Abstract
Progesterone action contributes to the signaling of many growth factor pathways relevant to breast cancer tumor biology, including the insulin-like growth factor (IGF) system. Previous work has shown that insulin receptor substrate-2 (IRS-2) but not IRS-1 levels were regulated by progestin in progesterone receptor-B (PR-B) isoform expressing MCF-7 cells (C4-12 PR-B). Furthermore, type 1 IGF receptor (IGF1R) signaling via IRS-2 correlated with the increased cell migration observed in a number of breast cancer cell lines. Consequently, in this study, we examined whether the elevation of IRS-2 protein induced by progestin was sufficient to promote IGF-I–stimulated cell motility. Treatment of C4-12 PR-B cells with progestin shifted the balance of phosphorylation from IRS-1 to IRS-2 in response to IGF-I. This shift in IRS-2 activation was associated with enhanced migration in C4-12 PR-B cells pretreated with progestin, but had no effect on cell proliferation or survival. Treatment of C4-12 PR-B cells with RU486, an antiprogestin, inhibited IGF-induced cell migration. Attenuation of IRS-2 expression using small interfering RNA resulted in decreased IGF-stimulated motility. In addition, IRS-2 knockdown resulted in an abrogation of PKB/Akt phosphorylation but not mitogen-activated protein kinase. Consequently, LY294002, a phosphoinositide-3-kinase inhibitor, abolished IGF-induced cell motility in progestin-treated C4-12 PR-B cells. These data show a role for the PR in functionally promoting growth factor signaling, showing that levels of IRS proteins can determine IGF-mediated biology, PR-B signaling regulates IRS-2 expression, and that IRS-2 can mediate IGF-induced cell migration via phosphoinositide-3-kinase in breast cancer cells.
Introduction
Approximately 70% to 80% of primary breast cancers are estrogen receptor (ER) and/or progesterone receptor (PR)–positive and the majority of these tumors are hormone responsive (1). The hormone dependence of ER-positive tumors is more often seen when both ER and PR are expressed. Clinical trial data underscore progesterone’s role in breast cancer; patients who took hormonal replacement therapy (HRT) preparations containing estrogen plus progesterone were at an increased risk of developing later stage breast cancer compared with patients treated with placebo or estrogen alone (2). Indeed, the hormonal regulation of breast cancer is critical to cancer progression and patient survival.
In normal breast tissue, hormones act in concert with growth factors to maintain the milieu of this organ system. In addition to the role of steroids and growth factors in normal mammary gland biology, it is clear that alterations in the function of steroid receptors and/or growth factor pathways both contribute to tumorigenesis and cancer progression. In addition, steroid pathways encourage growth factor signaling to promote tumor biology by directly modulating the expression of ligands, receptors, and signaling intermediates (3). Although a substantial effort has been placed on understanding ER’s role in promoting growth factor signaling and function, there has been less effort focused on understanding PR’s role in this context.
The insulin-like growth factor (IGF) system is critical in the function of normal and tumorigenic tissues. IGF has been shown to promote and maintain cancer progression (reviewed in ref. 4). In addition, IGF is mitogenic and promotes cancer metastasis (5, 6). Activation of the type 1 IGF receptor (IGF1R) results in the phosphorylation of adaptor proteins, IRS-1 and IRS-2, and the activation of pathways such as phosphoinositide-3-kinase (PI3K) and mitogen-activated protein kinase (MAPK). In breast cancer, the IRS proteins are the primary mediators of the IGF1R signal, making them a critical regulatory step in the IGF signaling cascade (7). Because different phenotypes are stimulated by the IGF1R in breast cancer cells, a differential role for IRSs has been suggested (8). Specific knockdown of IRS-2 in the mammary gland of polyoma virus middle-T– infected female mice displayed significantly impaired tumor metastasis (9). In contrast, specific knockdown of IRS-1 in the mammary gland of polyoma virus middle-T–infected animals elevated IRS-2 function and promoted tumor metastasis. However, the overexpression of mouse mammary tumor virus IRS-1 or IRS-2 in the mammary gland resulted in tumorigenesis and mammary tumor metastases in both sets of animals (10). Although such differences in IRS function may exist in vivo, it is difficult to determine if these phenotypes are regulated by IGF1R activation or if other pathways are affected by the genetic manipulation of IRS expression in these mouse models. Cell line models indicate that IRS-1 and IRS-2 could mediate specific IGF-induced biology in breast cancer. We and others have found that cell lines expressing and signaling via IRS-1 proliferate in response to IGF-I, whereas cell lines expressing and signaling via IRS-2 migrate and invade in response to the same stimulus (8, 9, 11).
The presence of PR in growth factor–responsive breast cancer tissues and cell lines suggests a role for progesterone in functionally influencing growth factor pathways. In some breast cancers, growth factor pathways depend on hormonal influences to contribute to the cancer phenotype. The loss of ER in MCF-7 cells disrupts functional IGF signaling (12). In fact, it has been suggested that PR functions to prime cells for secondary factors such as growth factor signals (13). In response to progesterone, PR expressing cells up-regulate up to 94 genes, some of which are involved in cell adhesion, signal transduction, metabolism, cell cycling, and apoptosis (14). Two isoforms, PR-A and PR-B, are coexpressed in breast cancer tissues but can regulate different gene sets when expressed independently, with PR-B exhibiting enhanced recruitment of transcriptional coactivators (14). Gene regulation by the two PR isoforms is mediated by activation function (AF) domains with AF-3 in the B-upstream segment of PR-B dictating differential transcriptional regulation (15). Indeed, gene regulation by progesterone involves the expression of proteins important in signaling mediated by multiple pathways, including the IGF pathway because IRS-2 is a PR-B–regulated gene (16). To examine PR-B effects on the IGF system, we wanted to use a model in which a nonfunctional IGF system existed. To do this, MCF-7 cells were selected for a loss of ER and a concurrent loss of the PR (C4-12), and then PR-B was stably overexpressed (C4-12 PR-B). This model is ideal for evaluating the direct effects of PR-B on the IGF system because ER effects on the system are absent; C4-12 cells express IGF1R and IRS-1 in a non–PR-regulated manner, the cells treated with progestin have increased IRS-2 levels, and exhibit an enhancement in IGF signaling via PI3K and MAPK after progestin pretreatment (17). Therefore, we propose that PR function can regulate IGF action in breast cancer cells and alter the biological outcome mediated by the IGF pathway.
To investigate how the IGF system can be regulated in breast cancer, the effects of progesterone on altering IGF signaling and biology were examined. In this study, IGF-I treatment of progestin-pretreated C4-12 PR-B cells resulted in enhanced cell motility, but no changes in cell proliferation or survival were observed. The relative level of IRS-1 to IRS-2 was a determining factor in which species was activated and whether a migratory behavior was observed. Finally, IRS-2–associated PI3K signaling mediated IGF action. Therefore, our data show that the PR and IGF1R signaling cooperate to enhance breast cancer cell motility. Regulation of IRS-2 by PR-B links these two pathways.
Results
Progestin Alters IGF Signaling by Promoting IRS-2 Phosphorylation and Abrogating IRS-1 Activation
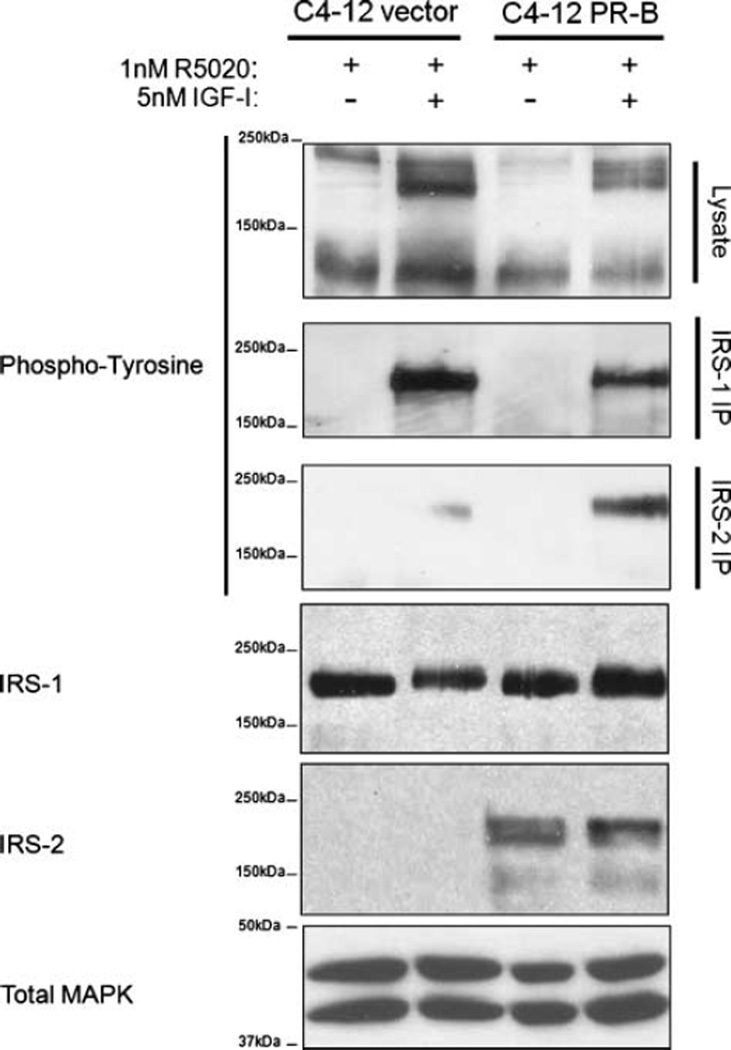
Progestin increases IRS-2 levels but does not alter the levels of either IRS-1 or the IGF1R (17). However, changes in total IRS-1 or IRS-2 are not indicative of adapter protein function; IRS activation must be directly assessed by examining tyrosine phosphorylation of the different IRS species (18, 19). Therefore, to address the function of IRS-1 and IRS-2 in breast cancer cells, C4-12 PR-B cells were pretreated with1 nmol/L of progestin for 24 hours followed by 5 nmol/L of IGF-I for 10 minutes and examined for tyrosine phosphorylation of IRS-1 and IRS-2 proteins by immunoprecipitation (Fig. 1). Progestin elevated IRS-2 protein, but not IRS-1. Activation of IGF1R by IGF-I resulted in the phosphorylation of both IRS-1 and IRS-2 in C4-12 cells. Although tyrosine phosphorylation of IRS-1 was seen in both vector-transfected and PR-B–transfected cells, IRS-2 phosphorylation was detected only in PR-B cells treated with R5020. In addition, these cells had a reduction in IRS-1 phosphorylation compared with the vector cells. Therefore, progestin can shift the relative balance of IRS protein activation in response to IGF from IRS-1 to IRS-2 in PR-B–expressing breast cancer cells. In the absence of progestin, IRS-2 phosphorylation remained at vector control levels (data not shown).
FIGURE 1.
Progestin alters IGF signaling by promoting IRS-2 phosphorylation and abrogating IRS-1 phosphorylation. Cells were serum-starved for 24 h, pretreated with 1 nmol/L of R5020 for another 24 h, and treated with 5 nmol/L of IGF-I for 10 min. Lysates were immunoprecipitated using antibodies specific for IRS-1 or IRS-2. Tyrosine phosphorylation of the IRS proteins was examined by immunoblot. Total IRS-1 and IRS-2 were examined by immunoblot. Total MAPK was used as a loading control.
Alteration of IRS-2 Protein Levels by Progesterone Conferred Biological Function to IGF-I
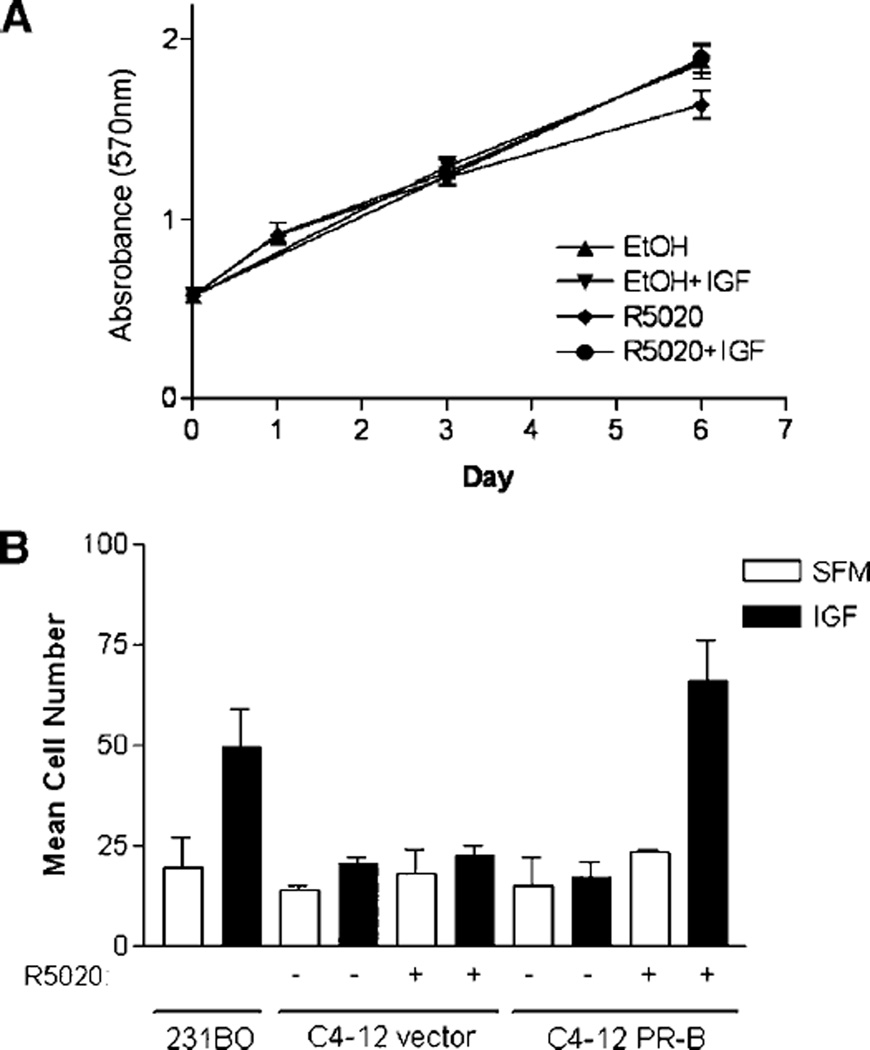
We had previously shown that IRS-2 is critical in the regulation of cell motility, but plays little role in IGF-mediated proliferation (11). We hypothesized that by elevating IRS-2 levels, C4-12 cells would respond to IGF-I by exhibiting a migratory phenotype only, and not a survival or proliferative phenotype.
To examine cell proliferation, C4-12 cells were pretreated with progestin for 24 hours followed by IGF-I and cell number was estimated over a period of 6 days using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay. Figure 2A illustrates that IGF-I and R5020 did not affect monolayer growth of C4-12 PR-B cells. Furthermore, IGF-I did not alter C4-12 vector cell growth (data not shown; ref. 12). These data show that neither progestin nor IGF-I stimulated cell proliferation of C4-12 cells. In addition, progestin’s effects on IGF-induced survival from chemotherapy were examined. Under these conditions, IGF-I treatment resulted in a slight reduction in PARP cleavage in both the C4-12 vector and PR-B–transfected cells, and was not significantly altered by progestin pretreatment (data not shown).
FIGURE 2.
A. Elevation of IRS-2 protein levels by progesterone does not promote IGF-I – induced cell proliferation. Cells were serum-starved for 24 h, pretreated with 1 nmol/L of progestin for another 24 h, and treated with 5 nmol/L of IGF-I. Changes in cell proliferation were examined using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay at days 0, 1, 3, and 6 following treatment with 5 nmol/L of IGF-I. B. PR-B promotes IGF-I – induced cell motility. Cells were serum-starved for 24 h and then pretreated with 1 nmol/L of progestin for another 24 h. Cell motility was examined using a modified Boyden chamber in SFM containing 2 µg/mL of fibronectin with 5 nmol/L of IGF-I for 6 h.
To address whether the elevation in IRS-2 levels altered IGF-induced cell motility, C4-12 cells were pretreated with progestin overnight and then placed in a modified Boyden chamber in the presence of 5 nmol/L of IGF-I for 6 hours (Fig. 2B). In this experiment, we used the migratory MDA-231BO cell line as a positive control for IGF-I–stimulated motility (11). The C4-12 vector cells were unresponsive to IGF-I stimulation in this assay. Furthermore, progestin alone did not enhance the migration of either the C4-12 vector or PR-B cells. However, C4-12 PR-B cells pretreated with progestin responded to IGF-I with increased migration, whereas IGF-I had no effect in R5020-treated vector cells. The Boyden chamber assay is dependent on cell adhesion. We showed that IGF-I and R5020 did not differentially affect cell adhesion in these cells (data not shown). However, to further confirm that these effects were due to the ability of IGF-I and R5020 to stimulate cell motility, we further confirmed these findings in a wound healing response of adherent monolayer cells. Results were consistent with data shown in Fig. 3 (data not shown). Therefore, ligand-dependent functions of PR-B enhanced IGF-I biology by promoting IGF-induced cell migration.
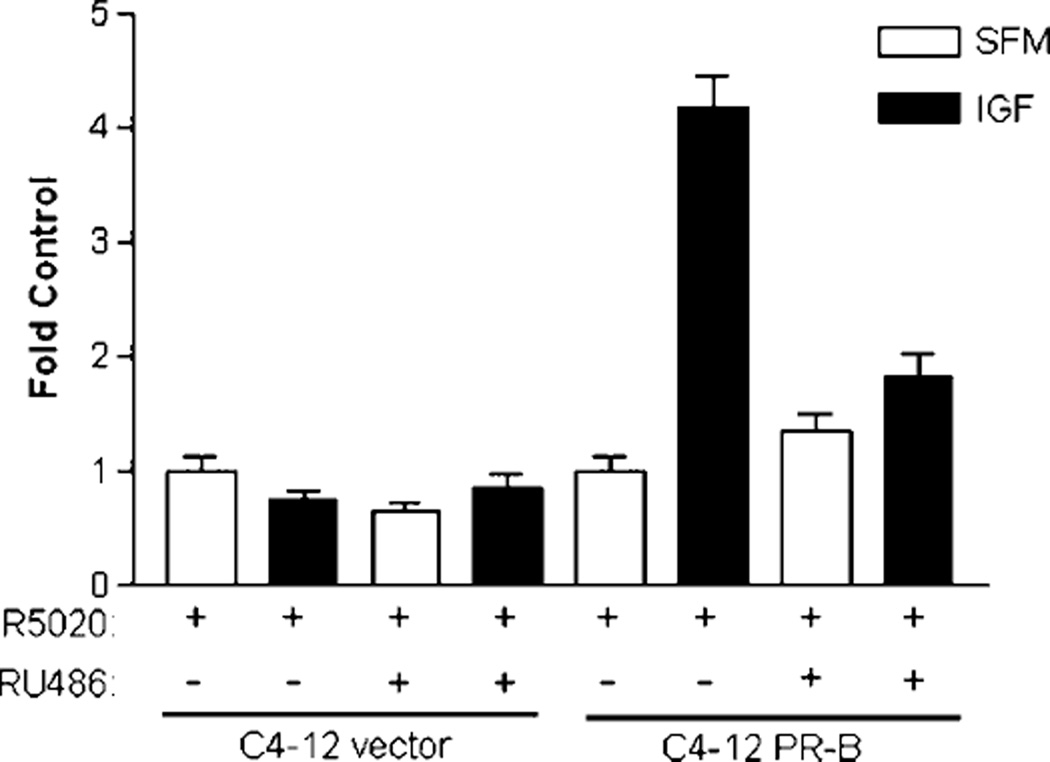
FIGURE 3.
PR-B function is required for progestin to promote IGF-induced cell migration. Cells were serum-starved for 24 h, pretreated with or without 1 µmol/L of RU486 in the presence of 1 nmol/L of R5020 for another 24 h, and treated with 5 nmol/L of IGF-I. Cell migration was examined on preadherent (4 µg/mL fibronectin) cells in a chambered glass-slide using time-lapse microscopy every 20 min over 12 h. The total distance migrated by single cells over the 12-h period was quantified and normalized to SFM control. Representative experiment is of n = 40–66 cells for each set.
Progesterone Receptor Function is Required for Progestin to Promote IGF-Induced Cell Migration
Because progestin was pivotal in promoting the IGF-induced cell migration of C4-12 PR-B cells, we decided to evaluate whether this effect was dependent on PR-B function. C4-12 cell migration was examined after treatment with the antiprogestin, RU486. To distinguish between IGF effects on adhesion versus migration on fibronectin, we examined time-lapse images of adherent cells pretreated with progestin/antiprogestin. It has been reported that integrin receptors are PR-regulated genes, and the IGF system has been linked to regulating migration specifically via the fibronectin binding receptor, α5β1 integrin, in breast cancer cells (14, 19). Neither integrin expression nor function were altered by progestin in C4-12 cells, however, IGF promoted adhesion to fibronectin (data not shown). Therefore, following adherence of untreated C4-12 cells to fibronectin, cells were pretreated with1 nmol/L of R5020 in the presence or absence of 1 µmol/L of the antiprogestin RU486 overnight, and then stimulated with 5 nmol/L of IGF-I. Neither progestin, antiprogestin, nor IGF-I altered the adherence of preadherent cells as observed by time-lapse imaging (data not shown). As observed previously, C4-12 vector cells were unresponsive to any of the treatments (Fig. 3). C4-12 PR-B cells had increased migration in the presence of R5020 and IGF-I. This effect was inhibited by RU486, confirming PR’s role in functionally altering IGF biology. Therefore, antiprogestin can block the effects of progestin on IGF-induced cell motility.
Progestin Promoted IGF-Induced Migration by Increasing IRS-2 Levels and Enhancing IGF-Induced PI3K Function
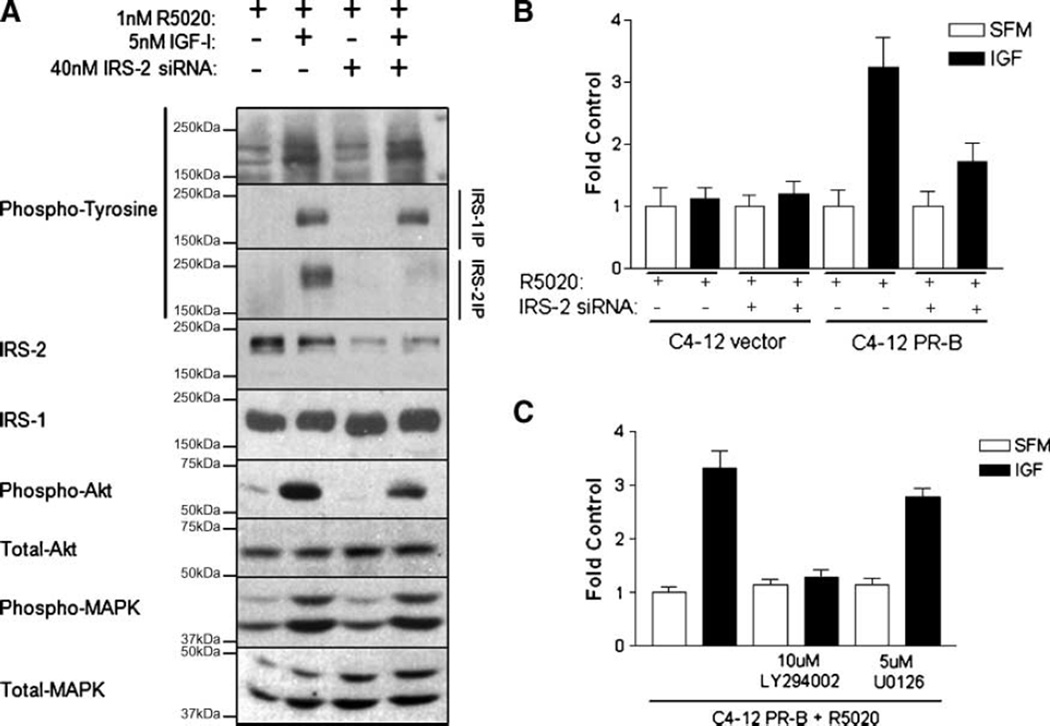
Elevated IRS-2 levels induced by progestin in C4-12 PR-B cells were associated with enhanced IGF-induced cell migration. To rule out the possibility that progestin enhanced IGF-induced cell migration via other pathways not dependent on IRS-2 expression, an IRS-2–specific knockdown approach was employed. Cells were grown to 70% confluence and serum-starved for 24 hours prior to transient transfection with small interfering RNA (siRNA) specifically targeting the IRS-2 sequence for an additional 24 hours in serum-free medium (SFM). Progestin was added posttransfection for another overnight period, at which time cells were treated with 5 nmol/L of IGF-I for 10 minutes and lysates were collected. As previously reported, IGF treatment of C4-12 PR-B cells activated the IGF pathway (Fig. 4A). Transient transfection with IRS-2 siRNA specifically reduced IRS-2 protein levels, but not IRS-1. A parallel reduction in IRS-2 phosphorylation was also observed, but IRS-1 phosphorylation was not changed by IRS-2 siRNA. Interestingly, IRS-2 siRNA-treated cells exhibited a reduction in PKB/Akt activation in response to IGF-I as compared with mock-transfected controls, whereas MAPK activation was unaltered. Therefore, IGF-mediated signaling via IRS-2 is preferentially linked to PI3K signaling in C4-12 PR-B cells.
FIGURE 4.
A. IRS-2 knockdown disrupts PI3K signaling. Cells were serum-starved for 24 h, transfected with 40 nmol/L of IRS-2 siRNA overnight, pretreated with 1 nmol/L of progestin for another 24 h, and treated with 5 nmol/L of IGF-I. Cell signaling was examined by immunoprecipitation of IRS-1 and IRS-2, followed by immunoblotting for phosphotyrosine, IRS-1, IRS-2, phospho-Akt, total Akt, phospho-MAPK, and total MAPK. B. Progestin-mediated enhancement of IGF-induced cell migration was dependent on IRS-2. Cells were serum-starved for 24 h, transfected with 40 nmol/L of IRS-2 siRNA overnight, pretreated with 1 nmol/L of progestin for another 24 h. Cell motility was examined using a modified Boyden chamber in SFM containing 2 µg/mL of fibronectin with 5 nmol/L of IGF-I for 6 h. C. PI3K mediates IGF-induced cell motility of progestin-treated cells. Cells were serum-starved for 24 h, pretreated with 1 nmol/L of progestin for another 24 h, and then placed in suspension. Cells were pretreated with 10 µmol/L of LY294002 or 5 µmol/L of U0126 for 30 min in suspension. Cell motility was examined using a modified Boyden chamber in SFM containing 2 µg/mL of fibronectin with 5 nmol/L of IGF-I for 6 h.
To test whether IRS-2 function was required for IGF-induced cell motility, cells were transiently transfected with IRS-2 siRNA as described above. Following progestin pretreatment, cells were detached and examined for migration using a modified Boyden chamber assay (Fig. 4B). Similar to the data in Fig. 2B, IGF did not stimulate the migration of progestin-pretreated C4-12 vector cells. Furthermore, IRS-2 siRNA did not alter the biological response of the C4-12 vector cells. C4-12 PR-B cells treated with progestin responded to IGF-I with increased migration, whereas pretreatment of these cells with IRS-2 siRNA abrogated this response. In contrast, increasing concentrations of IRS-1 siRNA in non–progestin-treated C4-12 cells resulted in a migratory response to IGF-I, however, the magnitude of this effect was less than that achieved by IGF-I in non-siRNA, progestin-treated C4-12 PR-B cells (data not shown). IRS-1 siRNA did not alter IRS-2 protein levels in the C4-12 vector cells (data not shown). Therefore, the enhanced migration induced by progestin and IGF-I was dependent on IRS-2 expression.
Because IRS-2 knockdown abrogated the IGF-induced phosphorylation of PKB/Akt and not MAPK, the role of PI3K in progestin-promoted IGF-induced motility was examined. Following progestin pretreatment, cells were detached and pretreated with PI3K inhibitor (10 µmol/L LY294002) or MAPK inhibitor (5 µmol/L U0126) for 30 minutes, and then placed in a modified Boyden chamber in the presence of 5 nmol/L of IGF-I (Fig. 4C). As observed previously, progestin-pretreated cells migrated in response to IGF-I. Inhibition of MAPK did not alter IGF-I–induced motility of C4-12 PR-B cells. In contrast, inhibition of PI3K abrogated IGF-induced motility to basal levels. LY294002-mediated disruption of IGF-induced motility was not a result of changes in IRS protein expression or tyrosine phosphorylation (data not shown). Thus, PI3K functions mediate IGF-induced motility of progestin-treated cells.
Discussion
A number of studies have established IRS proteins as the primary mediators of IGF signaling in breast cancer. Here, we show how PR can modulate IGF responsiveness by altering IRS adapter protein expression. Because IGF signaling is mediated through both IRS-1 and IRS-2, and IGF action leads to multiple biological phenotypes, it has been hypothesized that IRS-1 and IRS-2 have distinct roles in IGF action. Our data show that the progestin-induced expression of IRS-2 led to enhanced IGF signaling and an increase in cell migration. To show that IRS proteins could in fact regulate such distinct IGF-induced phenotypes, IRS-1 and IRS-2 were overexpressed in T47D-YA breast cancer cells which lack endogenous IRS-1 or IRS-2 expression (8). In this model, IRS-1 coupled IGF signaling to cell proliferation and colony formation, whereas IRS-2 coupled IGF signaling to cell migration.
The role of IRS proteins in IGF-induced biology is also supported by studies in animal models of breast cancer. Dearth et al. used a mouse mammary tumor virus targeting model to overexpress IRS-1 or IRS-2 in the mammary gland of female mice to show that IRS proteins could promote tumor growth and metastasis (10). Although both animal groups developed tumors and metastases, the kinetics of these studies are suggestive of differences in biological function of IRS-1 and IRS-2. Nagle et al. also showed the importance of IRS-2 in mediating tumor metastasis of mouse mammary tumor virus/polyoma virus middle-T–induced tumors. In these studies, IRS-2–null animals displayed significantly impaired tumor metastasis to the lungs, whereas primary tumor growth was unchanged (9). Furthermore, tumors from both wild-type and IRS-2–null animals displayed similar mitotic indices, suggesting that IRS-2 does not play a role in tumor growth. In a similar manner, IRS-2 overexpression in C4-12 PR-B cells did not enhance cell proliferation in the presence or absence of IGF-I. In contrast, IGF-induced cell growth of these cells is regulated by IRS-1 and requires ER (12). Because Nagle et al.’s studies involved tumor induction by polyoma virus middle-T, future studies using this model will help decipher IRS-2’s contribution to the later stages of cancer progression that lead to tumor metastasis. Taken together, these studies examine the function of IRS proteins in animal models and support a role for both IRS-1 and IRS-2 in breast cancer progression.
Because IRS-1 and IRS-2 are often both expressed in breast cancer, there must be a mechanism for regulating which IRS protein is mediating IGF action. To address this issue, some studies have suggested that the ratio of IRS proteins can dictate IGF-induced biology (8, 9). Ablation of IRS-1 in animals infected with the mouse mammary tumor virus/polyoma virus middle-T vector did not alter tumor onset but resulted in an enhanced metastatic potential (8). These IRS-1–null tumors also exhibited an elevation in IRS-2 signaling, demonstrating that IRS-2 is important in promoting the metastatic phenotype. In addition, we have also observed that the loss of IRS-1 in vivo by targeted knockdown results in enhanced IRS-2 phosphorylation in the mammary gland after mice are given a bolus of insulin (20). Our studies also show that IGF-I predominantly stimulated IRS-2 signaling in progestin-pretreated C4-12 PR-B cells, whereas IRS-1 signaling was reduced. These data show that the available IRS pool can determine downstream signaling from the IGF1R, and potentially dictate IGF-mediated cancer phenotypes.
Phosphorylation of both IRS-1 and IRS-2 resulted in the activation of the PI3K and MAPK pathways. However, the specificity of downstream signaling may be dependent on distinct signaling pathways. Byron et al. showed that MAPK signaling by IRS-1 is crucial for IGF-I’s mitogenic effect in T47D-YA breast cancer cells (8). The absence of a proliferative response to IGF-I in C4-12 cells is due to a dependence of the IGF system on ER expression, despite the activation of PI3K and MAPK pathways by IRS-1 in C4-12 cells (21). In a similar manner, IRS-1 expression in ER-negative MDA-MB-468, MDA-MB-435A, and MDA-231BO cells does not link IGF1R signaling to a mitogenic response (6, 22). These cells are unable to proliferate in response to IGF-I, yet have IGF-stimulated motility. Furthermore, it seems that multiple signaling pathways are involved in IRS-2–mediated cell migration (23).
In support of this, IRS-2 signals via the PI3K pathway to mediate cell migration in both the C4-12 PR-B and T47D-YA IRS-2 cells. Additionally, we observed that LY294002 treatment did not reduce IRS-1 or IRS-2 protein expression in the C4-12 cells (data not shown). If anything, pretreatment with the PI3K inhibitor maintained a slightly elevated level of IRS protein, indicating that PI3K effects on migration were not at the level of IRS-2 expression but downstream of IRS-2. However, both PI3K and MAPK can mediate the mitogenic effects of IGF-I in ER-positive breast cancer cells (24). Although the above studies indicate that distinct functions by IRS-1 and IRS-2 seem to be elucidated, the mechanisms by which IRS’s link the same pathways to different biology are still being examined. At the biochemical level, it will be important to elucidate which downstream pathways are preferentially linked to specific phenotypes stimulated by the IGF system, despite the activation of multiple pathways by both IRS proteins. Furthermore, an understanding of how IRS-1 or IRS-2 limit the function of signaling pathways that are capable of promoting multiple phenotypes to only promoting distinct phenotypes will need to be addressed. Potential molecular regulation may be at the level of differential IRS serine/threonine and tyrosine phosphorylation and subsequent recruitment of different positive and negative regulators, as well as distinct compartmental cross-talk with biologically relevant pathways (25, 26). In the case of IRS-1, compartmental crosstalk with ER is suggested, whereas IRS-2 may be linked to integrins at the plasma membrane (27, 28).
In this study, we sought to evaluate how the PR could functionally influence the IGF system. Several reports have shown ER’s role on the IGF system, however, none have shown a role for the PR in promoting IGF-mediated biology (3, 21). We have previously shown that progestin treatment of C4-12 PR-B cells could elevate IRS-2 levels, and promote IGF signaling (17). Because C4-12 cells do not have an IGF-driven phenotype, the C4-12 PR-B cell line is an ideal model to study the role of the PR-B, independent of the ER, on the IGF system. These cells also allow us to examine the effect of overexpressing IRS-2 to levels that would be similar to progesterone-responsive breast cancers. Indeed, progestin promoted IGF-I responsiveness through the PR. In addition, progestin did not alter integrin expression or function in the context of fibronectin, indicating that its effects on IGF action solely promoted cell migration.
The regulation of adapter protein expression to alter growth factor signaling is not unique to progesterone and IRS-2; estrogen also does this. Estrogen regulates IRS-1 levels, promoting IGF-proliferation (21). Not only is estrogen synergistic with IGF-I on cell proliferation, but ER is required for IGF1R and IRS-1 to stimulate proliferation (12). Therefore, PR function is linked to IRS levels, similar to the link between ER and IRS proteins. In this study, we show that PR elevates IRS-2 levels to alter IGF signaling from IRS-1 to IRS-2 and promote cell migration.
This work has several important implications about the therapeutic targeting of breast cancer. First, the IGF system is a bona fide breast cancer target (29). It is clear that with the development of new targeted therapies, patient selection will be critical for the success of such agents (30). Indeed, our studies have shown that the IGF system is not exclusively linked to mitogenic signaling by IRS-1, but is also linked to migratory signaling by IRS-2. Because response to cancer therapy is typically measured by primary tumor response, clinical response to therapies that target the IGF system in breast cancers signaling via IRS-2 would not be easily detected in clinical trials designed to measure response rates. Second, the PR can functionally promote the IGF system, leading cells towards an aggressive phenotype. PR action promotes the function of several growth factor pathways (13, 31). Our work suggests that PR function in breast cancer is needed for functional IGF1R signaling, similar to the role ER plays. However, whereas ER’s relationship with the IGF system is to promote cell proliferation, our data show that PR’s relationship with the IGF system is to promote cell migration. Therefore, PR and IRS-2 expression might identify tumors with high metastatic potential. Together, this work implies that IGF-driven breast cancers can exhibit a malignant phenotype, and that the selection of appropriate patient cohorts according to ER, PR, IRS-1, and IRS-2 expression should dictate anti-IGF study design. By providing a better understanding of how PR can influence IGF action, these pathways may offer more opportunities for therapeutic targeting of aggressive breast cancers.
Materials and Methods
Cells and Reagents
Generation of C4-12 vector and C4-12 PR-B cells was previously described (12, 17). These cells were routinely maintained in αMEM without phenol red (Invitrogen) + 5% charcoal/dextran-treated fetal bovine serum (Hyclone) + 2 mmol/L of glutamine + 50 IU/mL penicillin, 50 mg/mL of streptomycin. MDA-231BO is a bone-seeking metastatic variant of MDA-231, which was provided by Toshiyuki Yoneda (University of Texas Health Science Center, San Antonio, TX; ref. 32). These cells were maintained in DMEM with 10% FCS. The culture medium and fibronectin were purchased from Invitrogen Corporation. IGF-I was purchased from Gro Pep. Cells were serum-starved (SFM) in phenol red–free IMEM (improved MEM Zinc Option, supplemented with20 mmol/L HEPES, 1× trace elements, 2 µg/mL transferrin, and for IGF-I stimulation, 2 µg/mL fibronectin). All cells were grown at 37°C in a humidified atmosphere containing 5% CO2. Boyden chamber apparatus was purchased from NeuroProbe, Inc. HEMA3 (Fisher). Himatsu camera, Leica microscope, SimplePCI software were purchased from North Central Instruments, Inc. Glass-chambered slides were purchased from NUNC. Protein A agarose was purchased from Santa Cruz Biotechnology, Inc. Horseradish peroxidase–conjugated PY-20 antiphosphotyrosine antibody was purchased from Transduction Labs. Antibodies used for immunoprecipitation of IRS-1 or IRS-2 were produced by Alpha Diagnostics, and were protein A agarose–purified as described previously (7). IRS-2 antibody was purchased from Santa Cruz Biotechnology. Total ERK1 and ERK2 MAPK, phosphorylated ERK1 and ERK2 MAPK (Thr202/Tyr204), total Akt, and phosphorylated Akt (Ser473) antibodies were purchased from Cell Signaling Technology. Horseradishpe roxidase–linked anti-rabbit and horseradish peroxidase–linked FITC-linked anti-rabbit and anti-mouse antibodies were purchased from Amersham. IRS-1 and IRS-2 siRNA were synthesized by Dharmacon and used as described previously (8). Effectene transfection reagent was purchased from Qiagen. SuperSignal West Pico substrate and bicinchoninic acid kit were purchased form Pierce. LY294002 and U0126 were purchased from Calbiochem. Doxorubicin (Adriamycin) was purchased from BMS. RU486 was a kind gift from Carol A. Lange (University of Minnesota, Minneapolis, MN). All other chemicals were purchased from Sigma.
Lysate Preparation
Cells were grown to 70% confluency, incubated in SFM overnight, pretreated with 1 nmol/L of R5020 or an ethanol vector control for 24 h, then treated with 5 nmol/L of IGF-I for 10 min at 37°C. siRNA-treated samples were transiently transfected 24 hafter serum starvation, before progestin treatment. The IRS-1 and IRS-2 siRNA transfection was done for a 24-h period using Effectene transfection reagent, and medium was unchanged for progestin treatment. Cells were washed twice with ice-cold PBS on ice and lysed with 500 µL of TNESV lysis buffer [50 nmol/L Tris-Cl (pH 7.4), 1% NP40, 2 mmol/L EDTA (pH 8.0), 100 mmol/L NaCl, 10 mmol/L sodium orthovanadate, 1 mmol/L phenylmethylsulfonyl fluoride, 20 µg/mL leupeptin, and 20 µg/mL aprotinin]. Lysates were cleared by centrifugation at 12,000 × g at 4°C for 25 min. Protein concentrations were determined using the bicinchoninic acid kit.
Immunoblot
Total cell lysates (40 µg) in 1× Laemmli sample buffer were resolved on an 8% SDS-PAGE gel and transferred to a nitrocellulose membrane. Membranes were blocked with 5% nonfat dry milk in TBST (0.05% Tween in TBS w/v) for 1 hat room temperature. Primary antibodies were used according to the manufacturer’s direction, followed by six washes with TBST at 5-min intervals. Secondary antibodies were used according to the manufacturer’s direction. Membranes were washed six times for 5 min each and chemiluminesence was detected.
Immunoprecipitation
Total cellular lysates (1,000 µg) were precleared with50 µL of protein A agarose for 30 min at 4°C. Lysates were incubated overnight with IRS-1 or IRS-2 antibody at 4°C followed by incubation with protein A agarose for 4 h at 4°C. Samples were washed thrice with TNESV. Laemmli sample buffer (1×) was added to an immunoprecipitated sample and run on an 8% SDS-PAGE gel.
Cell Proliferation Assay
Cells were plated in 24-well plates with10,000 cells/well in charcoal-stripped serum-containing medium. Cells were switched to SFM for 24 h and then treated with 1 nmol/L of R5020 for another 24 h. Cells were then treated with or without 5 nmol/L of IGF-I for 6 days. All treatments were done in triplicate. Growth was measured 1 to 6 days after treatment. Growth was assayed by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay as described previously (33). Sixty microliters of 5 mg/mL 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide solution in SFM was added to each well. After incubation for 4 hat 37°C, wells were aspirated and formazan crystals were lysed with 500 µL of solubilization solution (95% DMSO + 5% IMEM). Absorbance was measured with a plate reader at 570 nm using a 650 nm differential filter.
Boyden Chamber Cell Motility Assay
Cell motility was measured by a modified Boyden chamber assay as described previously (11). Cells were serum-starved for 24 h, then pretreated with 1 nmol/L of R5020 for another 24-h period. Cells were briefly detached by trypsin-EDTA, washed twice with SFM, then resuspended in SFM. Cells (150,000) were placed in the upper chamber of a 10-well Boyden chamber apparatus. Upper and lower chambers were separated by a polycarbonate polyvinylpyrrolidone–free filter with12-µm pores. SFM (0.4 mL) with or without IGF (5 nmol/L) was placed in the bottom wells of the chamber. After 6 h of incubation at 37°C in a humidified atmosphere containing 5% CO2, cells remaining on the topside of the filter were removed with cotton swabs. The filter was then removed from the chamber and the cells that had migrated to the underside of the filter were fixed and stained in HEMA3. The filter was then mounted onto a glass microscope slide and cells were counted (in duplicate) in five different areas using a light microscope. For siRNA experiments, cells were serum-starved overnight, transiently transfected for 24 h, then pretreated with 1 nmol/L of R5020 for another 24 h.
Adhesion Assay
Integrin function was assessed using an adhesion assay to fibronectin as described previously (28). Briefly, 24-well plates were precoated with 8 µg/mL of fibronectin overnight. Serum-starved cells pretreated with1 nmol/L of progestin (24 h) were placed in suspension for 30 min (kept in suspension by vortexing every 5 min), and then treated with 5 nmol/L of IGF-I for 10 min. Cells were then plated on fibronectin for 5, 10, 20, 25, or 30 min. Nonadherent cells were washed off and the remaining adherent cells stained and quantified.
Time-lapse Microscopy of Single Cell Migration
Transfection or progestin/antiprogestin treatments were conducted as explained in Results. Cells were plated at a density of 1 × 105 cells/mL (375 µL) on precoated (2 µg/mL fibronectin) chamber slides for 2 h, then serum-starved overnight. Cells were placed in a humidified CO2 chamber designed for microscope imaging and images were taken using a Himatsu camera attached to an inverted Leica microscope at 20× bright-field every 10 min for 24 h (150 frames/field; five fields in duplicate). Distance clearance was analyzed using SimplePCI software. Images were analyzed manually for the migration of single cells coinciding with20-min intervals for a total of 12 h.
Apoptosis
Cells were grown to 70% confluence, incubated in SFM overnight, and then either untreated (SFM) or treated with 5 nmol/L of IGF-I for 24 h. Cells were then treated with 1,500 ng/mL of doxorubicin with or without IGF-I for 48 h. Cell lysates were collected and analyzed using Western immunoblot methods for PARP and MAPK.
Spot Densitometry
Integrated densitometry values of immunoblot bands were generated using Fluorochem V.3.04SA by Alpha Innotech Corporation.
Statistical Analysis
Two-way ANOVA with Bonferroni post-test and Student’s t test were done using GraphPad Prism version 3.02 for Windows (GraphPad Software). Error bars represent the SE. All experiments were repeated three to five times.
Acknowledgments
We thank Drs. Carol Lange and Deepali Sachdev for critical review of this manuscript.
Grant support: National Cancer Institute Cancer Biology Training grant NIH T32 CA09138 (Y. Ibrahim), NIH R01 CA074285 (D. Yee), and NIH P30 CA077598.
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Keen JC, Davidson NE. The biology of breast carcinoma. Cancer. 2003;97:825–833. doi: 10.1002/cncr.11126. [DOI] [PubMed] [Google Scholar]
- 2.Chlebowski RT, Hendrix SL, Langer RD, et al. Influence of estrogen plus progestin on breast cancer and mammography in healthy postmenopausal women: the Women’s Health Initiative Randomized Trial. JAMA. 2003;289:3243–3253. doi: 10.1001/jama.289.24.3243. [DOI] [PubMed] [Google Scholar]
- 3.Dupont J, Le Roith D. Insulin-like growth factor 1 and oestradiol promote cell proliferation of MCF-7 breast cancer cells: new insights into their synergistic effects. Mol Pathol. 2001;54:149–154. doi: 10.1136/mp.54.3.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yu H, Rohan T. Role of the insulin-like growth factor family in cancer development and progression. J Natl Cancer Inst. 2000;92:1472–1489. doi: 10.1093/jnci/92.18.1472. [DOI] [PubMed] [Google Scholar]
- 5.Wu Y, Yakar S, Zhao L, et al. Circulating insulin-like growth factor-I levels regulate colon cancer growth and metastasis. Cancer Res. 2002;62:1030–1035. [PubMed] [Google Scholar]
- 6.Sachdev D, Hartell JS, Lee AV, et al. A dominant negative type I insulin-like growth factor receptor inhibits metastasis of human cancer cells. J Biol Chem. 2004;279:5017–5024. doi: 10.1074/jbc.M305403200. [DOI] [PubMed] [Google Scholar]
- 7.Jackson JG, White MF, Yee D. Insulin receptor substrate-1 is the predominant signaling molecule activated by insulin-like growth factor-I, insulin, and interleukin-4 in estrogen receptor-positive human breast cancer cells. J Biol Chem. 1998;273:9994–10003. doi: 10.1074/jbc.273.16.9994. [DOI] [PubMed] [Google Scholar]
- 8.Byron SA, Horwitz KB, Richer JK, et al. Insulin receptor substrates mediate distinct biological responses to insulin-like growth factor receptor activation in breast cancer cells. Br J Cancer. 2006;95:1220–1228. doi: 10.1038/sj.bjc.6603354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nagle JA, Ma Z, Byrne MA, et al. Involvement of insulin receptor substrate 2 in mammary tumor metastasis. Mol Cell Biol. 2004;24:9726–9735. doi: 10.1128/MCB.24.22.9726-9735.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dearth RK, Cui X, Kim HJ, et al. Mammary tumorigenesis and metastasis caused by overexpression of insulin receptor substrate 1 (IRS-1) or IRS-2. Mol Cell Biol. 2006;26:9302–9314. doi: 10.1128/MCB.00260-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jackson JG, Zhang X, Yoneda T, et al. Regulation of breast cancer cell motility by insulin receptor substrate-2 (IRS-2) in metastatic variants of human breast cancer cell lines. Oncogene. 2001;20:7318–7325. doi: 10.1038/sj.onc.1204920. [DOI] [PubMed] [Google Scholar]
- 12.Oesterreich S, Zhang P, Guler RL, et al. Re-expression of estrogen receptor α in estrogen receptor α-negative MCF-7 cells restores both estrogen and insulin-like growth factor-mediated signaling and growth. Cancer Res. 2001;61:5771–5777. [PubMed] [Google Scholar]
- 13.Lange CA, Richer JK, Horwitz KB. Hypothesis: progesterone primes breast cancer cells for cross-talk with proliferative or antiproliferative signals. Mol Endocrinol. 1999;13:829–836. doi: 10.1210/mend.13.6.0290. [DOI] [PubMed] [Google Scholar]
- 14.Richer JK, Jacobsen BM, Manning NG, et al. Differential gene regulation by the two progesterone receptor isoforms in human breast cancer cells. J Biol Chem. 2002;277:5209–5218. doi: 10.1074/jbc.M110090200. [DOI] [PubMed] [Google Scholar]
- 15.Tung L, Abdel-Hafiz H, Shen T, et al. Progesterone receptors (PR)-B and -A regulate transcription by different mechanisms: AF-3 exerts regulatory control over coactivator binding to PR-B. Mol Endocrinol. 2006;20:2656–2670. doi: 10.1210/me.2006-0105. [DOI] [PubMed] [Google Scholar]
- 16.Vassen L, Wegrzyn W, Klein-Hitpass L. Human insulin receptor substrate-2 (IRS-2) is a primary progesterone response gene. Mol Endocrinol. 1999;13:485–494. doi: 10.1210/mend.13.3.0256. [DOI] [PubMed] [Google Scholar]
- 17.Cui X, Lazard Z, Zhang P, et al. Progesterone crosstalks with insulin-like growth factor signaling in breast cancer cells via induction of insulin receptor substrate-2. Oncogene. 2003;22:6937–6941. doi: 10.1038/sj.onc.1206803. [DOI] [PubMed] [Google Scholar]
- 18.Zhang X, Kamaraju S, Hakuno F, et al. Motility response to insulin-like growth factor-I (IGF-I) in MCF-7 cells is associated with IRS-2 activation and integrin expression. Breast Cancer Res Treat. 2004;83:161–170. doi: 10.1023/b:brea.0000010709.31256.c6. [DOI] [PubMed] [Google Scholar]
- 19.Hoang CD, Zhang X, Scott PD, et al. Selective activation of insulin receptor substrate-1 and -2 in pleural mesothelioma cells: association with distinct malignant phenotypes. Cancer Res. 2004;64:7479–7485. doi: 10.1158/0008-5472.CAN-04-1898. [DOI] [PubMed] [Google Scholar]
- 20.Hadsell DL, Olea W, Lawrence N, et al. Decreased lactation capacity and altered milk composition in insulin receptor substrate null mice is associated with decreased maternal body mass and reduced insulin-dependent phosphorylation of mammary Akt. J Endocrinol. 2007;194:327–336. doi: 10.1677/JOE-07-0160. [DOI] [PubMed] [Google Scholar]
- 21.Lee AV, James GJ, Jennifer LG, et al. Enhancement of the insulin-like growth factor pathway by estrogen in human breast cancer cells. Mol Endocrinol. 1999;13:787–796. doi: 10.1210/mend.13.5.0274. [DOI] [PubMed] [Google Scholar]
- 22.Jackson JG, Yee D. IRS-1 expression and activation are not sufficient to activate downstream pathways and enable IGF-I growth response in estrogen receptor negative breast cancer cells. Growth Horm IGF Res. 1999;9:280–289. doi: 10.1054/ghir.1999.0113. [DOI] [PubMed] [Google Scholar]
- 23.Goel HL, Breen M, Zhang J, et al. β1A integrin expression is required for type 1 insulin-like growth factor receptor mitogenic and transforming activities and localization to focal contacts. Cancer Res. 2005;65:6692–6700. doi: 10.1158/0008-5472.CAN-04-4315. [DOI] [PubMed] [Google Scholar]
- 24.Bartucci M, Morelli C, Mauro L, et al. Differential insulin-like growth factor I receptor signaling and function in estrogen receptor (ER)-positive MCF-7 and ER-negative MDA-MB-231 breast cancer cells. Cancer Res. 2001;61:6747–6754. [PubMed] [Google Scholar]
- 25.Greene MW, Garofalo RS. Positive and negative regulatory role of insulin receptor substrate 1 and 2 (IRS-1 and IRS-2) serine/threonine phosphorylation. Biochemistry. 2002;41:7082–7091. doi: 10.1021/bi015992f. [DOI] [PubMed] [Google Scholar]
- 26.Sun XJ, Pons S, Wang LM, et al. The IRS-2 gene on murine chromosome 8 encodes a unique signaling adapter for insulin and cytokine action. Mol Endocrinol. 1997;11:251–262. doi: 10.1210/mend.11.2.9885. [DOI] [PubMed] [Google Scholar]
- 27.Morelli C, Garofalo C, Sisci D, et al. Nuclear insulin receptor substrate 1 interacts with estrogen receptor α at ERE promoters. Oncogene. 2004;23:7517–7526. doi: 10.1038/sj.onc.1208014. [DOI] [PubMed] [Google Scholar]
- 28.Zhang X, Lin M, van Golen KL, et al. Multiple signaling pathways are activated during insulin-like growth factor-I (IGF-I) stimulated breast cancer cell migration. Breast Cancer Res Treat. 2005;93:159–168. doi: 10.1007/s10549-005-4626-8. [DOI] [PubMed] [Google Scholar]
- 29.Sachdev D, Yee D. Disrupting insulin-like growth factor signaling as a potential cancer therapy. Mol Cancer Ther. 2007;6:1–12. doi: 10.1158/1535-7163.MCT-06-0080. [DOI] [PubMed] [Google Scholar]
- 30.Pegram MD, et al. Targeted therapy: wave of the future. J Clin Oncol. 2005;23:1776–1781. doi: 10.1200/JCO.2005.11.029. [DOI] [PubMed] [Google Scholar]
- 31.Faivre EJ, Lange CA. Progesterone receptors upregulate Wnt-1 to induce epidermal growth factor receptor transactivation and c-Src-dependent sustained activation of Erk1/2 mitogen-activated protein kinase in breast cancer cells. Mol Cell Biol. 2007;27:466–480. doi: 10.1128/MCB.01539-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yoneda T, Williams PJ, Hiraga T, et al. A bone-seeking clone exhibits different biological properties from the MDA-MB-231 parental human breast cancer cells and a brain-seeking clone in vivo and in vitro. J Bone Miner Res. 2001;16:1486–1495. doi: 10.1359/jbmr.2001.16.8.1486. [DOI] [PubMed] [Google Scholar]
- 33.Twentyman PR, Luscombe M. A study of some variables in a tetrazolium dye (MTT) based assay for cell growth and chemosensitivity. Br J Cancer. 1987;56:279–285. doi: 10.1038/bjc.1987.190. [DOI] [PMC free article] [PubMed] [Google Scholar]