanism for neuronal death in Alzheimer's disease. Ann N Y Acad Sci. 1994 Dec 15;747:256–266. doi: 10.1111/j.1749-6632.1994.tb44414.x. [DOI] [PubMed] [Google Scholar]
- Arispe N., Pollard H. B., Rojas E. beta-Amyloid Ca(2+)-channel hypothesis for neuronal death in Alzheimer disease. Mol Cell Biochem. 1994 Nov 23;140(2):119–125. doi: 10.1007/BF00926750. [DOI] [PubMed] [Google Scholar]
- Arispe N., Rojas E., Pollard H. B. Alzheimer disease amyloid beta protein forms calcium channels in bilayer membranes: blockade by tromethamine and aluminum. Proc Natl Acad Sci U S A. 1993 Jan 15;90(2):567–571. doi: 10.1073/pnas.90.2.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker A. B., Roth R. A. Identification of glutamate-169 as the third zinc-binding residue in proteinase III, a member of the family of insulin-degrading enzymes. Biochem J. 1993 May 15;292(Pt 1):137–142. doi: 10.1042/bj2920137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brzović P. S., Choi W. E., Borchardt D., Kaarsholm N. C., Dunn M. F. Structural asymmetry and half-site reactivity in the T to R allosteric transition of the insulin hexamer. Biochemistry. 1994 Nov 8;33(44):13057–13069. doi: 10.1021/bi00248a015. [DOI] [PubMed] [Google Scholar]
- Bush A. I., Multhaup G., Moir R. D., Williamson T. G., Small D. H., Rumble B., Pollwein P., Beyreuther K., Masters C. L. A novel zinc(II) binding site modulates the function of the beta A4 amyloid protein precursor of Alzheimer's disease. J Biol Chem. 1993 Aug 5;268(22):16109–16112. [PubMed] [Google Scholar]
- Bush A. I., Pettingell W. H., Jr, Paradis M. D., Tanzi R. E. Modulation of A beta adhesiveness and secretase site cleavage by zinc. J Biol Chem. 1994 Apr 22;269(16):12152–12158. [PubMed] [Google Scholar]
- Ciszak E., Smith G. D. Crystallographic evidence for dual coordination around zinc in the T3R3 human insulin hexamer. Biochemistry. 1994 Feb 15;33(6):1512–1517. doi: 10.1021/bi00172a030. [DOI] [PubMed] [Google Scholar]
- Durell S. R., Guy H. R., Arispe N., Rojas E., Pollard H. B. Theoretical models of the ion channel structure of amyloid beta-protein. Biophys J. 1994 Dec;67(6):2137–2145. doi: 10.1016/S0006-3495(94)80717-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederickson C. J. Neurobiology of zinc and zinc-containing neurons. Int Rev Neurobiol. 1989;31:145–238. doi: 10.1016/s0074-7742(08)60279-2. [DOI] [PubMed] [Google Scholar]
- Gehm B. D., Kuo W. L., Perlman R. K., Rosner M. R. Mutations in a zinc-binding domain of human insulin-degrading enzyme eliminate catalytic activity but not insulin binding. J Biol Chem. 1993 Apr 15;268(11):7943–7948. [PubMed] [Google Scholar]
- Goldgaber D., Lerman M. I., McBride O. W., Saffiotti U., Gajdusek D. C. Characterization and chromosomal localization of a cDNA encoding brain amyloid of Alzheimer's disease. Science. 1987 Feb 20;235(4791):877–880. doi: 10.1126/science.3810169. [DOI] [PubMed] [Google Scholar]
- Gross L., Dunn M. F. Spectroscopic evidence for an intermediate in the T6 to R6 allosteric transition of the Co(II)-substituted insulin hexamer. Biochemistry. 1992 Feb 11;31(5):1295–1301. doi: 10.1021/bi00120a004. [DOI] [PubMed] [Google Scholar]
- Hardy J. A., Higgins G. A. Alzheimer's disease: the amyloid cascade hypothesis. Science. 1992 Apr 10;256(5054):184–185. doi: 10.1126/science.1566067. [DOI] [PubMed] [Google Scholar]
- Hyman B. T., Van Hoesen G. W., Kromer L. J., Damasio A. R. Perforant pathway changes and the memory impairment of Alzheimer's disease. Ann Neurol. 1986 Oct;20(4):472–481. doi: 10.1002/ana.410200406. [DOI] [PubMed] [Google Scholar]
- Jacoby E., Krüger P., Karatas Y., Wollmer A. Distinction of structural reorganisation and ligand binding in the T<==>R transition of insulin on the basis of allosteric models. Biol Chem Hoppe Seyler. 1993 Sep;374(9):877–885. doi: 10.1515/bchm3.1993.374.7-12.877. [DOI] [PubMed] [Google Scholar]
- Joachim C. L., Duffy L. K., Morris J. H., Selkoe D. J. Protein chemical and immunocytochemical studies of meningovascular beta-amyloid protein in Alzheimer's disease and normal aging. Brain Res. 1988 Nov 22;474(1):100–111. doi: 10.1016/0006-8993(88)90673-7. [DOI] [PubMed] [Google Scholar]
- Kawahara M., Muramoto K., Kobayashi K., Mori H., Kuroda Y. Aluminum promotes the aggregation of Alzheimer's amyloid beta-protein in vitro. Biochem Biophys Res Commun. 1994 Jan 28;198(2):531–535. doi: 10.1006/bbrc.1994.1078. [DOI] [PubMed] [Google Scholar]
- Kowall N. W., McKee A. C., Yankner B. A., Beal M. F. In vivo neurotoxicity of beta-amyloid [beta(1-40)] and the beta(25-35) fragment. Neurobiol Aging. 1992 Sep-Oct;13(5):537–542. doi: 10.1016/0197-4580(92)90053-z. [DOI] [PubMed] [Google Scholar]
- Kuroda Y., Kawahara M. Aggregation of amyloid beta-protein and its neurotoxicity: enhancement by aluminum and other metals. Tohoku J Exp Med. 1994 Nov;174(3):263–268. doi: 10.1620/tjem.174.263. [DOI] [PubMed] [Google Scholar]
- Malouf A. T. Effect of beta amyloid peptides on neurons in hippocampal slice cultures. Neurobiol Aging. 1992 Sep-Oct;13(5):543–551. doi: 10.1016/0197-4580(92)90054-2. [DOI] [PubMed] [Google Scholar]
- Masters C. L., Simms G., Weinman N. A., Multhaup G., McDonald B. L., Beyreuther K. Amyloid plaque core protein in Alzheimer disease and Down syndrome. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4245–4249. doi: 10.1073/pnas.82.12.4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLachlan D. R., Fraser P. E., Dalton A. J. Aluminium and the pathogenesis of Alzheimer's disease: a summary of evidence. Ciba Found Symp. 1992;169:87–108. doi: 10.1002/9780470514306.ch6. [DOI] [PubMed] [Google Scholar]
- Neve R. L., Dawes L. R., Yankner B. A., Benowitz L. I., Rodriguez W., Higgins G. A. Genetics and biology of the Alzheimer amyloid precursor. Prog Brain Res. 1990;86:257–267. doi: 10.1016/s0079-6123(08)63182-9. [DOI] [PubMed] [Google Scholar]
- Perlman R. K., Rosner M. R. Identification of zinc ligands of the insulin-degrading enzyme. J Biol Chem. 1994 Dec 30;269(52):33140–33145. [PubMed] [Google Scholar]
- Pollard J. R., Arispe N., Rojas E., Pollard H. B. A geometric sequence that accurately describes allowed multiple conductance levels of ion channels: the "three-halves (3/2) rule". Biophys J. 1994 Aug;67(2):647–655. doi: 10.1016/S0006-3495(94)80525-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth M., Tomlinson B. E., Blessed G. Correlation between scores for dementia and counts of 'senile plaques' in cerebral grey matter of elderly subjects. Nature. 1966 Jan 1;209(5018):109–110. doi: 10.1038/209109a0. [DOI] [PubMed] [Google Scholar]
- Selkoe D. J. The molecular pathology of Alzheimer's disease. Neuron. 1991 Apr;6(4):487–498. doi: 10.1016/0896-6273(91)90052-2. [DOI] [PubMed] [Google Scholar]
- Tanzi R. E., Gusella J. F., Watkins P. C., Bruns G. A., St George-Hyslop P., Van Keuren M. L., Patterson D., Pagan S., Kurnit D. M., Neve R. L. Amyloid beta protein gene: cDNA, mRNA distribution, and genetic linkage near the Alzheimer locus. Science. 1987 Feb 20;235(4791):880–884. doi: 10.1126/science.2949367. [DOI] [PubMed] [Google Scholar]
- Yankner B. A. Commentary and perspective on studies of beta amyloid neurotoxicity. Neurobiol Aging. 1992 Sep-Oct;13(5):615–616. doi: 10.1016/0197-4580(92)90067-8. [DOI] [PubMed] [Google Scholar]
- Yankner B. A., Duffy L. K., Kirschner D. A. Neurotrophic and neurotoxic effects of amyloid beta protein: reversal by tachykinin neuropeptides. Science. 1990 Oct 12;250(4978):279–282. doi: 10.1126/science.2218531. [DOI] [PubMed] [Google Scholar]



