Abstract
Platelets are well-known for their critical role in hemostasis, i.e. the prevention of blood loss at sites of mechanical vessel injury. Inappropriate platelet activation and adhesion, however, can lead to thrombotic complications, such as myocardial infarction and stroke. To fulfill its role in hemostasis, the platelet is equipped with various G protein-coupled receptors (GPCRs) that mediate the response to soluble agonists such as thrombin, ADP, and thromboxane A2. In addition to GPCRs, platelets express three glycoproteins (GP) that belong to the family of immunoreceptor tyrosine-based activation motif (ITAM) receptors: Fc receptor (FcR) γ chain, which is non-covalently associated with the GPVI collagen receptor, C-type lectin 2 (CLEC2), the receptor for podoplanin, and FcγRIIA, a low-affinity receptor for immune complexes. While both genetic and chemical approaches have documented a critical role for platelet GPCRs in hemostasis, the contribution of ITAM receptors to this process is less defined. Studies performed over the last decade, however, have identified new roles for platelet ITAM signaling in vascular integrity in utero and at sites of inflammation. The purpose of this review is to summarize recent findings on how platelet ITAM signaling controls vascular integrity, both in the presence and absence of mechanical injury.
Keywords: platelets, signaling, hemostasis, inflammation, vascular integrity
1. Platelet ITAM signaling in hemostasis and thrombosis
A monolayer of endothelial cells separates the lumen of blood vessels from the extracellular matrix (ECM). Upon mechanical disruption of this monolayer, blood gets into contact with the various components of the ECM 1. Proteins soluble in blood, such as von Willebrand factor (VWF) and clotting factors, are rapidly deposited in the ECM. As a consequence, platelets are recruited to the site of injury, followed by their activation and firm adhesion. Transient adhesion of platelets (tethering) depends largely on VWF and its receptor, the GPIb-V-IX complex. Firm adhesion requires the inside-out activation of integrin receptors such as αIIbβ3, α2β1, and α6β1, followed by interaction with their respective ligands in the ECM (see below). Critical to the activation process is signaling provided by GPCRs, agonist receptors triggered by soluble mediators such as thrombin (a main protease of the coagulation cascade) or thromboxane (Tx)A2 and ADP (agonists released by activated platelets and red blood cells 2). The molecular mechanisms underlying the various stages of thrombus formation at sites of vascular injury have been reviewed extensively 3, 4 and will thus not be discussed in detail. Instead, we will summarize important findings on the contribution of ITAM signaling to platelet adhesion at sites of vascular injury.
1.1 GPVI
Collagen has long been known to be a potent activator of platelets as well as a strong adhesive ligand by which activated platelets may adhere to an injured vessel wall in the face of arterial shear forces. These two platelet responses to collagen are mediated by structurally and functionally distinct receptors, GPVI and integrin α2β15, 6. GPVI is an Ig domain-containing transmembrane protein that is structurally homologous to immune-type receptors. Its short cytoplasmic tail does not have signaling activity but facilitates the non-covalent association with the ITAM-containing FcRγ chain. Collagen is an abundant protein in the body that is secreted into the ECM predominantly by fibroblasts 7. The basement membrane underneath the EC layer contains mostly type IV collagen. Unlike the fibrillar collagens type I, III, and V, which are found in deeper layers of the ECM, type IV collagen forms 2-dimensional networks. Compared to fibrillar collagens, type IV collagen shows lower platelet activating activity8. However, collagen type IV is important for GPVI-dependent platelet activation at sites of superficial vascular injury 9. Interestingly, GPVI is less critical for thrombus formation at sites of more severe injury as documented in various (mouse) models of thrombotic disease 9. Consistent with this finding, the bleeding times in humans 10 and mice 11 with defective GPVI function are only minimally prolonged. The likely explanation for this unexpected result is that thrombin, produced via the tissue factor-dependent coagulation pathway, provides a strong enough stimulus for platelet activation in the absence of GPVI engagement. It is important to note, however, that the reverse is not true. Deficiency in PAR4, the main GPCR expressed on mouse platelets, cannot be compensated for by signaling via GPVI 12, 13. The hemITAM receptor CLEC2 may also contribute to platelet activation and thrombus formation in the deeper layers of the ECM (see below). In addition to collagen, GPVI also binds laminins, heterotrimeric glycoproteins consisting of α, β, and γ chains 7. Laminins, in particular α4 and α5 laminins, are predominantly found in the basement membrane. GPVI has an ~10-fold lower affinity for laminin when compared to collagen 14, a fact that may explain why binding of platelets to laminin via integrin α6β1 is a prerequisite for the GPVI-laminin interaction. Elegant studies by Mangin and colleagues recently confirmed the critical role of α6β1 for platelet adhesion at sites of vascular injury 15. Using α6-deficient mice, the authors demonstrated that α6β1 is critical for platelet adhesion to α4 and α5 laminins in vitro and for normal thrombus formation in vivo. In contrast, platelets deficient in GPVI were normal in their ability to adhere to laminin in vitro, while they showed a minor activation defect specifically to α5 laminins. However, binding of GPVI to laminin may play a bigger role in vivo, where laminin is found together with type IV collagen in the basement membrane.
1.2 CLEC2
CLEC2 was identified in platelets as a receptor for the snake venom toxin rhodocytin and for the transmembrane glycoprotein Podoplanin (PDPN; aka T1alpha, gp38 and Aggrus) 16. CLEC2 is highly expressed on platelets but it is also found at a lower level on immune cells (dendritic cells, monocyte/macrophages, neutrophils) 17, 18. The intracellular domain of CLEC2 contains a single YxxL motif (known as a hemiITAM motif) 6. Clustering of CLEC2 by PDPN triggers a powerful platelet signaling response similar to that established for GPVI and FcγRIIA. Podoplanin is a heavily glycosylated type 1 transmembrane protein expressed in a variety of tissues: kidney podocytes, type-1 lung alveolar cells, lymphatic endothelial cells, the nervous sytem and metastatic tumour cells 19. The importance of CLEC2 in hemostasis remains controversial. Various studies using mice deficient in CLEC2 showed normal hemostasis in these mice 20, 21, 22. One study in mice depleted of CLEC2 by infusion of a monoclonal antibody to the receptor observed a significant prolongation of the tail bleeding time 23, while a similar treatment did not affect hemostasis in a different study 22. The study by Bender et al. further investigated the effect of combined deficiency in CLEC2 and GPVI on hemostasis 22. While antibody-induced depletion of both receptors from circulating platelets led to a marked prolongation of the tail bleeding time in mice, the defect in hemostasis was much more subtle in animals with a genetic deficiency in both receptors. It is important to remember that both approaches to eliminate CLEC2 from platelets have limitations. Antibody-induced depletion of CLEC2 may affect platelet activation in ways that are independent of CLEC2. Such off-target effects were shown for antibodies to GPVI, which when injected into mice transiently affect thrombin activation of platelets 24. On the other hand, genetic deletion of CLEC2 affects the integrity of blood vessels and allows blood to enter the lymphatic circulation (see below), alterations that could certainly affect the bleeding time in mice. Assuming there is a role for CLEC2 in hemostasis, the obvious question is how platelet CLEC2 signaling is initiated at the site of vascular injury. Expression of podoplanin, the only known endogenous ligand of CLEC2, has not been documented in cells of the vascular wall 25. Blood cells, including platelets, also lack podoplanin. Thus, a critical role for CLEC2 in hemostasis would suggest the existence of a CLEC2 ligand other than podoplanin, or a homotypic interaction between CLEC2 receptors as recently suggested 26. Further studies are needed to clarify the underlying mechanism.
1.3 FcγRIIA
Another ITAM receptor expressed on human platelets is a low affinity member of the Fcγ (IgG binding) family of Fc receptors, FcγRIIA (CD32A). Mice lack the genetic equivalent of human FcγRIIA, but transgenic mice expressing the human receptor 27 have been used to study its biology in vivo. FcγRIIA allows platelets to play a role in innate immunity by binding to and becoming activated by pathogens opsinized with antibody, thus speeding their clearance 28, 29, 30, 31. Platelet activation through the platelet Fcγ receptor, FcγRIIA, also plays a critical role in the pathogenesis of various immune-mediated thrombocytopenia and thrombosis (ITT) syndromes, including heparin-induced thrombocytopenia and thrombosis (HIT) 32–34, bacterial sepsis–associated thrombocytopenia and disseminated intravascular coagulation (DIC) 28, 29 and the varied thrombotic manifestations in the antiphospholipid syndromes (APLSS) 35.
At sites of vascular injury, FcγRIIA supports thrombus formation via its contribution to integrin outside-in signaling. As shown by Newman and colleagues 36, 37, increased integrin signaling in platelets expressing human FcγRIIA leads to significantly better spreading, aggregation and adhesion to collagen under flow in vitro. Consistently, thrombus formation was significantly increased in mice expressing human FcγRIIA when compared to WT controls.
1.4 Signaling downstream of (hem)ITAM receptors
Critical to the signaling activity of (hem)ITAM receptors are the cytosolic YXXL motifs which when phosphorylated serve as a docking site for SH2 domain containing signaling molecules 38, 39. Typical ITAM receptors such as GPVI/Fcγ chain and FcγRIIA contain two cytosolic YXXL motifs, separated by 6–12 residues, which facilitate the binding of the two SH2 domains of the non-receptor tyrosine kinase Syk 40, 6. CLEC2 contains only one such motif in its cytoplasmic tail. To enable Syk binding to two phosphorylated hemITAM motifs, CLEC2 exists as a homodimer on the cell surface 41. The binding and activation of Syk is a critical event in the formation of a signalosome consisting of various adapter and effector proteins. Central to the formation of the signalosome is linker for activation of T cells (LAT), which is localized to lipid rafts 42, 43. Phosphorylated LAT recruits the adapter proteins Grb2, Gads, and SLP-7644. Both LAT and SLP-76 45 contribute to the binding of phospholipase Cγ2 (PLCγ2), the enzyme required for the generation of the second messengers calcium (Ca2+) and diacylglycerol (DAG). Genetic deficiency in mice of any of the proteins mentioned above leads to severely impaired ITAM signaling in platelets. Other adapters and effectors that associate with the ITAM signalosome, such as Signal Transducer and Activator of Transcription 3 (STAT3) 46, the small GTPase Rac1 47, 48, 49 and its exchange factors Vav1 and Vav3 50–52, the tyrosine kinases Btk 53 and Tec 54, or various PI3 kinase isoforms 55 also contribute to effective signaling. Downstream of PLCγ2, the small GTPase Rap1 orchestrates various cellular responses, including integrin activation 56, 57, 58 TxA2 formation 59, and granule release 49, 60. The guanine nucleotide exchange factor, CalDAG-GEFI (RasGRP2), senses increased levels of cytosolic Ca2+ and facilitates the rapid but reversible activation of Rap1. DAG leads to delayed Rap1 activation via stimulation of protein kinase C-dependent granule release and feedback activation through the Gi-coupled receptor for ADP, P2Y12. Platelet ITAM signaling strongly depends on the Ca2+/CalDAG-GEFI/Rap1 pathway as deficiency in CalDAG-GEFI protects mice from collagen- and immune complex-induced thrombosis 57, 61. It is important to remember, however, that signaling molecules downstream of PLCγ2 such as CalDAG-GEFI and PKC are also critical for PLCβ-dependent GPCR signaling in platelets. Thus, we focused on literature that evaluated hemostasis and thrombosis in humans and animals with defects in signaling proteins upstream of PLCγ2 (table 1). Interestingly, genetic deletion or inhibition of Syk, a molecule central to signaling by all ITAM receptors on platelets, protects from thrombosis but does not affect hemostasis in mice 62, 63. In contrast, mice deficient in PLCγ2 are protected from experimental thrombosis but also exhibit a marked defect in hemostasis 64, 65, 66. A comprehensive analysis of the hemostasis and thrombosis phenotypes in mice with defects in signaling molecules upstream of PLCγ2 is shown in Table 1. This unexpected discrepancy in results may be explained by different experimental approaches used in the respective labs or it may reflect the well-documented role of PLCγ2 signaling downstream of ligand binding to GPIbα 66, 67, 68. In summary, these studies document that ITAM signaling plays a minor role for platelet adhesion at sites of vascular injury when compared to signaling via platelet GPCRs.
Table 1.
Table: A comprehensive analysis of the hemostasis and thrombosis phenotypes in mice with defects in signaling molecules upstream of PLCγ2. (↓ mild, ↓↓ moderate, ↓↓↓ severe platelet defect response)
| Molecule | Approach | Ca2+ mobilization | Secretion | Aggregation | Thrombosis | Bleeding time | references |
|---|---|---|---|---|---|---|---|
| Fyn/Lyn | Fyn−/− | ↓ | ↓ alpha granules ↓ dense granules |
↓ (only to low dose agonist) | normal thombus formation on collagen in vitro | normal | 126,127,128 |
| Lyn−/− | ↑ (delay) | ↑ alpha granules ↑ dense granules |
↑ (delay) | ↓ thrombus formation on collagen in vitro ↓ thrombosis after laser injury in arterioles |
normal | ||
| Fyn−/− Lyn−/− | ↓ dense granules | ↓ | ↓ thrombus formation on collagen in vitro | ||||
| Syk | Syk−/− | ↓↓↓ dense granules | ↓↓↓ | ↓↓↓ thrombus formation on collagen in vitro | normal | ||
| inhibitor | ↓↓↓ | instable thrombi on collagen in vitro protective in several models of thrombosis, including photochemical injury to carotid artery, pulmonary thromboembolism, and HIT | normal | 129,62,16,130,131, 132, 63 | |||
| LAT | Lat−/− | ↓↓ dense granules | ↓ (only to low dose agonist) | ↓ thrombus formation on collagen in vitro | normal | 133,134,135,136, 16 | |
| Gad | Gad−/− | ↓↓ dense granules | ↓ (only to low dose agonist) | normal thrombus formation on collagen in vitro | 134, 135 | ||
| Vav | Vav1−/− Vav2−/− Vav3−/− |
Vav2−/−: no defect Vav3−/−: no defect Vav1−/−: ↓ Vav1−/−/Vav2 −/−: ↓ Vav1−/−/Vav3−/−: ↓↓ |
52, 51, 50,137,16 | ||||
| Vav1/2/3−/− | ↓↓↓ | ↓ thrombus formation on collagen in vitro delayed time to occlusion after FeCl3 injury to the carotid artery | |||||
| Slp76 | Slp76−/− | ↓↓↓ | ↓↓↓ alpha granules | ↓↓↓ (overcome at high agonist concentrations) | ↓↓↓ thrombus formation on collagen in vitro | 45,138 | |
| PLCγ2 | PLCγ2−/− | ↓↓ | ↓↓↓ alpha granules ↓↓↓ dense granules |
↓↓↓ | ↓↓↓ thrombus formation on collagen in vitro ↓↓↓ thrombosis in a superficial laser injury model |
↑↑ | 66,139,64,140, 141, 65, 16, 142 |
| Rac1 | Rac1−/− | ↓ | ↓↓↓ alpha granules ↓↓↓ dense granules |
↓ (only to low dose agonist) | ↓↓↓ thrombus formation on collagen in vitro ↓↓↓ arterial thrombosis (laser injury, cremaster) ↓↓↓ arterial thrombosis (carotid artery ligation) |
↑ | 47, 48, 49 |
| inhibitor | ↓ | ↓↓↓ alpha granules ↓↓↓ dense granules |
↓ (only to low dose agonist) | ||||
| STAT3 | inhibitor | ↓↓ (only to low dose agonist) | ↓↓ thrombus formation on collagen in vitro | 46 |
2. Platelet ITAM signaling and blood-lymphatic separation
2.1. Lymphatics in the cardiovascular system
Cardiovascular function requires distinct blood and lymphatic vascular networks to circulate blood and drain interstitial fluid from the periphery 69. The partitioning of these two vascular compartments represents a fundamental adaptation underlying the physiology of mammals and related vertebrates; however, our understanding of the biological processes that give rise to two distinct networks remains incomplete. The lymphatic system performs additional functions that include dietary fat absorption, where it transports chylomicrons from the small intestine to the blood, and adaptive immune responses, where it transports antigens and antigen presenting cells to lymph nodes, connects lymph nodes together, and returns lymphocytes to the blood. Lymphatics form an extensive vascular network that originates during embryonic development from a subset of venous endothelial cells in both the cardinal vein and intersomitic vessels that acquire lymphatic identity. Starting at E9.75 in mice, induction of the transcription factor PROX1 in these venous endothelial cells activates lymphatic identity and these newly specified LECs bud out of the cardinal vein and intersomitic vessels with the help of vascular endothelial growth factor-C (VEGF-C) to form the primary lymph sacs, early structures that give rise to the entire lymphatic network 70, 71. The structure and function of the lymphatic system differ significantly from that of the blood vascular system. The blood vascular system propels blood at high pressures generated by mechanical pumping of the heart in order to circulate blood in a closed loop through pulmonary and systemic vessels. In contrast, lymph fluid is acquired from tissues through a permeable vascular network in which lymph flow is maintained by the contraction of collecting vessel walls, external compression, and valves. Blind ended lymphatic capillaries with loose endothelial junctions absorb interstitial fluid and coalesce into larger collecting vessels that then drain lymph into lymphatic ducts where protein-rich lymph fluid and immune cells are returned to venous blood. A series of intraluminal valves divide lymphatic vessels into functional units called lymphangions that promote forward lymph flow. In addition, a bicuspid lympho-venous (LV) valve is present at the site of connection where lymph drains into blood 72. The LV valves are thought to prevent blood from entering the low pressure lymphatic system, and in humans these valves are found at the right lymphatic duct-subclavian vein junction and the thoracic duct-subclavian vein junction.
2.2. Blood-lymphatic separation during embryonic development
Beginning almost 20 years ago, genetic studies in mice have revealed an unexpected role for ITAM signaling during embryonic vascular development. Mice lacking the essential ITAM signaling effectors Syk, SLP-76, or PLCα2 73, 74, 75, 76, 77 display blood-filled lymphatic vessels during embryonic stages and die postnatally due to impaired lymphatic function. Tissue-specific deletion in the megakaryocyte lineage revealed that ITAM signaling in the platelet mediates blood-lymphatic separation, with upstream components identified through deletion of the platelet CLEC2 receptor and its ligand PDPN expressed on lymphatic endothelial cells (LECs). Mice deficient in CLEC2 or PDPN exhibit blood-filled lymphatics during fetal life and die shortly after birth like mice lacking Syk or SLP-76. While these phenotypes all arise due to loss of platelet activation by lymphatic endothelial cells, it has been unclear precisely how activated platelets affect lymphatic development in embryos. Some studies have used in vitro approaches to suggest that activated platelets may release granule contents that regulate lymphatic endothelial cell growth and that this is therefore an angiogenic role for platelets 76, 78. However, we have recently demonstrated that the entry of blood into the developing lymphatic network in late gestation embryos is through the lympho-venous junction and that the role of platelets is to mediate an unexpected form of inter-vascular hemostasis that supports the lympho-venous valve and prevents blood from entering the thoracic duct 79. An important outstanding question is whether platelet function at the lympho-venous junction or valve can explain the early blood-lymphatic mixing observed in animals lacking platelets or PDPN-CLEC2 signaling to activate them. Deficient embryos exhibit blood-filled lymph sacs as early as E11.5, a timepoint prior to when the lympho-venous junction has been demonstrated to arise during lymphatic development 80, 81. Thus it is possible that platelet CLEC2 signaling mediates inter-vascular hemostasis in a distinct manner at early timepoints in lymphatic growth. While additional studies are needed to address this developmental role, it is clear that the role of platelets in this context is to perform a unique form of hemostasis and not to regulate lymphangiogenesis.
2.3. Blood-lymphatic separation in the adult
Lethally irradiated mature mice transplanted with bone marrow deficient in CLEC2 78, Syk 74 or SLP-76 80 develop blood-filled lymphatic vessels and die due to lymphatic dysfunction, revealing a lifelong requirement for platelet ITAM signaling to maintain blood-lymphatic separation. This role is also supported by inducible postnatal deletion of the O-glycan synthase enzyme required for PDPN expression on LECs, which also produces the blood-filled lymphatic phenotype 80, 82, 74. Platelets and LECs would not be predicted to interact; however, the genetic experiments described above identify a critical interaction that begins in the embryo and continues throughout life where platelets in circulating blood come into contact with LECs to initiate platelet ITAM signaling and regulate blood-lymphatic separation. The site where platelet CLEC2 signaling mediates blood-lymphatic separation was recently determined by examining induced CLEC2-deficiency states in both neonatal and mature animals 79. These studies reveal that mice lacking this signaling pathway develop blood-filled lymphatic vessels through retrograde filling of the lymphatic network with blood despite the presence of LV and lymphatic valves, and also identify the terminal thoracic duct as the location where blood first enters the lymphatic vascular system following loss of CLEC2. The LV valve has been thought to function alone in preventing the back-flow of blood into the LV junction; however, these data suggest that an additional platelet-dependent mechanism has escaped notice. To search for platelets at the terminal thoracic duct, the LV junction was examined and fibrin-containing platelet thrombi were observed within the lymphatic vascular environment of this site (approximately 25%) in wild-type but not CLEC2-deficient mice 79. To study the relationship between valves and platelets at the LV junction, mice heterozygous for Prospero Homeobox Protein 1 (Prox1) or mice lacking the Integrin α9 subunit (Itga9) were analyzed. Recent studies demonstrated that Prox1+/− embryos exhibit lympho-venous valve defects, while Itgα9−/− embryos are characterized by severe lymphatic valve developmental defects 83, 84, 85. These mice were found to have an augmented frequency of LV thrombi (approx. 100%) and clots that extend deeper within the thoracic duct than those observed in wild-type controls, suggesting that platelets can compensate for impaired valve function 79. To stress the LV junction by disabling platelet-mediated hemostasis, Itgb3−/− mice lacking integrin-mediated platelet aggregation 86 were examined. These animals still form clots within the lymphatic vascular environment yet have marked filling of the thoracic duct with blood, suggesting that integrin-mediated platelet aggregation through αIIbβ3 is not needed for thrombus formation in the lymphatic system yet is essential in preventing LV backflow. Together, these data support a hemostatic mechanism of platelet function at the LV junction that maintains blood-lymphatic separation throughout life. Unlike canonical hemostasis which limits hemorrhage from damaged vessels, LV hemostasis operates within an uninjured intravascular environment under low flow, low shear conditions; therefore, the contribution of coagulation and platelet degranulation may differ from arterial or venous thrombosis. Preliminary studies of LV hemostasis have identified a divergent role for integrin-mediated platelet aggregation compared to arterial hemostasis where αIIbβ3 is not required for thrombus growth but does contribute to thrombus stability in the prevention of LV backflow.
These studies highlight an unexpected platelet-dependent hemostatic response that functions alongside the lympho-venous valve to maintain the lymphatic system. The activation of platelet CLEC2 receptors by lymphatic endothelial Podoplanin is first observed during lymphatic development where it prevents blood from entering the immature system at a time when valves are not yet formed. However, genetic and pharmacologic studies demonstrate that the requirement for this hemostatic pathway extends throughout life, including in mature animals in which the lympho-venous valves are fully functional. The basis for this requirement is not yet established, but it is likely that this hemostatic mechanism is necessary to prevent pressure gradients from driving venous blood into lymphatic vessels. Compared to central venous pressure (5–10 mm Hg) the lymphatic pressure is low (1–2 mm Hg). Changes in body position, fluid status or disease states such as congestive heart failure (CHF) can further increase this pressure gradient and thus lead to backflow of blood into lymphatic vessels. Importantly, LV valve insufficiency and reflux of blood into the thoracic duct was recently described for patients with congestive heart failure 87. The identification of this platelet-dependent “safety mechanism” may have clinical implications. First, application of antiplatelet therapies to CHF patients in order to reduce the risk of myocardial infarction and stroke may have detrimental effects on lymphatic function. Since these patients have chronically elevated pulmonary venous pressures it is likely that lymphatic drainage in the lung plays an important role in preventing pulmonary edema. Thus anti-platelet therapies may protect against arterial thrombosis at the expense of lympho-venous hemostasis and worsen CHF symptoms. Second, new drugs targeting the Syk kinase that are intended to treat chronic inflammatory conditions such rheumatoid arthritis, may impair lymphatic function. The fact that these patients are expected to take anti-Syk agents for extended periods of time raises this risk. Our ability to predict the impact of anti-platelet and anti-Syk agents is limited at this time since this pathway has been explored almost exclusively in mouse models. Extending these studies to patients and clinically relevant scenarios will provide needed insight into whether and how impairment of this hemostatic pathway affects human health and disease.
3. Platelet ITAM signaling and vascular integrity in inflammation
3.1 GPVI, atherosclerosis and enhanced vascular permeability
In addition to hemostasis and thrombosis, platelets are important modulators of inflammatory reactions. Their role in inflammation is partially explained by their ability to interact and communicate with leukocytes and vascular cells. These interactions are mediated by various receptor-ligand pairs, including P-selectin – PSGL-1, GPIbα - Mac1, CD40L -CD40, and αIIbβ3 - ICAM1 88, 89. Platelets also deposit chemokines on activated endothelium, thereby enhancing leukocyte recruitment and promoting the progression and propagation of chronic inflammation 90. GPVI is also critically involved in platelet adhesion to activated endothelium. Endothelial dysfunction is considered to be a predictive sign of atherosclerosis in patients and correlates with the progression of the disease 91. GPVI can interact with the activated atherosclerotic endothelium in the absence of plaque rupture. Intravital microscopy studies demonstrated that administration of soluble GPVI-Fc and anti-GPVI (JAQ1) antibody inhibited platelet adhesion to the activated endothelium in ApoE−/− mice 92, 93. As a consequence of this inhibition, endothelial function was improved in atherosclerotic rabbits 93. The role of GPVI in the absence of plaque rupture in atheroprogression is unlikely to be attributed to the interaction with collagen/laminin but was suggested to occur through fibronectin which is secreted by activated platelets or endothelial cells at vascular lesions 93. Similarly, in an experimental animal model of myocardial infarction, recombinant GPVI-Fc molecules bound to activated endothelium via vitronectin and prevented platelet/endothelial interaction thereby reducing infarct size and preserving cardiac function 94.
Platelet ITAM signaling is also critical in inflammatory arthritis. While it was long recognized that platelets from arthritis patients are hyper-responsive 95, it was not clear whether platelets contribute to the progression of the disease. Studies in a mouse model of rheumatoid arthritis, done by Boilard and colleagues, demonstrated such a causal relationship, as mice depleted of all circulating platelets were protected from joint inflammation 96. Interestingly, mice lacking GPVI were also protected from experimental arthritis, suggesting that platelet ITAM signaling is critical for disease progression. Additional work proposed the following underlying mechanisms: exposure of platelets to collagen and laminin at sites of inflammation leads to the production of IL-1-rich, proinflammatory microparticles (MPs) 96, platelet fragments that are small enough to diffuse into the synovial fluid. Alternatively, platelet MPs may be transported into the joint by inflammatory cells. Furthermore, activated platelets may actively weaken the endothelial barrier at sites of inflammation, a process dependent on the release of serotonin from their dense granules 97, 98. Consistent with these findings, GPVI is important for platelet and leukocyte recruitment to the inflamed capillaries in experimental glomerulonephritis 99. Taken together, these studies suggest that GPVI is an attractive therapeutic target in inflammatory diseases beyond hemostasis and thrombosis. To date, the contribution of platelet CLEC2 and/or FcγRIIA to the pathogenesis of these diseases has not been evaluated. Confirmatory studies in mice defective in signaling molecules downstream of GPVI are also missing. Genetic targeting or inhibition in platelets of ITAM signaling molecules like Syk, however, is difficult as they are also critical for proper immune cell function. Furthermore, even platelet-specific targeting such as achieved in megakaryocyte/platelet-specific conditional knockout mice is complicated by the marked defects in vascular development documented for these animals (see above).
3.2 Platelets and maintenance of vascular integrity in inflammation
Platelets have long been recognized to support the integrity of the vasculature 100. Structural endothelial abnormalities such as thinning, fenestration and increased permeability have been shown for severely thrombocytopenic humans as well as animals depleted of virtually all circulating platelets 101, 102. Importantly, however, thrombocytopenia is often not associated with hemorrhage, suggesting that an additional trigger such as inflammation is required for bleeding to occur. This multi-hit concept was confirmed in elegant studies by Wagner and colleagues 103, who showed that acute severe thrombocytopenia in mice does not lead to hemorrhage unless these animals are challenged by inflammation. These studies also showed (a) that thrombocytopenia resulted in hemorrhage only at the site of inflammation, and (b) that important platelet adhesion receptors such as GPIb-V-IX and αIIbβ3 integrin were not required for this platelet function. Thus, the contribution of platelets to the maintenance of vascular integrity in inflammation does not depend on the platelet’s ability to form a hemostatic plug. At this point, very little is known about how platelets protect the inflamed vasculature. Vasoactive factors released from activated platelets may prevent hemorrhage by strengthening EC barrier function or by dampening the inflammatory response 104. Numerous candidate factors, including ADP released from dense granules 105, serpins 106,107 and metalloproteinase inhibitors 108 released from alpha granules, reactive oxygen scavengers 109, TREM-like transcript (TLT)-1 proteolytically shed from the platelets surface 110, 111 and the vasoactive lipid sphingosine-1 phosphate (S1P) 112 have been identified. The extent to which these factors contribute to inflammatory hemostasis, however, is not well-defined.
In addition to our lack of understanding with regard to the platelet protective activity, very little is known about what triggers platelet activation at sites of inflammation and what signaling response is required. Addressing these questions requires mice with platelet-specific signaling defects, as the pathways regulating cellular activation are very similar between platelets, inflammatory and vascular cells. This can be achieved by conditional deletion of genes in the megakaryocyte lineage, an approach that depends on the availability of mice with loxP-flanked genes that can be crossed to mice expressing Cre recombinase under the PF4 promoter 113. As an alternative approach, we recently described a method for the adoptive transfer of platelets into thrombocytopenic mice. In this approach thrombocytopenia is induced in transgenic mice with antibodies that recognize hIL4R, a heterologous antigen expressed on circulating platelets in these animals 114, 115. These mice are then transfused with genetically or chemically inhibited platelets, which are not destroyed by the circulating anti hIL4R antibodies. The main characteristics of this novel tool are: (1) a fast and reliable method to generate mice with platelet-specific signaling defects, (2) the ability to combine genetic and pharmacologic approaches to loss of function studies, and (3) an increased sensitivity for platelet defects due to the ability to establish a lower peripheral platelet count in experimental animals. It is also important to remember that deletion of genes in megakaryocytes/platelets only can lead to marked vascular changes (see above). This limitation is not relevant for the adoptive transfer system. Using this approach, we identified a critical role for GPVI, CLEC2, and the downstream adapter protein SLP-76 in the maintenance of vascular integrity at sites of immune complex-induced inflammation in the skin as well as LPS-induced inflammation in the lung 115. Surprisingly, our studies further demonstrated that platelet GPCR signaling is not required for the maintenance of vascular integrity in inflammation. Unlike platelets lacking functional CLEC2 and/or GPVI, platelets defective in signaling via the major GPCRs responding to thrombin, ADP and TxA2 were fully capable of supporting vascular integrity at sites of inflammation.
Together, these findings confirm the notion that hemostasis at sites of inflammation requires a platelet response(s) different to that important at sites of vascular injury (see Figure 1). Various questions arise from these unexpected findings. For example, while the main ligands for GPVI, collagen and laminin, are highly expressed in the vessel wall, it is not so clear what triggers the activation of CLEC2 at sites of inflammation. Some organs that are particularly vulnerable to hemorrhage such as the lung, the brain and the kidney contain cell types that express high levels of PDPN 19. Other tissues, however, such as the skin do not contain PDPN-positive cells. One possibility is that PDPN is “delivered” to the extravascular space by infiltrating PDPN-positive macrophages 116. Alternatively, a hitherto unrecognized ligand other than PDPN may trigger platelet CLEC2 signaling in these situations. Conditional deletion of PDPN in various tissues will be required to clarify the underlying mechanism. A second important question to be answered is why this novel form of hemostasis depends so strongly on signaling via the ITAM but not the GPCR pathway. It is difficult to imagine that this pathway selectivity simply reflects the availability of agonists at sites of inflammation. Weakening of EC barrier function leads to plasma leakage into the inflamed tissue, followed by the activation of coagulation and the generation of thrombin 117. Furthermore, platelet activation via ITAM receptors and/or PAR4 leads to the release of the second wave mediators ADP and TxA2. Thus, it appears more likely that ITAM receptors trigger a unique platelet response, which is crucial in the setting of inflammation. For example, there is increasing evidence that individual platelet agonist receptors can trigger very distinct granule release reactions, although so far these studies have been focused on platelets activated via GPCRs 118. Better established is the critical role for ITAM signaling in platelet microparticle release and surface phosphatidylserine exposure 119, 120 as well as shedding of surface receptors 121. Both microparticles and soluble glycoproteins could serve as diffusible mediators in a low flow environment such as the inflamed tissue. The identification of the required platelet response(s) will be a critical first step for a better understanding of how these cells safeguard vascular integrity at sites of inflammation.
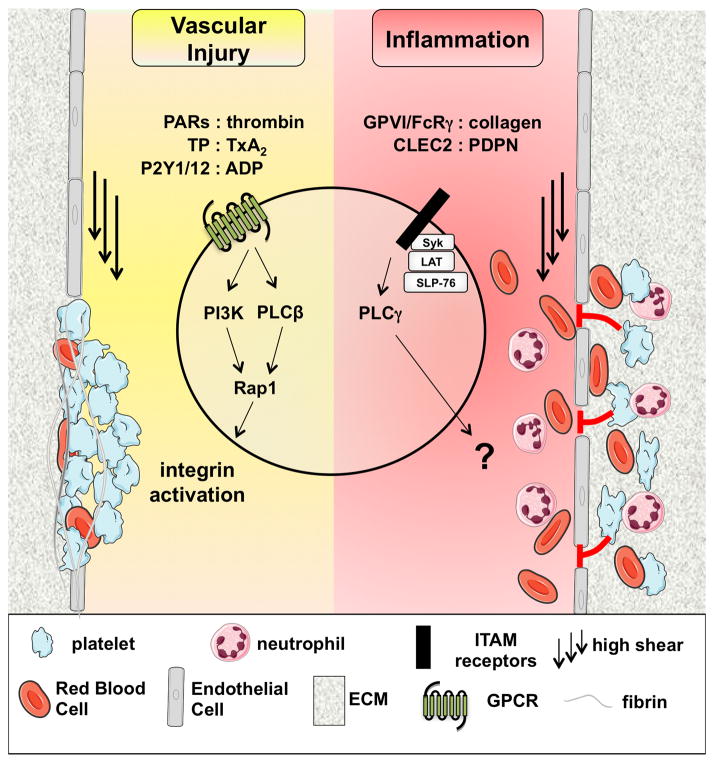
Figure 1. Platelet-dependent hemostasis after vascular injury and at sites of inflammation.
Schematic representation of important molecular mechanisms regulating platelet-dependent hemostasis. At sites of vascular injury, platelet activation and adhesion is strongly dependent on soluble agonists and their respective G protein-coupled receptors (GPCRs) expressed on the platelet surface. Engagement of GPCRs leads to the rapid activation of phospholipase (PL)Cβ2 and PI3 kinase, events that are critical for the activation of the small GTPase Rap1, affinity regulation in platelet integrins, and platelet aggregate formation. The contribution of immunoreceptor tyrosine-based activation motif (ITAM)-coupled receptors to platelet activation at sites of vascular injury is weak when compared to GPCRs. In contrast, hemostasis at sites of inflammation depends primarily on platelet ITAM signaling and is independent of major platelet adhesion receptors. These findings suggest a model where platelets get activated under low/no flow conditions in the extravascular space, leading to the release of soluble factors that secure vascular integrity. Both the signaling response downstream of PLCγ2 and the platelet-derived mediator(s) critical for vascular integrity in inflammation are currently unknown.
PAR: protease activated receptor; TxA2: thromboxane A2; TP: TxA2 receptor; FcRγ: Fc receptor γ-chain; CLEC2: C-type lectin 2; PDPN: podoplanin; PI3K: PI3 kinase; PLC: phospholipase C; ECM: extracellular matrix
In humans, marked thrombocytopenia, such as observed in Idiopathic Thrombocytopenia Purpura, does not necessarily lead to bleeding 122. However, hemorrhage is frequent in patients suffering from Wiskott-Aldrich syndrome, a clinical complication characterized by marked thrombocytopenia, recurrent infections, and an elevated risk for development of autoimmune disease 123, 124. Thus, it seems likely that both thrombocytopenia and an additional “trigger” such as inflammation are required to compromise the integrity of the vasculature in humans. Moreover, patients deficient in GPVI expression and/or function often present with ecchymoses, i.e. hematomas that are not caused by trauma 125, 10, suggesting that humans also depend on platelet ITAM signaling for maintenance of vascular integrity. To date, no patients with defects in CLEC-2 expression/function have been described.
Conclusions
Platelet-mediated hemostasis has classically been defined in the context of plug formation at sites of vessel injury and high fluid shear forces. Platelet integrins and G protein-coupled receptor activation pathways are essential in this context and form the foundation of modern anti-platelet therapies. In contrast, studies of ITAM-coupled platelet receptors are revealing new aspects of hemostasis that extend far beyond classic arterial injury responses. These include inflammatory and lympho-venous hemostasis, processes that depend on platelet ITAM signaling and platelet responses that have yet to be defined. In addition to our deficit in understanding of the basic mechanisms by which platelets safeguard vascular integrity, we lack information on when these inflammatory and LV hemostatic responses are most utilized in normal physiology and under pathophysiologic conditions? Without this knowledge, it will be difficult to predict which patients taking anti-platelet agents are at an increased risk of inflammatory or LV hemorrhage. Future studies addressing these questions have the potential to reveal new roles for platelets in common diseases, and new effects -both good and bad - of anti-platelet therapies in patients.
Supplementary Material
Acknowledgments
The authors thank David S. Paul for help with figure design and formatting.
Sources of funding
This work was funded by a fellowship by the American Heart Association (12POST12040088 to Y. Boulaftali) and by the National Heart, Lung, and Blood Institute, NIH, grants R01 HL106009 (to W. Bergmeier) and HL072798 to (M.L. Kahn)
Nonstandard Abbreviations and Acronyms
- ADP
adenosine diphosphate
- APLSS
anti-phospholipid syndrome
- ApoE
apolipoprotein E
- Btk
Bruton’s tyrosine kinase
- CHF
congestive heart failure
- CLEC2
C-type lectin 2
- DAG
diacylglycerol
- DIC
disseminated intravascular coagulation
- EC
endothelial cell
- ECM
extracellular matrix
- FcRγIIA
Fc receptor γ II A
- Gad
Grb2 adaptor downstream of Shc
- GPIbα
glycoprotein Ib subunitα
- GPCR
G protein-coupled receptors
- GPVI
glycoprotein VI
- Grb2
growth factor receptor bound protein-2
- HIT
heparin-induced thrombocytopenia and thrombosis
- hIL4R
human interleukin 4 receptor
- ITAM
immunoreceptor tyrosine-based activation motif
- ITT
immune-mediated thrombocytopenia and thrombosis
- IL-1
interleukin 1
- LAT
linker for activation of T cells
- LEC
lymphatic endothelial cells
- LV
lympho-venous
- PAR
protease activated receptor
- PDPN
podoplanin
- PI3K
phosphatidyl inositol-3 kinase
- PKC
protein kinase C
- PLC β/γ
phospholipase C β/γ
- PROX1
prospero homeobox protein 1
- SLP76
SH2 containing leukocyte protein of 76 kDa
- TxA2
thromboxane A2
- TP
TxA2 receptor
- VEGF-C
vascular endothelial growth factor C
- VWF
Von Willebrand factor
Footnotes
Disclosures
None
References
- 1.Ruggeri ZM. Platelets in atherothrombosis. Nat Med. 2002;8:1227–1234. doi: 10.1038/nm1102-1227. [DOI] [PubMed] [Google Scholar]
- 2.Helms CC, Marvel M, Zhao W, Stahle M, Vest R, Kato GJ, Lee JS, Christ G, Gladwin MT, Hantgan RR, Kim-Shapiro DB. Mechanisms of hemolysis-associated platelet activation. J Thromb Haemost. 2013 doi: 10.1111/jth.12422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sachs UJ, Nieswandt B. In vivo thrombus formation in murine models. Circ Res. 2007;100:979–991. doi: 10.1161/01.RES.0000261936.85776.5f. [DOI] [PubMed] [Google Scholar]
- 4.Versteeg HH, Heemskerk JW, Levi M, Reitsma PH. New fundamentals in hemostasis. Physiol Rev. 2013;93:327–358. doi: 10.1152/physrev.00016.2011. [DOI] [PubMed] [Google Scholar]
- 5.Kuijpers MJ, Schulte V, Bergmeier W, Lindhout T, Brakebusch C, Offermanns S, Fassler R, Heemskerk JW, Nieswandt B. Complementary roles of glycoprotein vi and alpha2beta1 integrin in collagen-induced thrombus formation in flowing whole blood ex vivo. Faseb J. 2003;17:685–687. doi: 10.1096/fj.02-0381fje. [DOI] [PubMed] [Google Scholar]
- 6.Watson SP, Herbert JM, Pollitt AY. Gpvi and clec-2 in hemostasis and vascular integrity. J Thromb Haemost. 2010;8:1456–1467. doi: 10.1111/j.1538-7836.2010.03875.x. [DOI] [PubMed] [Google Scholar]
- 7.Bergmeier W, Hynes RO. Extracellular matrix proteins in hemostasis and thrombosis. Cold Spring Harb Perspect Biol. 2012:4. doi: 10.1101/cshperspect.a005132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jung SM, Takemura Y, Imamura Y, Hayashi T, Adachi E, Moroi M. Collagen-type specificity of glycoprotein vi as a determinant of platelet adhesion. Platelets. 2008;19:32–42. doi: 10.1080/09537100701609027. [DOI] [PubMed] [Google Scholar]
- 9.Hechler B, Nonne C, Eckly A, Magnenat S, Rinckel JY, Denis CV, Freund M, Cazenave JP, Lanza F, Gachet C. Arterial thrombosis: Relevance of a model with two levels of severity assessed by histologic, ultrastructural and functional characterization. J Thromb Haemost. 2009;8:173–184. doi: 10.1111/j.1538-7836.2009.03666.x. [DOI] [PubMed] [Google Scholar]
- 10.Arthur JF, Dunkley S, Andrews RK. Platelet glycoprotein vi-related clinical defects. Br J Haematol. 2007;139:363–372. doi: 10.1111/j.1365-2141.2007.06799.x. [DOI] [PubMed] [Google Scholar]
- 11.Kato K, Kanaji T, Russell S, Kunicki TJ, Furihata K, Kanaji S, Marchese P, Reininger A, Ruggeri ZM, Ware J. The contribution of glycoprotein vi to stable platelet adhesion and thrombus formation illustrated by targeted gene deletion. Blood. 2003;102:1701–1707. doi: 10.1182/blood-2003-03-0717. [DOI] [PubMed] [Google Scholar]
- 12.Sambrano GR, Weiss EJ, Zheng YW, Huang W, Coughlin SR. Role of thrombin signalling in platelets in haemostasis and thrombosis. Nature. 2001;413:74–78. doi: 10.1038/35092573. [DOI] [PubMed] [Google Scholar]
- 13.Cornelissen I, Palmer D, David T, Wilsbacher L, Concengco C, Conley P, Pandey A, Coughlin SR. Roles and interactions among protease-activated receptors and p2ry12 in hemostasis and thrombosis. Proc Natl Acad Sci U S A. 2010;107:18605–18610. doi: 10.1073/pnas.1013309107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Inoue O, Suzuki-Inoue K, McCarty OJ, Moroi M, Ruggeri ZM, Kunicki TJ, Ozaki Y, Watson SP. Laminin stimulates spreading of platelets through integrin alpha6beta1-dependent activation of gpvi. Blood. 2006;107:1405–1412. doi: 10.1182/blood-2005-06-2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schaff M, Tang C, Maurer E, Bourdon C, Receveur N, Eckly A, Hechler B, Arnold C, de Arcangelis A, Nieswandt B, Denis CV, Lefebvre O, Georges-Labouesse E, Gachet C, Lanza F, Mangin PH. Integrin alpha6beta1 is the main receptor for vascular laminins and plays a role in platelet adhesion, activation, and arterial thrombosis. Circulation. 2013;128:541–552. doi: 10.1161/CIRCULATIONAHA.112.000799. [DOI] [PubMed] [Google Scholar]
- 16.Suzuki-Inoue K, Fuller GL, Garcia A, Eble JA, Pohlmann S, Inoue O, Gartner TK, Hughan SC, Pearce AC, Laing GD, Theakston RD, Schweighoffer E, Zitzmann N, Morita T, Tybulewicz VL, Ozaki Y, Watson SP. A novel syk-dependent mechanism of platelet activation by the c-type lectin receptor clec-2. Blood. 2006;107:542–549. doi: 10.1182/blood-2005-05-1994. [DOI] [PubMed] [Google Scholar]
- 17.Acton SE, Astarita JL, Malhotra D, Lukacs-Kornek V, Franz B, Hess PR, Jakus Z, Kuligowski M, Fletcher AL, Elpek KG, Bellemare-Pelletier A, Sceats L, Reynoso ED, Gonzalez SF, Graham DB, Chang J, Peters A, Woodruff M, Kim YA, Swat W, Morita T, Kuchroo V, Carroll MC, Kahn ML, Wucherpfennig KW, Turley SJ. Podoplanin-rich stromal networks induce dendritic cell motility via activation of the c-type lectin receptor clec-2. Immunity. 2012;37:276–289. doi: 10.1016/j.immuni.2012.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mourao-Sa D, Robinson MJ, Zelenay S, Sancho D, Chakravarty P, Larsen R, Plantinga M, Van Rooijen N, Soares MP, Lambrecht B, Reis e Sousa C. Clec-2 signaling via syk in myeloid cells can regulate inflammatory responses. Eur J Immunol. 2011;41:3040–3053. doi: 10.1002/eji.201141641. [DOI] [PubMed] [Google Scholar]
- 19.Astarita JL, Acton SE, Turley SJ. Podoplanin: Emerging functions in development, the immune system, and cancer. Front Immunol. 2012;3:283. doi: 10.3389/fimmu.2012.00283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hughes CE, Navarro-Nunez L, Finney BA, Mourao-Sa D, Pollitt AY, Watson SP. Clec-2 is not required for platelet aggregation at arteriolar shear. J Thromb Haemost. 2010;8:2328–2332. doi: 10.1111/j.1538-7836.2010.04006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Suzuki-Inoue K, Inoue O, Ding G, Nishimura S, Hokamura K, Eto K, Kashiwagi H, Tomiyama Y, Yatomi Y, Umemura K, Shin Y, Hirashima M, Ozaki Y. Essential in vivo roles of the c-type lectin receptor clec-2: Embryonic/neonatal lethality of clec-2-deficient mice by blood/lymphatic misconnections and impaired thrombus formation of clec-2-deficient platelets. J Biol Chem. 2010;285:24494–24507. doi: 10.1074/jbc.M110.130575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bender M, May F, Lorenz V, Thielmann I, Hagedorn I, Finney BA, Vogtle T, Remer K, Braun A, Bosl M, Watson SP, Nieswandt B. Combined in vivo depletion of glycoprotein vi and c-type lectin-like receptor 2 severely compromises hemostasis and abrogates arterial thrombosis in mice. Arterioscler Thromb Vasc Biol. 2013;33:926–934. doi: 10.1161/ATVBAHA.112.300672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.May F, Hagedorn I, Pleines I, Bender M, Vogtle T, Eble J, Elvers M, Nieswandt B. Clec-2 is an essential platelet-activating receptor in hemostasis and thrombosis. Blood. 2009;114:3464–3472. doi: 10.1182/blood-2009-05-222273. [DOI] [PubMed] [Google Scholar]
- 24.Schulte V, Reusch HP, Pozgajova M, Varga-Szabo D, Gachet C, Nieswandt B. Two-phase antithrombotic protection after anti-glycoprotein vi treatment in mice. Arterioscler Thromb Vasc Biol. 2006;26:1640–1647. doi: 10.1161/01.ATV.0000225697.98093.ed. [DOI] [PubMed] [Google Scholar]
- 25.Hatakeyama K, Kaneko MK, Kato Y, Ishikawa T, Nishihira K, Tsujimoto Y, Shibata Y, Ozaki Y, Asada Y. Podoplanin expression in advanced atherosclerotic lesions of human aortas. Thromb Res. 2012;129:e70–76. doi: 10.1016/j.thromres.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 26.Suzuki-Inoue K, Inoue O, Ozaki Y. Novel platelet activation receptor clec-2: From discovery to prospects. J Thromb Haemost. 2011;9 (Suppl 1):44–55. doi: 10.1111/j.1538-7836.2011.04335.x. [DOI] [PubMed] [Google Scholar]
- 27.McKenzie SE, Taylor SM, Malladi P, Yuhan H, Cassel DL, Chien P, Schwartz E, Schreiber AD, Surrey S, Reilly MP. The role of the human fc receptor fc gamma riia in the immune clearance of platelets: A transgenic mouse model. J Immunol. 1999;162:4311–4318. [PubMed] [Google Scholar]
- 28.Clawson CC, Rao GH, White JG. Platelet interaction with bacteria. Iv. Stimulation of the release reaction. Am J Pathol. 1975;81:411–420. [PMC free article] [PubMed] [Google Scholar]
- 29.Cines DB, Schreiber AD. Immune thrombocytopenia. Use of a coombs antiglobulin test to detect igg and c3 on platelets. N Engl J Med. 1979;300:106–111. doi: 10.1056/NEJM197901183000302. [DOI] [PubMed] [Google Scholar]
- 30.Fitzgerald JR, Foster TJ, Cox D. The interaction of bacterial pathogens with platelets. Nat Rev Microbiol. 2006;4:445–457. doi: 10.1038/nrmicro1425. [DOI] [PubMed] [Google Scholar]
- 31.Cox D, Kerrigan SW, Watson SP. Platelets and the innate immune system: Mechanisms of bacterial-induced platelet activation. J Thromb Haemost. 2011;9:1097–1107. doi: 10.1111/j.1538-7836.2011.04264.x. [DOI] [PubMed] [Google Scholar]
- 32.Davoren A, Aster RH. Heparin-induced thrombocytopenia and thrombosis. Am J Hematol. 2006;81:36–44. doi: 10.1002/ajh.20490. [DOI] [PubMed] [Google Scholar]
- 33.Warkentin TE. Heparin-induced thrombocytopenia: Pathogenesis and management. Br J Haematol. 2003;121:535–555. doi: 10.1046/j.1365-2141.2003.04334.x. [DOI] [PubMed] [Google Scholar]
- 34.Reilly MP, McKenzie SE. Insights from mouse models of heparin-induced thrombocytopenia and thrombosis. Curr Opin Hematol. 2002;9:395–400. doi: 10.1097/00062752-200209000-00002. [DOI] [PubMed] [Google Scholar]
- 35.Sammaritano LR, Gharavi AE. Antiphospholipid antibody syndrome. Clin Lab Med. 1992;12:41–59. [PubMed] [Google Scholar]
- 36.Zhi H, Rauova L, Hayes V, Gao C, Boylan B, Newman DK, McKenzie SE, Cooley BC, Poncz M, Newman PJ. Cooperative integrin/itam signaling in platelets enhances thrombus formation in vitro and in vivo. Blood. 2013;121:1858–1867. doi: 10.1182/blood-2012-07-443325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Boylan B, Gao C, Rathore V, Gill JC, Newman DK, Newman PJ. Identification of fcgammariia as the itam-bearing receptor mediating alphaiibbeta3 outside-in integrin signaling in human platelets. Blood. 2008;112:2780–2786. doi: 10.1182/blood-2008-02-142125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barrow AD, Trowsdale J. You say itam and i say itim, let’s call the whole thing off: The ambiguity of immunoreceptor signalling. Eur J Immunol. 2006;36:1646–1653. doi: 10.1002/eji.200636195. [DOI] [PubMed] [Google Scholar]
- 39.Underhill DM, Goodridge HS. The many faces of itams. Trends Immunol. 2007;28:66–73. doi: 10.1016/j.it.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 40.Reth M. Antigen receptor tail clue. Nature. 1989;338:383–384. [PubMed] [Google Scholar]
- 41.Hughes CE, Pollitt AY, Mori J, Eble JA, Tomlinson MG, Hartwig JH, O’Callaghan CA, Futterer K, Watson SP. Clec-2 activates syk through dimerization. Blood. 2010;115:2947–2955. doi: 10.1182/blood-2009-08-237834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wonerow P, Obergfell A, Wilde JI, Bobe R, Asazuma N, Brdicka T, Leo A, Schraven B, Horejsi V, Shattil SJ, Watson SP. Differential role of glycolipid-enriched membrane domains in glycoprotein vi- and integrin-mediated phospholipase cgamma2 regulation in platelets. Biochem J. 2002;364:755–765. doi: 10.1042/BJ20020128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Quinter PG, Dangelmaier CA, Quinton TM, Kunapuli SP, Daniel JL. Glycoprotein vi agonists have distinct dependences on the lipid raft environment. J Thromb Haemost. 2007;5:362–368. doi: 10.1111/j.1538-7836.2007.02309.x. [DOI] [PubMed] [Google Scholar]
- 44.Asazuma N, Wilde JI, Berlanga O, Leduc M, Leo A, Schweighoffer E, Tybulewicz V, Bon C, Liu SK, McGlade CJ, Schraven B, Watson SP. Interaction of linker for activation of t cells with multiple adapter proteins in platelets activated by the glycoprotein vi-selective ligand, convulxin. J Biol Chem. 2000;275:33427–33434. doi: 10.1074/jbc.M001439200. [DOI] [PubMed] [Google Scholar]
- 45.Gross BS, Lee JR, Clements JL, Turner M, Tybulewicz VL, Findell PR, Koretzky GA, Watson SP. Tyrosine phosphorylation of slp-76 is downstream of syk following stimulation of the collagen receptor in platelets. J Biol Chem. 1999;274:5963–5971. doi: 10.1074/jbc.274.9.5963. [DOI] [PubMed] [Google Scholar]
- 46.Zhou Z, Gushiken FC, Bolgiano D, Salsbery BJ, Aghakasiri N, Jing N, Wu X, Vijayan KV, Rumbaut RE, Adachi R, Lopez JA, Dong JF. Signal transducer and activator of transcription 3 (stat3) regulates collagen-induced platelet aggregation independently of its transcription factor activity. Circulation. 2013;127:476–485. doi: 10.1161/CIRCULATIONAHA.112.132126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McCarty OJ, Larson MK, Auger JM, Kalia N, Atkinson BT, Pearce AC, Ruf S, Henderson RB, Tybulewicz VL, Machesky LM, Watson SP. Rac1 is essential for platelet lamellipodia formation and aggregate stability under flow. J Biol Chem. 2005;280:39474–39484. doi: 10.1074/jbc.M504672200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pleines I, Elvers M, Strehl A, Pozgajova M, Varga-Szabo D, May F, Chrostek-Grashoff A, Brakebusch C, Nieswandt B. Rac1 is essential for phospholipase c-gamma2 activation in platelets. Pflugers Arch. 2009;457:1173–1185. doi: 10.1007/s00424-008-0573-7. [DOI] [PubMed] [Google Scholar]
- 49.Stefanini L, Boulaftali Y, Ouellette TD, Holinstat M, Desire L, Leblond B, Andre P, Conley PB, Bergmeier W. Rap1-rac1 circuits potentiate platelet activation. Arterioscler Thromb Vasc Biol. 2012;32:434–441. doi: 10.1161/ATVBAHA.111.239194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pearce AC, McCarty OJ, Calaminus SD, Vigorito E, Turner M, Watson SP. Vav family proteins are required for optimal regulation of plcgamma2 by integrin alphaiibbeta3. Biochem J. 2007;401:753–761. doi: 10.1042/BJ20061508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pearce AC, Senis YA, Billadeau DD, Turner M, Watson SP, Vigorito E. Vav1 and vav3 have critical but redundant roles in mediating platelet activation by collagen. J Biol Chem. 2004;279:53955–53962. doi: 10.1074/jbc.M410355200. [DOI] [PubMed] [Google Scholar]
- 52.Pearce AC, Wilde JI, Doody GM, Best D, Inoue O, Vigorito E, Tybulewicz VL, Turner M, Watson SP. Vav1, but not vav2, contributes to platelet aggregation by crp and thrombin, but neither is required for regulation of phospholipase c. Blood. 2002;100:3561–3569. doi: 10.1182/blood.V100.10.3561. [DOI] [PubMed] [Google Scholar]
- 53.Oda A, Ikeda Y, Ochs HD, Druker BJ, Ozaki K, Handa M, Ariga T, Sakiyama Y, Witte ON, Wahl MI. Rapid tyrosine phosphorylation and activation of bruton’s tyrosine/tec kinases in platelets induced by collagen binding or cd32 cross-linking. Blood. 2000;95:1663–1670. [PubMed] [Google Scholar]
- 54.Atkinson BT, Ellmeier W, Watson SP. Tec regulates platelet activation by gpvi in the absence of btk. Blood. 2003;102:3592–3599. doi: 10.1182/blood-2003-04-1142. [DOI] [PubMed] [Google Scholar]
- 55.Gibbins JM, Briddon S, Shutes A, van Vugt MJ, van de Winkel JG, Saito T, Watson SP. The p85 subunit of phosphatidylinositol 3-kinase associates with the fc receptor gamma-chain and linker for activitor of t cells (lat) in platelets stimulated by collagen and convulxin. J Biol Chem. 1998;273:34437–34443. doi: 10.1074/jbc.273.51.34437. [DOI] [PubMed] [Google Scholar]
- 56.Chrzanowska-Wodnicka M, Smyth SS, Schoenwaelder SM, Fischer TH, White GC., 2nd Rap1b is required for normal platelet function and hemostasis in mice. J Clin Invest. 2005;115:680–687. doi: 10.1172/JCI22973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Crittenden JR, Bergmeier W, Zhang Y, Piffath CL, Liang Y, Wagner DD, Housman DE, Graybiel AM. Caldag-gefi integrates signaling for platelet aggregation and thrombus formation. Nat Med. 2004;10:982–986. doi: 10.1038/nm1098. [DOI] [PubMed] [Google Scholar]
- 58.Cifuni SM, Wagner DD, Bergmeier W. Caldag-gefi and protein kinase c represent alternative pathways leading to activation of integrin alphaiibbeta3 in platelets. Blood. 2008;112:1696–1703. doi: 10.1182/blood-2008-02-139733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stefanini L, Roden RC, Bergmeier W. Caldag-gefi is at the nexus of calcium-dependent platelet activation. Blood. 2009;114:2506–2514. doi: 10.1182/blood-2009-04-218768. [DOI] [PubMed] [Google Scholar]
- 60.Xiang B, Zhang G, Stefanini L, Bergmeier W, Gartner TK, Whiteheart SW, Li Z. The src family kinases and protein kinase c synergize to mediate gq-dependent platelet activation. J Biol Chem. 2012;287:41277–41287. doi: 10.1074/jbc.M112.393124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stolla M, Stefanini L, Andre P, Ouellette TD, Reilly MP, McKenzie SE, Bergmeier W. Caldag-gefi deficiency protects mice in a novel model of fcgamma riia-mediated thrombosis and thrombocytopenia. Blood. 2011;118:1113–1120. doi: 10.1182/blood-2011-03-342352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Law DA, Nannizzi-Alaimo L, Ministri K, Hughes PE, Forsyth J, Turner M, Shattil SJ, Ginsberg MH, Tybulewicz VL, Phillips DR. Genetic and pharmacological analyses of syk function in alphaiibbeta3 signaling in platelets. Blood. 1999;93:2645–2652. [PubMed] [Google Scholar]
- 63.Andre P, Morooka T, Sim D, Abe K, Lowell C, Nanda N, Delaney S, Siu G, Yan Y, Hollenbach S, Pandey A, Gao H, Wang Y, Nakajima K, Parikh SA, Shi C, Phillips D, Owen W, Sinha U, Simon DI. Critical role for syk in responses to vascular injury. Blood. 2011;118:5000–5010. doi: 10.1182/blood-2011-06-360743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Suzuki-Inoue K, Inoue O, Frampton J, Watson SP. Murine gpvi stimulates weak integrin activation in plcgamma2−/− platelets: Involvement of plcgamma1 and pi3-kinase. Blood. 2003;102:1367–1373. doi: 10.1182/blood-2003-01-0029. [DOI] [PubMed] [Google Scholar]
- 65.Nonne C, Lenain N, Hechler B, Mangin P, Cazenave JP, Gachet C, Lanza F. Importance of platelet phospholipase cgamma2 signaling in arterial thrombosis as a function of lesion severity. Arterioscler Thromb Vasc Biol. 2005;25:1293–1298. doi: 10.1161/01.ATV.0000163184.02484.69. [DOI] [PubMed] [Google Scholar]
- 66.Mangin P, Nonne C, Eckly A, Ohlmann P, Freund M, Nieswandt B, Cazenave JP, Gachet C, Lanza F. A plc gamma 2-independent platelet collagen aggregation requiring functional association of gpvi and integrin alpha2beta1. FEBS Lett. 2003;542:53–59. doi: 10.1016/s0014-5793(03)00337-5. [DOI] [PubMed] [Google Scholar]
- 67.Suzuki-Inoue K, Wilde JI, Andrews RK, Auger JM, Siraganian RP, Sekiya F, Rhee SG, Watson SP. Glycoproteins vi and ib-ix-v stimulate tyrosine phosphorylation of tyrosine kinase syk and phospholipase cgamma2 at distinct sites. Biochem J. 2004;378:1023–1029. doi: 10.1042/BJ20031430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ozaki Y, Suzuki-Inoue K, Inoue O. Platelet receptors activated via mulitmerization: Glycoprotein vi, gpib-ix-v, and clec-2. J Thromb Haemost. 2013;11 (Suppl 1):330–339. doi: 10.1111/jth.12235. [DOI] [PubMed] [Google Scholar]
- 69.Tammela T, Alitalo K. Lymphangiogenesis: Molecular mechanisms and future promise. Cell. 2010;140:460–476. doi: 10.1016/j.cell.2010.01.045. [DOI] [PubMed] [Google Scholar]
- 70.Yang Y, Garcia-Verdugo JM, Soriano-Navarro M, Srinivasan RS, Scallan JP, Singh MK, Epstein JA, Oliver G. Lymphatic endothelial progenitors bud from the cardinal vein and intersomitic vessels in mammalian embryos. Blood. 2012;120:2340–2348. doi: 10.1182/blood-2012-05-428607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Srinivasan RS, Dillard ME, Lagutin OV, Lin FJ, Tsai S, Tsai MJ, Samokhvalov IM, Oliver G. Lineage tracing demonstrates the venous origin of the mammalian lymphatic vasculature. Genes Dev. 2007;21:2422–2432. doi: 10.1101/gad.1588407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.El Zawahry MD, Sayed NM, El-Awady HM, Abdel-Latif A, El-Gindy M. A study of the gross, microscopic and functional anatomy of the thoracic duct and the lympho-venous junction. Int Surg. 1983;68:135–138. [PubMed] [Google Scholar]
- 73.Clements JL, Lee JR, Gross B, Yang B, Olson JD, Sandra A, Watson SP, Lentz SR, Koretzky GA. Fetal hemorrhage and platelet dysfunction in slp-76-deficient mice. J Clin Invest. 1999;103:19–25. doi: 10.1172/JCI5317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Abtahian F, Guerriero A, Sebzda E, Lu MM, Zhou R, Mocsai A, Myers EE, Huang B, Jackson DG, Ferrari VA, Tybulewicz V, Lowell CA, Lepore JJ, Koretzky GA, Kahn ML. Regulation of blood and lymphatic vascular separation by signaling proteins slp-76 and syk. Science. 2003;299:247–251. doi: 10.1126/science.1079477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ichise H, Ichise T, Ohtani O, Yoshida N. Phospholipase cgamma2 is necessary for separation of blood and lymphatic vasculature in mice. Development. 2009;136:191–195. doi: 10.1242/dev.025353. [DOI] [PubMed] [Google Scholar]
- 76.Finney BA, Schweighoffer E, Navarro-Nunez L, Benezech C, Barone F, Hughes CE, Langan SA, Lowe KL, Pollitt AY, Mourao-Sa D, Sheardown S, Nash GB, Smithers N, Reis e Sousa C, Tybulewicz VL, Watson SP. Clec-2 and syk in the megakaryocytic/platelet lineage are essential for development. Blood. 2012;119:1747–1756. doi: 10.1182/blood-2011-09-380709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bohmer R, Neuhaus B, Buhren S, Zhang D, Stehling M, Bock B, Kiefer F. Regulation of developmental lymphangiogenesis by syk(+) leukocytes. Dev Cell. 2010;18:437–449. doi: 10.1016/j.devcel.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 78.Osada M, Inoue O, Ding G, Shirai T, Ichise H, Hirayama K, Takano K, Yatomi Y, Hirashima M, Fujii H, Suzuki-Inoue K, Ozaki Y. Platelet activation receptor clec-2 regulates blood/lymphatic vessel separation by inhibiting proliferation, migration, and tube formation of lymphatic endothelial cells. J Biol Chem. 2012;287:22241–22252. doi: 10.1074/jbc.M111.329987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hess PR, Rawnsley DR, Jakus Z, Yang Y, Sweet DT, Fu J, Herzog B, Lu M, Nieswandt B, Oliver G, Makinen T, Xia L, Kahn ML. Platelets mediate lymphovenous hemostasis to maintain blood-lymphatic separation throughout life. J Clin Invest. 2014;124:273–284. doi: 10.1172/JCI70422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bertozzi CC, Schmaier AA, Mericko P, Hess PR, Zou Z, Chen M, Chen CY, Xu B, Lu MM, Zhou D, Sebzda E, Santore MT, Merianos DJ, Stadtfeld M, Flake AW, Graf T, Skoda R, Maltzman JS, Koretzky GA, Kahn ML. Platelets regulate lymphatic vascular development through clec-2-slp-76 signaling. Blood. 2010;116:661–670. doi: 10.1182/blood-2010-02-270876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Uhrin P, Zaujec J, Breuss JM, Olcaydu D, Chrenek P, Stockinger H, Fuertbauer E, Moser M, Haiko P, Fassler R, Alitalo K, Binder BR, Kerjaschki D. Novel function for blood platelets and podoplanin in developmental separation of blood and lymphatic circulation. Blood. 2010;115:3997–4005. doi: 10.1182/blood-2009-04-216069. [DOI] [PubMed] [Google Scholar]
- 82.Fu J, Gerhardt H, McDaniel JM, Xia B, Liu X, Ivanciu L, Ny A, Hermans K, Silasi-Mansat R, McGee S, Nye E, Ju T, Ramirez MI, Carmeliet P, Cummings RD, Lupu F, Xia L. Endothelial cell o-glycan deficiency causes blood/lymphatic misconnections and consequent fatty liver disease in mice. J Clin Invest. 2008;118:3725–3737. doi: 10.1172/JCI36077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Srinivasan RS, Oliver G. Prox1 dosage controls the number of lymphatic endothelial cell progenitors and the formation of the lymphovenous valves. Genes Dev. 2011;25:2187–2197. doi: 10.1101/gad.16974811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bazigou E, Xie S, Chen C, Weston A, Miura N, Sorokin L, Adams R, Muro AF, Sheppard D, Makinen T. Integrin-alpha9 is required for fibronectin matrix assembly during lymphatic valve morphogenesis. Dev Cell. 2009;17:175–186. doi: 10.1016/j.devcel.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bazigou E, Lyons OT, Smith A, Venn GE, Cope C, Brown NA, Makinen T. Genes regulating lymphangiogenesis control venous valve formation and maintenance in mice. J Clin Invest. 2011;121:2984–2992. doi: 10.1172/JCI58050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hodivala-Dilke KM, McHugh KP, Tsakiris DA, Rayburn H, Crowley D, Ullman-Cullere M, Ross FP, Coller BS, Teitelbaum S, Hynes RO. Beta3-integrin-deficient mice are a model for glanzmann thrombasthenia showing placental defects and reduced survival. J Clin Invest. 1999;103:229–238. doi: 10.1172/JCI5487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Seeger M, Bewig B, Gunther R, Schafmayer C, Vollnberg B, Rubin D, Hoell C, Schreiber S, Folsch UR, Hampe J. Terminal part of thoracic duct: High-resolution us imaging. Radiology. 2009;252:897–904. doi: 10.1148/radiol.2531082036. [DOI] [PubMed] [Google Scholar]
- 88.Projahn D, Koenen RR. Platelets: Key players in vascular inflammation. J Leukoc Biol. 2012;92:1167–1175. doi: 10.1189/jlb.0312151. [DOI] [PubMed] [Google Scholar]
- 89.Stokes KY, Granger DN. Platelets: A critical link between inflammation and microvascular dysfunction. J Physiol. 2012;590:1023–1034. doi: 10.1113/jphysiol.2011.225417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gawaz M, Langer H, May AE. Platelets in inflammation and atherogenesis. J Clin Invest. 2005;115:3378–3384. doi: 10.1172/JCI27196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lerman A, Zeiher AM. Endothelial function: Cardiac events. Circulation. 2005;111:363–368. doi: 10.1161/01.CIR.0000153339.27064.14. [DOI] [PubMed] [Google Scholar]
- 92.Schulz C, Penz S, Hoffmann C, Langer H, Gillitzer A, Schneider S, Brandl R, Seidl S, Massberg S, Pichler B, Kremmer E, Stellos K, Schonberger T, Siess W, Gawaz M. Platelet gpvi binds to collagenous structures in the core region of human atheromatous plaque and is critical for atheroprogression in vivo. Basic Res Cardiol. 2008;103:356–367. doi: 10.1007/s00395-008-0722-3. [DOI] [PubMed] [Google Scholar]
- 93.Bultmann A, Li Z, Wagner S, Peluso M, Schonberger T, Weis C, Konrad I, Stellos K, Massberg S, Nieswandt B, Gawaz M, Ungerer M, Munch G. Impact of glycoprotein vi and platelet adhesion on atherosclerosis--a possible role of fibronectin. J Mol Cell Cardiol. 2010;49:532–542. doi: 10.1016/j.yjmcc.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 94.Schonberger T, Ziegler M, Borst O, Konrad I, Nieswandt B, Massberg S, Ochmann C, Jurgens T, Seizer P, Langer H, Munch G, Ungerer M, Preissner KT, Elvers M, Gawaz M. The dimeric platelet collagen receptor gpvi-fc reduces platelet adhesion to activated endothelium and preserves myocardial function after transient ischemia in mice. Am J Physiol Cell Physiol. 2012;303:C757–766. doi: 10.1152/ajpcell.00060.2012. [DOI] [PubMed] [Google Scholar]
- 95.Boilard E, Blanco P, Nigrovic PA. Platelets: Active players in the pathogenesis of arthritis and sle. Nat Rev Rheumatol. 2012;8:534–542. doi: 10.1038/nrrheum.2012.118. [DOI] [PubMed] [Google Scholar]
- 96.Boilard E, Nigrovic PA, Larabee K, Watts GF, Coblyn JS, Weinblatt ME, Massarotti EM, Remold-O’Donnell E, Farndale RW, Ware J, Lee DM. Platelets amplify inflammation in arthritis via collagen-dependent microparticle production. Science. 2010;327:580–583. doi: 10.1126/science.1181928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cloutier N, Pare A, Farndale RW, Schumacher HR, Nigrovic PA, Lacroix S, Boilard E. Platelets can enhance vascular permeability. Blood. 2012;120:1334–1343. doi: 10.1182/blood-2012-02-413047. [DOI] [PubMed] [Google Scholar]
- 98.Duerschmied D, Suidan GL, Demers M, Herr N, Carbo C, Brill A, Cifuni SM, Mauler M, Cicko S, Bader M, Idzko M, Bode C, Wagner DD. Platelet serotonin promotes the recruitment of neutrophils to sites of acute inflammation in mice. Blood. 2013;121:1008–1015. doi: 10.1182/blood-2012-06-437392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Devi S, Kuligowski MP, Kwan RY, Westein E, Jackson SP, Kitching AR, Hickey MJ. Platelet recruitment to the inflamed glomerulus occurs via an alphaiibbeta3/gpvi-dependent pathway. Am J Pathol. 2010;177:1131–1142. doi: 10.2353/ajpath.2010.091143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Danielli JF. Capillary permeability and oedema in the perfused frog. J Physiol. 1940;98:109–129. doi: 10.1113/jphysiol.1940.sp003837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kitchens CS, Weiss L. Ultrastructural changes of endothelium associated with thrombocytopenia. Blood. 1975;46:567–578. [PubMed] [Google Scholar]
- 102.Kitchens CS, Pendergast JF. Human thrombocytopenia is associated with structural abnormalities of the endothelium that are ameliorated by glucocorticosteroid administration. Blood. 1986;67:203–206. [PubMed] [Google Scholar]
- 103.Goerge T, Ho-Tin-Noe B, Carbo C, Benarafa C, Remold-O’Donnell E, Zhao BQ, Cifuni SM, Wagner DD. Inflammation induces hemorrhage in thrombocytopenia. Blood. 2008;111:4958–4964. doi: 10.1182/blood-2007-11-123620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ho-Tin-Noe B, Demers M, Wagner DD. How platelets safeguard vascular integrity. J Thromb Haemost. 2011;9 (Suppl 1):56–65. doi: 10.1111/j.1538-7836.2011.04317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.McGarrity ST, Stephenson AH, Hyers TM, Webster RO. Inhibition of neutrophil superoxide anion generation by platelet products: Role of adenine nucleotides. J Leukoc Biol. 1988;44:411–421. doi: 10.1002/jlb.44.5.411. [DOI] [PubMed] [Google Scholar]
- 106.Booth NA, Simpson AJ, Croll A, Bennett B, MacGregor IR. Plasminogen activator inhibitor (pai-1) in plasma and platelets. Br J Haematol. 1988;70:327–333. doi: 10.1111/j.1365-2141.1988.tb02490.x. [DOI] [PubMed] [Google Scholar]
- 107.Boulaftali Y, Adam F, Venisse L, Ollivier V, Richard B, Taieb S, Monard D, Favier R, Alessi MC, Bryckaert M, Arocas V, Jandrot-Perrus M, Bouton MC. Anticoagulant and antithrombotic properties of platelet protease nexin-1. Blood. 2010;115:97–106. doi: 10.1182/blood-2009-04-217240. [DOI] [PubMed] [Google Scholar]
- 108.Villeneuve J, Block A, Le Bousse-Kerdiles MC, Lepreux S, Nurden P, Ripoche J, Nurden AT. Tissue inhibitors of matrix metalloproteinases in platelets and megakaryocytes: A novel organization for these secreted proteins. Exp Hematol. 2009;37:849–856. doi: 10.1016/j.exphem.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 109.McGarrity ST, Hyers TM, Webster RO. Inhibition of neutrophil functions by platelets and platelet-derived products: Description of multiple inhibitory properties. J Leukoc Biol. 1988;44:93–100. doi: 10.1002/jlb.44.2.93. [DOI] [PubMed] [Google Scholar]
- 110.Washington AV, Gibot S, Acevedo I, Gattis J, Quigley L, Feltz R, De La Mota A, Schubert RL, Gomez-Rodriguez J, Cheng J, Dutra A, Pak E, Chertov O, Rivera L, Morales J, Lubkowski J, Hunter R, Schwartzberg PL, McVicar DW. Trem-like transcript-1 protects against inflammation-associated hemorrhage by facilitating platelet aggregation in mice and humans. J Clin Invest. 2009;119:1489–1501. doi: 10.1172/JCI36175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Fong KP, Barry C, Tran AN, Traxler EA, Wannemacher KM, Tang HY, Speicher KD, Blair IA, Speicher DW, Grosser T, Brass LF. Deciphering the human platelet sheddome. Blood. 2011;117:e15–26. doi: 10.1182/blood-2010-05-283838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Yatomi Y, Igarashi Y, Yang L, Hisano N, Qi R, Asazuma N, Satoh K, Ozaki Y, Kume S. Sphingosine 1-phosphate, a bioactive sphingolipid abundantly stored in platelets, is a normal constituent of human plasma and serum. J Biochem. 1997;121:969–973. doi: 10.1093/oxfordjournals.jbchem.a021681. [DOI] [PubMed] [Google Scholar]
- 113.Tiedt R, Schomber T, Hao-Shen H, Skoda RC. Pf4-cre transgenic mice allow the generation of lineage-restricted gene knockouts for studying megakaryocyte and platelet function in vivo. Blood. 2007;109:1503–1506. doi: 10.1182/blood-2006-04-020362. [DOI] [PubMed] [Google Scholar]
- 114.Kanaji T, Russell S, Ware J. Amelioration of the macrothrombocytopenia associated with the murine bernard-soulier syndrome. Blood. 2002;100:2102–2107. doi: 10.1182/blood-2002-03-0997. [DOI] [PubMed] [Google Scholar]
- 115.Boulaftali Y, Hess PR, Getz TM, Cholka A, Stolla M, Mackman N, Owens AP, 3rd, Ware J, Kahn ML, Bergmeier W. Platelet itam signaling is critical for vascular integrity in inflammation. J Clin Invest. 2013;123:908–916. doi: 10.1172/JCI65154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kerrigan AM, Navarro-Nunez L, Pyz E, Finney BA, Willment JA, Watson SP, Brown GD. Podoplanin-expressing inflammatory macrophages activate murine platelets via clec-2. J Thromb Haemost. 2012;10:484–486. doi: 10.1111/j.1538-7836.2011.04614.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Dvorak HF, Senger DR, Dvorak AM, Harvey VS, McDonagh J. Regulation of extravascular coagulation by microvascular permeability. Science. 1985;227:1059–1061. doi: 10.1126/science.3975602. [DOI] [PubMed] [Google Scholar]
- 118.Battinelli EM, Markens BA, Italiano JE., Jr Release of angiogenesis regulatory proteins from platelet alpha granules: Modulation of physiologic and pathologic angiogenesis. Blood. 2011;118:1359–1369. doi: 10.1182/blood-2011-02-334524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Leo L, Di Paola J, Judd BA, Koretzky GA, Lentz SR. Role of the adapter protein slp-76 in gpvi-dependent platelet procoagulant responses to collagen. Blood. 2002;100:2839–2844. doi: 10.1182/blood-2002-04-1234. [DOI] [PubMed] [Google Scholar]
- 120.Heemskerk JW, Mattheij NJ, Cosemans JM. Platelet-based coagulation: Different populations, different functions. J Thromb Haemost. 2013;11:2–16. doi: 10.1111/jth.12045. [DOI] [PubMed] [Google Scholar]
- 121.Gardiner EE, Al-Tamimi M, Andrews RK, Berndt MC. Platelet receptor shedding. Methods Mol Biol. 2012;788:321–339. doi: 10.1007/978-1-61779-307-3_22. [DOI] [PubMed] [Google Scholar]
- 122.Aledort LM, Hayward CP, Chen MG, Nichol JL, Bussel J. Prospective screening of 205 patients with itp, including diagnosis, serological markers, and the relationship between platelet counts, endogenous thrombopoietin, and circulating antithrombopoietin antibodies. Am J Hematol. 2004;76:205–213. doi: 10.1002/ajh.20104. [DOI] [PubMed] [Google Scholar]
- 123.Sullivan KE, Mullen CA, Blaese RM, Winkelstein JA. A multiinstitutional survey of the wiskott-aldrich syndrome. J Pediatr. 1994;125:876–885. doi: 10.1016/s0022-3476(05)82002-5. [DOI] [PubMed] [Google Scholar]
- 124.Imai K, Morio T, Zhu Y, Jin Y, Itoh S, Kajiwara M, Yata J, Mizutani S, Ochs HD, Nonoyama S. Clinical course of patients with wasp gene mutations. Blood. 2004;103:456–464. doi: 10.1182/blood-2003-05-1480. [DOI] [PubMed] [Google Scholar]
- 125.Hermans C, Wittevrongel C, Thys C, Smethurst PA, Van Geet C, Freson K. A compound heterozygous mutation in glycoprotein vi in a patient with a bleeding disorder. J Thromb Haemost. 2009;7:1356–1363. doi: 10.1111/j.1538-7836.2009.03520.x. [DOI] [PubMed] [Google Scholar]
- 126.Quek LS, Pasquet JM, Hers I, Cornall R, Knight G, Barnes M, Hibbs ML, Dunn AR, Lowell CA, Watson SP. Fyn and lyn phosphorylate the fc receptor gamma chain downstream of glycoprotein vi in murine platelets, and lyn regulates a novel feedback pathway. Blood. 2000;96:4246–4253. [PubMed] [Google Scholar]
- 127.Severin S, Nash CA, Mori J, Zhao Y, Abram C, Lowell CA, Senis YA, Watson SP. Distinct and overlapping functional roles of src family kinases in mouse platelets. J Thromb Haemost. 2012;10:1631–1645. doi: 10.1111/j.1538-7836.2012.04814.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Severin S, Pollitt AY, Navarro-Nunez L, Nash CA, Mourao-Sa D, Eble JA, Senis YA, Watson SP. Syk-dependent phosphorylation of clec-2: A novel mechanism of hem-immunoreceptor tyrosine-based activation motif signaling. J Biol Chem. 2011;286:4107–4116. doi: 10.1074/jbc.M110.167502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Poole A, Gibbins JM, Turner M, van Vugt MJ, van de Winkel JG, Saito T, Tybulewicz VL, Watson SP. The fc receptor gamma-chain and the tyrosine kinase syk are essential for activation of mouse platelets by collagen. Embo J. 1997;16:2333–2341. doi: 10.1093/emboj/16.9.2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hughan SC, Hughes CE, McCarty OJ, Schweighoffer E, Soultanova I, Ware J, Tybulewicz VL, Watson SP. Gpvi potentiation of platelet activation by thrombin and adhesion molecules independent of src kinases and syk. Arterioscler Thromb Vasc Biol. 2007;27:422–429. doi: 10.1161/01.ATV.0000252826.96134.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Spalton JC, Mori J, Pollitt AY, Hughes CE, Eble JA, Watson SP. The novel syk inhibitor r406 reveals mechanistic differences in the initiation of gpvi and clec-2 signaling in platelets. J Thromb Haemost. 2009;7:1192–1199. doi: 10.1111/j.1538-7836.2009.03451.x. [DOI] [PubMed] [Google Scholar]
- 132.Reilly MP, Sinha U, Andre P, Taylor SM, Pak Y, Deguzman FR, Nanda N, Pandey A, Stolla M, Bergmeier W, McKenzie SE. Prt-060318, a novel syk inhibitor, prevents heparin-induced thrombocytopenia and thrombosis in a transgenic mouse model. Blood. 2011;117:2241–2246. doi: 10.1182/blood-2010-03-274969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Pasquet JM, Gross B, Quek L, Asazuma N, Zhang W, Sommers CL, Schweighoffer E, Tybulewicz V, Judd B, Lee JR, Koretzky G, Love PE, Samelson LE, Watson SP. Lat is required for tyrosine phosphorylation of phospholipase cgamma2 and platelet activation by the collagen receptor gpvi. Mol Cell Biol. 1999;19:8326–8334. doi: 10.1128/mcb.19.12.8326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hughes CE, Auger JM, McGlade J, Eble JA, Pearce AC, Watson SP. Differential roles for the adapters gads and lat in platelet activation by gpvi and clec-2. J Thromb Haemost. 2008;6:2152–2159. doi: 10.1111/j.1538-7836.2008.03166.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Judd BA, Myung PS, Obergfell A, Myers EE, Cheng AM, Watson SP, Pear WS, Allman D, Shattil SJ, Koretzky GA. Differential requirement for lat and slp-76 in gpvi versus t cell receptor signaling. J Exp Med. 2002;195:705–717. doi: 10.1084/jem.20011583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Rabie T, Varga-Szabo D, Bender M, Pozgaj R, Lanza F, Saito T, Watson SP, Nieswandt B. Diverging signaling events control the pathway of gpvi down-regulation in vivo. Blood. 2007;110:529–535. doi: 10.1182/blood-2006-11-058107. [DOI] [PubMed] [Google Scholar]
- 137.Chen K, Li W, Major J, Rahaman SO, Febbraio M, Silverstein RL. Vav guanine nucleotide exchange factors link hyperlipidemia and a prothrombotic state. Blood. 2011;117:5744–5750. doi: 10.1182/blood-2009-01-201970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Bezman NA, Lian L, Abrams CS, Brass LF, Kahn ML, Jordan MS, Koretzky GA. Requirements of slp76 tyrosines in itam and integrin receptor signaling and in platelet function in vivo. J Exp Med. 2008;205:1775–1788. doi: 10.1084/jem.20080240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Goncalves I, Hughan SC, Schoenwaelder SM, Yap CL, Yuan Y, Jackson SP. Integrin alpha iib beta 3-dependent calcium signals regulate platelet-fibrinogen interactions under flow. Involvement of phospholipase c gamma 2. J Biol Chem. 2003;278:34812–34822. doi: 10.1074/jbc.M306504200. [DOI] [PubMed] [Google Scholar]
- 140.Wonerow P, Pearce AC, Vaux DJ, Watson SP. A critical role for phospholipase cgamma2 in alphaiibbeta3-mediated platelet spreading. J Biol Chem. 2003;278:37520–37529. doi: 10.1074/jbc.M305077200. [DOI] [PubMed] [Google Scholar]
- 141.Rathore V, Wang D, Newman DK, Newman PJ. Phospholipase cgamma2 contributes to stable thrombus formation on vwf. FEBS Lett. 2004;573:26–30. doi: 10.1016/j.febslet.2004.07.048. [DOI] [PubMed] [Google Scholar]
- 142.van der Meijden PE, Munnix IC, Auger JM, Govers-Riemslag JW, Cosemans JM, Kuijpers MJ, Spronk HM, Watson SP, Renne T, Heemskerk JW. Dual role of collagen in factor xii-dependent thrombus formation. Blood. 2009;114:881–890. doi: 10.1182/blood-2008-07-171066. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.