Abstract
Zebrafish offer many advantages that complement classic mammalian models for the study of normal development as well as for the teratogenic effects of exposure to hazardous compounds. The clear chorion and embryo of the zebrafish allow for continuous visualization of the anatomical changes associated with development, which, along with short maturation times and the capability of complex behavior, makes this model particularly useful for measuring changes to the developing nervous system. Moreover, the rich array of developmental, behavioral, and molecular benefits offered by the zebrafish have contributed to an increasing demand for the use of zebrafish in behavioral teratology. Essential for this endeavor has been the development of a battery of tests to evaluate a spectrum of behavior in zebrafish. Measures of sensorimotor plasticity, emotional function, cognition and social interaction have been used to characterize the persisting adverse effects of developmental exposure to a variety of chemicals including therapeutic drugs, drugs of abuse and environmental toxicants. In this review, we present and discuss such tests and data from a range of developmental neurobehavioral toxicology studies using zebrafish as a model. Zebrafish provide a key intermediate model between high throughput in vitro screens and the classic mammalian models as they have the accessibility of in vitro models and the complex functional capabilities of mammalian models.
Keywords: Zebrafish, Development, Neurotoxicology, Behavior
Introduction
Zebrafish have been widely used by molecular geneticists and developmental biologists for studying mechanisms of vertebrate development. The transparency of the zebrafish embryo (and chorion), in addition to the ability to apply transgenic reporter lines, permits direct and continuous visualization of tissue morphogenesis in vivo. This is especially relevant when assessing gene function, but other characteristics of the developing zebrafish make it ideal for addressing questions of behavior as well. Consequently, within the last decade or so, the Zebrafish have been developed to be a useful tool for understanding not just the structural and chemical effects of neurotoxicants, but also for assessing the behavioral impairment associated with such exposure and as such, is valuable for the field of developmental neurobehavioral toxicology.
Among other advantages, the zebrafish embryo develops rapidly, outside of the mother’s body, and reaches adulthood within a few months. By just 6 days post fertilization (pf) the zebrafish has functioning organs (or counterparts to mammalian organs) including a complex circulatory system (Langheinrich, 2003). At adulthood, zebrafish are quite small (approximately 2.5 cm), permitting logistical and economic advantages over rodent models. Further, both the structural integrity and functional ability of zebrafish can be assessed over the entire lifespan (e.g. larval, adult, etc.), making this model ideally suited for understanding the teratogenic effects of compounds. Moreover, the elementary actions of a neurotoxicant can be followed in terms of disruptions of neural differentiation, proliferation, migration, outgrowth, synapse formation, and circuit development. Then, behavior of the zebrafish can be measured to determine the functional impact of a chemical exposure.
As such, the use of zebrafish in neurobehavioral teratology has expanded greatly in the past decade (Linney et al., 2004; Scalzo & Levin, 2004). Our group and others have used the zebrafish model to study the teratological risks posed by drugs of abuse such as nicotine and alcohol, therapeutic drugs such as methylphenidate and valproic acid and environmental toxicants such as methlymercury, chlorpyrifos and silver. A variety of behavioral tests have been developed to assess motor function, stress response, social behavior and learning/memory in zebrafish. The present review highlights the use of zebrafish for understanding the behavioral effects of developmental exposure to a variety of compounds.
Behavioral Assays
The range and complexity of paradigms that have been developed to measure behavior in adult zebrafish, while not yet comparable to those seen with rodent models, now includes tasks that tap both cognitive and sensorimotor domains. In fact, specific tasks have been developed, or modified from rodent versions, to measure behavior in zebrafish. Often, very simple measures of swimming and ability to capture/consume can be informative. However, complex behavioral assays have been employed to measure more subtle changes in behavior. These include tasks that measure behavior characteristic of addiction, anxiety, aggression, learning/memory, locomotion, social responses, antipredatory behavior and mate choice. These tasks have been employed for studies of drug abuse (for a review see Ninkovic & Bally-Cuif, 2006), drug development (Levin, 2011) and toxicant exposure (Levin et al., 2003; Powers et al., 2010; Powers et al., 2011).
When possible, the focus of this review will be on the long-term effects of developmental exposure to toxins/toxicants. However, endpoints assessed during embryonic or larval stages of development frequently constitute the extent of behavioral testing. The most common method for measuring the effects of developmental exposure in embryos or larvae is activity (e.g. swimming speed or distance) during alternating periods of light and dark. The typical zebrafish will swim faster and farther during the light cycle than during the dark (Gerlai et al., 2000; Cahill, 2002).
Some of the most commonly used cognitive and sensorimotor tasks used with adult zebrafish are described and clarified here. Included are startle response/habituation learning, anti-predatory behavior, social responses and spatial discrimination learning.
Startle Response/Habituation
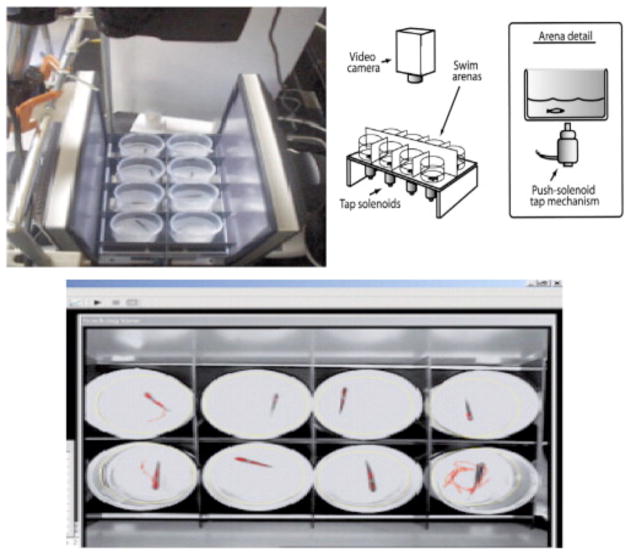
Often a “tap test” is used to elicit an unconditioned startle response to a stimulus (i.e. sudden movement of the tank environment resulting from pressure applied to the outside of the tank), which can measure both locomotor function and habituation with repeated closely timed stimuli (see Figure 1; Sledge et al., 2011). The unexposed, typically developed zebrafish will swim around the test arena rapidly following initial taps but will exhibit a reduction in this startle response as a function of repeated exposure to this stimulus.
Figure 1.
The tap startle/habituation task (Sledge et al., 2011) in which eight zebrafish are tested simultaneously. A solenoid beneath each arena provide taps at one-minute intervals for ten trials. Initial, vigorous swimming response is followed by gradual habituation of response.
Predatory Avoidance Behavior
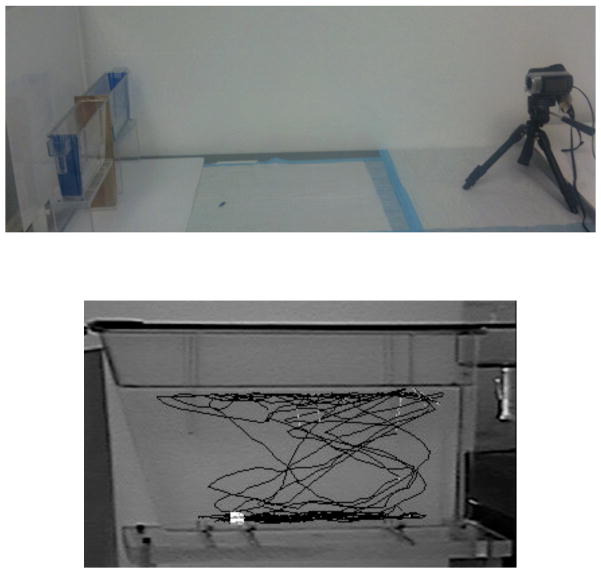
The novel tank dive test, which measures species-typical swimming patterns following submersion in a novel environment is thought to measure both a form of anti-predatory response and anxiety or stress-like behavior (see Figure 2 for representation of this task; Bencan et al., 2009). Here, the unexposed zebrafish will dive immediately to the tank floor but gradually (i.e. after 1–2 minutes) begin exploring the environment. Both the dive (predator avoidance) and the exploration behavior (food-seeking) are beneficial to the zebrafish and any aberration from this pattern is considered mal-adaptive. Another measure of anti-predatory behavior seen in the behavioral teratology literature is the species-typical “stress response” that occurs in the presence of visual or olfactory cues that a predator is near (Barcellos et al., 2007; Egan et al., 2009). Zebrafish will freeze or retreat from stimuli associated with predation.
Figure 2.
The novel tank diving task (Bencan et al., 2009) in which zebrafish are placed in a novel narrow tank and the vertical dimension of their swimming behavior is measured. Initially, during a five-minute session the fish dive to the bottom of the novel tank and later swim to explore other levels of the tank.
Spatial Discrimination
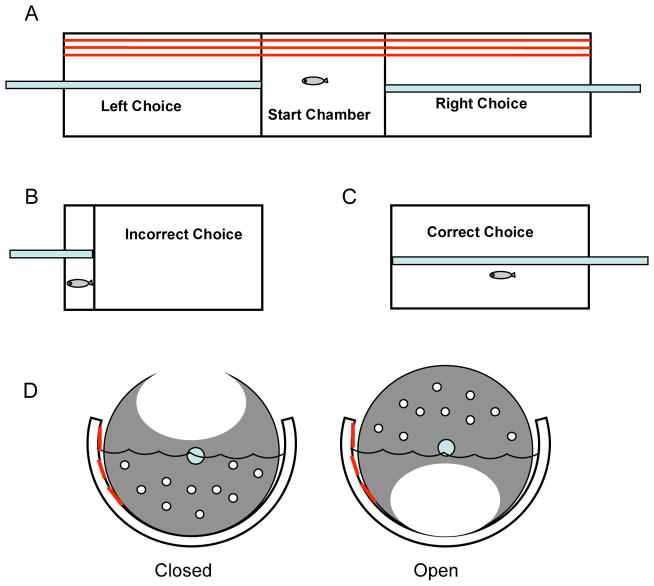
Levin et al. (2003) have measured learning ability following exposure to various teratogenic chemicals using a test of spatial discrimination in which zebrafish acquire a pattern of responding that avoids the delivery of an aversive stimulus and results in the delivery of reinforcement. Here, an animal is placed in the middle of a three-chamber testing apparatus. When two transparent dividers are lifted the animal is free to swim to either the left or right side of the chamber. Once side-preference is determined, a swimming response to the preferred side of the chamber results in a dramatic constriction of space whereas a swimming response to the non-preferred side results in an approximate doubling of swim-space (Figure 3). This allows for accuracy scores to be generated over repeated trials. An unexposed zebrafish typically learns the discrimination, as measured by an above chance accuracy score.
Figure 3.
The three-chamber task for testing learning and memory using a spatial discrimination procedure (Levin et al., 2003) in which the zebrafish is initially placed in the center chamber and then given the opportunity to swim to either of two side chambers. If the correct choice is made the fish is permitted to swim undisturbed in the choice chamber. If an incorrect choice is made the partition between chambers is moved toward the end to restrain the fish. Sequential trials are given to index learning. Spatial or nonspatial cues can be used for the discrimination.
Spatial Alternation
Spatial alternation procedures are similar to spatial discrimination in that spatially oriented responses are reinforced (or punished). However, here a response that is different from that which was previously emitted is reinforced. Often, an aquarium is divided into two equal sides with visual stimuli associated with each side. In some iterations of this procedure, a discriminative stimulus (tap on tank) signals the delivery of food to one side of the tank. On each subsequent trial the discriminative stimulus precedes food delivery to the side not previously associated with reinforcement. Thus, this task requires the animal alternate responding following delivery of the discriminative stimulus (Smith et al., 2010).
Social Response
Finally, social behavior is often assessed using a task in which an isolated animal is given access to images of shoaling zebrafish (Fernandes & Gerlai, 2009), usually displayed on a monitor located just outside the tank. However, the sight of live fish has also been presented (Saverino & Gerlai, 2008). Distance from shoal and other swimming characteristics (e.g. speed, direction) are the most common measures. Group preference (Gerlai et al., 2008) and tank area occupied (Dlugos & Rabin, 2003) have also been used to capture effects on social behavior.
Drugs of Abuse
Adult Effects
Alcohol
As one of the most widely-used drugs of abuse, the neuroteratogenic properties of alcohol are well characterized. Fetal alcohol syndrome (FAS) describes the most severe constellation of effects associated with exposure, which includes characteristic physical and cognitive abnormalities. FAS has been demonstrated in human (e.g. Streissguth et al., 1999; Riley et al., 2005; Kelly et al., 2000) as well as rodent (Schneider et al., 2001; Gil-Mohapel et al., 2002; for a review see Rout, 2005) and now zebrafish (Bilotta, 2003; Bilotta et al., 2004; Carvan et al., 2004) models. In fact, strikingly similar physical abnormalities occur across species that are exposed to alcohol during development, including cranial and facial malformations and retarded growth. Behaviorally, FAS and Fetal Alcohol Spectrum Disorders (FASD) are characterized by learning and attention difficulties, which have been demonstrated in humans, rodents and zebrafish. Interestingly, the effects of embryonic alcohol exposure on the developing brain occur at very low doses and have pronounced effects throughout the lifespan of the organism (e.g., West & Goodlett, 1990).
In zebrafish, the effects of embryonic exposure to alcohol on adult behavior have been characterized using a variety of endpoints. Carvan et al. (2004) exposed newly fertilized embryos to a range of ethanol concentrations from 3–300 mM. During adulthood the ability to acquire a spatial alternation task under appetitive control was used as a marker of cognitive function. Here, fish exposed to just 10 mM of ethanol for the first four hours pf made significantly more errors (i.e. did not alternate halves of the tank) and took more trials to reach criterion (75% correct) than the control fish. In a dose-dependent fashion, ethanol exposure increased errors on this task with the highest exposure group requiring nearly twice the number of trials as the control group to reach criterion (Carvan et al., 2004). Ethanol was also associated with increased latency to startle and decreased velocity of swimming following a tap (Carvan et al., 2004). Here, a zebrafish model was able to detect adult sensorimotor and learning deficits following embryonic ethanol exposure.
Importantly, this model of FAS has been extended to include impairment of social behavior in addition to learning and sensorimotor deficits. While not affecting general activity level, low concentrations of alcohol for two hours pf significantly increased the distance at which adults swam from computerized images of conspecifics engaging in shoaling behavior, and they did so in a dose-dependent fashion (Fernandes & Gerlai, 2009). Similarly, exposure at the same time point and for the same duration significantly increased inter-individual distance among adult members of a shoal (a measure of shoal cohesion), compared to shoals consisting of unexposed members (Buske & Gerlai, 2011). This behavior is atypical in zebrafish, as they prefer swimming in tight shoals with conspecifics.
Nicotine
The principal psychoactive ingredient of tobacco, nicotine, causes of variety of behavioral effects when consumed during adulthood. These effects are seen in humans, rodents and zebrafish and include enhanced cognitive function (Hahn et al., 2011; Levin & Chen, 2004; Levin et al., 2006; Warburton, 1992; Socci et al., 1995, for a review see Rezvani & Levin, 2001) and hyperactivity (O’Neill et al., 1991; Fung & Lau, 1989), especially at low to moderate doses. However, nicotine has adverse consequences to the developing nervous system, which can manifest as long-term behavioral deficits. Infants exposed to nicotine in utero have low birth weight, enhanced locomotor activity and general cognitive impairment (for a review see Ernst et al., 2001). This syndrome has also been observed in rodent models (Eriksson et al., 2011; Vaglenova et al., 2004).
Recently, there has been an effort in our lab to characterize the effects of embryonic nicotine exposure on adult behavior using a zebrafish model. In Eddins et al. (2010) adult zebrafish were exposed from 2 hours pf until 5 days pf to 15 or 25 μM nicotine ditartrate. Notably, these are doses that do not generate increases in mortality or malformations. Upon reaching adulthood, locomotor function and habituation learning were measured using the tap/startle test previously described. Embryonic nicotine exposure increased distance swam following the delivery of a vibratory stimulus. This occurred despite a nonsignificant interaction between tap trial and dose, suggesting a primary locomotor, not learning, effect. Moreover, nicotinic receptors are critical in axonal path finding in developing Zebrafish and embryonic nicotine exposure is associated with axonal path finding errors in both juvenile and adult zebrafish (Svoboda et al., 2002).
Larval Behavior
The use of the zebrafish to study the developmental behavioral toxicology of psychomotor stimulants is expanding rapidly, and embryonic and larval behavioral endpoints are often targeted. Caffeine was adopted early as a target of such studies (Chen et al., 2008; Capiotti et al., 2011). In one study, zebrafish embryos exposed to varying dosages of caffeine exhibited a reduction in sensitivity to touch-induced movement (Chen et al., 2008). These abnormalities were tied via immunohistochemistry to changes in the development of muscle fibers and axon projections of both primary and secondary motor neurons (Chen et al., 2008). A later study also examining developmental exposures to caffeine found increased expression of several adenosine receptor subtypes, as well as links in the adenosine signal transduction pathway and in brain-derived neurotrophic factor (BDNF) at levels too low to produce the touch-sensitivity outcomes described previously (Capiotti et al., 2011).
Other stimulants are becoming the focus of larval zebrafish neurodevelopmental models. For example, one study found that zebrafish larvae at 6 days pf were acutely sensitive to both amphetamine and cocaine, increasing locomotion at lower doses while decreasing it at higher doses (Irons et al., 2010). Since these effects reflect those found in mammalian models, it is likely that using zebrafish as a model for developmental neurobehavioral toxicology will continue to prove to be useful and relevant.
Therapeutic Drugs
Adult Behavior
Pilocarpine
Due to its function as a direct muscarinic cholinergic receptor agonist, pilocarpine has a number of clinical uses. It is also a useful experimental tool when addressing questions about the effects of elevated acetylcholine levels. Clinically, pilocarpine is used as an antidote to scopolamine and atropine toxicity, to treat dry mouth and glaucoma. However, interfering with acetylcholine levels in the developing organism can have profound neurotoxic effects (Slotkin et al., 2008). The effects of pilocarpine administration to larval zebrafish (0–5 days pf) were examined during adulthood using the startle response test. At the highest doses tested, adults exhibited a significantly greater startle response than did control animals. This effect was similar to that seen following nicotine and chlorpyrifos administration, both of which also affect the cholinergic system (Eddins et al., 2010).
Methylphenidate
The symptoms of Attention Deficit Hyperactivity Disorder (ADHD) are commonly treated with methylphenidate, also known as Ritalin, which inhibits the re-uptake of the monoaminergic neurotransmitters dopamine, norepinepherine and serotonin. It has been widely prescribed to children and adolescents diagnosed with ADHD. However, it is becoming more widely used in adults diagnosed with ADHD of the residual type, which makes embryonic and fetal exposure possible when women taking this medication become pregnant.
Using zebrafish, we found that exposure to methylphenidate (50 mg/L) during the first five days post fertilization caused a short-term elevation of dopamine, norepinepherine and serotonin concentrations (Levin et al., 2011). While these elevations in monoaminergic neurotransmitters were transient (disappearing by 25 days after embryonic exposure) behavioral effects persisted into adulthood. Here, a behavioral test battery consisting of novel tank dive, spatial discrimination and startle/habituation was used to assess the long-term effects of embryonic methylphenidate exposure. Unlike controls, when exposed fish were placed in a novel tank they swam faster and spent significantly less time at the bottom of the tank. Instead, exposed fish spent more time in the middle and top portions of the tank.
During the spatial discrimination procedure, exposed fish made significantly fewer correct responses than the control fish, thereby coming into contact with the aversive stimulus (extremely limited space in which to swim) more often. While this effect can be interpreted as a learning deficit, as the authors note, it might also speak to the efficacy of this “punisher” in methylphenidate-exposed fish. Moreover, these data might suggest decreased sensitivity to an aversive stimulus following methylphenidate exposure. This could be a useful distinction considering the increased exploration seen during the dive task in these fish. Regardless of which interpretation is supported by future work, early-life methylphenidate exposure profoundly altered the ability to acquire a spatial discrimination and to engage in an adaptive pattern of responding in a novel tank. Methylphenidate did not, however, disrupt startle response or habituation to repeated taps, indicating, among other things, that these doses do not disrupt sensorimotor function but do alter other normative behavior (Levin et al., 2011).
Larval Behavior
The potential teratogenic effects of a number of therapeutic drugs have been assessed using zebrafish in which embryonic or larval behavioral endpoints are measured. The anticonvulsant drug valproic acid is a known neuroteratogen. Exposure to valproic acid significantly alters the locomotor activity patterns of embryonic zebrafish and decreases retino-tectal projections (Cowden et al., 2012). Here, movement was measured under both light and dark conditions. Developmental valproate exposure was associated with a biphasic effect on larval locomotor activity in darkness, where low concentrations (≤60 μM) resulted in hyperactive larvae and high concentrations (≥80 μM) resulted in hypoactive larvae (Cowden et al., 2012). Embryonic exposure to the antipsychotic drugs haloperidol and fluphenazine caused significant hypoactivity in embryonic zebrafish (Giacomini et al., 2006). Modafinil, a narcolepsy medication, increased activity during the dark cycle, which was used as evidence for increased wakefulness in larval zebrafish (Sigurgeirsson et al., 2011).
In a model of hyperactivity, Ellis et al. (2012) showed zebrafish larvae could be used to distinguish the effects of three compounds associated with hyperactivity. Here, pentylenetetrazole (PTZ) exposure reversed the species-typical swimming pattern seen during conditions of light and dark. Aconitine increased activity during both conditions and PTZ and 4-aminopyridine affected behavior specifically during the transition between lighting conditions, which the authors note may be a useful marker of stress in this model (Ellis et al., 2012).
Using a more elaborate test of larval stress/anxiety, Richendrfer et al. (2012) exposed larvae to diazepam (Valium), fluoxetine (Prozac), and caffeine before administering an “edge preference” test in which zebrafish are placed in a well and shown an aversive stimulus (a red bouncing ball) which typically induces a stress response (i.e. preference for the edge of the well). Here, diazepam was associated with reduced time spent near the edge of the well whereas fluoxetine had no effect on time spent near the edge and caffeine increased edge preference.
Environmental Toxins and Toxicants
Adult Behavior
Chlorpyrifos
The widely used organophosphate pesticide, chlorpyrifos (CPF), has well characterized behavioral teratogenic effects in rats (Aldridge et al, 2005; Levin et al., 2001; Levin et al., 2002). One principal mechanism of CPF toxicity is thought to act via inactivation of acetylcholinesterase at nerve junctions. However, CPF impacts a variety of neurotransmitter systems including dopaminergic and serotonergic systems at CPF doses near or below the threshold for acetylcholinesterase inhibition (Slotkin and Seidler, 2007). Humans are exposed to CPF via both dietary and non-dietary sources and childhood exposure to contaminated toys has been a concern (Gurunathan et al., 1998). Studies using rodents have indicated behavioral changes associated with developmental exposure, including cognitive impairment (Aldridge et al, 2005; Levin et al., 2001; Levin et al., 2002). We have found that embryonic exposure of zebrafish to CPF causes long-term impairment of spatial learning, startle hyper-reactivity and impaired diving response in the novel tank diving test (Eddins et al., 2010; Levin et al., 2003; Sledge et al., 2011).
With respect to spatial discrimination learning during adulthood, developmental (0–5 dpf) CPF was associated with a dose-dependent decrease in accuracy, which was magnified in later sessions, and a biphasic effect on response latency with the low dose (10 ng/ml) increasing but the high dose (100 ng/ml) decreasing response latency (Levin et al., 2003). A similar effect was seen in a subsequent study (Sledge et al., 2011), in which 100 ng/ml CPF significantly reduced accuracy in adult zebrafish (exposed to CPF 1–5 dpf).
Similarly, developmental exposure to 100 ng/ml CPF caused hyperactivity during a startle response task (Eddins et al., 2010; Sledge et al., 2011). However, CPF (at similar doses and time of exposure) has had inconsistent effects on habituation, a type of learning, during this test with no effect (Eddins et al., 2010) and a reduction in habituation (Sledge et al., 2011) being reported. Finally, CPF has also been associated with an atypical response to submersion in a novel tank. Zebrafish adults exposed to 100 ng/ml 1–5 dpf spent significantly less time at the bottom of the tank and swam at a faster rate throughout the session compared to control fish.
Diazinon
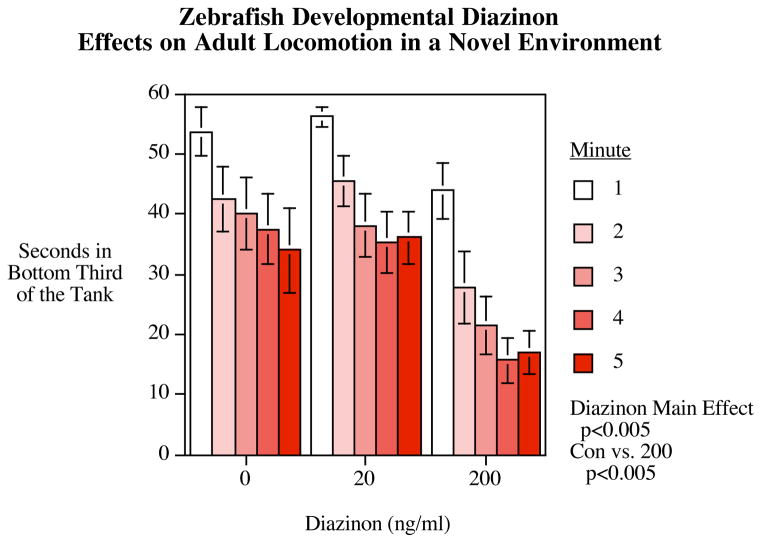
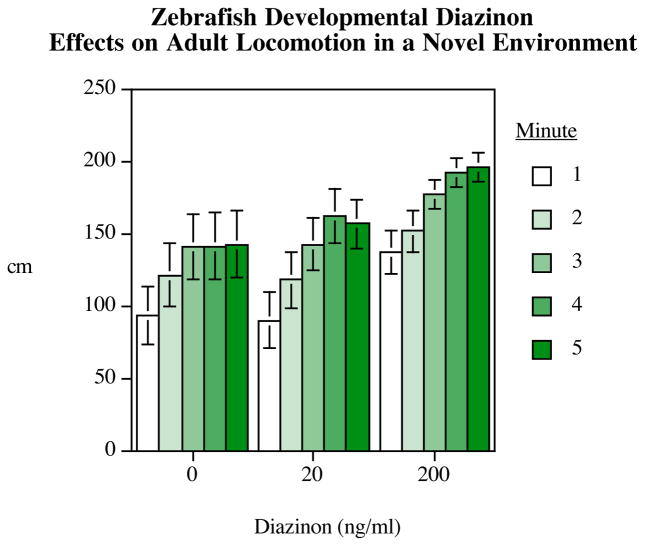
Another organophosphate insecticide, diazinon, also significantly alters novel tank behavior (Levin, 2010). Specifically, diazinon exposure during 0–5 dpf (200 ng/ml) significantly decreased the time spent in the bottom third of a novel test tank and increased total distance travelled when submerged in a novel tank. Taken together, these data suggest that hyperactivity is a primary effect associated with developmental diazanon exposure (see Figures 4–5).
Figure 4.
Developmental diazinon exposure in zebrafish (Levin et al., 2010) on the novel tank diving task mean±sem of time spent in bottom portion of the tank is plotted for each minute of the 5 minute session, for each exposure group.
Figure 5.
Developmental diazinon exposure in zebrafish (Levin et al., 2010), on the novel tank diving task, mean±sem of distance travelled is plotted for each minute of the 5 minute session, for each exposure group.
Silver
Humans are primarily exposed to silver (Ag+) via consumer goods (for a review see Wijnhoven et al., 2009) and concerns about health effects associated with exposure are rising. However, relatively few studies have examined the effects of developmental exposure to Ag+. High doses of Ag+ cause increased mortality while low doses are associated with morphological abnormalities in embryonic and larval zebrafish (Powers et al., 2010). When zebrafish embryos exposed to relatively low concentrations of Ag+ reached adulthood, significant differences in the acquisition of spatial discrimination appeared, compared to control animals (Powers et al., 2011). Specifically, adult zebrafish exposed to Ag+ during development made significantly fewer errors and acquired the spatial discrimination more rapidly than control animals. Interestingly, this was not a primary motor effect, as general swimming activity was not increased during this task. Improved acquisition of this spatial discrimination was coincident with elevations in DA and 5HT turnover (Powers et al., 2011). Because this task employs both aversive and appetitive stimuli, it is difficult to distinguish whether the effect seen here is the result of enhanced sensitivity to an aversive stimulus (i.e. the extreme reduction of tank space) or evidence of an improvement in associative mechanisms.
Cadmium
Exposure to metals often causes sensorimotor deficits (for review see Gobba, 2003) and cadmium is known to be especially toxic to olfaction. Sensory neurons appear to take up and transport cadmium towards the olfactory bulb (Bench et al., 2001) causing profound deficits in olfaction-mediated behavioral responses (e.g. feeding, foraging and avoiding predation) (Scott and Sloman 2004). Zebrafish have been useful in determining the long term effects of developmental exposure to cadmium. A species-typical alarm response to an olfactory-based predator-cue is markedly impaired in juvenile zebrafish exposed during development to cadmium (Blechinger et al., 2007). Cadmium-exposed larva and juveniles did not demonstrate the species-typical alarm response (predator avoidance behavior) to an olfactory cue that was seen in unexposed controls. Here, alarm responding was used primarily as an indirect marker of olfactory function and was coincident with increased cell death in the olfactory system. However, it is not clear to what extent, if any, deficits in associative mechanisms may contribute to this effect.
Methylmercury
Methlymercury (MeHg) is a ubiquitous environmental toxicant with known teratogenic effects. Like many compounds, MeHg appears to be far more harmful to the developing than to the mature nervous system. Evidence from environmental catastrophes affecting humans (i.e. widespread contamination in Japan, Iraq and Mexico), and various laboratory studies using mammalian models have described a constellation of severe sensorimotor and cognitive deficits associated with gestational exposure to MeHg that persists throughout the lifespan, often worsening with age (Reed et al., 2006; Newland et al., 2008).
Recently, the Zebrafish model has been employed to evaluate the long-term effects of MeHg exposure to the developing nervous system, the potential for dietary compounds to offer neuroprotection from MeHg exposure, and to validate the zebrafish model in studies of heavy metal toxicity. Zebrafish embryos exposed to various concentrations of MeHg (or selenium (Se), or a combination of MeHg and Se) from 2–24 hours pf were raised to adulthood and tested on a spatial alternation task (Smith et al., 2010). This task, like the simple spatial discrimination procedure, measures the ability to acquire a novel response. However, it also measures perseveration (as it requires the organism to respond differently than what was just reinforced) and memory (as the organism presumably must remember the side to which it previously responded). Here, zebrafish exposed during development to the highest dose of MeHg (0.30 μM) were unable to acquire the task altogether and lower doses caused significant impairment in acquisition (Smith et al., 2010). Moreover, treatment with Se, did not affect this MeHg-induced learning deficit. This deficit appeared to be mediated by a MeHg-induced decrease in cell body density in the telencephalon, a region associated with spatial learning and memory in fish (Smith et al., 2010).
Crude Oil
Exposure to crude oil is toxic to a wide variety of aquatic species, with high concentrations increasing mortality (Moles, 1998; Heintz et al., 1999; Hicken et al., 2011). Mortality is generally associated with the cardiotoxic effects of polycyclic aromatic hydrocarbons, found in petroleum. The long-term effects of sub-lethal exposure to crude oil during embryonic development have been assessed using zebrafish. Hicken et al. (2011) demonstrated that adult zebrafish were unable to engage in sustained swimming against a current following embryonic exposure to crude oil. Here, swimming performance is used as an indirect measure of cardiac function and not as a behavioral endpoint in its own right; however, this study demonstrates the utility of zebrafish in studies where large scale behavioral designs are needed.
Strychnine
While available over-the-counter as a medication in the US until 1962 (i.e. as a constituent of various tonics and laxatives), today human exposure to strychnine is primarily due to its continued use as a pesticide. Strychine is a direct glycine antagonist, blocking the binding of glycine to its post-synaptic chloride channel receptor (Hirata et al., 2005). Twenty-four hours pf, zebrafish were exposed to 1.5 mM strychnine for 18 or 29 hours, then raised to adulthood during which swimming ability and behavior in a novel environment were measured (Roy et al., 2012). Adult zebrafish exposed during development to strychnine spent significantly less time at the bottom of the tank than controls and swam faster during the trial than control fish (Roy et al., 2012). Strychnine also appeared to alter the expression of genes related to glycinergic, GABAergic and glutaminergic neuronal synapses.
Larval Behavior
The zebrafish model has proven a useful tool for assessing the effects of developmental exposure to toxic compounds on embryonic/larval behavior. Exposure of zebrafish embryos to the insecticide dichlorodiphenyltrichloroethane (DDT) increases susceptibility to seizures later in life and the marine toxin, domoic acid, has been found to increase susceptibility to convulsions after embryonic exposure (Tiedeken et al., 2009; Tiedeken et al., 2007; Tiedeken et al., 2005). Exposure to the insecticide fipronil is associated with notochord degeneration and impaired swimming activity in larval zebrafish (Stehr et al., 2006). Embryonic exposure of zebrafish to silver causes morphological abnormalities and short and long term behavioral perturbations which manifest as reduced swimming activity (Powers et al., 2011; Powers et al., 2010). MeHg exposure has been shown to disrupt startle response in larval zebrafish, increasing the latency to respond and prolonging the duration for which they swim following stimulus presentation (Weber, 2006). Chlorpyrifos causes hypoactivity in larval zebrafish on several measures of swimming activity (Levin et al., 2003). Finally, a species-typical alarm response to an olfactory-based predator-cue is markedly impaired in larval zebrafish exposed during development to cadmium (Kusch et al., 2008).
Discussion/Future Directions
In many ways the zebrafish model is poised to follow in the case of the mouse model, which emerged for many lines of research as a useful complementary model to classic rat models, due to the availability of genetic manipulations, economic advantages and the wide range of behavioral assays that were easily adapted to them. In a similar fashion, zebrafish are providing a useful complement to mouse models for investigations into the teratogenicity of compounds as behavioral and genetic assays become more widely assessable.
Currently, a range of behavioral assays are available for use in zebrafish that tap both sensorimotor and cognitive domains; moreover, several specific disease models have been well characterized (e.g. FAS) and drugs and toxicants appear to have analogous behavioral effects in zebrafish and mammalian species and, as shown here, zebrafish are a sensitive model for detecting the behavioral effects of embryonic exposures. Despite the progress made in adapting behavioral paradigms from rodents to zebrafish, it is desirable to push the boundaries of operant behavior in this species such that sensitive measures of learning or other cognitive processes are made more accessible. The zebrafish model will benefit from behavioral assays that measure timing behavior or fixed interval responding, as this has been especially illuminating in the mammalian toxicology and teratology literature (e.g. Rice, 1988; Rice & Gilbert, 1985; Rice et al., 1979) just as it will benefit from more sensitive assays of some of the most commonly studied constructs in psychology such as anxiety and depression, which have carefully constructed rodent models (e.g. learned helplessness, avoidance procedures) that have proven especially valuable (for reviews see Maier & Seligman, 1976; Treit, 1985).
Notably, zebrafish present several challenges for studies of complex behavior change. Primarily, many of the complex behavioral assays used in rodent models require repeated testing, which (because testing must span many days) requires unique identifiers for each animal. Individual housing is not preferred for a variety of reasons among which the fact that zebrafish are social animals (as described previously the mere sight of conspecifics is highly appetitive) ranks very high. But also, individual housing negates many of the logistical and economic advantages described earlier. To circumvent this problem we have used individual markers that allow for group housing (Levin et al., 2003), however, this process is very labor and time intensive and again negates some of the advantages of choosing this species. The development of behavioral assays that can employ repeated testing within a short window (i.e. within a single day) may prove the ideal compromise to this problem and some researchers have begin developing tasks that use many trials within a single session to measure acquisition (Pather & Gerlai, 2009). Zebrafish are proving to be sensitive, reliable and economic models for developmental neurobehavioral toxicology. They provide an important complementary model to in vitro cell assays and classic mammalian models, with much of the continuous access of the in vitro models and the temporal and anatomic complexity of mammalian models.
Acknowledgments
Supported by ES010356
References
- Aldridge JE, Levin ED, Seidler FJ, Slotkin TA. Developmental exposure of rats to chlorpyrifos leads to behavioral alterations in adulthood, involving serotonergic mechanisms and resembling animal models of depression. Environmental Health Perspectives. 2005;113:527–531. doi: 10.1289/ehp.7867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barcellos LJG, Ritter F, Kreutz LC, Quevedo RM, da Silva LB, Bedin AC, et al. Whole-body cortisol increases after direct and visual contact with a predator in zebrafish, Danio rerio. Aquaculture. 2007;272:774–8. [Google Scholar]
- Bencan Z, Sledge D, Levin ED. Buspirone, chlordiazepoxide and diazepam effects in a zebrafish model of anxiety. Pharmacology Biochemistry and Behavior. 2009;94:75–80. doi: 10.1016/j.pbb.2009.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bench G, Carlsen TM, Grant PG, Wollett JS, Martinelli RE, Lewis JL, et al. Olfactory Bulb Uptake and Determination of Biotransfer Factors in the California Ground Squirrel (Spermophilus beecheyi) Exposed to Manganese and Cadmium in Environmental Habitats. Environmental Science & Technology. 2000;35:270–7. doi: 10.1021/es0014180. [DOI] [PubMed] [Google Scholar]
- Bilotta J, Barnett JA, Hancock L, Saszik S. Ethanol exposure alters zebrafish development: A novel model of fetal alcohol syndrome. Neurotoxicology and Teratology. 2004;26:737–743. doi: 10.1016/j.ntt.2004.06.011. [DOI] [PubMed] [Google Scholar]
- Bilotta J. Zebrafish as a model for studying fetal alcohol syndrome. Neurotoxicology and Teratology. 2003;25(3):382. [Google Scholar]
- Blechinger SR, Kusch RC, Haugo K, Matz C, Chivers DP, Krone PH. Brief embryonic cadmium exposure induces a stress response and cell death in the developing olfactory system followed by long-term olfactory deficits in juvenile zebrafish. Toxicology and Applied Pharmacology. 2007;224:72–80. doi: 10.1016/j.taap.2007.06.025. [DOI] [PubMed] [Google Scholar]
- Buske C, Gerlai R. Early embryonic ethanol exposure impairs shoaling and the dopaminergic and serotoninergic systems in adult zebrafish. Neurotoxicology and Teratology. 2011;33:698–707. doi: 10.1016/j.ntt.2011.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill G. Clock mechanisms in zebrafish. Cell and Tissue Research. 2002;309:27–34. doi: 10.1007/s00441-002-0570-7. [DOI] [PubMed] [Google Scholar]
- Capiotti KM, Menezes FP, Nazario LR, Pohlmann JB, de Oliveira GMT, Fazenda L, et al. Early exposure to caffeine affects gene expression of adenosine receptors, DARPP-32 and BDNF without affecting sensibility and morphology of developing zebrafish (Danio rerio) Neurotoxicology and Teratology. 2011;33:680–5. doi: 10.1016/j.ntt.2011.08.010. [DOI] [PubMed] [Google Scholar]
- Carvan MJ, 3rd, Loucks E, Weber DN, Williams FE. Ethanol effects on the developing zebrafish: Neurobehavior and skeletal morphogenesis. Neurotoxicology and Teratology. 2004;26:757–68. doi: 10.1016/j.ntt.2004.06.016. [DOI] [PubMed] [Google Scholar]
- Chen YH, Huang YH, Wen CC, Wang YH, Chen WL, Chen LC, Tsay HJ. Movement disorder and neuromuscular change in zebrafish embryos after exposure to caffeine. Neurotoxicology and Teratology. 2008;30:440–7. doi: 10.1016/j.ntt.2008.04.003. [DOI] [PubMed] [Google Scholar]
- Cowden J, Padnos B, Hunter D, MacPhail R, Jensen K, Padilla S. Developmental exposure to valproate and ethanol alters locomotor activity and retino-tectal projection area in zebrafish embryos. Reprod Toxicol. 2012;33:165–73. doi: 10.1016/j.reprotox.2011.11.111. [DOI] [PubMed] [Google Scholar]
- Dlugos CA, Rabin RA. Ethanol effects on three strains of zebrafish: model system for genetic investigations. Pharmacology Biochemistry and Behavior. 2003;74:471–80. doi: 10.1016/s0091-3057(02)01026-2. [DOI] [PubMed] [Google Scholar]
- Eddins D, Cerutti D, Williams P, Linney E, Levin ED. Zebrafish provide a sensitive model of persisting neurobehavioral effects of developmental chlorpyrifos exposure: comparison with nicotine and pilocarpine effects and relationship to dopamine deficits. Neurotoxicology and Teratology. 2010;32:99–108. doi: 10.1016/j.ntt.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan RJ, Bergner CL, Hart PC, Cachat JM, Canavello PR, Elegante MF, et al. Understanding behavioral and physiological phenotypes of stress and anxiety in zebrafish. Behavioural Brain Research. 2009;205:38–44. doi: 10.1016/j.bbr.2009.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis LD, Seibert J, Soanes KH. Distinct models of induced hyperactivity in zebrafish larvae. Brain Research. 2012;1449:46–59. doi: 10.1016/j.brainres.2012.02.022. [DOI] [PubMed] [Google Scholar]
- Eriksson P, Ankarberg E, Fredriksson A. Exposure to nicotine during a defined period in neonatal life induces permanent changes in brain nicotinic receptors and in behaviour of adult mice. Brain Research. 2011;853:41–8. doi: 10.1016/s0006-8993(99)02231-3. [DOI] [PubMed] [Google Scholar]
- Ernst M, Moolchan ET, Robinson ML. Behavioral and neural consequences of prenatal exposure to nicotine. J Am Acad Child Adolesc Psychiatry. 2001;40:630–41. doi: 10.1097/00004583-200106000-00007. [DOI] [PubMed] [Google Scholar]
- Fernandes Y, Gerlai R. Long-Term Behavioral Changes in Response to Early Developmental Exposure to Ethanol in Zebrafish. Alcoholism: Clinical and Experimental Research. 2009;33:601–9. doi: 10.1111/j.1530-0277.2008.00874.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung YK, Lau YS. Effects of prenatal nicotine exposure on rat striatal dopaminergic and nicotinic systems. Pharmacology, Biochemistry & Behavior. 1989;33:1–6. doi: 10.1016/0091-3057(89)90419-x. [DOI] [PubMed] [Google Scholar]
- Gerlai R, Ahmad F, Prajapati S. Differences in Acute Alcohol-Induced Behavioral Responses Among Zebrafish Populations. Alcoholism: Clinical and Experimental Research. 2008;32:1763–73. doi: 10.1111/j.1530-0277.2008.00761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlai R, Lahav M, Guo S, Rosenthal A. Drinks like a fish: zebra fish (Danio rerio) as a behavior genetic model to study alcohol effects. Pharmacology Biochemistry and Behavior. 2000;67:773–82. doi: 10.1016/s0091-3057(00)00422-6. [DOI] [PubMed] [Google Scholar]
- Giacomini NJ, Rose B, Kobayashi K, Guo S. Antipsychotics produce locomotor impairment in larval zebrafish. Neurotoxicol Teratol. 2006;28:245–50. doi: 10.1016/j.ntt.2006.01.013. [DOI] [PubMed] [Google Scholar]
- Gil-Mohapel J, Boehme F, Kainer L, Christie BR. Hippocampal cell loss and neurogenesis after fetal alcohol exposure: Insights from different rodent models. Brain Research Reviews. 2002;64:283–303. doi: 10.1016/j.brainresrev.2010.04.011. [DOI] [PubMed] [Google Scholar]
- Gobba F. Occupational Exposure to Chemicals and Sensory Organs: A Neglected Research Field. NeuroToxicology. 2003;24:675–91. doi: 10.1016/S0161-813X(03)00038-X. [DOI] [PubMed] [Google Scholar]
- Gurunathan S, Robson M, Freeman N, Buckley B, Roy A, Meyer R, et al. Accumulation of Chlorpyrifos on Residential Surfaces and Toys Accessible to Children. Environmental Health Perspectives. 1998;106:9–16. doi: 10.1289/ehp.981069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn B, Shoaib M, Stolerman IP. Selective nicotinic receptor antagonists: effects on attention and nicotine-induced attentional enhancement. Psychopharmacology (Berl) 2011;217:75–82. doi: 10.1007/s00213-011-2258-8. [DOI] [PubMed] [Google Scholar]
- Heintz RA, Short JW, Rice SD. Sensitivity of fish embryos to weathered crude oil: Part II. Increased mortality of pink salmon (Oncorhynchus gorbuscha) embryos incubating downstream from weathered Exxon valdez crude oil. Environmental Toxicology and Chemistry. 1999;18:494–503. [Google Scholar]
- Hicken CE, Linbo TL, Baldwin DH, Willis ML, Myers MS, Holland L, et al. Sublethal exposure to crude oil during embryonic development alters cardiac morphology and reduces aerobic capacity in adult fish. PNAS. 2011;108:7086–90. doi: 10.1073/pnas.1019031108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata H, Saint-Amant L, Downes GB, Cui WW, Zhou W, Granato M, et al. Zebrafish bandoneon mutants display behavioral defects due to a mutation in the glycine receptor β-subunit. PNAS. 2005;102:8345–50. doi: 10.1073/pnas.0500862102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irons TD, MacPhail RC, Hunter DL, Padilla S. Acute neuroactive drug exposures alter locomotor activity in larval zebrafish. Neurotoxicology and Teratology. 2010;32:84–90. doi: 10.1016/j.ntt.2009.04.066. [DOI] [PubMed] [Google Scholar]
- Kelly SJ, Day N, Streissguth AP. Effects of prenatal alcohol exposure on social behavior in humans and other species. Neurotoxicology and Teratology. 2000;22:143–9. doi: 10.1016/s0892-0362(99)00073-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusch RC, Krone PH, Chivers DP. Chronic exposure to low concentrations of waterborne cadmium during embryonic and larval development results in the long-term hindrance of antipredator behavior in zebrafish. Environmental Toxicology and Chemistry. 2008;27:705–10. doi: 10.1897/07-273.1. [DOI] [PubMed] [Google Scholar]
- Langheinrich U. Zebrafish: A new model on the pharmaceutical catwalk. BioEssays. 2003;25:904–12. doi: 10.1002/bies.10326. [DOI] [PubMed] [Google Scholar]
- Levin ED, Addy N, Baruah A, Elias A, Christopher NC, Seidler FJ, et al. Prenatal chlorpyrifos exposure in rats causes persistent behavioral alterations. Neurotoxicology and Teratology. 2002;24:733–41. doi: 10.1016/s0892-0362(02)00272-6. [DOI] [PubMed] [Google Scholar]
- Levin ED, Addy N, Nakajima A, Christopher NC, Seidler FJ, Slotkin TA. Persistent behavioral consequences of neonatal chlorpyrifos exposure in rats. Developmental Brain Research. 2001;130:83–9. doi: 10.1016/s0165-3806(01)00215-2. [DOI] [PubMed] [Google Scholar]
- Levin ED, Chen E. Nicotinic involvement in memory function in zebrafish. Neurotoxicology and Teratology. 2004;26:731–5. doi: 10.1016/j.ntt.2004.06.010. [DOI] [PubMed] [Google Scholar]
- Levin ED, Chrysanthis E, Yacisin K, Linney E. Chlorpyrifos exposure of developing zebrafish: effects on survival and long-term effects on response latency and spatial discrimination. Neurotoxicol Teratol. 2003;25:51–7. doi: 10.1016/s0892-0362(02)00322-7. [DOI] [PubMed] [Google Scholar]
- Levin ED, Limpuangthip J, Rachakonda T, Peterson M. Timing of nicotine effects on learning in zebrafish. Psychopharmacology. 2006;184:547–52. doi: 10.1007/s00213-005-0162-9. [DOI] [PubMed] [Google Scholar]
- Levin ED, Sledge D, Roach S, Petro A, Donerly S, Linney E. Persistent behavioral impairment caused by embryonic methylphenidate exposure in zebrafish. Neurotoxicology and Teratology. 2010;33:668–73. doi: 10.1016/j.ntt.2011.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ED, Yen J, Cerutti D, Donerly S, Linney E. Persisting behavioral impairment in the novel tank diving task after embryonic exposure to diazinon in zebrafish. Paper presented at: Neurobehavioral Teratology Society, Annual Meeting; June 25–29, 2010; Coronado, CA.. [Google Scholar]
- Levin ED. Zebrafish assessment of cognitive improvement and anxiolysis: filling the gap between in vitro and rodent models for drug development. Reviews in the Neurosciences. 2011;22:75–84. doi: 10.1515/RNS.2011.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linney E, Upchurch L, Donerly S. Zebrafish as a neurotoxicological model. Neurotoxicol Teratol. 2004;26:709–18. doi: 10.1016/j.ntt.2004.06.015. [DOI] [PubMed] [Google Scholar]
- Maier SF, Seligman ME. Learned helplessness: Theory and evidence. Journal of Experimental Psychology: General. 1976;105:3–46. [Google Scholar]
- Moles A. Sensitivity of Ten Aquatic Species to Long-Term Crude Oil Exposure. Bulletin of Environmental Contamination and Toxicology. 1998;61:102–7. doi: 10.1007/s001289900735. [DOI] [PubMed] [Google Scholar]
- Newland MC, Paletz EM, Reed MN. Methylmercury and nutrition: Adult effects of fetal exposure in experimental models. NeuroToxicology. 2008;29:783–801. doi: 10.1016/j.neuro.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ninkovic J, Bally-Cuif L. The zebrafish as a model system for assessing the reinforcing properties of drugs of abuse. Methods. 2006;39:262–74. doi: 10.1016/j.ymeth.2005.12.007. [DOI] [PubMed] [Google Scholar]
- O’Neill MF, Dourish CT, Iversen SD. Evidence for an involvement of D1 and D2 dopamine receptors in mediating nicotine-induced hyperactivity in rats. Psychopharmacology. 1991;104:343–350. doi: 10.1007/BF02246034. [DOI] [PubMed] [Google Scholar]
- Pather S, Gerlai R. Shuttle box learning in zebrafish (Danio rerio) Behavioural Brain Research. 2009;196:323–7. doi: 10.1016/j.bbr.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers CM, Levin ED, Seidler FJ, Slotkin TA. Silver exposure in developing zebrafish produces persistent synaptic and behavioral changes. Neurotoxicology and Teratology. 2011;33:329–332. doi: 10.1016/j.ntt.2010.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers CM, Yen J, Linney EA, Seidler FJ, Slotkin TA. Silver exposure in developing zebrafish (Danio rerio): persistent effects on larval behavior and survival. Neurotoxicol Teratol. 2010;32:391–7. doi: 10.1016/j.ntt.2010.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed MN, Paletz EM, Newland MC. Gestational exposure to methylmercury and selenium: Effects on a spatial discrimination reversal in adulthood. NeuroToxicology. 2006;27:721–32. doi: 10.1016/j.neuro.2006.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezvani AH, Levin ED. Cognitive effects of nicotine. Biological Psychiatry. 2001;49:258–67. doi: 10.1016/s0006-3223(00)01094-5. [DOI] [PubMed] [Google Scholar]
- Rice DC, Gilbert SG, Willes RF. Neonatal low-level lead exposure in monkeys: Locomotor activity, schedule-controlled behavior, and the effects of amphetamine. Toxicology and Applied Pharmacology. 1979;51:503–13. doi: 10.1016/0041-008x(79)90375-2. [DOI] [PubMed] [Google Scholar]
- Rice DC, Gilbert SG. Low lead exposure from birth produces behavioral toxicity (DRL) in monkeys. Toxicology and Applied Pharmacology. 1985;80:421–6. doi: 10.1016/0041-008x(85)90386-2. [DOI] [PubMed] [Google Scholar]
- Rice DC. Schedule-controlled behavior in infant and juvenile monkeys exposed to lead from birth. Neurotoxicology. 1988;9:75–87. [PubMed] [Google Scholar]
- Richendrfer H, Pelkowski SD, Colwill RM, Creton R. On the edge: Pharmacological evidence for anxiety-related behavior in zebrafish larvae. Behav Brain Res. 2012;228:99–109. doi: 10.1016/j.bbr.2011.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley EP, McGee CL. Fetal Alcohol Spectrum Disorders: An Overview with Emphasis on Changes in Brain and Behavior. Exp Biol Med. 2005:357–65. doi: 10.1177/15353702-0323006-03. [DOI] [PubMed] [Google Scholar]
- Rout UK. Alcohol, GABA Receptors, and Neurodevelopmental Disorders. International Review of Neurobiology. 2005;71:217–37. doi: 10.1016/s0074-7742(05)71010-2. [DOI] [PubMed] [Google Scholar]
- Roy NM, Arpie B, Lugo J, Linney E, Levin ED, Cerutti D. Brief embryonic strychnine exposure in zebrafish causes long-term adult behavioral impairment with indications of embryonic synaptic changes. Neurotoxicology and Teratology. 2012;34:587–91. doi: 10.1016/j.ntt.2012.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saverino C, Gerlai R. The social zebrafish: Behavioral responses to conspecific, heterospecific, and computer animated fish. Behavioural Brain Research. 2008;191:77–87. doi: 10.1016/j.bbr.2008.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scalzo FM, Levin ED. The use of zebrafish (Danio rerio) as a model system in neurobehavioral toxicology. Neurotoxicol Teratol. 2004;26:707–8. doi: 10.1016/j.ntt.2004.06.008. [DOI] [PubMed] [Google Scholar]
- Schneider M, Moore C, Adkins M. The Effects of Prenatal Alcohol Exposure on Behavior: Rodent and Primate Studies. Neuropsychology Review. 2001;21:186–203. doi: 10.1007/s11065-011-9168-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott GR, Sloman KA. The effects of environmental pollutants on complex fish behaviour: integrating behavioural and physiological indicators of toxicity. Aquatic Toxicology. 2004;68:369–92. doi: 10.1016/j.aquatox.2004.03.016. [DOI] [PubMed] [Google Scholar]
- Sigurgeirsson B, Porsteinsson H, Arnardottir H, Johannesdottir IP, Karlsson K. Effects of modafinil on sleep-wake cycles in larval zebrafish. Zebrafish. 2011;8:133–40. doi: 10.1089/zeb.2011.0708. [DOI] [PubMed] [Google Scholar]
- Sledge D, Yen J, Morton T, Dishaw L, Petro A, Donerly S, et al. Critical duration of exposure for developmental chlorpyrifos-induced neurobehavioral toxicity. Neurotoxicology and Teratology. 2011;33:742–51. doi: 10.1016/j.ntt.2011.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA, Bodwell BE, Ryde IT, Seidler FJ. Adolescent nicotine treatment changes the response of acetylcholine systems to subsequent nicotine administration in adulthood. Brain Res Bull. 2008;76:152–165. doi: 10.1016/j.brainresbull.2007.12.009. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, Seidler FJ. Prenatal chlorpyrifos exposure elicits presynaptic serotonergic and dopaminergic hyperactivity at adolescence: critical periods for regional and sex-selective effects. Reproductive Toxicology. 2007;23:421–427. doi: 10.1016/j.reprotox.2006.07.010. [DOI] [PubMed] [Google Scholar]
- Smith LE, Carvan MJ, III, Dellinger JA, Ghorai JK, White DB, Williams FE, et al. Developmental selenomethionine and methylmercury exposures affect zebrafish learning. Neurotoxicology and Teratology. 2010;32:246–55. doi: 10.1016/j.ntt.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Socci DJ, Sanberg PR, Arendash GW. Nicotine enhances Morris water maze performance of young and aged rats. Neurobiol Aging. 1995;16:857–860. doi: 10.1016/0197-4580(95)00091-r. [DOI] [PubMed] [Google Scholar]
- Stehr CM, Linbo TL, Incardona JP, Scholz NL. The developmental neurotoxicity of fipronil: notochord degeneration and locomotor defects in zebrafish embryos and larvae. Toxicol Sci. 2006;92:270–8. doi: 10.1093/toxsci/kfj185. [DOI] [PubMed] [Google Scholar]
- Streissguth AP, Barr HM, Bookstein FL, Sampson PD, Olson HC. The Long-Term Neurocognitive Consequences of Prenatal Alcohol Exposure: A 14-Year Study. Psychological Science. 1999;10:186–90. [Google Scholar]
- Svoboda KR, Vijayaraghavan S, Tanguay RL. Nicotinic receptors mediate changes in spinal motoneuron development and axonal pathfinding in embryonic zebrafish exposed to nicotine. Journal of Neuroscience. 2002;22:10731–41. doi: 10.1523/JNEUROSCI.22-24-10731.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiedeken JA, Ramsdell JS, Ramsdell AF. Developmental toxicity of domoic acid in zebrafish (Danio rerio) Neurotoxicol Teratol. 2005;27:711–7. doi: 10.1016/j.ntt.2005.06.013. [DOI] [PubMed] [Google Scholar]
- Tiedeken JA, Ramsdell JS. DDT exposure of zebrafish embryos enhances seizure susceptibility: Relationship to fetal p,p′-DDE burden and domoic acid exposure of California sea lions. Environ Health Perspect. 2009;117:68–73. doi: 10.1289/ehp.11685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiedeken JA, Ramsdell JS. Embryonic exposure to domoic acid increases the susceptibility of zebrafish larvae to the chemical convulsant pentylenetetrazole. Environ Health Perspect. 2007;115:1547–52. doi: 10.1289/ehp.10344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treit D. Animal models for the study of anti-anxiety agents: A review. Neuroscience & Biobehavioral Reviews. 1985;9:203–22. doi: 10.1016/0149-7634(85)90046-6. [DOI] [PubMed] [Google Scholar]
- Vaglenova J, Birru S, Pandiella NM, Breese CR. An assessment of the long-term developmental and behavioral teratogenicity of prenatal nicotine exposure. Behavioural Brain Research. 2004;150:159–70. doi: 10.1016/j.bbr.2003.07.005. [DOI] [PubMed] [Google Scholar]
- Warburton DM. Nicotine as a cognitive enhancer. Prog Neuro-Psychopharmacol Biol Psychiat. 1992;16:181–191. doi: 10.1016/0278-5846(92)90069-q. [DOI] [PubMed] [Google Scholar]
- Weber DN. Dose-dependent effects of developmental mercury exposure on C-start escape responses of larval zebrafish Danio rerio. Journal of Fish Biology. 2006;69:75–94. [Google Scholar]
- West JR, Goodlett CR. Teratogenic Effects of Alcohol on Brain Development. Annals of Medicine. 1990;22:319–25. doi: 10.3109/07853899009147914. [DOI] [PubMed] [Google Scholar]
- Wijnhoven SWP, Peijnenburg WJGM, Herberts CA, Hagens WI, Oomen AG, Heugens EHW, et al. Nano-silver - a review of available data and knowledge gaps in human and environmental risk assessment. Nanotoxicology. 2009;3:109–38. [Google Scholar]