Abstract
Background
Neuroimaging studies of cocaine users have demonstrated white matter abnormalities associated with behavioral measures of impulsivity and decision-making deficits. The underlying bases for this dysregulation in white matter structure and function have yet to be determined. The aim of the present studies was to investigate the influence of prolonged cocaine self-administration on the levels of myelin-associated proteins and mRNAs in nonhuman primate white matter.
Methods
Rhesus monkeys (n=4) self-administered cocaine (0.3 mg/kg/inj, 30 reinforcers per session) for 300 sessions. Control animals (n=4) responded for food. Following the final session monkeys were euthanized and white matter tissue at three brain levels was processed for immunoblotting analysis of proteolipid protein (PLP) and myelin basic protein (MBP), as well as for in situ hybridization histochemical analysis of PLP and MBP mRNAs.
Results
Both MBP and PLP immunoreactivities in white matter at the level of the precommissural striatum were significantly lower in tissue from monkeys self-administering cocaine as compared to controls. No significant differences were seen for either protein at the levels of the prefrontal cortex or postcommissural striatum. In addition, no differences were observed in expression of mRNA for either protein.
Conclusions
These preliminary findings, in a nonhuman model of prolonged cocaine self-administration, provide further evidence that compromised myelin may underlie the deficits in white matter integrity described in studies of human cocaine users.
Keywords: Cocaine, self-administration, white matter, myelin basic protein, proteolipid protein, monkey
1. INTRODUCTION
Recent investigations have demonstrated alterations in the structural integrity and density of white matter in the brains of human cocaine users (Hanlon et al., 2011a; Lane et al., 2010; Moeller et al., 2005; Romero et al., 2010). These deficits have been associated with impaired behavioral measures of impulsivity and decision-making (Lane et al., 2010; Moeller et al., 2005), and correlate with duration of cocaine use (Lim et al., 2008). Cocaine-associated disruptions in functional connectivity have also been demonstrated (Hanlon et al., 2011b; Kelly et al., 2011; Ma et al., 2012; McHugh et al., 2013). Taken together, these reports suggest that compromised white matter integrity may contribute to the disruptions in connectivity and executive control typically observed in chronic cocaine abusers.
Despite the compelling evidence from human studies, however, the questions of both a causal relationship between cocaine use and altered white matter integrity, as well as the pathological processes underlying these deficits, remain largely unanswered. These questions are especially challenging given that white matter alterations have been observed in several conditions which frequently accompany cocaine abuse, such as depression and anxiety (Dolan et al., 1990; Wang et al., 2012), tobacco use (Hudkins et al., 2012; Paul et al., 2008), and alcohol abuse (de la Monte, 1988; Monnig et al., 2012). Rodent studies, however, have begun to address the issue of causality, with reports of dysregulated myelin-related proteins (Kovalevich et al., 2012; Narayana et al., 2009) and modification of myelin-related genes (Nielsen et al., 2012) following cocaine exposure, as well as deficits in white matter integrity, as measured by diffusion tensor imaging (DTI; Narayana et al., 2009).
DTI studies in cocaine-dependent subjects have shown reduced white matter integrity primarily in anterior portions of the corpus callosum and frontal fiber tracts (Ma et al., 2009; Moeller et al., 2005). The availability of callosal tissue from a monkey study of prolonged cocaine self-administration, a model closely homologous to human abusers, made possible a preliminary investigation into the impact of cocaine exposure on white matter, while eliminating the need to covary cocaine use with other confounding factors common in human studies. The aim of these studies, therefore, was to determine the influence of extended cocaine self-administration on levels of myelin-associated proteins and mRNAs in dorsal subcortical white matter tracts. White matter tissue from three rostro-caudal brain levels was processed for immunoblotting analysis of the two most abundant proteins in myelin, proteolipid protein (PLP) and myelin basic protein (MBP), as well as for in situ hybridization histochemical (ISHH) analysis of their corresponding mRNAs.
2. MATERIALS AND METHODS
2.1 Subjects
A total of 8 male rhesus monkeys (Macaca mulatta) served as subjects. All procedures were performed in accordance with the National Institutes of Health Guide for Care and Use of Laboratory Animals and were reviewed and approved by the Animal Care and Use Committee of Wake Forest University.
2.2 Cocaine self-administration
Details of the surgical and self-administration procedures have been described previously (Beveridge et al., 2005, 2009; Nader et al., 2002), with the exception of the cumulative duration of exposure. Briefly, monkeys were trained to respond under a fixed-interval 3-minute (FI-3-min) schedule of food reinforcement until stable performance was obtained. Animals were then randomly assigned to food-reinforced (N=4) or cocaine-reinforced (N=4) groups and continued to respond under an FI-3-min schedule for either food or cocaine (0.3 mg/kg per injection). Experimental sessions continued for a total of 300 sessions (2750 mg/kg total cocaine intake); sessions ended after 30 reinforcers were delivered. Following the final session animals were humanely euthanized with an overdose of pentobarbital (100 mg/kg, i.v.).
2.3 Tissue Processing
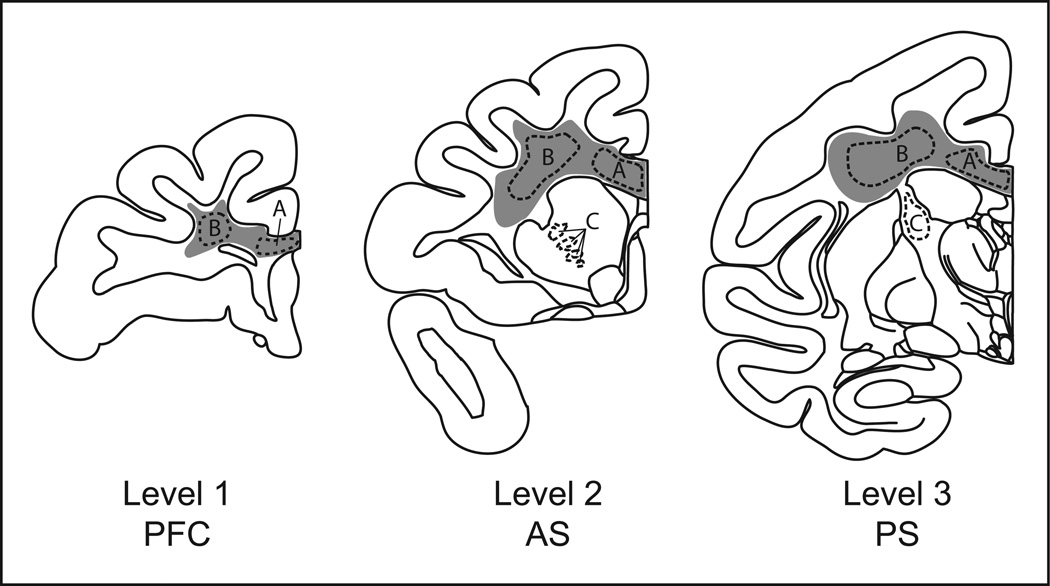
After euthanasia, brains were removed, flash-frozen, and stored at −80°C. Brains were cut in a cryostat at −20°C in the coronal plane into 20 µm sections onto electrostatically charged slides. White matter samples for western blot analysis were collected by scraping corona radiata and corpus callosum from frozen slide-mounted sections. This procedure was carried out in a freezer at −80°C, and specimens remained frozen until they were thawed for crude protein homogenate preparation. Slides for both western blotting and ISHH were selected at 3 rostro-caudal brain levels (Fig. 1) which correspond to Figures 24 (PFC), 37 (precommissural striatum) and 60 (postcommissural striatum) of the rhesus monkey atlas of Paxinos et al. (2000).
Figure 1.
Schematic of representative monkey brain levels selected for white matter analysis. Gray shaded areas represent the approximate extent of white matter collected for western blot analysis. Outlined areas represent regions of interest analyzed for MBP and PLP mRNA expression. A, Corpus callosum; B, Corona radiata; C, Internal capsule. Abbreviations: PFC, prefrontal cortical level; AS, Anterior (precommissural) striatal level; PS, Posterior (postcommissural) striatal level.
2.4 Western Blotting
Samples were homogenized in ice-cold water (20uL/mg) with the addition of protease inhibitors. Homogenates were centrifuged at 16,000 × g at 4°C for 10 minutes to remove insoluble material. The supernatant was collected and protein concentrations were determined by Pierce BCA protein assay kit (Thermo Fisher Scientific, Waltham, MA). For electrophoresis, samples were diluted 1:1 with sample buffer (2% SDS, 10% glycerol, 5% β-mercaptoethanol, and 62.5 mM Tris-HCL, PH 6.8). Samples (15 µg protein) were denatured at 70°C for 10 min, separated by 15% SDS polyacrylamide gel electrophoresis, and transferred to PVDF membrane (Bio-Rad; Hercules, CA). Membranes were blocked in 50% blocking buffer (Li-Cor; Lincoln, NE)/50% PBS for 1 hr at room temperature, incubated in primary antibodies against MBP (1:2000; Millipore, Billerica, MA) or PLP (1:1000; Millipore) in blocking buffer/0.1% Tween-20 overnight at 4°C, washed (PBS, 0.05% Tween-20), incubated in IRDye® 680RD goat anti-mouse secondary antibody (Li-Cor) for one hour, and washed again. Membranes were then scanned and bands were analyzed using an Odyssey® infrared imager (Li-Cor). Membranes were also probed with an antibody against β-actin (1:5,000; Abcam, Cambridge, MA) as a loading control. Data are expressed as a ratio of the protein of interest and the corresponding density of β-actin.
2.5 In situ hybridization histochemistry
For ISHH, 35S-labeled antisense oligonucleotide probes were used to hybridize to MBP and PLP mRNAs in the subcortical white matter. Probes complementary to published sequences for PLP (exon 2, bases 274 – 312; NCBI accession number NM_000533.3) and MBP (exon 1, bases 92–136; NCBI accession number NM_001025081.1) were synthesized by the DNA Synthesis Core Laboratory of Wake Forest School of Medicine. Probes were hybridized to tissue as previously described (Letchworth et al., 1999). In brief, probes were 3’-labeled with α-35S-deoxyadenosine triphosphate (1200 Ci/mmol; PerkinElmer, Waltham, MA) using terminal deoxynucleotidyl transferase (Promega, Madison, WI). Sections were incubated with ~6.0×106 cpm of labeled probe in 300µl hybridization buffer overnight at 37°C, washed, dried, and apposed to Kodak Biomax MR film (Fisher Scientific, Pittsburgh, PA) for 5 days in the presence of 14C microscale standards (GE Healthcare, Pittsburgh, PA). Densitometric analysis was carried out using a computer-assisted image-processing system (MCID; Interfocus Imaging, Cambridge, UK).
Data were averaged across three adjacent tissue sections per level. Regions of interest were corpus callosum, corona radiata, and internal capsule. Values for each region were determined from optical densities compared to calibrations of 14C standards, and converted from 14C nCi/mg of tissue to disintegrations per minute (dpms) of 35S/mg tissue using 35S brain paste standards, as previously described (Letchworth et al., 1999; Miller, 1991).
2.6 Statistical analysis
For both immunoblotting and ISHH studies differences between cocaine-treated and control groups were determined by Student’s t-tests with significance set at p < 0.05, corrected for multiple comparisons, and carried out with SPSS software (Version 18.0; IBM, New York).
3. RESULTS
3.1 Western Blotting
3.1.1 PLP and DM-20
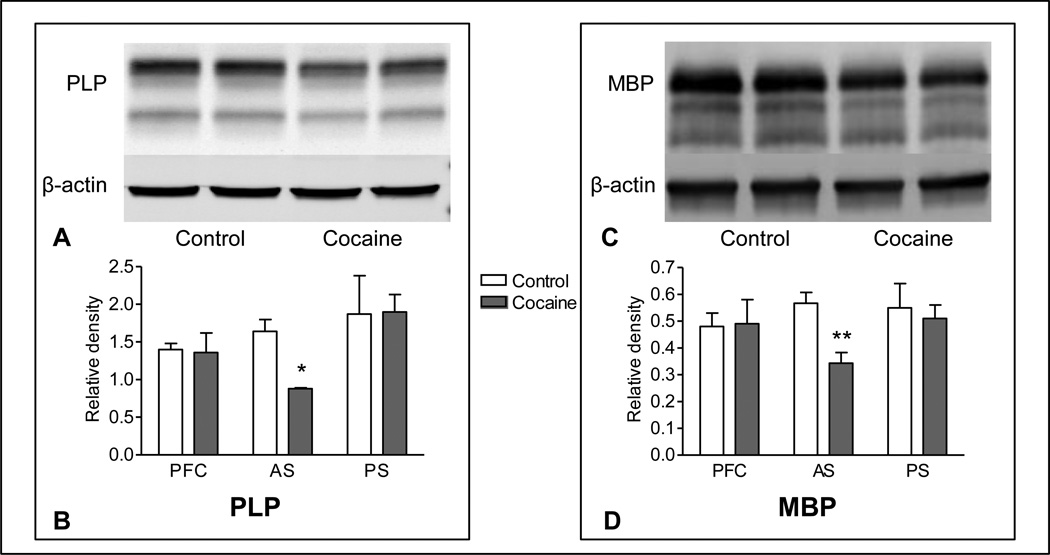
In white matter tissue from food-control monkeys two prominent bands of PLP-immunoreactive protein were observed, which correspond to the two major isoforms: PLP at 23 kDa and DM-20 at 20 kDa. PLP protein levels in the cocaine group were significantly lower than those in controls in white matter tissue collected at the level of the precommissural striatum (−47%, p=0.015; Figure 2), however no differences between groups were observed at any other brain level. No differences between groups were observed in DM-20 protein expression.
Figure 2.
PLP (left panel) and MBP (right panel) immunoreactivity in white matter of nonhuman primate brain following prolonged exposure to cocaine or food self-administration. Representative western blots (A, C) show immunoreactivity in white matter from cocaine and food animals at the level of the precommissural striatum. Graphic representations of western blot data (B, D) show that immunolabeling of PLP and MBP proteins was significantly lower in white matter from cocaine-exposed monkeys at the level of the precommissural striatum, while no differences in any marker were seen at either the prefrontal or posterior striatal levels. PFC, prefrontal cortical level (level 1); AS, Anterior (precommissural) striatal level (level 2); PS, Posterior (postcommissural) striatal level (level 3). * p<0.05, ** p<0.01.
3.1.2 MBP
Immunoreactivity to MBP in control animals contained one major band corresponding to the predominant 18.5 kDa isoform in humans, as well as two minor bands of approximately 17 and 17.5 kDa. Immunoreactivity of the 18.5 band in tissue from the level of the precommissural striatum was significantly lower in the cocaine group than in controls (−39%, p=0.008; Fig 2).
3.2 In situ hybridization histochemistry
Labeling of both PLP and MBP mRNAs was consistent with their expression in white matter in the rhesus monkey brain. While white matter tracts were densely labeled, expression in gray matter was sparse. Expression of PLP and MBP transcripts in white matter was not significantly different between groups at any level measured.
4. DISCUSSION
The findings of this preliminary study demonstrate that prolonged exposure to cocaine self-administration resulted in lower levels of expression of the two most abundant proteins in myelin, PLP and MBP, in white matter of nonhuman primates when compared to non-drug exposed controls, providing further evidence that compromised myelin may contribute to the deficits in frontal white matter integrity seen in human cocaine users. Furthermore, analysis of mRNA levels of these proteins revealed no cocaine-related differences, suggesting that their reduced expression in these white matter tracts may be due to factors other than perturbations in gene transcription.
The present findings are consistent with DTI disruptions that have been interpreted as indicative of compromised myelin in the corpus callosum of cocaine users (Moeller et al., 2007). The group differences in fractional anisotropy observed in this study were attributed to an increase in water diffusion perpendicular to the fiber tract, which previously has been linked to reductions in myelin (Song et al., 2002, 2005).
The anatomically restricted nature of the effects observed in both the PLP and MBP protein levels is also consistent with many previous findings in imaging studies of human addicts, in which compromised white matter has been observed primarily in frontal regions of the brain (Bell et al., 2011; Lane et al., 2010; Lim et al., 2002; Lyoo et al., 2004; Moeller et al., 2005; 2007; Romero et al., 2010). They stand in contrast to findings in rodents, however, where deficits have been restricted to the more posterior portions of the corpus callosum (Narayana et al., 2009), emphasizing the importance of nonhuman primate models.
The lack of effects of cocaine self-administration on PLP and MBP gene expression in white matter at the striatal level, although consistent with a study which failed to see MBP or PLP DNA methylation changesin cocaine-exposed rats (Nielsen et al., 2012), was surprising, given the results of previous studies in which these mRNAs were down-regulated in post-mortem studies of human cocaine abusers (Albertson et al., 2004; Bannon et al., 2005; Kristiansen et al., 2009; Lehrmann et al., 2003). These studies focused primarily on transcripts in striatal and prefrontal cortical tissue, however, and although in one report PLP mRNA was altered in the internal capsule (Kristiansen et al., 2009), measurements were not made in samples of callosal or other subcortical white matter, and this difference in the anatomical targets between these two studies and the present investigation may underlie the discrepant findings. In the case of MBP, for example, protein synthesis occurs at the oligodendritic/neuronal contact site, where multiple signaling mechanisms regulate translation, posttranslational modifications, and incorporation into the myelin sheath (Muller et al., 2013). Here, biosynthesis, modification, and trafficking are tightly regulated to respond in a regionally-specific manner to local axonal requirements.
Likewise, the lack of effects in PLP mRNA in white matter tracts seen in this study contrast with the aforementioned reports of reductions in PLP mRNA in striatal and cortical gray matter. Compared to the corpus callosum, however, these areas are rich in both dopaminergic and glutamatergic terminals, and since both transmitters can be toxic to oligodendrocytes (Dewar et al., 2003; Hemdan and Almazan, 2007; Khorchid et al., 2002; Matute et al., 2001; Rosin et al., 2005; Stys, 2004), it is possible that aberrant signaling due to cocaine exposure may have deleterious consequences for oligodendrocytes in these circuits, while callosal oligodendrocytes may be spared. Indeed, along with reductions in gene expression, Albertson and colleagues (2004) did observe a marked decrease in MBP immunoreactive cells in the nucleus accumbens of cocaine abusers.
At least in response to chronic cocaine exposure, then, protein synthesis may be differentially regulated depending upon the nature of regionally-specific neuroadaptations in different neurocircuits. Based on these findings it seems possible that the decreases in protein levels we observed in the corpus callosum and corona radiata may be due to perturbations in one or more of the post-transcriptional events leading up to, and including, protein insertion into the plasma membrane, rather than gene expression per se.
It must be stated that this study employed a very small number of both samples and anatomical levels. The significance of these preliminary findings, however, is that they support those from the human literature, suggesting that cocaine is, indeed, the causative factor for the white matter deficits reported elsewhere. Further investigations into the negative impact of cocaine on myelin are needed to address questions of mechanisms, persistence of effects, and functional consequences of compromised myelin.
ACKNOWLEDGMENTS
The authors wish to acknowledge Siwei Wang for her valuable technical assistance and Tonya Moore for her excellent work conducting the nonhuman primate behavioral experiments.
ROLE OF FUNDING SOURCE
This work was supported by NIH grants DA09085 and DA06634. The funding agencies (NIH/NIDA) had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONTRIBUTORS
LJP, TJRB, and MAN designed the nonhuman primate behavioral and neurobiological studies within the context of a collaborative investigation into the effects of prolonged exposure to cocaine self-administration.
HRS and LJP designed, and HRS conducted, the western blot and in situ hybridization experiments.
Image analysis, statistical analysis, creation of figures, and major manuscript drafting was conducted by HRS with significant input from LJP.
All authors have approved the manuscript.
CONFLICT OF INTEREST
No conflicts declared.
References
- Albertson DN, Pruetz B, Schmidt CJ, Kuhn DM, Kapatos G, Bannon MJ. Gene expression profile of the nucleus accumbens of human cocaine abusers: evidence for dysregulation of myelin. J. Neurochem. 2004;88:1211–1219. doi: 10.1046/j.1471-4159.2003.02247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannon M, Kapatos G, Albertson D. Gene expression profiling in the brains of human cocaine abusers. Addict. Biol. 2005;10:119–126. doi: 10.1080/13556210412331308921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell RP, Foxe JJ, Nierenberg J, Hoptman MJ, Garavan H. Assessing white matter integrity as a function of abstinence duration in former cocaine-dependent individuals. Drug Alcohol Depend. 2011;114:159–168. doi: 10.1016/j.drugalcdep.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beveridge TJ, Smith HR, Nader MA, Porrino LJ. Effects of chronic cocaine selfadministration on norepinephrine transporters in the nonhuman primate brain. Psychopharmacology (Berl.) 2005;180:781–788. doi: 10.1007/s00213-005-2162-1. [DOI] [PubMed] [Google Scholar]
- Beveridge TJ, Smith HR, Nader MA, Porrino LJ. Abstinence from chronic cocaine selfadministration alters striatal dopamine systems in rhesus monkeys. Neuropsychopharmacology. 2009;34:1162–1171. doi: 10.1038/npp.2008.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Monte SM. Disproportionate atrophy of cerebral white matter in chronic alcoholics. Arch. Neurol. 1988;45:990–992. doi: 10.1001/archneur.1988.00520330076013. [DOI] [PubMed] [Google Scholar]
- Dewar D, Underhill SM, Goldberg MP. Oligodendrocytes and ischemic brain injury. J. Cereb. Blood Flow Metab. 2003;23:263–274. doi: 10.1097/01.WCB.0000053472.41007.F9. [DOI] [PubMed] [Google Scholar]
- Dolan RJ, Poynton AM, Bridges PK, Trimble MR. Altered magnetic resonance white-matter T1 values in patients with affective disorder. Br. J. Psychiatry. 1990;157:107–110. doi: 10.1192/bjp.157.1.107. [DOI] [PubMed] [Google Scholar]
- Hanlon CA, Dufault DL, Wesley MJ, Porrino LJ. Elevated gray and white matter densities in cocaine abstainers compared to current users. Psychopharmacology (Berl.) 2011a;218:681–692. doi: 10.1007/s00213-011-2360-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanlon CA, Wesley MJ, Stapleton JR, Laurienti PJ, Porrino LJ. The association between frontal-striatal connectivity and sensorimotor control in cocaine users. Drug Alcohol Depend. 2011b;115:240–243. doi: 10.1016/j.drugalcdep.2010.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemdan S, Almazan G. Deficient peroxide detoxification underlies the susceptibility of oligodendrocyte progenitors to dopamine toxicity. Neuropharmacology. 2007;52:1385–1395. doi: 10.1016/j.neuropharm.2007.01.019. [DOI] [PubMed] [Google Scholar]
- Hudkins M, O'Neill J, Tobias MC, Bartzokis G, London ED. Cigarette smoking and white matter microstructure. Psychopharmacology (Berl.) 2012;221:285–295. doi: 10.1007/s00213-011-2621-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly C, Zuo XN, Gotimer K, Cox CL, Lynch L, Brock D, Imperati D, Garavan H, Rotrosen J, Castellanos FX, Milham MP. Reduced interhemispheric resting state functional connectivity in cocaine addiction. Biol. Psychiatry. 2011;69:684–692. doi: 10.1016/j.biopsych.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khorchid A, Fragoso G, Shore G, Almazan G. Catecholamine-induced oligodendrocyte cell death in culture is developmentally regulated and involves free radical generation and differential activation of caspase-3. Glia. 2002;40:283–299. doi: 10.1002/glia.10123. [DOI] [PubMed] [Google Scholar]
- Kovalevich J, Corley G, Yen W, Rawls SM, Langford D. Cocaine-induced loss of white matter proteins in the adult mouse nucleus accumbens is attenuated by administration of a beta-lactam antibiotic during cocaine withdrawal. Am. J. Pathol. 2012;181:1921–1927. doi: 10.1016/j.ajpath.2012.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristiansen LV, Bannon MJ, Meador-Woodruff JH. Expression of transcripts for myelin related genes in postmortem brain from cocaine abusers. Neurochem. Res. 2009;34:46–54. doi: 10.1007/s11064-008-9655-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane SD, Steinberg JL, Ma L, Hasan KM, Kramer LA, Zuniga EA, Narayana PA, Moeller FG. Diffusion tensor imaging and decision making in cocaine dependence. PLoS One. 2010;5:e11591. doi: 10.1371/journal.pone.0011591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrmann E, Oyler J, Vawter MP, Hyde TM, Kolachana B, Kleinman JE, Huestis MA, Becker KG, Freed WJ. Transcriptional profiling in the human prefrontal cortex: evidence for two activational states associated with cocaine abuse. Pharmacogenomics J. 2003;3:27–40. doi: 10.1038/sj.tpj.6500146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letchworth SR, Sexton T, Childers SR, Vrana KE, Vaughan RA, Davies HM, Porrino LJ. Regulation of rat dopamine transporter mRNA and protein by chronic cocaine administration. J. Neurochem. 1999;73:1982–1989. [PubMed] [Google Scholar]
- Lim KO, Choi SJ, Pomara N, Wolkin A, Rotrosen JP. Reduced frontal white matter integrity in cocaine dependence: a controlled diffusion tensor imaging study. Biol. Psychiatry. 2002;51:890–895. doi: 10.1016/s0006-3223(01)01355-5. [DOI] [PubMed] [Google Scholar]
- Lim KO, Wozniak JR, Mueller BA, Franc DT, Specker SM, Rodriguez CP, Silverman AB, Rotrosen JP. Brain macrostructural and microstructural abnormalities in cocaine dependence. Drug Alcohol Depend. 2008;92:164–172. doi: 10.1016/j.drugalcdep.2007.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyoo IK, Streeter CC, Ahn KH, Lee HK, Pollack MH, Silveri MM, Nassar L, Levin JM, Sarid-Segal O, Ciraulo DA, Renshaw PF, Kaufman MJ. White matter hyperintensities in subjects with cocaine and opiate dependence and healthy comparison subjects. Psychiatry Res. 2004;131:135–145. doi: 10.1016/j.pscychresns.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Ma L, Hasan KM, Steinberg JL, Narayana PA, Lane SD, Zuniga EA, Kramer LA, Moeller FG. Diffusion tensor imaging in cocaine dependence: regional effects of cocaine on corpus callosum and effect of cocaine administration route. Drug Alcohol Depend. 2009;104:262–267. doi: 10.1016/j.drugalcdep.2009.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L, Steinberg JL, Hasan KM, Narayana PA, Kramer LA, Moeller FG. Stochastic dynamic causal modeling of working memory connections in cocaine dependence. Hum. Brain Mapp. 2012 doi: 10.1002/hbm.22212. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matute C, Alberdi E, Domercq M, Perez-Cerda F, Perez-Samartin A, Sanchez-Gomez MV. The link between excitotoxic oligodendroglial death and demyelinating diseases. Trends Neurosci. 2001;24:224–230. doi: 10.1016/s0166-2236(00)01746-x. [DOI] [PubMed] [Google Scholar]
- McHugh MJ, Demers CH, Braud J, Briggs R, Adinoff B, Stein EA. Striatal-insula circuits in cocaine addiction: implications for impulsivity and relapse risk. Am. J. Drug Alcohol Abuse. 2013;39:424–432. doi: 10.3109/00952990.2013.847446. [DOI] [PubMed] [Google Scholar]
- Miller JA. The calibration of 35S or 32P with 14C-labeled brain paste or 14C-plastic standards for quantitative autoradiography using LKB Ultrofilm or Amersham Hyperfilm. Neurosci. Lett. 1991;121:211–214. doi: 10.1016/0304-3940(91)90687-o. [DOI] [PubMed] [Google Scholar]
- Moeller FG, Hasan KM, Steinberg JL, Kramer LA, Dougherty DM, Santos RM, Valdes I, Swann AC, Barratt ES, Narayana PA. Reduced anterior corpus callosum white matter integrity is related to increased impulsivity and reduced discriminability in cocaine-dependent subjects: diffusion tensor imaging. Neuropsychopharmacology. 2005;30:610–617. doi: 10.1038/sj.npp.1300617. [DOI] [PubMed] [Google Scholar]
- Moeller FG, Hasan KM, Steinberg JL, Kramer LA, Valdes I, Lai LY, Swann AC, Narayana PA. Diffusion tensor imaging eigenvalues: preliminary evidence for altered myelin in cocaine dependence. Psychiatry Res. 2007;154:253–258. doi: 10.1016/j.pscychresns.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Monnig MA, Caprihan A, Yeo RA, Gasparovic C, Ruhl DA, Lysne P, Bogenschutz MP, Hutchison KE, Thoma RJ. Diffusion tensor imaging of white matter networks in individuals with current and remitted alcohol use disorders and comorbid conditions. Psychol. Addict. Behav. 2012;27:455–465. doi: 10.1037/a0027168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller C, Bauer NM, Schafer I, White R. Making myelin basic protein -from mRNA transport to localized translation. Front. Cell. Neurosci. 2013;7:169. doi: 10.3389/fncel.2013.00169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nader MA, Daunais JB, Moore T, Nader SH, Moore RJ, Smith HR, Friedman DP, Porrino LJ. Effects of cocaine self-administration on striatal dopamine systems in rhesus monkeys: initial and chronic exposure. Neuropsychopharmacology. 2002;27:35–46. doi: 10.1016/S0893-133X(01)00427-4. [DOI] [PubMed] [Google Scholar]
- Narayana PA, Ahobila-Vajjula P, Ramu J, Herrera J, Steinberg JL, Moeller FG. Diffusion tensor imaging of cocaine-treated rodents. Psychiatry Res. 2009;171:242–251. doi: 10.1016/j.pscychresns.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen DA, Huang W, Hamon SC, Maili L, Witkin BM, Fox RG, Cunningham KA, Moeller FG. Forced abstinence from cocaine self-administration is associated with DNA methylation changes in myelin genes in the corpus callosum: a preliminary study. Front. Psychiatry. 2012;3:60. doi: 10.3389/fpsyt.2012.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul RH, Grieve SM, Niaura R, David SP, Laidlaw DH, Cohen R, Sweet L, Taylor G, Clark RC, Pogun S, Gordon E. Chronic cigarette smoking and the microstructural integrity of white matter in healthy adults: a diffusion tensor imaging study. Nicotine Tob. Res. 2008;10:137–147. doi: 10.1080/14622200701767829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Huang XF, Toga AW. The Rhesus Monkey Brain in Stereotaxic Coordinates. San Diego, CA: Academic Press; 2000. [Google Scholar]
- Romero MJ, Asensio S, Palau C, Sanchez A, Romero FJ. Cocaine addiction: diffusion tensor imaging study of the inferior frontal and anterior cingulate white matter. Psychiatry Res. 2010;181:57–63. doi: 10.1016/j.pscychresns.2009.07.004. [DOI] [PubMed] [Google Scholar]
- Rosin C, Colombo S, Calver AA, Bates TE, Skaper SD. Dopamine D2 and D3 receptor agonists limit oligodendrocyte injury caused by glutamate oxidative stress and oxygen/glucose deprivation. Glia. 2005;52:336–343. doi: 10.1002/glia.20250. [DOI] [PubMed] [Google Scholar]
- Song SK, Sun SW, Ramsbottom MJ, Chang C, Russell J, Cross AH. Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. Neuroimage. 2002;17:1429–1436. doi: 10.1006/nimg.2002.1267. [DOI] [PubMed] [Google Scholar]
- Song SK, Yoshino J, Le TQ, Lin SJ, Sun SW, Cross AH, Armstrong RC. Demyelination increases radial diffusivity in corpus callosum of mouse brain. Neuroimage. 2005;26:132–140. doi: 10.1016/j.neuroimage.2005.01.028. [DOI] [PubMed] [Google Scholar]
- Stys PK. White matter injury mechanisms. Curr. Mol. Med. 2004;4:113–130. doi: 10.2174/1566524043479220. [DOI] [PubMed] [Google Scholar]
- Wang Y, Jia Y, Xu G, Ling X, Liu S, Huang L. Frontal white matter biochemical abnormalities in first-episode, treatment-naive patients with major depressive disorder: a proton magnetic resonance spectroscopy study. J. Affect Disord. 2012;136:620–626. doi: 10.1016/j.jad.2011.10.020. [DOI] [PubMed] [Google Scholar]