Abstract
Tobacco smoking has been shown to be quite addictive in people. However, nicotine itself is a weak reinforcer compared to other commonly abused drugs, leading speculation that other factors contribute to the high prevalence of tobacco addiction in the human population. In addition to nicotine, there are over 5000 chemical compounds that have been identified in tobacco smoke, and more work is needed to ascertain their potential contributions to tobacco’s highly addictive properties, or as potential candidates for smoking cessation treatment. In this study, we examined seven non-nicotine tobacco constituent compounds (anabasine, anatabine, nornicotine, myosmine, harmane, norharmane, and tyramine) for their effects on nicotine self-administration behavior in rats. Young adult female Sprague-Dawley rats were allowed to self-administer nicotine (0.03 mg/kg/ 50 μl infusion) under a fixed ratio-1 schedule of reinforcement. Each self-administration session lasted 45 min. Doses of each tobacco constituent compound were administered subcutaneously 10 min prior to the start of each session in a repeated measures, counterbalanced order two times. Anabasine displayed a biphasic dose-effect function. Pretreatment with 0.02 mg/kg anabasine resulted in a 25% increase in nicotine self-administration, while 2.0 mg/kg of anabasine reduced nicotine infusions per session by over 50%. Pretreatment with 2.0 mg/kg anatabine also significantly reduced nicotine self-administration by nearly half. These results suggest that some non-nicotine tobacco constituents may enhance or reduce nicotine’s reinforcing properties. Also, depending upon the appropriate dose, some of these compounds may also serve as potential smoking cessation agents.
Keywords: Anabasine, Anatabine, Myosmine, Nornicotine, Harmane, Norharmane, Tyramine, Nicotine, Self-administration
1. INTRODUCTION
As the use of tobacco products constitutes the leading cause of preventable death worldwide, and at a cost of approximately 193 billion dollars annually in the United States alone, there continues to be a need to understand more intricately the nature of tobacco addiction as well as a need to develop a greater arsenal of pharmacological tools to effectively reduce its use among tobacco addicts. While much progress has been made in the development of pharmacotherapies to treat tobacco addiction, the majority of tobacco users are typically unsuccessful at remaining abstinent permanently, even after multiple cessation attempts (Benowitz, 2010). Many of the currently available smoking cessation agents focus on replacing the nicotine from tobacco products (nicotine patches, gum, nasal spray, lozenges), or targeting nicotinic acetylcholine receptors (varenicline). Bupropion, which inhibits monoamine reuptake, also has direct nicotinic effects (Lukas et al., 2010). This approach has been pragmatic and intuitive, as nicotine is widely accepted as the primary reinforcing agent found in tobacco that causes addiction in humans (Rose and Corrigall, 1997). However, there are over 5000 chemical compounds present in tobacco smoke, many of which likely exert their own effects on the brain (Rose, 2006). Indeed, previous studies have suggested that minor alkaloids found in the tobacco leaf augment the effects of nicotine in rats (Clemens et al., 2009), or are reinforcing in their own right (Bardo et al., 1999). These findings are made all the more intriguing when one considers that despite the high prevalence of nicotine addiction among the human population, in laboratory settings nicotine itself is viewed as a relatively weak reinforcer, particularly in comparison with other drugs of abuse (Bespalov et al., 1999, Manzardo et al., 2002). It remains a priority to elucidate which of these non-nicotine compounds found in tobacco may contribute to its highly addictive properties. Conversely, just as the nicotine itself contained in tobacco has been utilized as a treatment for nicotine addiction (Levin et al., 1994), these compounds should be evaluated for their potential to also serve as pharmacological treatment agents. Therefore, the purpose of this study was to determine the effects of acute treatment of seven of these non-nicotine tobacco compounds in a rat model of nicotine self-administration.
Anabasine, anatabine, nornicotine, and myosmine are all minor alkaloids present in the tobacco leaf (Huang and Hsieh, 2007). Each of these compounds shares a chemical structure closely related to nicotine, and most have been shown to have affinity for nicotinic acetylcholine receptors (nAChRs) (Crooks and Dwoskin, 1997, Maciuk et al., 2008). Anabasine and nornicotine have been the most studied of these four compounds, each having been shown to at least partially substitute for nicotine in drug discrimination tasks (Brioni et al., 1994, Desai et al., 1999), and evoke midbrain dopamine release from rat striatal slices (Dwoskin et al., 1993, Dwoskin et al., 1995). Nornicotine has also been found to support self-administration in rats on its own, although this was accomplished using very high concentrations of the compound (Bardo et al., 1999).
The β-carboline alkaloids harmane and norharmane are also found in the tobacco leaf and have been shown to bind to several neurotransmitter receptors in the brain (Adell et al., 1996, Husbands et al., 2001, Melchior and Collins, 1982). The two compounds may also be formed in cigarette smoke or in vivo by chemical reactions between indolamines and aldehydes (e.g., acetaldehyde) that are created by combustion of tobacco (poly)saccharides (Cao et al., 2007). Like the minor alkaloids discussed above, both compounds elicit dopamine efflux in mesolimbic dopamine neurons, in what has been described as a dose-dependent, U-shaped manner (Arib et al., 2010, Baum et al., 1996). However, with regard to tobacco use, harmane and norharmane have received much more attention for their ability to inhibit both isoforms of monoamine oxidase (MAO). It is generally accepted that harmane is an inhibitor of monoamine oxidase A (MAO-A) and norharmane inhibits monamine oxidase B (MAO-B), although there is evidence that norharmane is an inhibitor of both enzymes (for review, see van Amsterdam et al., 2006). It has previously been shown that monoamine oxidase inhibition enhances nicotine self-administration in rats (Guillem et al., 2005), and it is believed that the abrupt discontinuation of this inhibition potentiates withdrawal symptoms in smokers who attempt to quit. Interestingly, the monoamine tyramine, another compound present in the tobacco leaf (Songstad et al., 1991), is a substrate for MAO-A. The inhibition of MAO-A in tobacco users by the β-carboline alkaloids could lead to the potentiation of tyramine’s sympathomimetic effects in the periphery.
The current studies were conducted to determine the interactive effects of a group of tobacco constituents on nicotine self-administration in rats. The hypothesis was that compounds that impact nicotinic receptors directly or have effects of inhibiting MAO activity would significantly affect nicotine self-administration. The characterization of the interactions of nicotine with other tobacco compounds could help increase understanding of why tobacco use is so addictive.
2. MATERIALS AND METHODS
2.1 Subjects
Young adult female Sprague-Dawley rats (8 weeks old at the start of the study) were purchased from Taconic Laboratories (Germantown, NY, USA), and used in the self-administration studies. The rats were singly housed in a vivarium at Duke University adjacent to the testing room under standard laboratory conditions. Single housing for the rats was necessary to prevent catheter damage from cagemates. All animals were kept on a 12:12 reverse light/dark cycle so that behavioral testing was performed during the animals’ active phase. Animals were allowed unlimited access to water while in their home cages and were fed a restricted diet of standard rat chow after completing each testing session. All testing procedures in this study were approved by the Duke University Animal Care and Use Committee and conducted according to AAALAC guidelines.
2.2 Materials
Nicotine hydrogen tartrate, tyramine HCl, anabasine, myosmine, harmane, and norharmane were purchased from Sigma-Aldrich (St. Louis, MO, USA). D, L Nornicotine was purchased from Matrix Scientific (Columbia, SC, USA), and D,L anatabine was purchased from Fisher Scientific (Pittsburgh, PA, USA). Nicotine, nornicotine, tyramine HCl, myosmine, anabasine and anatabine were dissolved in 0.9% sterile saline (Hospira Inc, Lake Forest, IL, USA). Harmane and norharmane were both dissolved in a solution containing equal parts sterile saline and DMSO (Sigma-Aldrich), which also served as the vehicle for these two compounds. All tobacco constituent solutions were injected (s.c.) in a volume of 1.0 ml/kg body weight.
2.3 Surgical procedures
Using aseptic technique, a sterile catheter (SAI Infusion Technologies, Libertyville, IL, USA) was surgically implanted into the jugular vein of each animal. Animals were anesthetized with a combination of ketamine (60 mg/kg i.p.) and dexmedetomidine (0.15 mg/kg i.p.). Under general anesthesia, an incision was made lateral to the midline and the jugular vein isolated via dissection. The vein was then tied off distal to the desired area of nick incision. A small incision was then made in the jugular and the catheter inserted until just outside the heart. The cannula was sutured to deep muscle and the remaining portion was routed subcutaneously around the back such that it emerged between the scapulae. The cannula was then connected to an infusion harness (SAI Infusion Technologies, Libertyville, Ill). All surgical wounds were sutured using polypropylene sutures and treated with the topical analgesic bupivacaine. Each animal was administered ketoprofen (5.0 mg/kg, s.c.) for postoperative pain. After surgery the catheters were flushed daily with a combination of sterile saline and heparin (0.25 ml/day). Upon completion of each self-administration session, the nicotine remaining in the harness ports was removed and replaced with a sterile lock solution containing heparinized saline and gentamicin (8mg/ml, Butler Schein Animal Health, Dublin, OH, USA) as an antibiotic.
2.4 Behavioral procedures
Animals were initially trained to receive a food reward via lever response under an FR1 schedule of reinforcement. Once the animal reached criteria for lever response training (3 consecutive 30 min sessions of ≥ 50 pellets earned) catheterization surgery was performed and nicotine self-administration sessions begun. Sessions were conducted inside dual lever operant chambers (30.5 × 24.1 × 21.0 cm) (Med Associates, St. Albans, VT, USA) with a response on one of the levers resulting in the delivery of an infusion of nicotine (0.03 mg/kg/50 μl infusion) and a response on the other lever having no consequence. The dose of 0.03 mg/kg of nicotine per infusion is widely used and was chosen based on previous work demonstrating that this dose produced the most robust self-administration response to nicotine in rats (Corrigall and Coen, 1989). An illuminated cue light placed above the response lever served as a visual secondary reinforcer by indicating an active lever. The active lever for each animal was the same lever on which the animal was trained to respond for food rewards. A response on the active lever resulted in the delivery of a nicotine infusion (50-μl over less than 1 sec) and the activation of a feedback tone for 0.5 sec. Each infusion of nicotine was followed by a 1 min. timeout period in which the cue light was extinguished and lever responses were recorded but no infusions were delivered. Each session lasted 45 min. All sessions were programmed and recorded using MED-PC software.
2.5 Acute treatment with tobacco constituents
Acute treatment with each compound began after the animals completed 5 baseline sessions of nicotine self-administration. Each compound was tested in a separate cohort of animals. All compounds were injected subcutaneously (s.c.) 10 min prior to behavioral testing. Treatment with each dose of compound was given in a repeated measures, counterbalanced design twice, in a randomized order for each animal. Doses for each compound were chosen based on previous literature searches (Baum et al, 1996; Clemens et al, 2009; Arib et al, 2010) and were as follows: tyramine 0.3, 1.0, and 3.0 mg/kg; anabasine, anatabine, myosmine, and nornicotine 0.02, 0.2, 1.0 and 2.0 mg/kg; harmane and norharmane 0.1, 0.3, 1.0 and 3.0 mg/kg. All doses of each compound used in the study were evaluated for possible general suppressant effects on food motivated lever responding. No significant effects on food motivated responding were observed.
2.6 Data analysis
All averaged data sets are presented as the mean ± S.E.M. The data for each experiment were analyzed via repeated measures ANOVA using Supernova/Statview software (SAS, Cary, NC, USA). Statistical significance was defined by an α level of p < 0.05 (two-tailed) with planned comparisons made for each dose of compound with its respective vehicle control.
3. RESULTS
3.1 nAChR Ligands
3.1.1 Anabasine
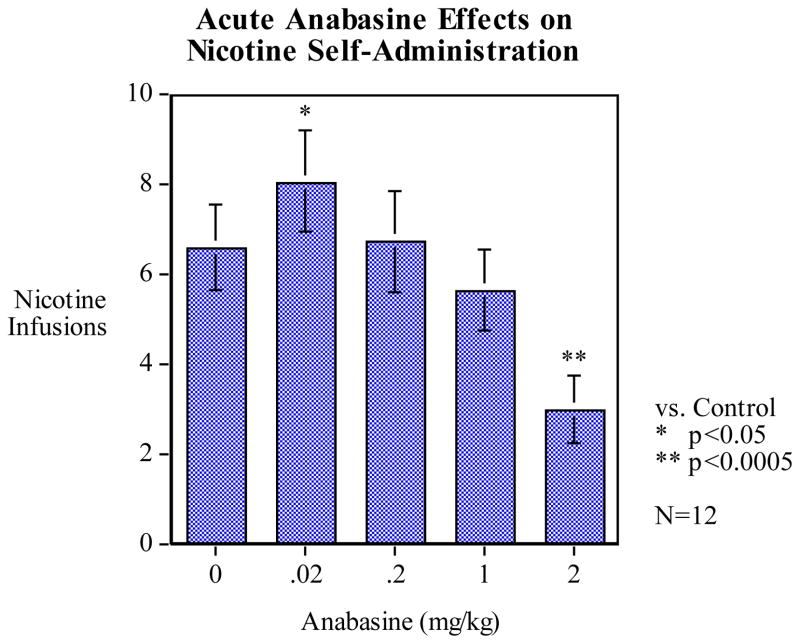
The results of acute anabasine treatment on nicotine self-administration are presented in Figure 1. Pretreatment with anabasine produced a significant main effect of dose (F4, 44 = 17.92, p < 0.01). Post-hoc analysis of anabasine treatment on nicotine self-administration revealed a biphasic effect of dose: the lowest dose given (0.02 mg/kg) significantly increased average nicotine infusions per session (p < 0.05) by nearly 25% (mean = 8.04 ± 0.95) compared to vehicle control (0.0 mg/kg, mean = 6.58 ± 0.83), while the highest dose (2.0 mg/kg) significantly decreased infusions (p < 0.01) compared to vehicle by more than half (mean = 2.96 ± 0.58). There were no significant differences in nicotine infusions per sessions for the 0.2 and 1.0 mg/kg doses of anabasine compared to vehicle.
Figure 1.
Effects of anabasine pretreatment on nicotine self-administration (mean ± S.E.M.). Statistical analysis revealed that anabasine treatment resulted in a non-linear, biphasic dose-response effect on nicotine infusions per session. Post-hoc analysis showed that the lowest dose of 0.02 mg/kg significantly increased average infusions per session (mean 8.04 ± 0.95, p < 0.05) compared to vehicle control (0.0 mg/kg, mean 6.58 ± 0.83). The highest dose of 2.0 mg/kg anabasine significantly reduced average infusions per session (mean 2.96 ± 0.58) compared to vehicle treatment. (n = 12)
3.1.2 Anatabine
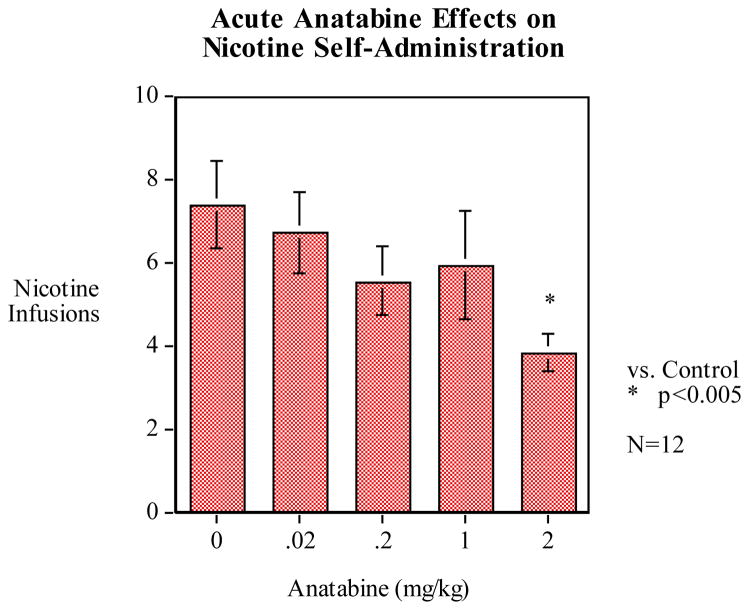
Analysis of variance showed a significant main effect of the dose of anatabine on nicotine self-administration (F4, 44 = 3.727, p < 0.05). Anatabine pretreatment resulted in a general trend toward a dose-dependent reduction of nicotine infusions per session compared to vehicle pretreatment (Fig. 2). Post-hoc analysis revealed a nearly significant reduction in average nicotine infusions per session at the 0.2 mg/kg dose (p = 0.08, mean = 5.54 ± 0.77), compared to vehicle control (mean = 7.38 ± 0.83) while the highest dose of 2.0 mg/kg significantly reduced infusions per session by nearly 50% (p < 0.01, mean = 3.83 ± 0.58).
Figure 2.
Pretreatment with anatabine trended toward a dose-dependent reduction of nicotine self-administration (mean ± S.E.M.). There was a nearly significant reduction of nicotine self-administration at the 0.2 mg/kg dose of anatabine (mean 5.54 ± 0.77, p = 0.08), compared to vehicle control (avg. 7.38 ± 0.83) while a statistically significant reduction of average infusions per session was observed at the 2.0 mg/kg dose (p < 0.005, mean 3.83 ± 0.58). (n = 12)
3.1.3 Nornicotine and Myosmine
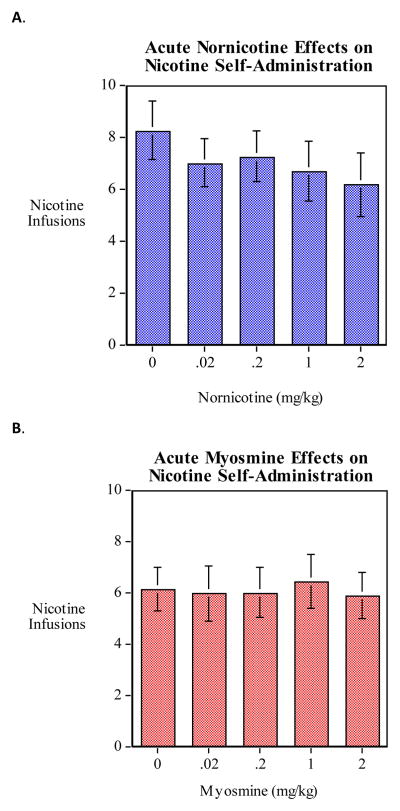
The results of pretreatment of nornicotine and myosmine on nicotine self-administration are presented in Fig. 3. There were no significant differences in average nicotine infusions per session observed between constituent pretreatment and vehicle pretreatment for either nornicotine or myosmine, although there was a trend toward a significant decrease in infusions (p < 0.07) for nornicotine.
Figure 3.
Neither pretreatment with nornicotine (A, n = 12) nor myosmine (B, n = 12) resulted in significant effects on nicotine self-administration.
3.2 MAO Inhibitors
3.2.1 Harmane and Norharmane
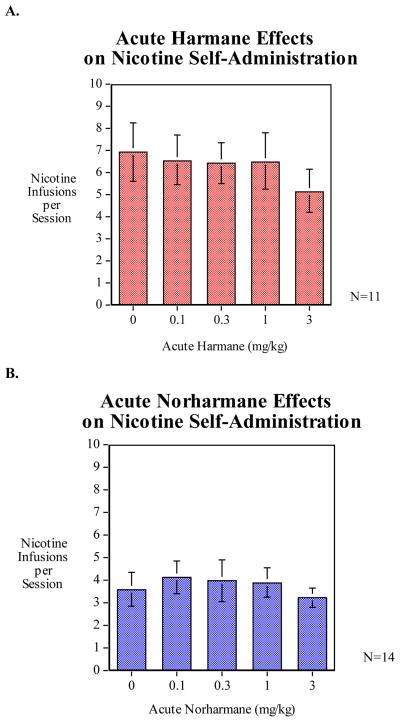
There was a trend (p < 0.10) toward a decrease in nicotine self-administration for harmane treatment that did not reach the level of significance (Fig. 4A). The average nicotine infusions per session for the 3.0 mg/kg group was 5.14 ± 0.693 compared to the vehicle treatment average of 6.96 ± 0.977. There were no significant differences in nicotine self-administration for animals pretreated with norharmane (Fig. 4B).
Figure 4.
No significant differences in nicotine infusions per sessions were observed after pretreatment with either harmane (A, n = 11) or norharmane (B, n = 14)
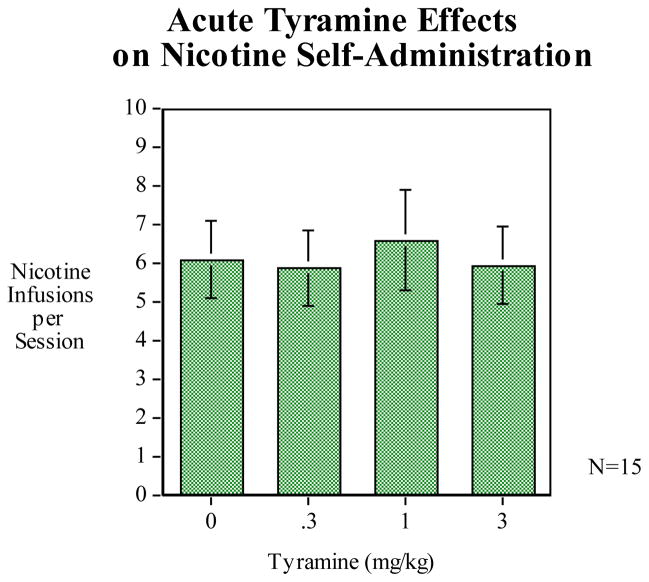
3.3 Tyramine
Pretreatment with tyramine under a dose range of 0.1, 0.3, and 3.0 mg/kg did not significantly affect nicotine self-administration in the rats (Fig. 5).
Figure 5.
Tyramine pretreatment did not produce any significant effects on nicotine self-administration (n = 15).
4. DISCUSSION
Surprisingly little research has been published concerning the behavioral effects of non-nicotine compounds contained in tobacco smoke. However, interest in these constituent compounds, and their possible contributions to tobacco addiction, is rapidly increasing. Recently, a review publication has highlighted much of what has been shown of the abuse potential of some of the most well-known of these compounds (Hoffman and Evans, 2013), but their effects on nicotine self-administration have still been largely ignored. In this study, we have elucidated some of those effects. The results of the present study will inform future studies designed to co-infuse solutions of non-nicotine tobacco constituent compounds with nicotine during self-administration, to better model the human condition of smoking.
Pretreatment with anabasine resulted in a non-linear dose effect in which the lowest tested dose significantly increased nicotine self-administration while the highest dose tested significantly decreased this behavior. As stated previously, nicotine itself is regarded as a relatively weak reinforcer compared to other drugs of abuse. Nonetheless, it has been demonstrated repeatedly that rats will reliably (if not necessarily robustly) self-administer nicotine. The most effective doses for acquisition of nicotine self-administration during short-access sessions have been shown to be 0.03 mg/kg and 0.06 mg/kg per infusion, although the patterns of behavior at these two doses may differ (Caille et al., 2012, Donny et al., 1998). Lower or higher doses of nicotine have been found to maintain even lower nicotine self-administration in rats due to either lack of reinforcing or possible aversive effects, respectively (Caille et al., 2012, Corrigall and Coen, 1989, Donny et al., 1998). We chose the dose of 0.03 mg/kg of nicotine as it is typically the most common dose used and the most optimal for behavior. Therefore, because this dose represents the top of the inverted U-shaped dose-response curve to nicotine, an increase in self-administration could be interpreted as increasing the reinforcing efficacy of nicotine, while conversely a decrease in this behavior could be interpreted as interfering with nicotine’s reinforcing effects or adding to its aversive effects.
An increase in nicotine infusions per-session by pretreatment with anabasine in this case was not entirely unanticipated, as our lowest dose chosen (0.02 mg/kg) was derived from a single study in which a cocktail of minor tobacco alkaloids + nicotine was shown to increase self-administration in rats when compared with nicotine alone (Clemens et al., 2009). Importantly, the doses for the minor alkaloids chosen in that study were based on published literature showing the concentrations of these compounds found in cigarette smoke (Liu et al., 2008), so these doses should be relevant to human smokers. Compared to nicotine, anabasine displays a lower EC50 for eliciting GABA release, and a higher IC50 for inducing desensitization of nAChRs (Lu et al., 1999). It is possible that greater agonist effects at lower concentrations of anabasine prime nicotine self-administration, resulting in the significant increase of nicotine infusions per session observed in our study. Considering these findings, it therefore follows that lower doses of anabasine on the level typically found in cigarette smoke likely significantly contribute to the reinforcing effects of tobacco, and further research into this minor alkaloid is warranted.
That the highest dose of anabasine (2.0 mg/kg) reduced nicotine self-administration could potentially be explained as a result of the compound’s own reinforcing effects. While no studies have been published regarding the self-administration of anabasine, as stated previously the compound has affinity for nAChRs and evokes DA release in the striatum. Previous studies have also shown that doses of anabasine similar to our highest dose can substitute for nicotine in drug discrimination procedures (Brioni et al., 1994, Pratt et al., 1983, Stolerman et al., 1984). It has long been accepted that in short-access self-administration paradigms, nicotine exhibits an inverted U-shaped dose-response curve, typically with our chosen dose of 0.03 mg/kg eliciting the greatest response (Corrigall and Coen, 1989). As previously mentioned, anabasine displays a higher IC50 for inducing desensitization of nAChRs (Lu et al., 1999). It is therefore possible that higher concentrations of anabasine desensitize nAChRs more effectively, resulting in a reduction of nicotine self-administration. It should be noted that 2.0 mg/kg of anabasine is a particularly high dose; the distribution of which in the body is likely higher than what would be experienced by active smoking. Somewhat similarly, it is also possible that at higher doses anabasine acts as an antagonist in the presence of nicotine, similar to what has been proposed regarding the effects of the α4β2 nAChR partial agonist varenicline in self-administration paradigms (Rollema et al., 2007).
Nicotine self-administration was significantly reduced by pretreatment with our highest dose of 2.0 mg/kg of anatabine, and there was also a nearly significant reduction of nicotine self-administration at our dose of 0.2 mg/kg. Indeed, pretreatment across our entire chosen dose range of anatabine resulted in an insignificant trend toward a dose-dependent reduction of nicotine infusions per session. While both anabasine and anatabine have been proposed as biomarkers for tobacco use (Jacob et al., 1999), anabasine has garnered more attention concerning abuse potential and behavioral effects. There is currently increasing interest in anatabine, meanwhile, for its potential anti-inflammatory properties (Paris et al., 2013). However, both compounds share very similar chemical structures (Rodgman, 2009), and like anabasine, anatabine has been shown to interact with nAChRs (Maciuk et al., 2008). Anatabine has also been shown to augment nicotine’s effects on locomotor activity (Clemens et al., 2009). As of this writing, no publications could be found regarding definitively anatabine’s ability to potentiate striatal DA release. However, considering the above evidence, this is well within the realm of possibility. All of this combined with the results of our current study suggests that anatabine could be an intriguing candidate compound possible development for smoking cessation.
Although there were no significantly different effects on nicotine self-administration for the other compounds we tested in this study, it would still nonetheless be unwise to dismiss these compounds as having no contributing role to tobacco addiction or potential as cessation treatments. To wit, pretreatment with nornicotine in our study resulted in a general trend toward a dose dependent reduction of nicotine self-administration, but did not reach the level of statistical significance. Moreover, previous studies have shown that at doses higher than those which we employed, nornicotine significantly reduces nicotine self-administration (Green et al., 2000), and alters locomotor response to nicotine (Dwoskin et al., 1999). Likewise, while pretreatment with either β-carboline alkaloid in this study was without a significant main effect, it has been shown that sufficient MAO-I activity can increase nicotine self-administration in rats (Guillem et al., 2005) and can also augment locomotor and reward responses to nicotine (Villegier et al., 2006). However, it should be noted that the effects were seen in these studies using irreversible MAO-I’s and that Villegier et al (2006) concluded that harmane and norharmane might not contribute to tobacco addiction because the inhibition of MAO-A and B by both compounds is reversible. There is also evidence that myosmine can enhance nicotine’s effects on locomotor activity (Clemens et al., 2009), indicating that this compound may also contribute, in some fashion, to nicotine’s effects. These findings, with the addition of our current study, might suggest an additive effect of each compound. However, given all this, the most intriguing evidence from Clemens et al. (2009) and Villegier et al. (2006) suggests that a synergistic relationship between these tobacco constituent compounds and nicotine results in greater reward potentiation than nicotine alone. As concluded by Clemens et al (2009), it is possible that the minor alkaloids in tobacco could act as allosteric modulators at nAChRs, producing partial desensitization of the receptors, a phenomenon proposed previously regarding the pharmacological actions of cotinine (Buccafusco et al., 2007). Future studies should determine whether this is the case. In any event, the results of this study show that non-nicotine tobacco constituent compounds may contribute to the addictive properties of tobacco and also may be viable options for development as smoking cessation agents.
Highlights.
Lower dose anabasine increases nicotine self-administration in rats
Higher dose anabasine decreases nicotine self-administration in rats
Higher dose anatabine decreases nicotine self-administration in rats
Non-nicotine tobacco alkaloids influence self-administration of nicotine.
Acknowledgments
This study was supported by a P50 center grant from the National Institute on Drug Abuse (NIDA) DA027840.
Footnotes
Conflicts of Interest
JER: Patent purchase and consulting agreements with Philip Morris International. Consultancy agreements with Targacept, Inc. and Novartis International AG.
EDL: Research sponsored by AstraZeneca Pharmaceuticals.
Other Authors: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adell A, Biggs TA, Myers RD. Action of harman (1-methyl-beta-carboline) on the brain: body temperature and in vivo efflux of 5-HT from hippocampus of the rat. Neuropharmacology. 1996;35:1101–7. doi: 10.1016/s0028-3908(96)00043-3. [DOI] [PubMed] [Google Scholar]
- Arib O, Rat P, Molimard R, Chait A, Faure P, de Beaurepaire R. Electrophysiological characterization of harmane-induced activation of mesolimbic dopamine neurons. European journal of pharmacology. 2010;629:47–52. doi: 10.1016/j.ejphar.2009.12.012. [DOI] [PubMed] [Google Scholar]
- Bardo MT, Green TA, Crooks PA, Dwoskin LP. Nornicotine is self-administered intravenously by rats. Psychopharmacology. 1999;146:290–6. doi: 10.1007/s002130051119. [DOI] [PubMed] [Google Scholar]
- Baum SS, Hill R, Rommelspacher H. Harman-induced changes of extracellular concentrations of neurotransmitters in the nucleus accumbens of rats. European journal of pharmacology. 1996;314:75–82. doi: 10.1016/s0014-2999(96)00543-2. [DOI] [PubMed] [Google Scholar]
- Benowitz NL. Nicotine addiction. The New England journal of medicine. 2010;362:2295–303. doi: 10.1056/NEJMra0809890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bespalov A, Lebedev A, Panchenko G, Zvartau E. Effects of abused drugs on thresholds and breaking points of intracranial self-stimulation in rats. European Neuropsychopharmacology. 1999;9:377–83. doi: 10.1016/s0924-977x(99)00008-5. [DOI] [PubMed] [Google Scholar]
- Brioni JD, Kim DJ, O’Neill AB, Williams JE, Decker MW. Clozapine attenuates the discriminative stimulus properties of (-)-nicotine. Brain research. 1994;643:1–9. doi: 10.1016/0006-8993(94)90002-7. [DOI] [PubMed] [Google Scholar]
- Buccafusco JJ, Shuster LC, Terry AV., Jr Disconnection between activation and desensitization of autonomic nicotinic receptors by nicotine and cotinine. Neuroscience letters. 2007;413:68–71. doi: 10.1016/j.neulet.2006.11.028. [DOI] [PubMed] [Google Scholar]
- Caille S, Clemens K, Stinus L, Cador M. Modeling nicotine addiction in rats. Methods in molecular biology. 2012;829:243–56. doi: 10.1007/978-1-61779-458-2_15. [DOI] [PubMed] [Google Scholar]
- Cao R, Peng W, Wang Z, Xu A. beta-Carboline alkaloids: biochemical and pharmacological functions. Current medicinal chemistry. 2007;14:479–500. doi: 10.2174/092986707779940998. [DOI] [PubMed] [Google Scholar]
- Clemens KJ, Caille S, Stinus L, Cador M. The addition of five minor tobacco alkaloids increases nicotine-induced hyperactivity, sensitization and intravenous self-administration in rats. International Journal of Neuropsychopharmacology. 2009;12:1355–66. doi: 10.1017/S1461145709000273. [DOI] [PubMed] [Google Scholar]
- Corrigall WA, Coen KM. Nicotine maintains robust self-administration in rats on a limited-access schedule. Psychopharmacology. 1989;99:473–8. doi: 10.1007/BF00589894. [DOI] [PubMed] [Google Scholar]
- Crooks PA, Dwoskin LP. Contribution of CNS nicotine metabolites to the neuropharmacological effects of nicotine and tobacco smoking. Biochem Pharmacol. 1997;54:743–53. doi: 10.1016/s0006-2952(97)00117-2. [DOI] [PubMed] [Google Scholar]
- Desai RI, Barber DJ, Terry P. Asymmetric generalization between the discriminative stimulus effects of nicotine and cocaine. Behavioral Pharmacology. 1999;10:647–56. doi: 10.1097/00008877-199911000-00011. [DOI] [PubMed] [Google Scholar]
- Donny EC, Caggiula AR, Mielke MM, Jacobs KS, Rose C, Sved AF. Acquisition of nicotine self-administration in rats: the effects of dose, feeding schedule, and drug contingency. Psychopharmacology. 1998;136:83–90. doi: 10.1007/s002130050542. [DOI] [PubMed] [Google Scholar]
- Dwoskin LP, Buxton ST, Jewell AL, Crooks PA. S(−)-nornicotine increases dopamine release in a calcium-dependent manner from superfused rat striatal slices. J Neurochem. 1993;60:2167–74. doi: 10.1111/j.1471-4159.1993.tb03502.x. [DOI] [PubMed] [Google Scholar]
- Dwoskin LP, Crooks PA, Teng LH, Green TA, Bardo MT. Acute and chronic effects of nornicotine on locomotor activity in rats: altered response to nicotine. Psychopharmacology. 1999;145:442–51. doi: 10.1007/s002130051079. [DOI] [PubMed] [Google Scholar]
- Dwoskin LP, Teng L, Buxton ST, Ravard A, Deo N, Crooks PA. Minor alkaloids of tobacco release [3H]dopamine from superfused rat striatal slices. European journal of pharmacology. 1995;276:195–9. doi: 10.1016/0014-2999(95)00077-x. [DOI] [PubMed] [Google Scholar]
- Green TA, Phillips SB, Crooks PA, Dwoskin LP, Bardo MT. Nornicotine pretreatment decreases intravenous nicotine self-administration in rats. Psychopharmacology. 2000;152:289–94. doi: 10.1007/s002130000524. [DOI] [PubMed] [Google Scholar]
- Guillem K, Vouillac C, Azar MR, Parsons LH, Koob GF, Cador M, et al. Monoamine oxidase inhibition dramatically increases the motivation to self-administer nicotine in rats. Journal of Neuroscience. 2005;25:8593–600. doi: 10.1523/JNEUROSCI.2139-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman AC, Evans SE. Abuse potential of non-nicotine tobacco smoke components: acetaldehyde, nornicotine, cotinine, and anabasine. Nicotine & Tobacco Research. 2013;15:622–32. doi: 10.1093/ntr/nts192. [DOI] [PubMed] [Google Scholar]
- Huang HY, Hsieh SH. Analyses of tobacco alkaloids by cation-selective exhaustive injection sweeping microemulsion electrokinetic chromatography. J Chromatogr A. 2007;1164:313–9. doi: 10.1016/j.chroma.2007.06.065. [DOI] [PubMed] [Google Scholar]
- Husbands SM, Glennon RA, Gorgerat S, Gough R, Tyacke R, Crosby J, et al. beta-carboline binding to imidazoline receptors. Drug and alcohol dependence. 2001;64:203–8. doi: 10.1016/s0376-8716(01)00123-5. [DOI] [PubMed] [Google Scholar]
- Jacob P, 3rd, Yu L, Shulgin AT, Benowitz NL. Minor tobacco alkaloids as biomarkers for tobacco use: comparison of users of cigarettes, smokeless tobacco, cigars, and pipes. American journal of public health. 1999;89:731–6. doi: 10.2105/ajph.89.5.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ED, Westman EC, Stein RM, Carnahan E, Sanchez M, Herman S, et al. Nicotine skin patch treatment increases abstinence, decreases withdrawal symptoms, and attenuates rewarding effects of smoking. Journal of clinical psychopharmacology. 1994;14:41–9. [PubMed] [Google Scholar]
- Liu B, Chen C, Wu D, Su Q. Enantiomeric analysis of anatabine, nornicotine and anabasine in commercial tobacco by multi-dimensional gas chromatography and mass spectrometry. Journal of Chromatography B, Analytical Technologies in the Biomedical and Life Sciences. 2008;865:13–7. doi: 10.1016/j.jchromb.2008.01.034. [DOI] [PubMed] [Google Scholar]
- Lu Y, Marks MJ, Collins AC. Desensitization of nicotinic agonist-induced [3H]gamma-aminobutyric acid release from mouse brain synaptosomes is produced by subactivating concentrations of agonists. J Pharmacol Exp Ther. 1999;291:1127–34. [PubMed] [Google Scholar]
- Lukas RJ, Muresan AZ, Damaj MI, Blough BE, Huang X, Navarro HA, et al. Synthesis and characterization of in vitro and in vivo profiles of hydroxybupropion analogues: aids to smoking cessation. Journal of medicinal chemistry. 2010;53:4731–48. doi: 10.1021/jm1003232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maciuk A, Moaddel R, Haginaka J, Wainer IW. Screening of tobacco smoke condensate for nicotinic acetylcholine receptor ligands using cellular membrane affinity chromatography columns and missing peak chromatography. J Pharmaceut Biomed. 2008;48:238–46. doi: 10.1016/j.jpba.2007.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzardo AM, Stein L, Belluzzi JD. Rats prefer cocaine over nicotine in a two-lever self-administration choice test. Brain research. 2002;924:10–9. doi: 10.1016/s0006-8993(01)03215-2. [DOI] [PubMed] [Google Scholar]
- Melchior C, Collins MA. The route and significance of endogenous synthesis of alkaloids in animals. Critical reviews in toxicology. 1982;9:313–56. doi: 10.3109/10408448209037496. [DOI] [PubMed] [Google Scholar]
- Paris D, Beaulieu-Abdelahad D, Abdullah L, Bachmeier C, Ait-Ghezala G, Reed J, et al. Anti-inflammatory activity of anatabine via inhibition of STAT3 phosphorylation. European journal of pharmacology. 2013;698:145–53. doi: 10.1016/j.ejphar.2012.11.017. [DOI] [PubMed] [Google Scholar]
- Pratt JA, Stolerman IP, Garcha HS, Giardini V, Feyerabend C. Discriminative stimulus properties of nicotine: further evidence for mediation at a cholinergic receptor. Psychopharmacology. 1983;81:54–60. doi: 10.1007/BF00439274. [DOI] [PubMed] [Google Scholar]
- Rodgman A, Perfetti TA. The chemical components of tobacco and tobacco smoke. Boca Raton, FL: Taylor & Francis Group, LLC; 2009. [Google Scholar]
- Rollema H, Chambers LK, Coe JW, Glowa J, Hurst RS, Lebel LA, et al. Pharmacological profile of the alpha4beta2 nicotinic acetylcholine receptor partial agonist varenicline, an effective smoking cessation aid. Neuropharmacology. 2007;52:985–94. doi: 10.1016/j.neuropharm.2006.10.016. [DOI] [PubMed] [Google Scholar]
- Rose JE. Nicotine and nonnicotine factors in cigarette addiction. Psychopharmacology. 2006;184:274–85. doi: 10.1007/s00213-005-0250-x. [DOI] [PubMed] [Google Scholar]
- Rose JE, Corrigall WA. Nicotine self-administration in animals and humans: similarities and differences. Psychopharmacology. 1997;130:28–40. doi: 10.1007/s002130050209. [DOI] [PubMed] [Google Scholar]
- Songstad DD, Kurz WGW, Nessler CL. Tyramine Accumulation in Nicotiana-Tabacum Transformed with a Chimeric Tryptophan Decarboxylase Gene. Phytochemistry. 1991;30:3245–6. [Google Scholar]
- Stolerman IP, Garcha HS, Pratt JA, Kumar R. Role of training dose in discrimination of nicotine and related compounds by rats. Psychopharmacology. 1984;84:413–9. doi: 10.1007/BF00555223. [DOI] [PubMed] [Google Scholar]
- van Amsterdam J, Talhout R, Vleeming W, Opperhuizen A. Contribution of monoamine oxidase (MAO) inhibition to tobacco and alcohol addiction. Life Sci. 2006;79:1969–73. doi: 10.1016/j.lfs.2006.06.010. [DOI] [PubMed] [Google Scholar]
- Villegier AS, Salomon L, Granon S, Changeux JP, Belluzzi JD, Leslie FM, et al. Monoamine oxidase inhibitors allow locomotor and rewarding responses to nicotine. Neuropsychopharmacol. 2006;31:1704–13. doi: 10.1038/sj.npp.1300987. [DOI] [PubMed] [Google Scholar]