Abstract
Background
Ventricular assist devices (VADs) are increasingly common and their surgical implantation predisposes patients to an increased risk of acute kidney injury (AKI). We sought to evaluate the incidence, risk factors, short and long term all cause mortality of patients with AKI following VAD implantation.
Methods
We identified all patients who underwent VAD implantation at the University of Chicago from January 1, 2008 to January 31, 2012. We evaluated the incidence of AKI, defined as a ≥50% increase in serum creatinine over the first 7 post-operative days (RIFLE Risk-Creatinine). A logistic regression model was used to identify risk factors for development of AKI and a cox proportional hazards model was used to examine factors associated with 30-day and 365-day all cause mortality.
Results
157 eligible patients had VAD implantations with 44 (28%) developing post-implantation AKI. In a multivariate analysis only diabetes mellitus [OR=2.25 (1.03-4.94), P=0.04] was identified as a significant predictor of postoperative AKI. Using a multivariable model censored for heart transplantation, only AKI [HR= 3.01(1.15-7.92), P=0.03] and cardiopulmonary bypass time [HR =1.01(1.001-1.02), P= 0.02] were independent predictors of 30-day mortality. Pre-operative Body Mass Index [HR=0.95(0.90-0.99), P=0.03], pre-operative diabetes mellitus [HR=1.89 (1.07-3.35), P=0.03] and post-implantation AKI [HR=1.85 (1.06-3.21); P=0.03] independently predicted 365-day mortality.
Conclusion
AKI is common following VAD implantation and is an independent predictor of 30-day and 1-year all cause mortality.
Keywords: Acute Kidney Injury, Cardiac Surgery, Mortality, Ventricular Assist Device, RIFLE
Introduction
Acute Kidney Injury (AKI) is common following traditional cardiac surgery [coronary artery bypass grafting (CABG) and/ or valve replacement] and is an independent risk factor for post-operative mortality [1,2]. Additionally, AKI has been associated with higher patient morbidity, longer length of stay, higher costs [1,3-5]. Multiple pre-operative risk stratification systems have been developed to predict the development of AKI following traditional cardiac surgery, however their role and application in the setting of ventricular assist device (VAD) implantation is unclear [6-9].
Since the Randomized Evaluation of Mechanical Assistance for the treatment of congestive heart failure (REMATCH) trial [10] there has been an increased number of VADs being implanted, with nearly 6000 VADs being implanted since June 2006 [11]. The first generation VADs were pulsatile and presumably more “physiologic”, however they were associated with higher infection and thrombosis rates, lower durability and even lower cardiac transplant rates in comparison to continuous flow devices [12]. This has lead to a transition away from pulsatile flow devices to continuous flow devices which now make up for > 90% implants in the United States [13]. While the increasing numbers of implants , have improved our understanding of the impact of AKI during the peri-operative period; its etiology, predictors and short and long term consequences require further investigation. Previous studies have been difficult to interpret, as many have not used consensus definitions of AKI [14-19]. Utilizing consensus definitions of AKI, we performed a single-center, retrospective, longitudinal cohort study to evaluate the incidence of AKI following VAD implantation and to identify risk factors as well as assessing its impact on 30 and 365-day survival.
Methods
The Institutional Review Board of The University of Chicago approved this study. We performed a single center, retrospective chart review, of all patients who underwent a ventricular assist device placement at our institution from January 1, 2008 through January 31, 2012. Patients with End Stage Renal Disease (ESRD), those who were already on renal replacement therapy (RRT) for AKI prior to VAD implantation and those who died intra-operatively were excluded.
Observations and Measurements
Demographic, biochemical and clinical profiles were obtained from the INTERMACS national registry , Society of Thoracic Surgeons (STS) database and the University of Chicago electronic medical record (EMR) [20]. We collected preoperative data on age, race, sex, body mass index (BMI), baseline renal function (estimated glomerular filtration rate (eGFR) using MDRD) [21], past medical history, hemodynamic numbers; intraoperative data including cardiopulmonary bypass time and receipt of blood products, post-operative data including length of stay, post-operative complications (e.g. sepsis, thromboembolic events), need for RRT and in-hospital mortality. Baseline creatinine was defined as the listing creatinine data obtained from the INTERMACS registry, if INTERMACS did not contain a preoperative serum creatinine then the last serum creatinine in the EMR prior to device implantation was used . We collected information about the indication for VAD implantation [Bridge to Transplant (BTT) or Destination Therapy (DT)] as well as the type of device implanted.
Outcomes
The primary outcome of AKI was defined as a 50% rise in serum creatinine over the pre-operative baseline during the first 7 postoperative days as per the creatinine based RIFLE “Risk” Criteria [22]. The Acute Kidney Injury Network (AKIN) creatinine criteria [23,24] and urine output criteria were utilized for secondary analyses. All cause mortality was monitored over 1-year post implantation, with data reported at 30 and 365 days.
Statistical Methods
Qualitative data was recorded in a categorical fashion and quantitative covariates were measured as continuous variables. Differences between characteristics were analyzed either by an unpaired t-test or the Wilcoxon signed rank test as appropriate. Categorical variables were assessed by chi square analysis or the Fischer's exact test. Categorical variables were reported with their corresponding odds ratio (OR), hazards ratio (HR) and 95 percent confidence intervals. Continuous variables are represented with mean and their 95% CI values and hazards ratio (HR) when pertaining to survival data.
To assess determinants of AKI we first performed a univariate logistic regression analysis. Significant predictors (p<0.10) were then included in a multivariate model.
Survival data was analyzed using cox proportional hazards modeling. All survival data was censored for cardiac transplantation. We analyzed survival at 30 and 365 days. Significant variables (p<0.10) were then included in a multivariate model. All statistical tests were 2- sided with the alpha set at 0.05 for statistical significance. STATA 11.2 (StataCorp LP, College Station TX) was used for all data analysis.
Results
Baseline Characteristics of those with acute kidney Injury
We identified 168 patients who underwent VAD implantation at our institution during the study period. Eleven subjects were excluded from the analysis with 8 patients having previous ESRD, 2 requiring pre-implant RRT for AKI and 1 subject with intra-operative mortality. In the final cohort of 157 a total of 44 (28%) patients developed AKI. Table 1a and 1b demonstrates the pre-, intra- and post-operative characteristics of those with and without AKI. Prior to implantation, there was no difference in age, baseline renal function (serum creatinine and eGFR), NYHA CHF class, preoperative hemodynamics, INTERMACS score, the presence of cardiogenic shock or inotrope use in those with and without AKI. However those with AKI tended to have more diabetes and cerebrovascular disease (CVD) and had higher pre-operative hematocrits (Table 1a). Intra-operatively there was no difference in cardiopulmonary bypass time, need for packed red blood cell (PRBC) transfusion or units of PRBC transfused between the two groups. Post-operatively there was no difference in complications including bleeding, cardiac tamponade, prolonged ventilation, postoperative sepsis, ischemic strokes (symptoms lasting greater than 24 hours) or pneumonia, however those with AKI had longer ICU stays and a higher in-hospital mortality (Table 1b). Both groups had a net negative fluid balance by day 7. However patients with AKI had a significantly less net negative fluid balance by day 7 in comparison to the no AKI group (p=0.02).
Table 1A. Baseline Clinical Characteristics of VAD Patients by AKI Status.
| No AKI (N=113) |
AKI (N=44) |
P value | |
|---|---|---|---|
|
| |||
| Operative Age (years) | 58.3±12.7 | 56.4±12.7 | 0.4 |
|
| |||
| Weight (kg) | 89.2±23.3 | 84.3±25.3 | 0.25 |
|
| |||
| Race N (%) | |||
| Caucasian | 68(60.2) | 23(52.3) | 0.39 |
| African American | 36(31.9) | 18(40.9) | |
| Asian | 4(3.5) | 0 (0) | |
| Unknown | 5(4.4) | 3(6.8) | |
|
| |||
| Males: N (%) | 89(78.8) | 34(77.3) | 0.84 |
|
| |||
| Body mass index (kg/m2) | 28.9±6.3 | 28.4±7.3 | 0.62 |
|
| |||
| Baseline Serum Creatinine (mg/dl) | 1.67± 1.01 | 1.58±0.71 | 0.57 |
|
| |||
| eGFR MDRD (ml/min) | 49.3±26.7 | 50.4±22.1 | 0.82 |
|
| |||
| Pre-operative Hematocrit | 34.2±6.2 | 36.5±5.3 | 0.05 |
|
| |||
| HbA1c | 6.9±1.2 | 6.9±1.2 | 0.84 |
|
| |||
| Diabetes N(%) | 35(31.0) | 23(52.3) | 0.01 |
|
| |||
| Peripheral Vascular Disease N(%) | 7(6.2) | 3(6.8) | 0.85 |
|
| |||
| Hypertension N(%) | 48(42.5) | 23(52.2) | 0.27 |
|
| |||
| Cerebro-Vascular Disease N(%) | 9(8.0) | 9(20.5) | 0.03 |
|
| |||
| Hyperlipidemia N(%) | 61(54) | 21(47.7) | 0.48 |
|
| |||
| CHF N(%) | 101(89.4) | 43(97.7) | 0.09 |
|
| |||
| Ejection Fraction | 17.7 ± 7.6 | 16.5 ±7.9 | 0.57 |
|
| |||
| Cardiogenic Shock N(%) | 34(30.1) | 16 (36.36) | 0.68 |
|
| |||
| VAD Device (N%) | |||
| HeartMate II | 88(77.9) | 35(79.6) | |
| Heartware | 19(16.8) | 5(11.4) | |
| BiVAD | 3(2.7) | 1(2.2) | 0.48 |
| PVAD/RVAD | 0(0) | 1(2.2) | |
| HeartMate XVE | 3(2.6) | 2(4.6) | |
|
| |||
| Goal of Implant N (%) | |||
| Bridge to Transplant | 54(47.8) | 21(48.8) | 0.90 |
| Destination Therapy | 59(52.2) | 22(51.2) | |
|
| |||
| NYHA classification N(=133) (%) | |||
| Class I | 1(1.1) | 1(2.6) | 0.58 |
| Class II | 1(1.1) | 1(2.6) | |
| Class III | 9(9.4) | 4(10.5) | |
| Class IV | 84(88.4) | 32(84.2) | |
|
| |||
| INTERMACS Score (N=147) | |||
| 1 | 18 (17 %) | 11 (26.82 %) | |
| 2 | 46(43.39 %) | 14 (34.14 %) | 0.63 |
| 3 | 25 (23.58 %) | 10 (24.39 %) | |
| 4 | 15(14.15%) | 6(14.63 %) | |
| ≥5 | 2(1.88 %) | 0 | |
|
| |||
| Preoperative Hemodynamics (N=111) | |||
| RAP (mm-hg) | 13.7±6.6 | 14.5±7.5 | 0.60 |
| RVSP (mm-hg) | 53.3±14.8 | 51± 12.7 | 0.45 |
| PASP (mm-hg) | 54.3±15.1 | 51.6±13.9 | 0.40 |
| PADP (mm-hg) | 26.9±8.5 | 26.5±7.2 | 0.84 |
| PCWP | 25.9±8.7 | 23.6±10.2 | 0.25 |
| Cardiac Output (L/min) | 3.78±1.7 | 3.60±1.4 | 0.62 |
| Cardiac Index | 1.84±0.6 | 1.82±0.7 | 0.87 |
eGFR – Estimated Glomerular Filtration Rate
RAP- Right Atrial Pressures
RVSP- Right Ventricle Systolic pressure
PASP – Pulmonary artery Systolic Pressure.
PADP- Pulmonary artery diastolic pressure
PCWP – Pulmonary capillary wedge pressure.
PVAD/RVAD- Paracorporeal ventricular /Right ventricular assist device.
Data presented as either mean ± SD or median (inter-quartile range) as appropriate
Table 1B. Intra- and Post-operative Outcomes in Patients with and without AKI following VAD Implantation.
| No AKI (N=113) |
AKI (N=44) |
P value | |
|---|---|---|---|
| Intra-operative Outcomes | |||
| CPB time (minutes) | 131.0±36.5 | 133.9±49.4 | 0.69 |
| Patients who received Intraoperative Blood Products N (%) | 88(77.9) | 26(59.1) | 0.02 |
| Patients who received Intraoperative PRBC N(%) | 61(54.0) | 24(54.6) | 0.94 |
| Units of Intraoperative PRBC used (n) | 1(0–3) | 1(0–6) | 0.45 |
| Post-operative Outcomes | |||
| Net fluid gain at 7 days (Liters) | −6.65± 5.62 | −4.06 ± 5.78 | 0.02 |
| ICU length of stay (Days) | 6.4± 7.7 | 11.2±11.0 | 0.006 |
| Total length of stay (Days) | 29.2±59.4 | 34.5±46.1 | 0.60 |
| Pneumonia N(%) | 4(3.5) | 4(9.1) | 0.16 |
| Re-Operation for bleeding N(%) | 11(9.7) | 3(6.8) | 0.57 |
| Gastro-Intestinal Bleeding N(%) | 6(5.3) | 3(6.8) | 0.71 |
| Blood product transfusion N(%) | 70(68.6) | 23(57.5) | 0.21 |
| Units of PRBC transfused | 3(2–6) | 7(4–16) | 0.002 |
| In House Mortality N(%) | 9(8.0) | 14(31.8) | <0.001 |
| 30 day mortality N(%) | 8(7.1) | 11(25.0) | 0.002 |
| RRT < 30 days N(%) | 2(1.8) | 9(20.5) | <0.001 |
| Postoperative Sepsis (including driveline during implantation hospitalization) | 7 (6.19) | 6 (13.64) | 0.13 |
| Postoperative Ischemic Stroke (during implantation hospitalization) | 1(0.88) | 2 (4.55) | 0.13 |
CPB- Cardiopulmonary bypass
PRBC- Packed red blood cells
RRT- Renal replacement therapy
Data presented as either mean ± SD or median (inter-quartile range) as appropriate
Table 2 demonstrates the varied AKI event rates in the complete cohort of 157 subjects, utilizing RIFLE and AKIN criteria. The incidence varied greatly depending of the AKI criteria (creatinine, urine output or both). (Table 2).
Table 2. Prevalence of AKI following VAD Implantation – Across differing definitions.
| N (%) | |
|---|---|
|
| |
| RIFLE SCr -Risk | 44(28.0) |
| RIFLE SCr -Injury | 22(14.0) |
| RIFLE SCr -Failure | 6(3.8) |
|
| |
| AKIN SCr –Stage 1 | 67(42.7) |
| AKIN SCr –Stage 2 | 13(8.3) |
| AKIN SCr –Stage 3 | 7(4.46) |
|
| |
| AKIN/RIFLE Urine Output –Stage 1 | 119(75.8) |
| AKIN/RIFLE Urine Output – Stage 2 | 113(72.0) |
| AKIN Urine Output – Stage 3 | 24(15.3) |
Predictors of AKI following VAD implantation
Utilizing univariate logistic regression modeling, diabetes and prior cerebrovascular disease were identified as significant predictors of the development of AKI (Table 3). However on a multivariate analysis only diabetes [OR= 2.27 (1.03-4.97), P=0.04] was identified as a significant predictor of postoperative AKI with prior cerebro-vascular disease and pre-operative hematocrit demonstrating a trend towards significance (Table 3).
Table 3. Predictors of Acute Kidney Injury following VAD Implantation.
| UNIVARIATE PREDICTORS | OR (95 %CI) | P value | MULTIVARIATE PREDICTORS | OR (95% CI) | P value |
|---|---|---|---|---|---|
| Age | 0.99(0.96–1.01) | 0.4 | Diabetes | 2.27(1.03–4.97) | 0.04 |
| Male Sex | 0.91(0.39–2.12) | 0.84 | Prior CVD | 2.84(0.95–8.6) | 0.06 |
| African American Race | 1.48(0.72–3.04) | 0.29 | Preoperative Hematocrit | 1.06(0.99–1.13)) | 0.06 |
| Weight | 0.99(0.98–1.01) | 0.25 | |||
| BMI | 0.99(0.93–1.04) | 0.62 | |||
| Creatinine | 0.89(0.59–1.34) | 0.57 | |||
| Hypertension | 1.48(0.73–2.98) | 0.27 | |||
| CHF | 5.1 (0.64–40.5) | 0.12 | |||
| Diabetes | 2.44(1.20–4.98) | 0.01 | |||
| Prior CVD | 3.12(1.09–8.07) | 0.03 | |||
| PVD | 1.1(0.27–4.49) | 0.88 | |||
| Preoperative Hematocrit | 1.07(0.99–1.13) | 0.05 | |||
| Perfusion Time | 1.00(0.99–1.01) | 0.69 | |||
| Intraoperative PRBC use | 1.02(0.51–2.06) | 0.95 | |||
| Right Atrial Pressure | 1.02(0.95–1.08) | 0.6 | |||
| Pulmonary capillary wedge pressure | 0.98(0.94–1.02) | 0.40 | |||
| Cardiac Output | 0.95(0.73–1.25) | 0.76 |
BMI – Body Mass Index
eGFR – estimated glomerular filtration rate
PRBC- Packed red blood cells
PVD- Peripheral vascular disease
CVD- cerebrovascular disease
CHF- congestive heart failure
AKI and All-cause mortality: 30 day and 365 days
At 30 days, 11(26 %) of those with AKI had died compared to 8 (7%) without AKI (p=0.002). At 365 days, 21(48 %) of those with AKI had died compared to 34 (30%) without AKI (p=0.03). Figure 1 demonstrates the Kaplan Meier Survival Curves for those with and without AKI, with data censored for cardiac transplantation.
Figure 1.
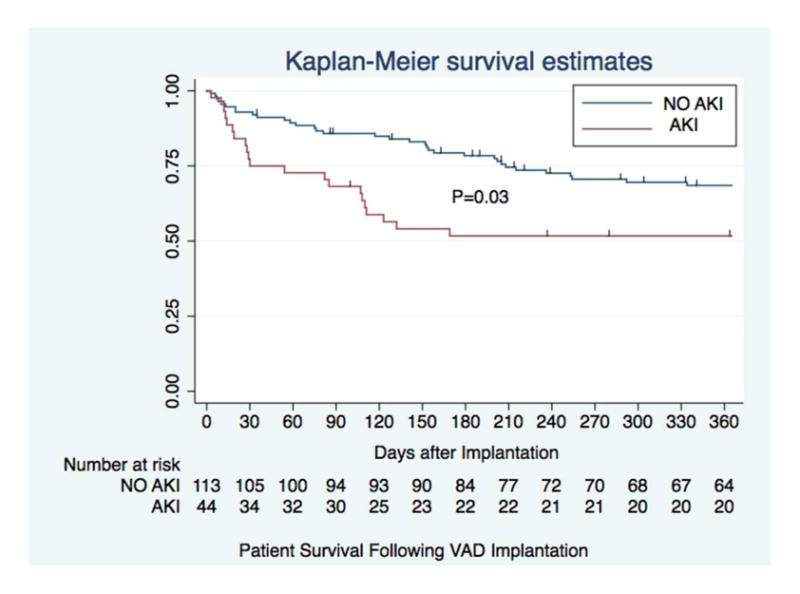
The figure demonstrates the 1-year survival curves for patients with and without AKI. The hash marks denote censoring. At 365 days, 21(48%) of those with AKI had died compared to 34 (30.1%) of those without AKI p=0.03.
In a univariate model to identify predictors of 30 and 365-day mortality several variables were determined to be significantly associated with patient survival (Table 4 and 5). Using a multivariable model AKI [HR=3.01(1.15-7.91), p=0.03] and longer bypass time [1.01(1.001-1.02), P=0.02] were independent predictor of a higher 30-day mortality (Table 4). While AKI [HR=1.85(1.06-3.22); p=0.03] and diabetes mellitus [HR= 1.9(1.07-3.35), P=0.03] were associated with higher mortality at 365 days (Table 5). Higher preoperative BMIs were protective, and were associated with lower mortality at 365 days [0.95 (0.90-0.99), p=0.03].
Table 4. Hazard Ratios for 30-day All-Cause Mortality following VAD Implantation.
| UNIVARIATE ANALYSIS | HR (95%CI) | P value |
|---|---|---|
| Age | 1.04(0.99–1.09) | 0.07 |
| Male Sex | 1.50(0.44–5.16) | 0.52 |
| Black Race | 0.34(0.10–1.17) | 0.09 |
| eGFR | 0.99(0.98–1.02) | 0.77 |
| AKI | 3.74(1.50–9.30) | 0.005 |
| BMI | 0.92(0.84–1.00) | 0.06 |
| Diabetes | 2.47(0.99–6.15) | 0.051 |
| CHF | 0.42(0.12–1.44) | 0.17 |
| Hypertension | 0.89(0.36–2.21) | 0.80 |
| Cardiogenic Shock | 0.96(0.37–2.54) | 0.95 |
| Cerebrovascular Disease | 2.25(0.75–6.79) | 0.15 |
| Perfusion Time | 1.01(1.00–1.02) | <0.001 |
| Hematocrit | 1.00(0.92–1.10) | 0.87 |
| Intraoperative PRBC use | 3.42(1.13–10.32) | 0.03 |
| Rt. Atrial pressure | 0.98 (0.90–1.07) | 0.70 |
| PCWP | 1.02(0.97–1.13) | 0.49 |
| Cardiac Output | 1.04 (0.74–1.45) | 0.81 |
| MULTIVARIATE ANALYSIS | ||
| Age | 1.01(0.97–1.07) | 0.56 |
| Black Race | 0.50(0.13–1.99) | 0.33 |
| AKI | 3.01(1.15–7.91) | 0.03 |
| BMI | 0.92(0.84–1.00) | 0.07 |
| Diabetes | 2.76(0.98–7.72) | 0.054 |
| Perfusion Time | 1.01(1.001–1.02) | 0.02 |
| Intraoperative PRBC use | 2.34(0.75–7.31) | 0.14 |
eGFR – estimated glomerular filtration rate
PRBC- Packed red blood cells
PVD- Peripheral vascular disease
CVD- Cerebrovascular disease
CHF- Congestive heart failure
BMI- Body Mass Index
PCWP- Pulmonary capillary wedge pressure
Table 5. Hazard Ratios for 365-day All-Cause Mortality following VAD Implantation.
| UNIVARIATE COX PROPORTIONAL HAZARDS MODEL | HR (95%CI) | P-value |
|---|---|---|
| Age | 1.00(0.99–1.03) | 0.53 |
| Male Sex | 1.12(0.58–2.19) | 0.72 |
| Black Race | 0.99(0.56–1.72) | 0.96 |
| eGFR | 0.99(0.98–1.00) | 0.29 |
| AKI | 2.01(1.16–3.48) | 0.01 |
| BMI | 0.96(0.92–1.00) | 0.08 |
| Diabetes | 1.66(0.97–2.84) | 0.06 |
| CHF | 0.63(0.27–1.48) | 0.29 |
| Hypertension | 1.26(0.74–2.16) | 0.31 |
| Cardiogenic Shock | 1.06(0.60–1.88) | 0.81 |
| Cerebrovascular Disease | 1.25(0.57–2.77) | 0.58 |
| Perfusion Time | 1.00(1.00–1.01) | 0.04 |
| Hematocrit | 1.00(0.95–1.05) | 0.99 |
| Intraoperative PRBC use | 1.25(0.73–2.16) | 0.40 |
| Right Atrial Pressure | 0.99(0.95–1.04) | 0.86 |
| PCWP. | 1.00(0.97–1.04) | 0.92 |
| Cardiac Output | 1.02(0.85–1.23) | 0.80 |
| MULTIVARIATE COX PROPORTIONAL HAZARDS | ||
| AKI | 1.85(1.06–3.22) | 0.03 |
| BMI | 0.95(0.90–0.99) | 0.03 |
| Diabetes | 1.90 (1.07–3.35) | 0.02 |
| Perfusion Time | 1.00(0.99–1.01) | 0.07 |
eGFR – estimated glomerular filtration rate.
PRBC- Packed red blood cells.
PVD- Peripheral vascular disease.
CVD- Cerebrovascular disease.
CHF- Congestive heart failure.
BMI- Body Mass Index.
AKI- Acute Kidney Injury.
PCWP- Pulmonary capillary wedge pressure.
Discussion
Current literature on incidence of AKI in patients with VAD implantation and its outcomes are limited. Prior studies have defined AKI based the need for RRT while others have used non-standardized definitions [14-19,25-28]. Conducting a single center retrospective cohort study utilizing internationally accepted consensus definitions we identified the incidence of AKI as 28%(n=44) with a high portion of these patients receiving RRT (n= 9; 5.7% of the total cohort). Additionally, our study demonstrates that AKI is associated with short and long-term mortality following VAD implantation [29] .
Diabetes has been shown to increase the risk of AKI following traditional cardiac surgery [6,7]. In a multivariate analysis we identified diabetes as the sole predictor of AKI after VAD implantation. Previous studies in VADs had identified age, baseline eGFR and perfusion time as other risk factors for the development of post implant AKI [30,31]. However similar to the Borgi and colleagues [32] we were unable to demonstrate perfusion time as a risk factor for AKI. Our inability to replicate differences in baseline eGFR may stem from our utilization of the standardized creatinine based definition of AKI rather than more subjective definitions [14-19].
In our study, predictors of 30-day mortality included AKI and perfusion time. Our findings mirror those of Borgi et al., a smaller single center study (n=100), and demonstrate that post-VAD AKI is also a predictor of 1-year mortality [32]. After our adjusted analyses these findings further strengthen the pre-existing literature demonstrating the long-term consequences of AKI in a variety of clinical settings[33,34]. Additionally, we observed that patients with a higher pre-operative BMI had lower 1-year mortality; corroborating the findings of Butler et al who demonstrated that in a univariate analyses the 180 and 365-day survival after VAD implantation was significantly higher among patients with higher BMIs. However, unlike our study this effect was no longer significant following multivariate analysis. [35]
Age, baseline eGFR, gender and African American race have been previously reported to have an impact on AKI and mortality. However we did not identify these to be significant AKI risk factors (Tables 4,5). The recently published fifth INTERMACS report: identified age to be a predictor of mortality although the actuarial survival of patients older than 70 years was reported to be only modestly inferior to those older than age 50 [13] .Similarly we were unable to identify baseline eGFR as predictor for short and long term survival. Even after stratifying pre-implant eGFR to < 60 ml/minute and ≥ 60 ml/minute we found no significant difference in long-term mortality (Data not shown). This was contrary to the study done by Sandner et al, which reported worse survival for group with eGFR < 60 ml/minute [36]. Our inability to demonstrate eGFR as a risk factor may stem from the lower than previously published eGFR in our cohort (49.7 ml/min). Traditionally African Americans have been noted to have worse outcomes after cardiac surgeries [37,38], but our study as well two recently published studies showed no difference in outcomes in patients who had a VAD placed [39,40]. Women have also been reported to be at higher risk for mortality after traditional cardiothoracic surgery, but we did not appreciate that in any of our analysis [6,41,42].
Haase et al demonstrated no difference in diagnosing AKI following traditional cardiac surgery when comparing the RIFLE and AKIN criteria [43]. However, unlike our cohort, in their prospective observational study, less than 25% of all patients had an eGFR less than 60 ml/min. Our study demonstrated clear differences between the AKIN and RIFLE criteria defined AKI; with over 67 (42.7%) subjects developing AKIN serum creatinine based Stage-1, compared to 28% for RIFLE-Risk. In a secondary analysis the use of AKIN-stage-1 (creatinine criteria) was not associated with a significant risk of mortality 30-day [HR=1.88 (0.76-4.67, P=0.17)] or 365-day [HR=1.41 (0.83-2.41, P=0.2)] survival. This analysis suggests the use of a 0.3 mg/dl increase in serum creatinine to identify post-VAD AKI may be inferior to RIFLE- Risk (50% increase) from a prognostic perspective. Similarly despite the high proportion of patients with AKI defined by urine output, n=119(75.8%), Stage-1 <0.5 ml/kg/h for 6 hours) (Table 2) we did not note any statistically significant impact on 30 and 365-day survival (data not shown). A recent study in a general ICU cohort has raised the question whether the urine output criteria for defining AKI is too liberal. Similar to our observation the authors were unable to show an impact of AKI as defined by urine output criteria to have a significant impact on 1 year mortality[44]. However these findings need to be validated in larger multicenter prospective trials.
Questions remain about the effects of pulsatile versus continuous flow devices on renal function. While pulsatile flow is physiologic and theoretically better, based on the limited published human data, renal outcomes have not differed across the 2 modalities [25]. Perhaps counter-intuitively, continuous flow devices have been associated with lower device related complications and improved outcomes [28,45,46]. The further investigation of this issue has proven difficult, with greater than 90% of U.S. VAD implantations being continuous flow devices [13].
Our study has several strengths in that to our knowledge this is the largest cohort study of VAD patients to employ a consensus definition of AKI, including analyzing AKI endpoints defined by urine output. Additionally compared to previously published papers we have a significant number of African American patients as well as DT VAD patients. Both these sub-populations that are steadily increasing [11]. Our study suffers from all the inherent limitations of a single center retrospective review. However it is these same single-center factors that allowed us to have excellent patient follow-up and complete, demographic, postoperative serum creatinine and urine output data. Additional strengths include utilization of our single center electronic medical record and access to INTERMACS to validate the true pre-implantation baseline creatinine/eGFR. Included in our limitations is the low numbers of patients who developed severe AKI (RIFLE I or F), and this limits our ability to make any conclusions about severe AKI.
In conclusion, we identified the incidence of AKI following VAD implantation to be 28% and those who developed AKI had increased risk for both short and long term mortality. Further investigations are needed to tease out the differences between the risks of AKI following traditional cardiac surgery versus VAD implantations. Additionally, given high AKI event rates and the mounting evidence linking AKI to mortality following VAD implantation, the use of biomarkers to identify those at risk may have a role. Larger prospective multicenter trials are needed to develop a risk stratification system to identify patients at risk for developing post VAD implantation AKI.
Acknowledgments
JLK was supported by K23 DK081616
VJ reports receiving consulting fees from Thoratec, Heartware, SAA reports receiving research funding from Thoratec.
We wish to thank Ms. Elizabeth Johnson for her assistance in working with the INTERMACs database
Footnotes
All other authors have nothing to disclose
References
- 1.Chertow GM, Levy EM, Hammermeister KE, Grover F, Daley J. Independent association between acute renal failure and mortality following cardiac surgery. Am J Med. 1998;104:343–348. doi: 10.1016/s0002-9343(98)00058-8. [DOI] [PubMed] [Google Scholar]
- 2.Parikh CR, Coca SG, Thiessen-Philbrook H, Shlipak MG, Koyner JL, Wang Z, Edelstein CL, Devarajan P, Patel UD, Zappitelli M, Krawczeski CD, Passik CS, Swaminathan M, Garg AX, Consortium TA. Postoperative biomarkers predict acute kidney injury and poor outcomes after adult cardiac surgery. J Am Soc Nephrol. 2011;22:1748–1757. doi: 10.1681/ASN.2010121302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cooper WA, O'Brien SM, Thourani VH, Guyton RA, Bridges CR, Szczech LA, Petersen R, Peterson ED. Impact of renal dysfunction on outcomes of coronary artery bypass surgery: Results from the society of thoracic surgeons national adult cardiac database. Circulation. 2006;113:1063–1070. doi: 10.1161/CIRCULATIONAHA.105.580084. [DOI] [PubMed] [Google Scholar]
- 4.Dasta JF, Kane-Gill SL, Durtschi AJ, Pathak DS, Kellum JA. Costs and outcomes of acute kidney injury (aki) following cardiac surgery. Nephrol Dial Transplant. 2008;23:1970–1974. doi: 10.1093/ndt/gfm908. [DOI] [PubMed] [Google Scholar]
- 5.Lenihan CR, Montez-Rath ME, Mora Mangano CT, Chertow GM, Winkelmayer WC. Trends in acute kidney injury, associated use of dialysis, and mortality after cardiac surgery, 1999 to 2008. Ann Thorac Surg. 2013;95:20–28. doi: 10.1016/j.athoracsur.2012.05.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wijeysundera DN, Karkouti K, Dupuis JY, Rao V, Chan CT, Granton JT, Beattie WS. Derivation and validation of a simplified predictive index for renal replacement therapy after cardiac surgery. JAMA. 2007;297:1801–1809. doi: 10.1001/jama.297.16.1801. [DOI] [PubMed] [Google Scholar]
- 7.Mehta RH, Grab JD, O'Brien SM, Bridges CR, Gammie JS, Haan CK, Ferguson TB, Peterson ED. Society of Thoracic Surgeons National Cardiac Surgery Database I: Bedside tool for predicting the risk of postoperative dialysis in patients undergoing cardiac surgery. Circulation. 2006;114:2208–2216. doi: 10.1161/CIRCULATIONAHA.106.635573. quiz 2208. [DOI] [PubMed] [Google Scholar]
- 8.Eriksen BO, Hoff KR, Solberg S. Prediction of acute renal failure after cardiac surgery: Retrospective cross-validation of a clinical algorithm. Nephrol Dial Transplant. 2003;18:77–81. doi: 10.1093/ndt/18.1.77. [DOI] [PubMed] [Google Scholar]
- 9.Thakar CV, Arrigain S, Worley S, Yared JP, Paganini EP. A clinical score to predict acute renal failure after cardiac surgery. J Am Soc Nephrol. 2005;16:162–168. doi: 10.1681/ASN.2004040331. [DOI] [PubMed] [Google Scholar]
- 10.Rose EA, Gelijns AC, Moskowitz AJ, Heitjan DF, Stevenson LW, Dembitsky W, Long JW, Ascheim DD, Tierney AR, Levitan RG, Watson JT, Meier P, Ronan NS, Shapiro PA, Lazar RM, Miller LW, Gupta L, Frazier OH, Desvigne-Nickens P, Oz MC, Poirier VL. Randomized Evaluation of Mechanical Assistance for the Treatment of Congestive Heart Failure Study G: Long-term use of a left ventricular assist device for end-stage heart failure. The New England journal of medicine. 2001;345:1435–1443. doi: 10.1056/NEJMoa012175. [DOI] [PubMed] [Google Scholar]
- 11. http://www.Uab.Edu/intermacs/images/federal_quarterly_report/intermacs_federal_partners_quarterly_report_03_2012_website_all.Pdf.
- 12.Slaughter MS, Rogers JG, Milano CA, Russell SD, Conte JV, Feldman D, Sun B, Tatooles AJ, Delgado RM, 3rd, Long JW, Wozniak TC, Ghumman W, Farrar DJ, Frazier OH. HeartMate III: Advanced heart failure treated with continuous-flow left ventricular assist device. The New England journal of medicine. 2009;361:2241–2251. doi: 10.1056/NEJMoa0909938. [DOI] [PubMed] [Google Scholar]
- 13.Kirklin JK, Naftel DC, Kormos RL, Stevenson LW, Pagani FD, Miller MA, Timothy Baldwin J, Young JB. Fifth intermacs annual report: Risk factor analysis from more than 6,000 mechanical circulatory support patients. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2013;32:141–156. doi: 10.1016/j.healun.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 14.McCarthy PM, Smedira NO, Vargo RL, Goormastic M, Hobbs RE, Starling RC, Young JB. One hundred patients with the heartmate left ventricular assist device: Evolving concepts and technology. J Thorac Cardiovasc Surg. 1998;115:904–912. doi: 10.1016/S0022-5223(98)70373-3. [DOI] [PubMed] [Google Scholar]
- 15.Kaltenmaier B, Pommer W, Kaufmann F, Hennig E, Molzahn M, Hetzer R. Outcome of patients with ventricular assist devices and acute renal failure requiring renal replacement therapy. ASAIO J. 2000;46:330–333. doi: 10.1097/00002480-200005000-00017. [DOI] [PubMed] [Google Scholar]
- 16.Haddad M, Hendry PJ, Masters RG, Mesana T, Haddad H, Davies RA, Mussivand TV, Struthers C, Keon WJ. Ventricular assist devices as a bridge to cardiac transplantation: The ottawa experience. Artif Organs. 2004;28:136–141. doi: 10.1111/j.1525-1594.2003.47331.x. [DOI] [PubMed] [Google Scholar]
- 17.Feller ED, Sorensen EN, Haddad M, Pierson RN, 3rd, Johnson FL, Brown JM, Griffith BP. Clinical outcomes are similar in pulsatile and nonpulsatile left ventricular assist device recipients. Ann Thorac Surg. 2007;83:1082–1088. doi: 10.1016/j.athoracsur.2006.10.034. [DOI] [PubMed] [Google Scholar]
- 18.Demirozu ZT, Etheridge WB, Radovancevic R, Frazier OH. Results of heartmate ii left ventricular assist device implantation on renal function in patients requiring post-implant renal replacement therapy. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2011;30:182–187. doi: 10.1016/j.healun.2010.08.019. [DOI] [PubMed] [Google Scholar]
- 19.Yoshioka D, Sakaguchi T, Saito S, Miyagawa S, Nishi H, Yoshikawa Y, Fukushima S, Saito T, Daimon T, Ueno T, Kuratani T, Sawa Y. Predictor of early mortality for severe heart failure patients with left ventricular assist device implantation: Significance of intermacs level and renal function. Circ J. 2012;76:1631–1638. doi: 10.1253/circj.cj-11-1452. [DOI] [PubMed] [Google Scholar]
- 20.Intermacs user' guide. [Accessed on october 25,2012];Manual of operation. 2.3:66. http://www.Uab.Edu/intermacs/ [Google Scholar]
- 21.Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, Kusek JW, Van Lente F. Chronic Kidney Disease Epidemiology C: Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145:247–254. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 22.Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P. Acute Dialysis Quality Initiative w: Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: The second international consensus conference of the acute dialysis quality initiative (adqi) group. Critical care. 2004;8:R204–212. doi: 10.1186/cc2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, Levin A. Acute Kidney Injury N: Acute kidney injury network: Report of an initiative to improve outcomes in acute kidney injury. Critical care. 2007;11:R31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kellum JALN, Aspelin P, Barsoum RS, Burdmann EA, Goldstein SL, Herzog CA, Joannidis M, Kribben A, MacLeod AM, Mehta RL, Murray PT, Naicker S, Opal SM, Schaefer F, Schetz M, Uchino S. KDIGO Clinical Practice Guideline for Acute Kidney Injury 2012. Kidney International Supplements. 2012 [Google Scholar]
- 25.Sandner SE, Zimpfer D, Zrunek P, Dunkler D, Schima H, Rajek A, Grimm M, Wolner E, Wieselthaler GM. Renal function after implantation of continuous versus pulsatile flow left ventricular assist devices. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2008;27:469–473. doi: 10.1016/j.healun.2007.12.012. [DOI] [PubMed] [Google Scholar]
- 26.Hasin T, Topilsky Y, Schirger JA, Li Z, Zhao Y, Boilson BA, Clavell AL, Rodeheffer RJ, Frantz RP, Edwards BS, Pereira NL, Joyce L, Daly R, Park SJ, Kushwaha SS. Changes in renal function after implantation of continuous-flow left ventricular assist devices. J Am Coll Cardiol. 2012;59:26–36. doi: 10.1016/j.jacc.2011.09.038. [DOI] [PubMed] [Google Scholar]
- 27.Deng MC, Edwards LB, Hertz MI, Rowe AW, Keck BM, Kormos R, Naftel DC, Kirklin JK, Taylor DO. International Society for H, Lung T: Mechanical circulatory support device database of the international society for heart and lung transplantation: Third annual report--2005. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2005;24:1182–1187. doi: 10.1016/j.healun.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 28.Miller LW, Pagani FD, Russell SD, John R, Boyle AJ, Aaronson KD, Conte JV, Naka Y, Mancini D, Delgado RM, MacGillivray TE, Farrar DJ, Frazier OH. HeartMate IICI: Use of a continuous-flow device in patients awaiting heart transplantation. The New England journal of medicine. 2007;357:885–896. doi: 10.1056/NEJMoa067758. [DOI] [PubMed] [Google Scholar]
- 29.Chertow G, Levy E, Hammermeister K, Grover F, Daley J. Independent association between acute renal failure and mortality following caridac surgery. Am J Med. 1998;104:343–348. doi: 10.1016/s0002-9343(98)00058-8. [DOI] [PubMed] [Google Scholar]
- 30.Alba AC, Rao V, Ivanov J, Ross HJ, Delgado DH. Predictors of acute renal dysfunction after ventricular assist device placement. J Card Fail. 2009;15:874–881. doi: 10.1016/j.cardfail.2009.05.015. [DOI] [PubMed] [Google Scholar]
- 31.Sandner SE, Zimpfer D, Zrunek P, Rajek A, Schima H, Dunkler D, Zuckermann AO, Wieselthaler GM. Age and outcome after continuous-flow left ventricular assist device implantation as bridge to transplantation. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2009;28:367–372. doi: 10.1016/j.healun.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 32.Borgi J, Tsiouris A, Hodari A, Cogan CM, Paone G, Morgan JA. Significance of postoperative acute renal failure after continuous-flow left ventricular assist device implantation. Ann Thorac Surg. 2013;95:163–169. doi: 10.1016/j.athoracsur.2012.08.076. [DOI] [PubMed] [Google Scholar]
- 33.Amdur RL, Chawla LS, Amodeo S, Kimmel PL, Palant CE. Outcomes following diagnosis of acute renal failure in u.S. Veterans: Focus on acute tubular necrosis. Kidney Int. 2009;76:1089–1097. doi: 10.1038/ki.2009.332. [DOI] [PubMed] [Google Scholar]
- 34.Lo LJ, Go AS, Chertow GM, McCulloch CE, Fan D, Ordonez JD, Hsu CY. Dialysis-requiring acute renal failure increases the risk of progressive chronic kidney disease. Kidney Int. 2009;76:893–899. doi: 10.1038/ki.2009.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Butler J, Howser R, Portner PM, Pierson RN., 3rd Body mass index and outcomes after left ventricular assist device placement. Ann Thorac Surg. 2005;79:66–73. doi: 10.1016/j.athoracsur.2004.06.047. [DOI] [PubMed] [Google Scholar]
- 36.Sandner SE, Zimpfer D, Zrunek P, Rajek A, Schima H, Dunkler D, Grimm M, Wolner E, Wieselthaler GM. Renal function and outcome after continuous flow left ventricular assist device implantation. Ann Thorac Surg. 2009;87:1072–1078. doi: 10.1016/j.athoracsur.2009.01.022. [DOI] [PubMed] [Google Scholar]
- 37.Bridges CR, Edwards FH, Peterson ED, Coombs LP. The effect of race on coronary bypass operative mortality. J Am Coll Cardiol. 2000;36:1870–1876. doi: 10.1016/s0735-1097(00)00956-6. [DOI] [PubMed] [Google Scholar]
- 38.Allen JG, Weiss ES, Arnaoutakis GJ, Russell SD, Baumgartner WA, Conte JV, Shah AS. The impact of race on survival after heart transplantation: An analysis of more than 20,000 patients. Ann Thorac Surg. 2010;89:1956–1963. doi: 10.1016/j.athoracsur.2010.02.093. discussion 1963-1954. [DOI] [PubMed] [Google Scholar]
- 39.Tsiouris A, Brewer RJ, Borgi J, Nemeh H, Paone G, Morgan JA. Continuous-flow left ventricular assist device implantation as a bridge to transplantation or destination therapy: Racial disparities in outcomes. The Journal of heart and lung transplantation : the official publication of the International Society for Heart Transplantation. 2013;32:299–304. doi: 10.1016/j.healun.2012.11.017. [DOI] [PubMed] [Google Scholar]
- 40.Bhat Gea. Racial differences in over 1,000 patients with continuous flow left ventricular assist devices. Journal of the American College of Cardiology. 2013;61 doi: 10.1016/j.jacc.2012.09.055. [DOI] [PubMed] [Google Scholar]
- 41.Roques F, Nashef SA, Michel P, Gauducheau E, de Vincentiis C, Baudet E, Cortina J, David M, Faichney A, Gabrielle F, Gams E, Harjula A, Jones MT, Pintor PP, Salamon R, Thulin L. Risk factors and outcome in european cardiac surgery: Analysis of the euroscore multinational database of 19030 patients. Eur J Cardiothorac Surg. 1999;15:816–822. doi: 10.1016/s1010-7940(99)00106-2. discussion 822-813. [DOI] [PubMed] [Google Scholar]
- 42.Hannan EL, Racz M, Culliford AT, Lahey SJ, Wechsler A, Jordan D, Gold JP, Higgins RS, Smith CR. Risk score for predicting in-hospital/30-day mortality for patients undergoing valve and valve/coronary artery bypass graft surgery. Ann Thorac Surg. 2013;95:1282–1290. doi: 10.1016/j.athoracsur.2012.11.019. [DOI] [PubMed] [Google Scholar]
- 43.Haase M, Bellomo R, Matalanis G, Calzavacca P, Dragun D, Haase-Fielitz A. A comparison of the rifle and acute kidney injury network classifications for cardiac surgery-associated acute kidney injury: A prospective cohort study. J Thorac Cardiovasc Surg. 2009;138:1370–1376. doi: 10.1016/j.jtcvs.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 44.Md Ralib A, Pickering JW, Shaw GM, Endre ZH. The urine output definition of acute kidney injury is too liberal. Critical care. 2013;17:R112. doi: 10.1186/cc12784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Slaughter MS. Long-term continuous flow left ventricular assist device support and end-organ function: Prospects for destination therapy. J Card Surg. 2010;25:490–494. doi: 10.1111/j.1540-8191.2010.01075.x. [DOI] [PubMed] [Google Scholar]
- 46.Pagani FD, Miller LW, Russell SD, Aaronson KD, John R, Boyle AJ, Conte JV, Bogaev RC, MacGillivray TE, Naka Y, Mancini D, Massey HT, Chen L, Klodell CT, Aranda JM, Moazami N, Ewald GA, Farrar DJ, Frazier OH. HeartMate III: Extended mechanical circulatory support with a continuous-flow rotary left ventricular assist device. J Am Coll Cardiol. 2009;54:312–321. doi: 10.1016/j.jacc.2009.03.055. [DOI] [PubMed] [Google Scholar]


