Abstract
Signaling processes between various immune cells involve large scale spatial reorganization of receptors and signaling molecules within the cell-cell junction. These structures, now collectively referred to as immune synapses, interleave physical and mechanical processes with the cascades of chemical reactions that constitute signal transduction systems. Molecular level clustering, spatial exclusion, and long range directed transport are all emerging as key regulatory mechanisms. The study of these processes is drawing researchers from physical sciences to join the effort and represents a rapidly growing branch of biophysical chemistry. Recent advances in the physical and quantitative analysis of signaling within the immune synapses will be reviewed here.
Keywords: Immune synapse, cytoskeleton, signal transduction, signaling cluster, spatial mutation
Background and Introduction
The immune synapse was first conceptualized for interactions between cells of the adaptive immune system, T and B cells, but the concept has since been applied to innate immune cells like natural killer (NK) cells and more recently phagocytes (5, 11, 23, 24, 48, 50). As such, the immunological synapses constitute a class of junctions and we will refer to data from phagocytic, T cell, B cell and NK cell synapses as specific subtypes. Common to each of these intercellular junctions is a highly organized reaction environment in which numerous receptors and cell surface ligands engage in juxtacrine fashion. A number of recent review articles cover various aspects of the immunological synapse in comprehensive detail and the reader is referred to these for fundamental background on the system (17-20, 44, 60).
TCR microclusters are a substructure within the T cell immunological synapse, which have now emerged as the primary functional signaling units (Figure 1). In this review, we focus on these signal transduction machines with emphasis on recent studies characterizing physical and quantitative aspects of TCR clusters during antigen triggering. Activation of T cell receptors (TCR) on T cells by antigen peptide majorhistocompatibility (pMHC) proteins on APCs involves a multi-scale choreography of macromolecular assembly and directed movement. Fluorescence and electron microscopy data reveal that TCR assemble into clusters containing tens to a hundred or more receptors upon recognition of antigen at high concentrations (10, 44, 65). Other molecules including lck, Lat, SLP76 and actin are recruited to the clusters, creating a microenvironment in which signal transduction and regulation occur. Actin association leads to directed transport of the TCR clusters over micron distances within the immunological synapse, which is readily observed (Figure 2), as they become coupled to flow of the cortical actin network (13, 37, 66).
Figure 1.
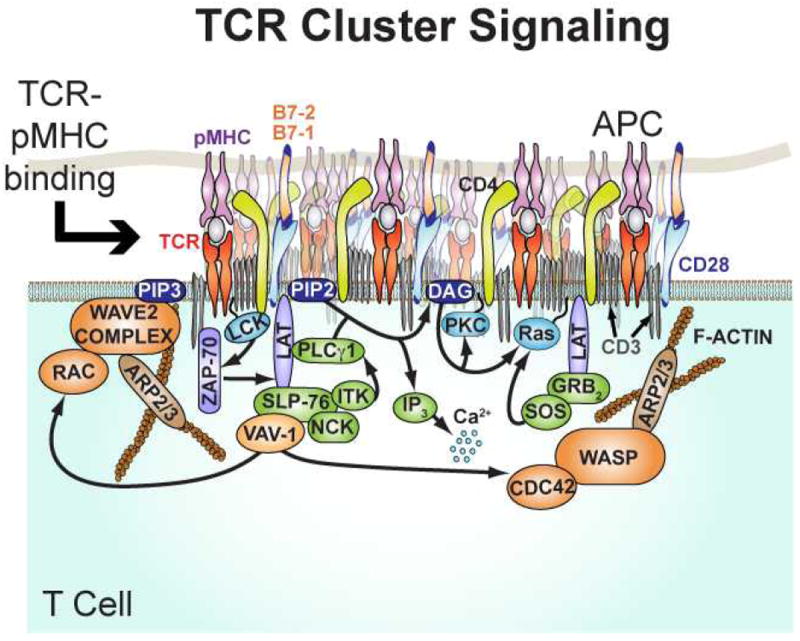
Schematic of a T cell receptor microcluster, which is densely packed with a diverse set of signaling molecules. Activation of actin assembly initiates centripetal transport. Adapted from Hartman & Groves Curr. Op. Cell Biol 2011, 23: 370-376
Figure 2.
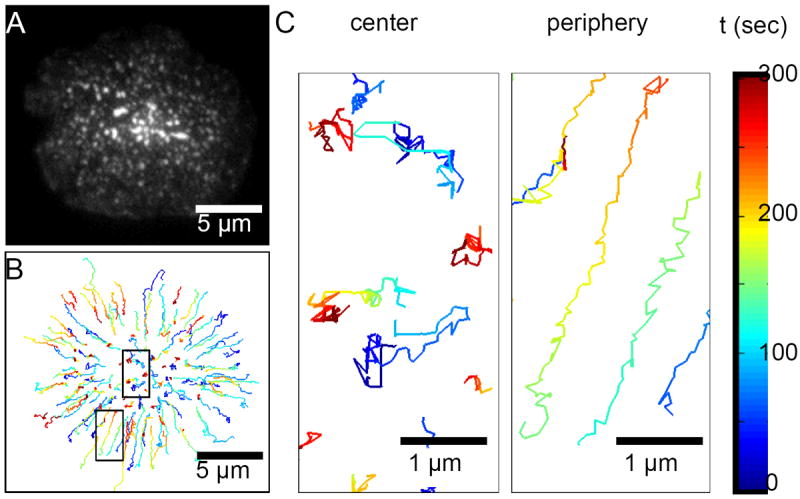
(A) Total internal reflection fluorescence (TIRF) image of T cell receptors (TCRs) labeled with H57 αTCR Fab (Alexa Fluor 594) at 60 seconds after the initial T cell-bilayer contact. (B) Trajectories of all TCR microclusters show their highly confined motion in the central area (left image in panel C) and centripetal movement in the cell periphery (right image in panel C) during the immunological synapse formation. Color bar corresponds to the elapsed time after the initial cell-bilayer contact (t = 0).
Among the most significant questions about TCR microclusters concerns what functional properties emerge as a result of clustering, and what are the consequences of this to overall T cell behavior. Another line of recent work has begun to probe this issue directly. Supported membranes have a long history of use as surrogate APC surfaces for T cell activation (26). The resulting hybrid live cell – supported membrane immunological synapse can be physically manipulated by structures, prefabricated onto the underlying solid substrate, which guide and restrict the movement of molecules in the supported membrane as well as any cognate receptors with which they are engaged on the living T cell (25, 47) (Figure 3). Experimental observations using this spatial mutation strategy suggest that antigen triggering of Ca2+ flux in T cells is determined by the number of antigen within individual TCR clusters rather than the amount encountered by the whole cell (45). TCR microclusters thus appear to function with a high degree of internal cooperativity but with little or no cooperativity between separate clusters.
Figure 3.
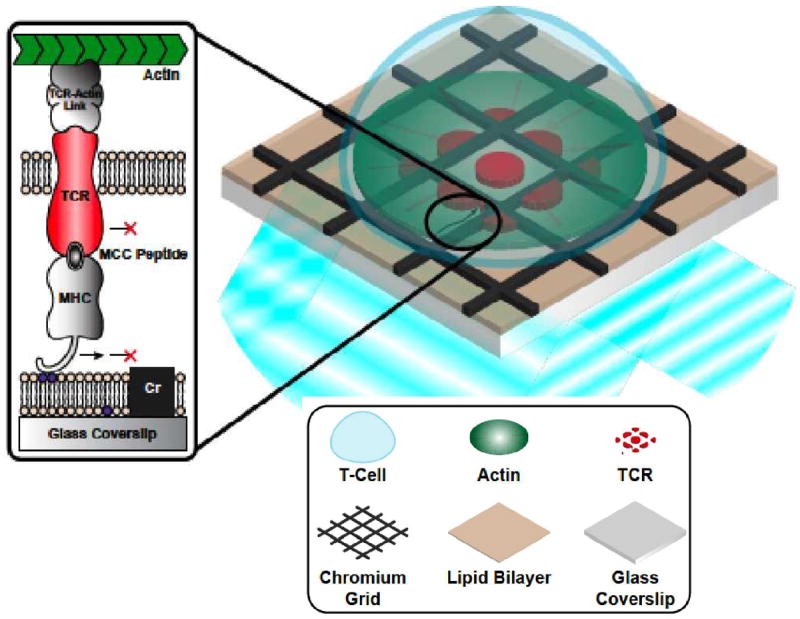
Schematic of a hybrid live cell – supported membrane junction. pMHC, ICAM1, and possibly other molecules such as CD80 can be incorporated into a supported membrane where they are free to diffuse laterally and engage their cognate receptors on the live T cell. Structures, such as nanometer scale metal lines, may be fabricated onto the underlying substrate to corral and guide the motion of these supported membrane molecules. Then, through specific receptor-ligand interactions, molecules within the living T cell become subject to the same physical constraints. This type of manipulation is referred to as a spatial mutation. Adapted from Smoligovets A. 2011. J. Cell Sci. in press
Molecular physiology of the immune synapse
Among all of the various types of immunological synapse, phagocytic synapses might serve as an ancestral template. Phagocytosis evolved from early efforts for single cell organisms to more efficiently compete for nutrients in the environment and use receptor system similar to those utilized by innate immune cells of mammals (3). While the term phagocytic synapse could have been utilized in a general sense based on early studies of junctions driven by phagocytic receptors (62), the specific current point of convergence that led to the proposal of a phagocytic synapse this year is the first effort to address how phagocytosis is selectively triggered by particulate, but not polyvalent soluble ligands engaging the same receptors (23). The threshold is around 0.5 μm diameter and this same submicron threshold is similar to the size of antigen receptor microclusters that drive signaling in T cells (61). This scale of microclusters may result from biophysical processes that are fundamental to many types of cell-cell communication (29). In the progression from innate to adaptive immune response, at least five distinct immunological synapses are triggered.
A common element in all of these synapses is that the key triggering signals are accompanied by phosphatase exclusion- a means of enabling activation of kinases by removing an inhibitor. Phosphatase exclusion models for immune cell triggering typically focus on the hematopoietic phosphatase CD45, which is a type I transmembrane protein with a large extracellular domain and a cytoplasmic tyrosine phosphatase domain. T cell antigen receptors (TCR) and NK cells activating receptors all utilize the Src family tyrosine kinase Lck to mediate early phosphorylation events. The maintenance of Lck in an active state requires CD45 due to its ability to remove a C-terminal inhibitory phosphorylation that is generated by Csk. Thus, CD45 must be expressed in the cell to maintain activity of Lck. However, CD45 also deactivates several targets of Lck at antigen receptors and thus it was proposed, first in a speculative mode by Springer and later with experimental support by van der Merwe and Dustin that CD45 exclusion would be a key initial event in TCR triggering.
A planar interface that enables high-resolution imaging has been necessary to investigate phosphatase exclusion. In early investigations, T cells were activated by antibodies to the TCR complex (a component known as CD3ε is most common) and anti-CD28, a co-stimulatory receptor. Although substrates coated with these antibodies completely exclude CD45 (16, 21), the experimental configuration may not be representative of physiological conditions. Presentation of MHC-peptide ligands and the adhesion ligand ICAM-1 on supported planar bilayers activates T cells (24) in a more physiological manner. Confocal imaging in these systems also initially indicated that CD45 was excluded from a large fraction of the T cell synapse (36). A significant increase in sensitivity was achieved utilizing total internal reflection fluorescence microscopy (TIRFM), which revealed faint f-actin dependent TCR microclusters that were responsible for sustained signaling (8, 61). In TIRFM, only the TCR microclusters exclude CD45, which otherwise fills the synapse (61). Clusters of B cell antigen receptors (BCR) also excluded CD45 in the same spatially restricted fashion when visualized by TIRFM (15). A significant artifact in confocal and deconvolution methods is that actin rich protrusions on flat surface (lamellipodia) have two membrane layers closely apposed to each other that generate the appearance of two-fold more CD45.
Phosphatase exclusion has not been fully addressed for NK cells since activating NK cell synapses were found to include CD45 by confocal microscopy (46). However, this method is not sufficient to observe sub-micron exclusion within localized signaling clusters.
The exclusion of CD45 from Dectin-1 rich clusters in the cells phagocytosing yeast cell walls has been observed by confocal microscopy (23). Dectin-1, a β-glucan receptor, has similar kinase recruitment motifs as T and B cell antigen receptors. The CD45 exclusion zones in this study were defined by contacts with β-glucan-rich yeast cell walls of ~5 μm diameter, which generated >2 μm regions of CD45 exclusion. These results are exciting and suggest a unifying mechanism for triggering synapses, but further study of how Dectin-1 forms signaling complexes using systems that enable TIRFM or super-resolution imaging methods would be of great value.
TCR signaling microcluster
Kupfer first described the micron-scale bull’s eye pattern of the T-B synapses with a ring of LFA-1, an integrin family adhesion molecule, surrounding a central cluster of TCR (40). Parallel studies with MHC-peptide complexes and LFA-1 ligand ICAM-1 presented in a supported planar bilayer with CD2 as an early marker for TCR rich domains, demonstrated that active processes in the T cells generate the pattern (24, 64). Kupfer described the LFA-1 rich ring as a peripheral supramolecular activation cluster (pSMAC) and the central TCR rich region as a central supramolecular activation cluster (cSMAC). Many of the ideas originally ascribed to the cSMAC may be more applicable to the TCR microclusters; the cSMAC itself is not required for T cell signaling (40, 41).
The initial contact area between a T cell and an APC is formed by a rapid, f-actin driven spreading that is mediated by the Rac effector WAVE2 to activate Arp2/3 complex and formins (33, 34). Cdc42 and Wiscott-Aldrich syndrome protein also play a role in this process, but are not needed for this initial spreading phase (37). TIRFM on the bilayer system has revealed that the SMACs are assembled by centripetal transport of LFA-1 and TCR microclusters. The TCR microclusters are well established to incorporate both the CD2-CD58 adhesion system and the CD28-CD80 costimulatory pathway. Negative regulators like CTLA-4 and PD-1 may also be incorporated into these microclusters. Although segregated spatially, the LFA-1-ICAM-1 interaction improves the sensitivity of the TCR for ligand by 100-fold and increases the duration of Ca2+ signaling (1, 2, 58). The LFA-1 accumulates in a ring associated with the adapter protein talin, whereas TCR microclusters translocate through spaces in this ring en route to the center of the synapse (30, 49) in a manner dependent upon TSG101, an early component in the endosomal complexes required for transport (ESCRT) (6).
TSG101 recognizes receptors with monoubiquitin groups. The TCR is ubiquitinated by c-Cbl and Cbl-b ubiquitin ligases that are recruited and activated under stimulation with agonist MHC-peptide complexes (7, 32). In fact, the very robust tyrosine phosphorylation due to CD45 exclusion may paradoxically promote TCR ubiquitination and rapid signal termination. TCR signaling is terminated by the TSG101 dependent step, which also sorts out the CD28-CD80 interactions into a distinct signaling structure rich in PKC-θ (6, 22).
A significant effect of this system on TCR microclusters is that while signaling, these structures are continuously being buffeted by centripetal actin flow and myosin IIA dependent contractions. These effects decrease the duration of the TCR-MHC-peptide interaction by 10-fold, and at the same time are required to achieve full signaling activity (31, 53). The stable immunological synapse is dependent upon a continual centripetal actin flow and the synapse breaks and re-locates whenever the symmetry of the pSMAC structure is broken (9, 37). While most of these observations have been made using the supported planar bilayer model system, there is evidence for similar events in T cell-dendritic cell synapses in vivo and in vitro (37, 39). Dendritic cells add another dimension to the T cell synapse as the dendritic cell cytoskeleton plays an important role in T cell activation (14, 28, 56). Each element in the multifocal T-dendritic cell immunological synapse appears to be a SMAC like assembly of multiple microclusters, rather than single microclusters (38, 39).
One very apparent property of TCR microclusters is their directed movement. Within a matter of seconds after formation, TCR clusters begin to move in concert with the retrograde flow of the cortical actin cytoskeleton and ultimately accumulate in the cSMAC. TCR signaling activity appears to occur prior to and during cluster transport, which also raises the question of the degree to which physical translocation of the clusters may contribute to or regulate TCR signaling. Cluster transport is clearly driven by actin polymerization processes. This is readily confirmed by treatment with the actin polymerization blocking drug, latrunculin, causes them to stop in their tracks (8). Precisely how TCR clusters are mechanically associated with the cortical actin cytoskeleton is less clear, but recent physical observations are revealing some salient characteristics.
TCR microclusters move in the direction of actin flow but their speed and even direction can differ (8, 13, 37, 65). Among the most visually compelling observations of this was made using a patterned supported membrane substrate in the configuration that acts as a molecular maze (Figure 4). This substrate consists of an array of unconnected metal barriers (100nm wide and 1.5 μm long), which present obstacles to TCR cluster transport, but don’t permanently trap the clusters. Trajectories of TCR are observed to encounter the barriers, and then translate along them until they reach the end of the barrier, at which time cluster trajectories realign with the actin flow direction (13). In a different set of experiments, in both Jurkat and primary T cells, direct visualization of actin flow speeds were measured by speckle or more advanced gradient imaging on patterned substrates (54, 66) (Figure 5). In these experimental configurations, the continuous barriers stably trap TCR in certain regions, enabling comparison of actin flow across the barriers with and without trapped TCR. Fluorescent speckles from actin-GFP or utrophin-GFP (which provides better imaging of actin dynamics due to less perturbation from the fluorescent protein fusion), were observed to slow and bunch up over trapped TCR, but the flow was not blocked. Actin flow is unperturbed crossing barriers without trapped TCR, providing important confirmation that substrate barriers only influence the T cell through their effect on proteins in the supported membrane.
Figure 4.
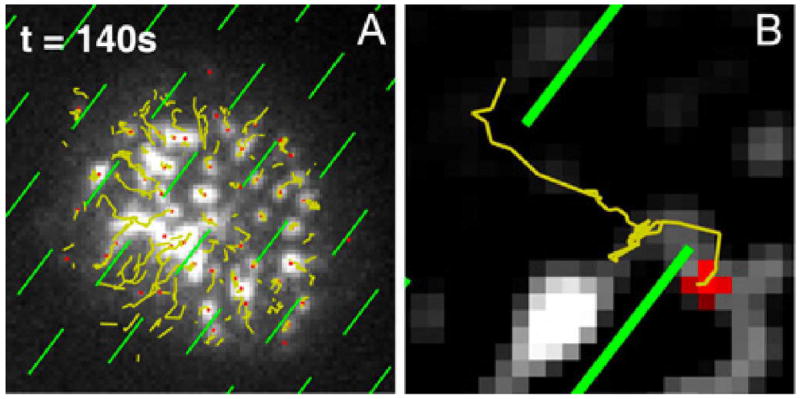
Molecular maze experiment. A pattern of barriers in a upported membrane substrate (1.5 μm long, 100 nm wide, and less than 10 nm high) impose obstacles to TCR cluster transport. TCR clusters are observer to percolate through the array of barriers by moving along barriers, at angles to the actin flow, until the edge is reached and the cluster rejoins the centripital flow. These observation led to the suggestion that TCR clusters were frictionally coupled to the flowing actin. Adapted from DeMond AL, Mossman KD, Starr T, Dustin ML, Groves JT. 2008. T cell receptor microcluster transport through molecular mazes reveals mechanism of translocation. Biophys. J. 94: 3286-92
Figure 5.
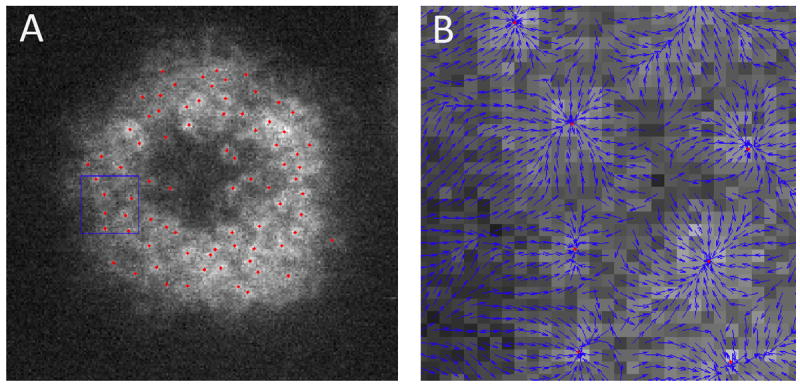
Example of gradient flow tracking analysis actin movement in live primary T cell. (A) Actin is imaged using a fluorescent fusion of the actin binding protein, utrophin. Image analysis is used to identify intensity gradients. The convergence points of these gradients can be tracked reliably. An important feature of this method is that it does not require well resolved objects; irregularly shaped density waves can tracked with precision. This type of analysis has proven more effective in studies of actin flow in primary T cells than speckle microscopy. Adapted from Smoligovets A. 2011. J. Cell Sci. in press
The type movement observed in the experiments mentioned above is consistent with a dissipative or frictional coupling between the TCR cluster and the actin. An interesting corollary of this observation is that it is possible that even imperceptibly weak binding interactions between TCR or other adaptor proteins and the actin could operate in concert within the cluster to produce the frictional drag. Importantly, these binding interactions could be very difficult to detect between dispersed molecules in vitro. In vivo coupling mechanisms of this sort can be difficult to predict from in vitro molecular binding data.
Another consequence of frictional coupling is that the strength of coupling is likely to scale with receptor clustering state nonlinearly. This follows for the simple reason that the actin itself is organized into polymerized structures, thus cooperative binding between clustered receptors and actin is expected due to the forced proximity. Such nonlinear coupling suggests that clustering state of receptors could be a contributing factor for how proteins are differentially sorted within the immune synapse. Such clustering state-based sorting has been directly observed for the case of LFA on the T cell. Upon strong antigen triggering, ICAM-LFA complexes become sorted into and largely define the pSMAC, which surrounds the cSMAC. If ICAM is crosslinked with externally applied antibodies, the resulting LFA-ICAM complexes are transported to progressively more inwardly radial positions within the synapse. The more clustered LFA-ICAM is apparently able to physically displace less clustered LFA-ICAM to occupy the more downstream positions in close analogy to a buoyancy effect (30).
Spatial mutation of TCR microcluster
T cells exhibit an exquisite ability to recognize extremely low densities of agonist peptide antigen. Some studies have suggested that T cells may react to even a few individual agonist peptide molecules(35, 55, 56). At the same time, T cells maintain this extreme sensitivity without spontaneous triggering in the absence of agonist, which could lead to autoimmune disorders(63). Since their first identification, the appearance TCR microclusters has been observed to correlate with the onset of intracellular calcium flux, a definitive measure of T cell triggering(8, 65). Indeed, this led to the conclusion that the microsclusters, rather than larger features of the immune synapse, were centers for signaling activity. Various mechanisms for how high sensitivity to agonist with apparent immunity to stochastic noisemight be achieved within TCR microsclusters have been proposed. Some suggestions include TCR monomer triggering by co-receptor or self pMHC heterodimerization(39, 57) or force-induced conformational changes(42). Extensive cooperativity among multiple receptors within the TCR clusters is also implicated(61), which may result from conformation induced clustering(43), kinetic segregation(12), or lipid-mediated assemble (59). However, a detailed physical description of the signaling reactions at play within TCR microclusters remains largely unresolved. Key to ultimately determining, and experimentally verifying, the molecular mechanism of TCR triggering is the ability to manipulate TCR cluster assembly in living T cells.
Recent experiments that directly probe the consequences of differentially partitioning agonist, coagonist, and null pMHC among TCR clusters in primary T cells are have illuminated intriguing aspects of the system. The strategy is based on the hybrid live cell – supported membrane configuration, in which a solid supported lipid bilayer takes the place of the antigen presenting cell(24, 47, 49) (Figure 4). Histidine-tagged variants of MHC class II, intercellular adhesion molecule 1 (ICAM-1) are linked to the membrane through Ni2+-chelating lipid groups(26, 49). The lipids and proteins diffuse freely and as monomers on the supported membrane(27). The confirmed monomeric properties of histidine-linked proteins is a crucial part of this experiment. Many earlier supported membrane experiments, including those that originally identified TCR clusters, utilized GPI-linked MHC. While still functional, GPI-linkages to supported membranes are problematic in that they can lead to high levels of intrinsic clustering (45), which greatly undermines their utility for studies of natural receptor clustering.
In supported membranes, peptide composition and pMHC density are under direct experimental control. TCR clustering is controlled through grid patterns of metal lines, which have been prefabricated onto the underlying solid substrate. These structures, known as diffusion or mobility barriers(26, 27, 47), block lateral transport of lipids and supported membrane associated proteins. Molecules diffuse freely within each corral, but are unable to hop between separate corrals. The number of pMHC within each supported membrane corral determines the maximum pMHC content of the corresponding TCR cluster that may assemble on the T cell.
Thus adjusting the grid size at constant pMHC density titrates the maximum number of pMHC per TCR cluster without changing the number of antigen engaged by the T cell (Figure 6). We refer to this physical manipulation of molecular organization within living cells as a spatial mutation(25, 47, 52). In the present application, T cells differing only in the peptide agonist distribution among TCR clusters are generated and compared side-by-side.
Figure 6.
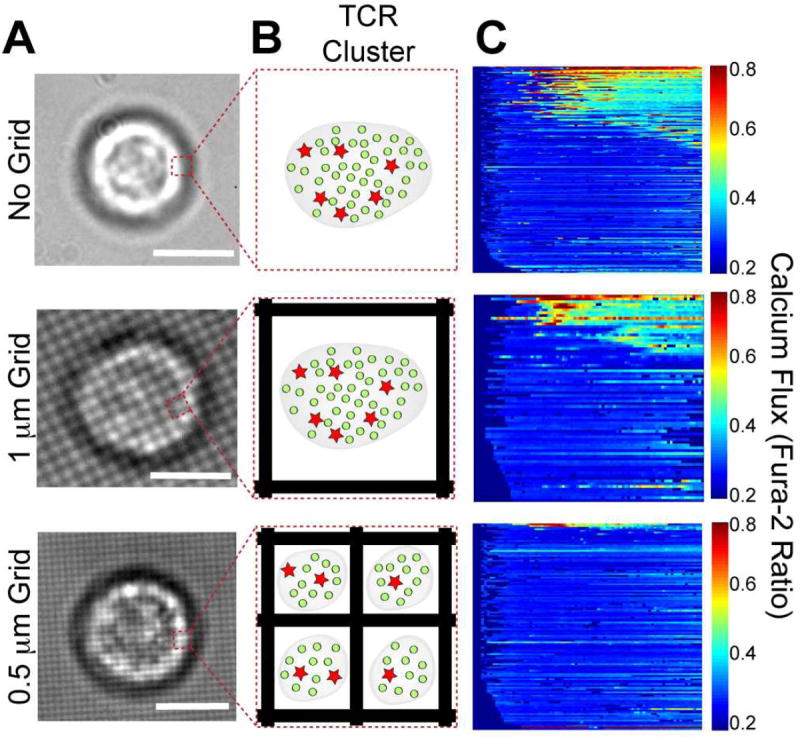
TCR cluster titration experiment. (a) Brightfield images of cells off and on the indicated grid pattern size (scale bar = 5 mm). (b) Schematic of TCR microcluster within indicated area in (a) with pMHC bound to activating agonists (stars) and non-activating null (circles) peptides. (c) Corresponding heat maps that display calcium flux for a population of cells on and off the grids, with each cell shown as a horizontal line and >100 cells per heat map. Adapted from Manz BN, Jackson BL, Petit RS, Dustin ML, Groves J. 2011. Proc. Natl. Acad. Sci. U. S. A. 108: 9089-94
In this experiment, a two parameter titration is performed in which the overall antigen peptide surface density as well as its partitioning among TCR clusters are independently controlled in living T cells. The results indicate that the threshold antigen densities for triggering Ca2+ flux (in terms of the number of antigen per cell) are dependent on agonist partitioning among TCR clusters. Most significantly, when antigen dose-response functions obtained on different grid partition sizes are analyzed in terms of the maximum number of antigens per TCR cluster (determined by pMHC content within individual membrane corrals), they collapse onto a single curve. T cells trigger at an average agonist density of ~two per TCR cluster, irrespective of the total number of agonist engaged by the cell. The term triggering threshold is used here to describe the average density at which half-maximum response is achieved. Observed triggering thresholds were not changed by inclusion of the coagonist null peptide ER60. Interpretation of this invariant activation response in terms of the Poisson distribution of agonist pMHC among TCR clusters indicates that the most likely activation threshold is at least four agonist peptides within a single TCR cluster in this system.
Observations with CD80 – CD28 costimulation reveal that TCR triggering thresholds are not absolute. CD80 – CD28 costimulation is one of perhaps multiple ways that triggering thresholds may be tuned in vivo (9, 22, 31-33, 39, 64). The one truly invariant observation among all of the results presented here is that triggering thresholds are determined on a per TCR cluster basis, and not on a per T cell basis.
A number of estimates of T cell sensitivity to pMHC have been made over the past two decades, with increasing precision(14, 28, 38, 51, 56). Single agonist sensitivity has been reported by fluorescence, in a cell-cell interface where multiple ligands, protein complexes and coagonist endogenous peptides are present(35, 39, 56). There are several factors to consider when interpreting the seemingly different conclusions from these various reports and the TCR cluster size titration experiments described above. First, the definition of triggering threshold as the average antigen number at which half maximum response is achieved may differ from earlier interpretations. This definition was chosen because it converges to an informative value in the limit of a large number of experiments. Triggering threshold cannot properly be defined as the lowest level of antigen at which any triggering is observed since there is a non-zero probability of a cell triggering with no antigen due to stochastic fluctuations. These fluctuations are intrinsically random physical events and cannot be distinguished from causal triggering on a cell-by-cell basis. Secondly, supported membranes differ from live antigen presenting cell (APC) experiments since costimulatory molecule and peptide antigen distributions are directly controllable. These are intrinsically unknown in live APCs, and may alter triggering thresholds(4, 39).
Conclusions
Studies of the immune synapses, and especially the T cell immune synapse, are providing a view into the mechanistic inner workings of an elaborate signal transduction system with unprecedented clarity. Especially noteable are the multi-scale spatial organization processes, which interleave physical, mechanical, and biochemical processes seamlessly into a functional unit. In spite of the vast amount of progress made over the last decade, we are really only beginning to realize how little we actually know about how intracellular signal transduction networks actually function. Particularly exciting, however, is the way researchers from various fields are becoming attracted to these problems and bringing different tools and perspectives to the research table.
References
- 1.Al-Alwan MM, Liwski RS, Haeryfar SMM, Baldridge WH, Hoskin DW, et al. Cutting edge: dendritic cell actin cytoskeletal polarization during immunological synapse formation is highly antigen-dependent. J Immunol. 2003;171:4479–83. doi: 10.4049/jimmunol.171.9.4479. [DOI] [PubMed] [Google Scholar]
- 2.Al-Alwan MM, Rowden G, Lee TD, West KA. The dendritic cell cytoskeleton is critical for the formation of the immunological synapse. J Immunol. 2001;166:1452–56. doi: 10.4049/jimmunol.166.3.1452. [DOI] [PubMed] [Google Scholar]
- 3.Allen PG, Dawidowicz EA. Phagocytosis in Acanthamoeba: I. A mannose receptor is responsible for the binding and phagocytosis of yeast. J Cell Physiol. 1990;145:508–13. doi: 10.1002/jcp.1041450317. [DOI] [PubMed] [Google Scholar]
- 4.Anderson HA, Hiltbold EM, Roche PA. Concentration of MHC class II molecules in lipid rafts facilitates antigen presentation. Nat Immunol. 2000;1:156–62. doi: 10.1038/77842. [DOI] [PubMed] [Google Scholar]
- 5.Batista FD, Iber D, Neuberger MS. B cells acquire antigen from target cells after synapse formation. Nature. 2001;411:489–94. doi: 10.1038/35078099. [DOI] [PubMed] [Google Scholar]
- 6.Benvenuti F, Hugues S, Walmsley M, Ruf S, Fetler L, et al. Requirement of Rac1 and Rac2 expression by mature dendritic cells for T cell priming. Science. 2004;305:1150–53. doi: 10.1126/science.1099159. [DOI] [PubMed] [Google Scholar]
- 7.Brossard C, Feuillet V, Schmitt A, Randriamampita C, Romao M, et al. Multifocal structure of the T cell – dendritic cell synapse. Eur J Immunol. 2005;35:1741–53. doi: 10.1002/eji.200425857. [DOI] [PubMed] [Google Scholar]
- 8.Campi G, Varma R, Dustin ML. Actin and agonist MHC-peptide complex-dependent T cell receptor microclusters as scaffolds for signaling. J Exp Med. 2005;202:1031–6. doi: 10.1084/jem.20051182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carreño LJ, Riquelme EM, González PA, Espagnolle N, Riedel CA, et al. T-cell antagonism by short half-life pMHC ligands can be mediated by an efficient trapping of T-cell polarization toward the APC. Proc Natl Acad Sci U S A. 2010;107:210–15. doi: 10.1073/pnas.0911258107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Choudhuri K, Dustin ML. Signaling microdomains in T cells. FEBS Lett. 2010;584:4823–31. doi: 10.1016/j.febslet.2010.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davis DM, Chiu I, Fassett M, Cohen GB, Mandelboim O, Strominger JL. The human natural killer cell immune synapse. Proc Natl Acad Sci U S A. 1999;96:15062–67. doi: 10.1073/pnas.96.26.15062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davis SJ, van der Merwe PA. The kinetic-segregation model: TCR triggering and beyond. Nat Immunol. 2006;7:803–09. doi: 10.1038/ni1369. [DOI] [PubMed] [Google Scholar]
- 13.DeMond AL, Mossman KD, Starr T, Dustin ML, Groves JT. T cell receptor microcluster transport through molecular mazes reveals mechanism of translocation. Biophys J. 2008;94:3286–92. doi: 10.1529/biophysj.107.119099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Demotz S, Grey HM, Sette A. The minimal number of class II MHC-antigen complexes needed for T cell activation. Science. 1990;249:1028–30. doi: 10.1126/science.2118680. [DOI] [PubMed] [Google Scholar]
- 15.Depoil D, Fleire S, Treanor BL, Weber M, Harwood NE, et al. CD19 is essential for B cell activation by promoting B cell receptor-antigen microcluster formation in response to membrane-bound ligand. Nat Immunol. 2008;9:63–72. doi: 10.1038/ni1547. [DOI] [PubMed] [Google Scholar]
- 16.Douglass AD, Vale RD. Single-molecule microscopy reveals plasma membrane microdomains created by protein-protein networks that exclude or trap signaling molecules in T cells. Cell. 2005;121:937–50. doi: 10.1016/j.cell.2005.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dustin ML. T-cell activation through immunological synapses and kinapses. Immunol Rev. 2008;221:77–89. doi: 10.1111/j.1600-065X.2008.00589.x. [DOI] [PubMed] [Google Scholar]
- 18.Dustin ML. The Cellular Context of T Cell Signaling. Immunity. 2009;30:482–92. doi: 10.1016/j.immuni.2009.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dustin ML. Insights into function of the immunological synapse from studies with supported planar bilayers. Curr Top Microbiol Immunol. 2010;340:1–24. doi: 10.1007/978-3-642-03858-7_1. [DOI] [PubMed] [Google Scholar]
- 20.Fooksman DR, Vardhana S, Vasiliver-Shamis G, Liese J, Blair DA, et al. Functional Anatomy of T Cell Activation and Synapse Formation. Annu Rev Immunol. 2010;28:79–105. doi: 10.1146/annurev-immunol-030409-101308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Freiberg BA, Kupfer H, Maslanik W, Delli J, Kappler J, et al. Staging and resetting T cell activation in SMACs. Nat Immunol. 2002;3:911–17. doi: 10.1038/ni836. [DOI] [PubMed] [Google Scholar]
- 22.González PA, Carreño LJ, Coombs D, Mora JE, Palmieri E, et al. T cell receptor binding kinetics required for T cell activation depend on the density of cognate ligand on the antigen-presenting cell. Proc Natl Acad Sci U S A. 2005;102:4824–29. doi: 10.1073/pnas.0500922102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goodridge HS, Reyes CN, Becker CA, Katsumoto TR, Ma J, et al. Activation of the innate immune receptor Dectin-1 upon formation of a ‘phagocytic synapse’. Nature. 2011;472:471–75. doi: 10.1038/nature10071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grakoui A. The Immunological Synapse: A Molecular Machine Controlling T Cell Activation. Science. 1999;285:221–27. doi: 10.1126/science.285.5425.221. [DOI] [PubMed] [Google Scholar]
- 25.Groves JT. Spatial mutation of the T cell immunological synapse. Curr Opin Chem Biol. 2006;10:544–50. doi: 10.1016/j.cbpa.2006.10.021. [DOI] [PubMed] [Google Scholar]
- 26.Groves JT, Dustin ML. Supported planar bilayers in studies on immune cell adhesion and communication. J Immunol Methods. 2003;278:19–32. doi: 10.1016/s0022-1759(03)00193-5. [DOI] [PubMed] [Google Scholar]
- 27.Groves JT, Ulman N, Boxer SG. Micropatterning Fluid Lipid Bilayers on Solid Supports. Science. 1997;275:651–53. doi: 10.1126/science.275.5300.651. [DOI] [PubMed] [Google Scholar]
- 28.Harding CV, Unanue ER. Quantitation of antigen-presenting cell MHC class II/peptide complexes necessary for T-cell stimulation. Nature. 1990;346:574–76. doi: 10.1038/346574a0. [DOI] [PubMed] [Google Scholar]
- 29.Hartman NC, Groves JT. Signaling clusters in the cell membrane. Curr Opin Cell Biol. 2011:1–7. doi: 10.1016/j.ceb.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hartman NC, Nye JA, Groves JT. Cluster size regulates protein sorting in the immunological synapse. Proc Natl Acad Sci U S A. 2009;106:12729–34. doi: 10.1073/pnas.0902621106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Henrickson SE, Mempel TR, Mazo IB, Liu B, Artyomov MN, et al. T cell sensing of antigen dose governs interactive behavior with dendritic cells and sets a threshold for T cell activation. Nat Immunol. 2008;9:282–91. doi: 10.1038/ni1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang J, Zarnitsyna VI, Liu B, Edwards LJ, Jiang N, et al. The kinetics of two-dimensional TCR and pMHC interactions determine T-cell responsiveness. Nature. 2010;464:932–36. doi: 10.1038/nature08944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huppa JB, Axmann M, Mörtelmaier MA, Lillemeier BF, Newell EW, et al. TCR-peptide-MHC interactions in situ show accelerated kinetics and increased affinity. Nature. 2010;463:963–67. doi: 10.1038/nature08746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ilani T, Vasiliver-Shamis G, Vardhana S, Bretscher A, Dustin ML. T cell antigen receptor signaling and immunological synapse stability require myosin IIA. Nat Immunol. 2009;10:531–39. doi: 10.1038/ni.1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Irvine DJ, Purbhoo MA, Krogsgaard M, Davis MM. Direct observation of ligand recognition by T cells. Nature. 2002;419:845–49. doi: 10.1038/nature01076. [DOI] [PubMed] [Google Scholar]
- 36.Johnson KG, Bromley SK, Dustin ML, Thomas ML. A supramolecular basis for CD45 tyrosine phosphatase regulation in sustained T cell activation. Proc Natl Acad Sci U S A. 2000;97:10138–43. doi: 10.1073/pnas.97.18.10138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kaizuka Y, Douglass AD, Varma R, Dustin ML, Vale RD. Mechanisms for segregating T cell receptor and adhesion molecules during immunological synapse formation in Jurkat T cells. Proc Natl Acad Sci U S A. 2007;104:20296–301. doi: 10.1073/pnas.0710258105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kimachi K, Croft M, Grey HM. The minimal number of antigen-major histocompatibility complex class II complexes required for activation of naive and primed T cells. Eur J Immunol. 1997;27:3310–17. doi: 10.1002/eji.1830271230. [DOI] [PubMed] [Google Scholar]
- 39.Krogsgaard M, Li Q-j, Sumen C, Huppa JB, Huse M, Davis MM. Agonist/endogenous peptide-MHC heterodimers drive T cell activation and sensitivity. Nature. 2005;434:238–43. doi: 10.1038/nature03391. [DOI] [PubMed] [Google Scholar]
- 40.Lee K-H, Dinner AR, Tu C, Campi G, Raychaudhuri S, et al. The immunological synapse balances T cell receptor signaling and degradation. Science. 2003;302:1218–22. doi: 10.1126/science.1086507. [DOI] [PubMed] [Google Scholar]
- 41.Lee K-H, Holdorf AD, Dustin ML, Chan AC, Allen PM, Shaw AS. T Cell Receptor Signaling Precedes Immunological Synapse Formation. Science. 2002;295:1539–42. doi: 10.1126/science.1067710. [DOI] [PubMed] [Google Scholar]
- 42.Li Y-C, Chen B-M, Wu P-C, Cheng T-L, Kao L-S, et al. Cutting Edge: mechanical forces acting on T cells immobilized via the TCR complex can trigger TCR signaling. J Immunol. 2010;184:5959–63. doi: 10.4049/jimmunol.0900775. [DOI] [PubMed] [Google Scholar]
- 43.Lillemeier BF, Mortelmaier MA, Forstner MB, Huppa JB, Groves JT, Davis MM. TCR and Lat are expressed on separate protein islands on T cell membranes and concatenate during activation. Nat Immunol. 2010;11:90–96. doi: 10.1038/ni.1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Manz BN, Groves JT. Spatial organization and signal transduction at intercellular junctions. Nat Rev Mol Cell Biol. 2010;11:342–52. doi: 10.1038/nrm2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Manz BN, Jackson BL, Petit RS, Dustin ML, Groves J. T-cell triggering thresholds are modulated by the number of antigen within individual T-cell receptor clusters. Proc Natl Acad Sci U S A. 2011;108:9089–94. doi: 10.1073/pnas.1018771108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McCann FE, Vanherberghen B, Eleme K, Carlin LM, Newsam RJ, et al. The size of the synaptic cleft and distinct distributions of filamentous actin, ezrin, CD43, and CD45 at activating and inhibitory human NK cell immune synapses. J Immunol. 2003;170:2862–70. doi: 10.4049/jimmunol.170.6.2862. [DOI] [PubMed] [Google Scholar]
- 47.Mossman KD, Campi G, Groves JT, Dustin ML. Altered TCR Signaling from Geometrically Repatterned Immunological Synapses. Science. 2005;310:1191–93. doi: 10.1126/science.1119238. [DOI] [PubMed] [Google Scholar]
- 48.Norcross MA. A synaptic basis for T-lymphocyte activation. Annales dimmunologie. 1984;135D:113–34. doi: 10.1016/s0769-2625(84)81105-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nye JA, Groves JT. Kinetic Control of Histidine-Tagged Protein Surface Density on Supported Lipid Bilayers. Langmuir. 2008;24:4145–49. doi: 10.1021/la703788h. [DOI] [PubMed] [Google Scholar]
- 50.Paul WE, Seder RA. Lymphocyte responses and cytokines. Cell. 1994;76:241–51. doi: 10.1016/0092-8674(94)90332-8. [DOI] [PubMed] [Google Scholar]
- 51.Reay PA, Matsui K, Haase K, Wulfing C, Chien Y-H, Davis MM. Determination of the Relationship Between T Cell Responsiveness and the Number of MHC-Peptide Complexes Using Specific Monoclonal Antibodies. J Immunol. 2000;164:5626–34. doi: 10.4049/jimmunol.164.11.5626. [DOI] [PubMed] [Google Scholar]
- 52.Salaita K, Nair PM, Petit RS, Neve RM, Das D, et al. Restriction of Receptor Movement Alters Cellular Response: Physical Force Sensing by EphA2. Science. 2010;327:1380–85. doi: 10.1126/science.1181729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schmits R, Kündig TM, Baker DM, Shumaker G, Simard JJ, et al. LFA-1-deficient mice show normal CTL responses to virus but fail to reject immunogenic tumor. J Exp Med. 1996;183:1415–26. doi: 10.1084/jem.183.4.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Smoligovets A. 2011 in press. [Google Scholar]
- 55.Stefanova I, Dorfman JR, Germain RN. Self-recognition promotes the foreign antigen sensitivity of naive T lymphocytes. Nature. 2002;420:429–34. doi: 10.1038/nature01146. [DOI] [PubMed] [Google Scholar]
- 56.Sykulev Y, Joo M, Vturina I, Tsomides TJ, Eisen HN. Evidence that a Single Peptide–MHC Complex on a Target Cell Can Elicit a Cytolytic T Cell Response. Immunity. 1996;4:565–71. doi: 10.1016/s1074-7613(00)80483-5. [DOI] [PubMed] [Google Scholar]
- 57.Trautmann A, Randriamampita C. Initiation of TCR signalling revisited. Trends Immunol. 2003;24:425–28. doi: 10.1016/s1471-4906(03)00182-0. [DOI] [PubMed] [Google Scholar]
- 58.Tseng S-Y, Waite JC, Liu M, Vardhana S, Dustin ML. T cell-dendritic cell immunological synapses contain TCR-dependent CD28-CD80 clusters that recruit protein kinase C theta. J Immunol. 2008;181:4852–63. doi: 10.4049/jimmunol.181.7.4852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Václav H. Lipid rafts and their roles in T-cell activation. Microbes Infect. 2005;7:310–16. doi: 10.1016/j.micinf.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 60.van der Merwe PA, Dushek O. Mechanisms for T cell receptor triggering. Nat Rev Immunol. 2011;11:47–55. doi: 10.1038/nri2887. [DOI] [PubMed] [Google Scholar]
- 61.Varma R, Campi G, Yokosuka T, Saito T, Dustin ML. T Cell Receptor-Proximal Signals Are Sustained in Peripheral Microclusters and Terminated in the Central Supramolecular Activation Cluster. Immunity. 2006;25:117–27. doi: 10.1016/j.immuni.2006.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wright SD, Silverstein SC. Phagocytosing macrophages exclude proteins from the zones of contact with opsonized targets. Nature. 1984;309:359–61. doi: 10.1038/309359a0. [DOI] [PubMed] [Google Scholar]
- 63.Wylie DC, Das J, Chakraborty AK. Sensitivity of T cells to antigen and antagonism emerges from differential regulation of the same molecular signaling module. Proc Natl Acad Sci U S A. 2007;104:5533–38. doi: 10.1073/pnas.0611482104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yokosuka T, Kobayashi W, Sakata-Sogawa K, Takamatsu M, Hashimoto-Tane A, et al. Spatiotemporal regulation of T cell costimulation by TCR-CD28 microclusters and protein kinase C theta translocation. Immunity. 2008;29:589–601. doi: 10.1016/j.immuni.2008.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yokosuka T, Sakata-Sogawa K, Kobayashi W, Hiroshima M, Hashimoto-Tane A, et al. Newly generated T cell receptor microclusters initiate and sustain T cell activation by recruitment of Zap70 and SLP-76. Nat Immunol. 2005;6:1253–62. doi: 10.1038/ni1272. [DOI] [PubMed] [Google Scholar]
- 66.Yu C-h, Wu H-J, Kaizuka Y, Vale RD, Groves JT. Altered Actin Centripetal Retrograde Flow in Physically Restricted Immunological Synapses. PLoS ONE. 2010;5:e11878. doi: 10.1371/journal.pone.0011878. [DOI] [PMC free article] [PubMed] [Google Scholar]


