Abstract
Myeloid-derived suppressor cells (MDSC) are myeloid cells that suppress the immune response, a definition that reflects both their origin and their function. As negative regulators of the immune response, MDSC represent a novel therapeutic approach for manipulating the immune system toward tolerance or immunity. MDSC are present in cancer patients and tumor-bearing mice and are in part responsible for the inhibition of the cell-mediated immune response against the tumor. Our laboratories investigate the immunologic mechanisms of tumor acceptance mediated by MDSC, which can be exploited to prevent allograft rejection in transplantation. A better understanding of MDSC biology will open new avenues for therapeutic intervention, either by inhibiting their function (i.e. in cancer patients), or by enhancing their suppressive effects and promoting their expansion (i.e. in organ transplantation and alloimmune responses). In this review, we summarize some of the critical aspects of the immunoregulatory function of MDSC in cancer and transplantation and discuss their potential clinical applications.
Keywords: MDSC, Transplantation, Cancer
Introduction
Progressive tumor development is normally accompanied by defects in the immune system in the form of immunosuppression [1], which is mediated by the activation of suppressor cells induced by a growing tumor [2]. While there is considerable interest in defining alterations in the immune system that precede tumor growth and their development, little is known about the suppressive mechanisms that protect the solid tumor from the immune response. Myeloid cells with specific inhibitory activity have emerged as negative regulators of the immune response, and the term myeloid-derived suppressor cells (MDSC) has recently been proposed to define cells of myeloid origin with suppressor function [3]. Although initially described in cancer patients, MDSC are also present in other inflammatory settings including solid organ transplantation. Pioneer studies from Bernard Vanhove demonstrated that transplantation tolerance is dependent on MDSC that accumulate in the allografts of tolerant recipients [4]. Since MDSC are in part responsible for protecting tumors against rejection despite the recognition of tumor-associated antigens, we hypothesize that a better understanding of the immunologic mechanisms of tumor acceptance mediated by MDSC can provide critical information to prevent allograft rejection in transplantation. Indeed, Argyris [5, 6] suggested that spleen cells from tumor-bearing mice secreted a “suppressor factor,” which could be used to prolong skin allograft survival. Further, Muller and colleagues prolonged graft survival by transferring spleen cells from tumor-bearing mice into skin transplant recipients [7]. However, while there is an increasing interest in developing MDSC as a cell-based therapy, several concerns need to be addressed before MDSC can be taken into the clinic. Before proposing therapeutic targeting of MDSC, we must understand the mechanisms by which specific MDSC subsets mediate their suppressive function and their inhibitory effect. Here, we discuss the immune regulatory function of MDSC and comment on the similarities of MDSC in tumor-bearing mice, cancer patients, and transplant recipients.
MDSC subsets
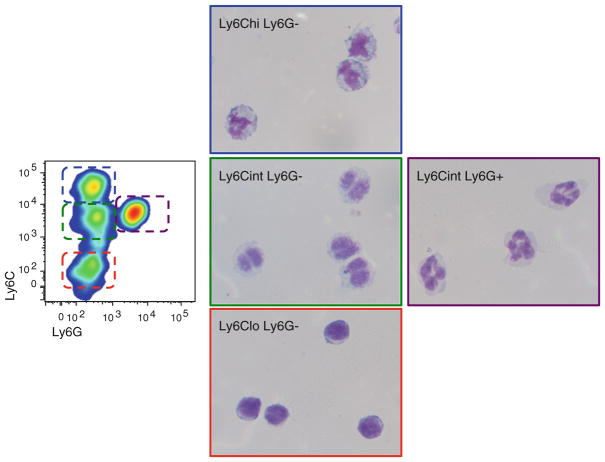
A major concern regarding MDSC is the difficulty to define their specific lineage. The term MDSC describes their origin and function and includes a morphologically and functionally heterogeneous population of myeloid progenitor cells, dendritic cells (DC), and immature myeloid cells (IMC) at different stages of differentiation [8]. Given the wide range of cell types that may be included in this category, finding a phenotypic profile that characterizes all of them has been a difficult task (Table 1). In mice, all MDSC express the cell surface markers CD11b+Gr-1+ [9, 10]. CD11b is a subunit of the b2 integrin Mac-1, which is expressed in granulocytes, DC, monocytes, and macrophages, and regulates leukocyte adhesion and cell migration. The Gr-1 antigen is predominantly expressed on the surface of monocytes/macrophages and granulocytes and is recognized by the RB6-8C5 antibody, which binds to the cell surface molecules Ly6C and Ly6G [11]. The expression of Gr-1 antigen (Ly6C/Ly6G) and nuclear morphology have been used to characterize two major populations of MDSC: granulocytic MDSC (G-MDSC) expressing CD11b+ Ly6G+ Ly6Cint CD115lo, and monocytic MDSC (M-MDSC) expressing CD11b+ Ly6G− Ly6C+ CD115+ [12–14]. In addition to Gr-1 and CD11b, other markers have been used to characterize specific subsets of MDSC. In tumor-bearing mice, Gr-1+ CD11b+ cells also express the immature myeloid antigens CD31 and ER-MP58 [9, 15]. Additional markers related to their suppressive function, activation or developmental stage include CD80, CD40, PD-L1, the cytokine receptor CD124 (alpha chain of IL-4 and IL-13 receptors), CD49d, F4/80, CD16/32, low expression of MHC class II and CD11c [12, 16–20]. Based on these cell surface molecules, the presence of MDSC in transplant recipient mice has been described [21–26]. However, the specific contribution of monocytic and granulocytic MDSC that develops in transplant recipients remains. Using CD11b, Ly6C, and Ly6G, our laboratories have identified different populations of MDSC. At least four different populations of myeloid cells with potential suppressive function can be identified by flow cytometry, which include Ly6G− Ly6Chi, Ly6G− Ly6Clo, Ly6G−Ly6Cint, and Ly6G+ Ly6Cint populations (Fig. 1).
Table 1.
MDSC subsets
| MDSC subset | Cell markers | Reference(s) |
|---|---|---|
| Mouse G-MDSC | CD11b+, Ly6Cint, Ly6G+ | [14] |
| Mouse M-MDSC | CD11b+, CD115+, Ly6C+, Ly6G− | [12–14, 24] |
| Mouse N1-like MDSC | CD11b+, Ly6G+, ICAM1+, ROS+, TNFα+ | [17] |
| Mouse N2-like MDSC | CD11b+, Ly6G+, CCL2+, CCL5+, ARG+ | [105] |
| Mouse M1-like MDSC | CD11b+, Ly6C+, iNOS+, TNFα+, CCR7+, CXCL10+ | [18, 104] |
| Mouse M2-like MDSC | CD11b+, Ly6C+, F4/80+, CD206+, ARG+, C124+ | [94] |
| Human G-MDSC | CD11b+, CD33+, CD15+ | [31] |
| Human M-MDSC | CD11b+, CD33+, CD14+, CD16+, MHClo | [29] |
| Human bone marrow MDSC | CD11blo, CD16− | [36] |
| Rat MDSC | CD11b+, NKRP1+, CD172a+, His48+, CD80/86+ |
Fig. 1.
MDSC subsets. Cytospin preparations of CD11b-expressing Ly6Chi Ly6G−, Ly6Cint Ly6G−, Ly-6Clo Ly6G−, and Ly6Cint Ly6G+ myeloid cells from tolerant allografts
It is important to clarify that the presence of myeloid cells expressing CD11b+Gr-1+ is not necessarily detrimental. Under steady-state conditions, CD11b+ Gr-1+ cells account for 20–30 % of bone marrow cells and 2–4 % of splenocytes, and these cells are not suppressive [8]. Therefore, before different myeloid subsets may be identified as MDSC, the specific suppressive function of these subpopulations must be investigated.
Unlike mouse MDSC, the human counterpart does not have a universal marker and their phenotype is less well defined. Both monocytic and granulocytic human MDSC express CD33 and CD11b [27]. M-MDSC are characterized by their additional expression of CD14+ and low/negative levels of HLA-DR [28–30], while G-MDSC express higher levels of CD15 [14, 31]. Other common markers include CD66b and VEGFR1 [32, 33], and CD34 in agreement with the immature phenotype of MDSC [34, 35]. More recently, it has been reported that the suppressive activity of human MDSC resides in a CD11blo CD16− bone marrow myeloid cell population [36]. In transplantation, the presence of MDSC in human recipients has not yet been reported.
Suppressive mechanisms of MDSC
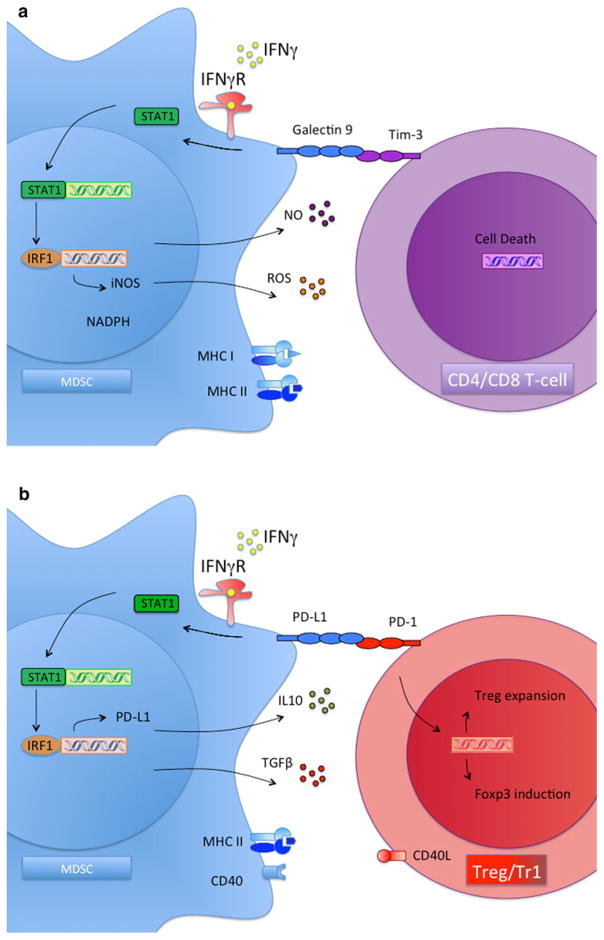
The most important function of MDSC is to inhibit the cytotoxic response mediated by T lymphocytes and NK cells [37–39]. G-MDSC and M-MDSC differ in their ability to suppress T-cell responses. Data from Dmitry Gabrilovich’s laboratory demonstrated that tumor-derived G-MDSC inhibit T-cell responses though reactive oxygen species (ROS) [22, 25]. In addition, data from our laboratory and Vincenzo Bronte’s laboratory demonstrated that M-MDSC inhibit T-cell responses through depletion of L-arginine, via both arginase-1 and iNOS [12, 19, 40, 41]. In transplantation, the critical role of iNOS was demonstrated by blocking iNOS with aminoguanidine in vivo, which resulted in rapid rejection of all kidney donor allografts in long-term tolerant recipients (>120 days post-transplant) [4]. Interestingly, production of iNOS in the transplanted allograft may be triggered in an antigen-specific manner. Otto and colleagues demonstrated that macrophages that accumulate in non-vascularized allografts produce iNOS following contact with antigen-specific CD4 T cells, in comparison with syngeneic control grafts [42]. In addition to the direct inhibitory effects of iNOS, large amounts of NO can also react with superoxide anion to form peroxynitrite, which is highly toxic [43]. In a tumor model, it has been shown that peroxynitrite alters the peptide recognition ability of T-cell receptor, so that T cells no longer respond to a specific antigen, providing a mechanism of tumor evasion [44]. Data from our laboratories indicated that MDSC are required for the induction of graft tolerance though iNOS-dependent mechanisms [24] and that IL-10 but not iNOS, is required for tumor tolerance and Treg induction [12]. Using recipient transgenic mice in which tolerance cannot be induced due to their deficiency in numbers of Gr1+-expressing myeloid cells [45], we were able to induce tolerance following adoptive transfer of Gr1+ myeloid precursors from the bone marrow under CD40 ligand blockade. However, Gr1+ myeloid precursors from iNOS- or STAT-1-deficient mice failed to induce tolerance when adoptively transferred. This suggests a critical role for IFN-γ signaling in the induction of MDSC-mediated transplantation tolerance (Fig. 2).
Fig. 2.
Suppressive mechanisms of MDSC. a MDSC-mediated T-cell suppression. IFN-gamma signaling mediates the induction of tolerance mediated by MDSC through activating STAT-1-dependent pathways, including iNOS activation and ROS production. b MDSC-mediated Treg development. IFN-gamma signaling mediates the induction of tolerance mediated by MDSC through activating STAT-1-dependent pathways, including PDL-1 expression and IL-10 plus TGF-β secretion
In addition to directly suppressing the immune response, MDSC have indirect mechanisms for actively suppressing T-cell-mediated cytotoxicity. Our groups have demonstrated that MDSC mediate the development of regulatory T cells (Treg) that suppress T-cell responses against tumor and transplanted grafts [12, 24, 44]. MDSC-dependent development of Treg seems to be mediated through IFN-γ-dependent pathways [24]. Although the protective role of IFN-γ in transplantation tolerance remains controversial, accumulating evidence indicates that a functioning IFN-γ signaling pathway is necessary to achieve indefinite allograft survival. Transplantation tolerance is not induced in IFN-γ-deficient recipient mice due to an exaggerated expansion of alloreactive effector T lymphocytes [46]. These tolerogenic effects of IFN-γ occur within the allograft, which may explain the necessity for M-MDSC cells in this anatomic compartment. In further support of the tolerogenic effects of IFN-γ at the transplanted site, it has been reported that IFN-γ and NO synthase gene expression are upregulated in infiltrating cells of tolerated heart allografts [47], which is associated with Treg cell development at the transplanted site [48]. Consistent with this finding, Kathryn Wood and colleagues demonstrated that development of alloantigen reactive Treg is impaired in the absence of IFN-γ and iNOS within the allograft [49]. Therefore, expression of IFN-γ and iNOS in the transplanted graft precedes Treg development and the induction of transplantation tolerance. Supporting this hypothesis, Vanhove and colleagues reported that iNOS-expressing MDSC stimulate IFN-γ secretion in Treg and are necessary for indefinite allograft survival [4]. However, we should clarify that the precise mechanism by which iNOS mediates Treg development has not yet been reported. It is possible that iNOS expression by MDSC may be selectively causing effector T-cell death, thus indirectly promoting Treg survival. On the other hand, we have shown that MDSC-mediated development of antigen-specific Treg in tumor-bearing mice requires IL-10, IL-4R, and arginase but not iNOS [12, 50].
MDSC development and therapeutic applications
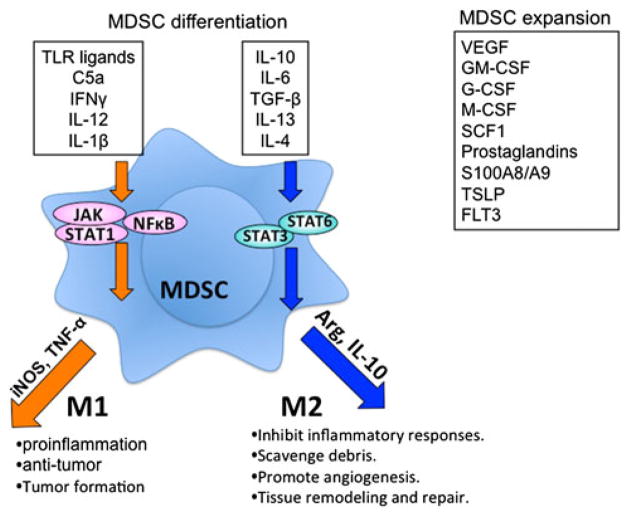
Myeloid-derived suppressor cells develop under acute and chronic inflammatory conditions. In a mouse model of inflammation, acute-phase proteins were shown to mediate the development of MDSC in a STAT-3-dependent manner [51]. In a mouse model of chronic inflammation, Wang and colleagues demonstrated that the pro-inflammatory cytokine IL1-β activates MDSC that accumulate in the stomach of gastric tumor-bearing mice though NF-KB signaling pathways [52]. In transplantation, the ischemic donor allograft and the surgical procedure during engraftment induces various inflammation signaling processes that mediate the mobilization of bone marrow CD11b+Gr-1+ cells [24]. Therefore, cytokines and soluble factors that are associated with inflammatory responses through signaling pathways such as NF-κB, JAK, and STAT control the survival, proliferation, and differentiation of MDSC. It has become clear that the suppressive activity of MDSC requires not only factors that promote their expansion, but also factors that activate MDSC. These factors include inflammatory cytokines (e.g. IL-1β, IL-12, TNF-α, and IFN-γ), ligands of Toll-like receptors, and complement (C5a), which are produced by activated T cells, tumor stromal cells, or as a result of tumor cell death [13, 19, 53–57]. On the other hand, anti-inflammatory cytokines (e.g. IL-10, IL-4, IL-13, IL-6, and TGF-β) can activate MDSC to mediate immune suppression. Additional factors that are involved in the development of MDSC include M-CSF, GM-CSF, G-CSF, SCF, S100A8/A9, PGE2, COX-2, and TSLP1 [58–64]. Activation of M-MDSC by these factors leads to the signaling of various pathways in differentiation of MDSC as summarized in Fig. 3.
Fig. 3.
M-MDSC activation and differentiation in tumor microenvironment. M-MDSC can differentiate into M1-like phenotype (controlled by TLR ligands, C5a, INFγ, IL-12, and IL-1β), which have antitumor effect through the production of iNOS. Alternatively, M-MDSC can differentiate into M2-like phenotype (controlled by IL-10, IL-6, TGF-β, IL-13, and IL-4), which promotes tumor growth, tissue remodeling and angiogenesis through producing IL-10 and arginase. In addition, the expansion of MDSC population can be controlled by VEGF, GM-CSF, G-CSF, M-CSF, SCF1, prostaglandins, S100A8/A9, and FLT3
Interleukins are involved in the development of MDSC. IL-1β increases ROS expression in MDSC, which enhances their suppressive activity [65–67], and promotes the secretion of IL-10 and IL-12 in the tumor environment [68]. In addition, signaling though IL-4 or IL-13 was shown to increase arginase-1 expression and the activation of MDSC [53, 69]. In transplantation, it has been reported that IL-13 in combination with G-CSF and GM-CSF generates MDSC expressing high levels of arginase-1 that inhibit graft versus host disease (GvHD) [70]. This is consistent with previous observations which demonstrated that IL-13 prolongs cardiac graft survival [71]. More recently, Thomson and colleagues have reported that IL-33 expands splenic MDSC expressing intermediate levels of Gr-1 and F4/80 that potently inhibit allogeneic T-cell responses and prolong graft survival [72]. In addition, IL-6-induced C/EBP beta transcription factor was shown to be critical for the induction of MDSC suppressive activity and, in combination with GM-CSF, generated MDSC that induced tolerance to islet allografts [73].
Prostaglandin E2 (PGE2) and cyclooxygenase-2 (COX-2) are inflammatory mediators produced by different tumors [74–76]. Cyclooxygenase (COX) 2 is the inducible isoform of rate-limiting enzymes involved in the synthesis of PGE2. PGE2 upregulates arginase-1 levels and suppressive activity in human MDSC [77], while blockade of COX-2 in bone marrow cell cultures prevents the development of MDSC [78]. Therefore, elevated levels of PGE2 promote tumor progression through non-immune mechanisms and through the induction of MDSC expansion that inhibits antitumor immunity [61, 62, 79]. Recent studies suggest that tumor-infiltrated MDSC frequently exhibit upregulated COX-2 expression and have enhanced PGE2 metabolism. These changes can skew GM-CSF-driven differentiation of M1-oriented myeloid APCs into M2-oriented arginase-expressing macrophages in the tumor microenvironment. Tumors impair intracellular PGE2 catabolism in myeloid cells through simultaneous stimulation of PGE2-forming enzyme, and inhibition of PGE2-degrading systems could be the potential mechanism for MDSC activation [78]. The proper control of MDSC differentiation may play an important role in how MDSC are able to modulate the immune response. In transplantation, upregulation of PEG2 is associated with prolonged skin allograft survival [80]. The positive feedback between COX-2 and PGE2 highlights a potential mechanism for the development of MDSC to manipulate immune responses in cancer and transplantation [81]. Since COX-2 blockade inhibits MDSC development and prevents tumor development [82], specific COX-2 blockade by celecoxib reduces the levels of PGE2 in vivo and prevents the expansion of MDSC [83]. Other strategies aim at blocking PGE2 production using COX-2 inhibitors, which reduce the expansion of MDSC [84] and inhibit tumor growth in mice [62].
Growth factors are also involved in the development of MDSC. Vascular endothelial growth factor (VEGF) is secreted by many tumors and mediates the formation and maintenance of vasculature. In addition to its well-characterized role in angiogenesis, VEGF inhibits dendritic cell differentiation and promotes the accumulation of functional MDSC [58, 85, 86]. MDSC accumulation depends on VEGFR2 [87], which is increased following GM-CSF production [88]. HIF-1α is transcriptional factor involved in VEGF expression and regulates the development of MDSC [89]. In transplantation, hypoxia-induced VEGF enhances engraftment and prolongs islet allograft survival [90, 91]. Anti-VEGF treatment has shown promising results in tumor-bearing mice by reducing MDSC accumulation and preventing T-cell anergy and Treg development [92]. In addition, our laboratory has demonstrated that MDSC can be efficiently generated in vitro from ES cells or hematopoietic stem cells (HSC) in the presence of FLT3 ligand and VEGF that are able to prevent GvHD [93].
Inhibition of the paired immunoglobulin-like receptor B (PIR-B), also known as leukocyte immunoglobulin-like receptor subfamily B member 3 (LILRB3), prevents MDSC differentiation. MDSC genetically ablated for PIR-B (Lilrb3−/−) underwent a specific transition to M1-like cells when entering the periphery from bone marrow, resulting in decreased suppressive function, regulatory T-cell activation activity, primary tumor growth, and lung metastases. Activation of Toll-like receptor (TLR), signal transducers and activator of transcription 1 (STAT1), and nuclear factor-kappa B (NF-κB) signaling in Lilrb3−/−MDSC promoted the acquisition of an M1 phenotype [94]. In transplantation, MDSC can be generated in vitro using immunoglobulin-like transcript 2 (ILT2) to promote long-term allograft survival to skin allografts [26].
Final remarks
MDSC develop from bone marrow myeloid progenitors conditioned by tumor-derived cytokines and soluble factors, express the surface markers CD11b and Gr-1, and inhibit T-lymphocyte proliferation in cancer patients and tumor-bearing mice [95]. The understanding of MDSC development in tumor models provides an important means by which to control undesirable immune responses against transplant allografts. Bertie Argyris isolated a “suppressor factor” secreted from the spleen cells of tumor-bearing mice that was able to prolong skin allograft survival [5, 6]. More recently, Vinvenzo Bronte and our laboratory reported that adoptive transfer of MDSC was able to induce tolerance to autoimmune T cells against islet antigen, islet allograft and prevent graft versus host disease in bone marrow-transplanted mice [50, 73, 93]. Overall, these studies suggest that MDSC can be used as a cell-based therapy to prevent T-cell-mediated allograft rejection.
Different types of MDSC can be generated in vitro from mouse bone marrow in the presence of specific cytokines and growth factors [96, 97] and conditioned media [78, 98]. In addition, subsets of MDSC including monocytic (Ly6G−Ly6C+ CD115+) and granulocytic (Ly6G+ Ly6CInt CD115−) MDSC, generated from mouse embryonic stem (ES) cells and hematopoietic stem cells, suppress T-cell proliferation by IL-10 and NO production [93]. However, the specific proportions and suppressive functions of varying MDSC subsets generated in vitro remain to be characterized. In addition, the similarities and differences between the different types of MDSC generated using different protocols, and the relationship between MDSC and other types of myeloid regulatory cells such as tumor-associated macrophages, alternatively activated macrophages (M2), or immature myeloid DC need to be fully established using specific cell markers and functional assays. In the tumor microenvironment, TAM constitute the majority of tumor-infiltrating leukocytes. Two distinct TAM subpopulations have been defined. Classical or M1 macrophages are characterized by the expression of high amounts of iNOS and TNF-α, whereas alternatively activated M2 macrophages typically produce arginase-1 and IL-10 [18]. At the tumor site, TAM are predominantly M2-like macrophages, which are primarily responsible for suppressing T-cell-mediated antitumor responses and promoting tumor progression, metastasis, and angiogenesis [99–102], while M1 macrophages exhibit a tumoricidal effect [55, 56, 94, 103]. M1-like MDSC express CCR7, MHC class II, CD86, and iNOS, while M2-like MDSC express the mannose receptor (CD206) and IL-10 [104]. In addition, tumor-associated neutrophils (TAN) expressing CD11b+Ly6G+ have also been described in tumor-bearing mice [105]. In transplantation, lacto-N-fucopentaose III promotes the development of macrophages with high levels of PD-L1 that prolongs graft survival of both vascularized and non-vascularized allografts [106]. Lacto-N-fucopentaose III induces M2 macrophages that express high levels of arginase-1 and produce high levels of IL-10 and TGF-β [107]. In addition, since NO and ROS production are IFN-γ dependent [108, 109], blocking IFN-γ using neutralizing antibodies or disrupting IFN-γ pathway signaling using STAT1-deficient mice abolishes MDSC-mediated T-cell suppression [13]. Geissler and colleagues recently reported that differentiation of monocytes in the presence of IFN-γ results in the development of suppressive macrophages in vivo with T-cell suppressive function [110]. Similar results have been obtained with human cells in vitro, in which IFN-γ-mediated development of tolerogenic DCs from blood monocytes leads to the ability to promote Treg development [111]. More recently, the ONE study (a multinational clinical trial of immunomodulatory cell therapy in renal transplantation) demonstrated the clinical application of IFN-γ-induced regulatory macrophages in human kidney recipients [112]. Therefore, the efficacy of MDSC generated from a clinically applicable source depends on our ability to generate specific suppressive cell subsets, and their manipulation represents a novel therapeutic approach to achieving tolerance or immunity in the clinic. Furthermore, MDSC trafficking to the site of inflammation through chemokine-mediated migration may provide a potential tool for using MDSC to deliver therapeutic agents to these sites for immune suppression or tumor targeting or to prolong graft survival [113]. These concerns must be addressed before taking MDSC into the clinic.
Acknowledgments
This work was supported by NIH grants to S.H.C. J.C.O. is a recipient of American Society of Transplantation/Pfizer basic sciences faculty development grant. We like to thank Dr. Ge Ma’s helpful discussion and editing.
Contributor Information
Jordi C. Ochando, Email: jordi.ochando@mssm.edu, Department of Nephrology, Mount Sinai School of Medicine, New York, NY, USA. Immunology Institute, Mount Sinai School of Medicine, New York, NY, USA
Shu Hsia Chen, Department of Oncological Sciences, Mount Sinai School of Medicine, New York, NY, USA. Immunology Institute, Mount Sinai School of Medicine, New York, NY, USA.
References
- 1.Kamo I, Friedman H. Immunosuppression and the role of suppressive factors in cancer. Adv Cancer Res. 1977;25:271–321. doi: 10.1016/s0065-230x(08)60636-3. [DOI] [PubMed] [Google Scholar]
- 2.Kirchner H, Holden HT, Herberman RB. Splenic suppressor macrophages induced in mice by injection of Corynebacterium parvum. J Immunol. 1975;115:1212–6. [PubMed] [Google Scholar]
- 3.Gabrilovich DI, Bronte V, Chen SH, Colombo MP, Ochoa A, Ostrand-Rosenberg S, Schreiber H. The terminology issue for myeloid-derived suppressor cells. Cancer Res. 2007;67:425. doi: 10.1158/0008-5472.CAN-06-3037. author reply 426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dugast AS, Haudebourg T, Coulon F, Heslan M, Haspot F, Poirier N, Vuillefroy de Silly R, Usal C, Smit H, Martinet B, et al. Myeloid-derived suppressor cells accumulate in kidney allograft tolerance and specifically suppress effector T cell expansion. J Immunol. 2008;180:7898–906. doi: 10.4049/jimmunol.180.12.7898. [DOI] [PubMed] [Google Scholar]
- 5.Argyris BF. Suppressor factor produced by neonatal mouse spleen cells. Cell Immunol. 1981;62:412–24. doi: 10.1016/0008-8749(81)90342-7. [DOI] [PubMed] [Google Scholar]
- 6.Argyris BF. Suppressor factor from tumor-allosensitized spleen cells—its effect on in vitro proliferation of tumor cells and in vivo skin allograft survival. Cell Immunol. 1981;57:62–72. doi: 10.1016/0008-8749(81)90120-9. [DOI] [PubMed] [Google Scholar]
- 7.Odling KA, Halliday GM, Muller HK. Acceptance of class II major histocompatibility complex disparate skin grafts associated with suppressor cells and elevated Langerhans cell numbers. Pathology. 1992;24:184–9. doi: 10.3109/00313029209063170. [DOI] [PubMed] [Google Scholar]
- 8.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–74. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bronte V, Apolloni E, Cabrelle A, Ronca R, Serafini P, Zamboni P, Restifo NP, Zanovello P. Identification of a CD11b(+)/Gr-1(+)/CD31(+) myeloid progenitor capable of activating or suppressing CD8(+) T cells. Blood. 2000;96:3838–46. [PMC free article] [PubMed] [Google Scholar]
- 10.Bronte V, Wang M, Overwijk WW, Surman DR, Pericle F, Rosenberg SA, Restifo NP. Apoptotic death of CD8+ T lymphocytes after immunization: induction of a suppressive population of Mac-1+/Gr-1+ cells. J Immunol. 1998;161:5313–20. [PMC free article] [PubMed] [Google Scholar]
- 11.Gumley TP, McKenzie IF, Sandrin MS. Tissue expression, structure and function of the murine Ly-6 family of molecules. Immunol Cell Biol. 1995;73:277–96. doi: 10.1038/icb.1995.45. [DOI] [PubMed] [Google Scholar]
- 12.Huang B, Pan PY, Li Q, Sato AI, Levy DE, Bromberg J, Divino CM, Chen SH. Gr-1+CD115+ immature myeloid suppressor cells mediate the development of tumor-induced T regulatory cells and T-cell anergy in tumor-bearing host. Cancer Res. 2006;66:1123–31. doi: 10.1158/0008-5472.CAN-05-1299. [DOI] [PubMed] [Google Scholar]
- 13.Movahedi K, Guilliams M, Van den Bossche J, Van den Bergh R, Gysemans C, Beschin A, De Baetselier P, Van Ginderachter JA. Identification of discrete tumor-induced myeloid-derived suppressor cell subpopulations with distinct T cell-suppressive activity. Blood. 2008;111:4233–44. doi: 10.1182/blood-2007-07-099226. [DOI] [PubMed] [Google Scholar]
- 14.Youn JI, Nagaraj S, Collazo M, Gabrilovich DI. Subsets of myeloid-derived suppressor cells in tumor-bearing mice. J Immunol. 2008;181:5791–802. doi: 10.4049/jimmunol.181.8.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rossner S, Voigtlander C, Wiethe C, Hanig J, Seifarth C, Lutz MB. Myeloid dendritic cell precursors generated from bone marrow suppress T cell responses via cell contact and nitric oxide production in vitro. Eur J Immunol. 2005;35:3533–44. doi: 10.1002/eji.200526172. [DOI] [PubMed] [Google Scholar]
- 16.Pan PY, Ma G, Weber KJ, Ozao-Choy J, Wang G, Yin B, Divino CM, Chen SH. Immune stimulatory receptor CD40 is required for T-cell suppression and T regulatory cell activation mediated by myeloid-derived suppressor cells in cancer. Cancer Res. 2010;70:99–108. doi: 10.1158/0008-5472.CAN-09-1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang R, Cai Z, Zhang Y, Yutzy WH, Roby KF, Roden RB. CD80 in immune suppression by mouse ovarian carcinoma-associated Gr-1+CD11b+ myeloid cells. Cancer Res. 2006;66:6807–15. doi: 10.1158/0008-5472.CAN-05-3755. [DOI] [PubMed] [Google Scholar]
- 18.Umemura N, Saio M, Suwa T, Kitoh Y, Bai J, Nonaka K, Ouyang GF, Okada M, Balazs M, Adany R, et al. Tumor-infiltrating myeloid-derived suppressor cells are pleiotropic-inflamed monocytes/macrophages that bear M1- and M2-type characteristics. J Leukoc Biol. 2008;83:1136–44. doi: 10.1189/jlb.0907611. [DOI] [PubMed] [Google Scholar]
- 19.Gallina G, Dolcetti L, Serafini P, De Santo C, Marigo I, Colombo MP, Basso G, Brombacher F, Borrello I, Zanovello P, et al. Tumors induce a subset of inflammatory monocytes with immunosuppressive activity on CD8+ T cells. J Clin Invest. 2006;116:2777–90. doi: 10.1172/JCI28828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haile LA, Gamrekelashvili J, Manns MP, Korangy F, Greten TF. CD49d is a new marker for distinct myeloid-derived suppressor cell subpopulations in mice. J Immunol. 2010;185:203–10. doi: 10.4049/jimmunol.0903573. [DOI] [PubMed] [Google Scholar]
- 21.Adeegbe D, Serafini P, Bronte V, Zoso A, Ricordi C, Inverardi L. In vivo induction of myeloid suppressor cells and CD4(+)Foxp3(+) T regulatory cells prolongs skin allograft survival in mice. Cell Transplant. 2011;20:941–54. doi: 10.3727/096368910X540621. [DOI] [PubMed] [Google Scholar]
- 22.Cheng P, Corzo CA, Luetteke N, Yu B, Nagaraj S, Bui MM, Ortiz M, Nacken W, Sorg C, Vogl T, et al. Inhibition of dendritic cell differentiation and accumulation of myeloid-derived suppressor cells in cancer is regulated by S100A9 protein. J Exp Med. 2008;205:2235–49. doi: 10.1084/jem.20080132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.De Wilde V, Van Rompaey N, Hill M, Lebrun JF, Lemaitre P, Lhomme F, Kubjak C, Vokaer B, Oldenhove G, Charbonnier LM, et al. Endotoxin-induced myeloid-derived suppressor cells inhibit alloimmune responses via heme oxygenase-1. Am J Transplant. 2009;9:2034–47. doi: 10.1111/j.1600-6143.2009.02757.x. [DOI] [PubMed] [Google Scholar]
- 24.Garcia MR, Ledgerwood L, Yang Y, Xu J, Lal G, Burrell B, Ma G, Hashimoto D, Li Y, Boros P, et al. Monocytic suppressive cells mediate cardiovascular transplantation tolerance in mice. J Clin Investig. 2010;120:2486–96. doi: 10.1172/JCI41628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kusmartsev S, Nefedova Y, Yoder D, Gabrilovich DI. Antigen-specific inhibition of CD8+ T cell response by immature myeloid cells in cancer is mediated by reactive oxygen species. J Immunol. 2004;172:989–99. doi: 10.4049/jimmunol.172.2.989. [DOI] [PubMed] [Google Scholar]
- 26.Zhang W, Liang S, Wu J, Horuzsko A. Human inhibitory receptor immunoglobulin-like transcript 2 amplifies CD11b+ Gr1+ myeloid-derived suppressor cells that promote long-term survival of allografts. Transplantation. 2008;86:1125–34. doi: 10.1097/TP.0b013e318186fccd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mirza N, Fishman M, Fricke I, Dunn M, Neuger AM, Frost TJ, Lush RM, Antonia S, Gabrilovich DI. All-trans-retinoic acid improves differentiation of myeloid cells and immune response in cancer patients. Cancer Res. 2006;66:9299–307. doi: 10.1158/0008-5472.CAN-06-1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Filipazzi P, Valenti R, Huber V, Pilla L, Canese P, Iero M, Castelli C, Mariani L, Parmiani G, Rivoltini L. Identification of a new subset of myeloid suppressor cells in peripheral blood of melanoma patients with modulation by a granulocyte-macrophage colony-stimulation factor-based antitumor vaccine. J Clin Oncol. 2007;25:2546–53. doi: 10.1200/JCO.2006.08.5829. [DOI] [PubMed] [Google Scholar]
- 29.Hoechst B, Ormandy LA, Ballmaier M, Lehner F, Kruger C, Manns MP, Greten TF, Korangy F. A new population of myeloid-derived suppressor cells in hepatocellular carcinoma patients induces CD4(+)CD25(+)Foxp3(+) T cells. Gastroenterology. 2008;135:234–43. doi: 10.1053/j.gastro.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 30.Zea AH, Rodriguez PC, Atkins MB, Hernandez C, Signoretti S, Zabaleta J, McDermott D, Quiceno D, Youmans A, O’Neill A, et al. Arginase-producing myeloid suppressor cells in renal cell carcinoma patients: a mechanism of tumor evasion. Cancer Res. 2005;65:3044–8. doi: 10.1158/0008-5472.CAN-04-4505. [DOI] [PubMed] [Google Scholar]
- 31.Eruslanov E, Neuberger M, Daurkin I, Perrin GQ, Algood C, Dahm P, Rosser C, Vieweg J, Gilbert SM, Kusmartsev S. Circulating and tumor-infiltrating myeloid cell subsets in patients with bladder cancer. Int J Cancer. 2012;130:1109–19. doi: 10.1002/ijc.26123. [DOI] [PubMed] [Google Scholar]
- 32.Schmielau J, Finn OJ. Activated granulocytes and granulocyte-derived hydrogen peroxide are the underlying mechanism of suppression of t-cell function in advanced cancer patients. Cancer Res. 2001;61:4756–60. [PubMed] [Google Scholar]
- 33.Rodriguez PC, Ernstoff MS, Hernandez C, Atkins M, Zabaleta J, Sierra R, Ochoa AC. Arginase I-producing myeloid-derived suppressor cells in renal cell carcinoma are a subpopulation of activated granulocytes. Cancer Res. 2009;69:1553–60. doi: 10.1158/0008-5472.CAN-08-1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pak AS, Wright MA, Matthews JP, Collins SL, Petruzzelli GJ, Young MR. Mechanisms of immune suppression in patients with head and neck cancer: presence of CD34(+) cells which suppress immune functions within cancers that secrete granulocyte-macrophage colony-stimulating factor. Clin Cancer Res. 1995;1:95–103. [PubMed] [Google Scholar]
- 35.Young MR, Wright MA, Lozano Y, Prechel MM, Benefield J, Leonetti JP, Collins SL, Petruzzelli GJ. Increased recurrence and metastasis in patients whose primary head and neck squamous cell carcinomas secreted granulocyte-macrophage colony-stimulating factor and contained CD34+ natural suppressor cells. Int J Cancer. 1997;74:69–74. doi: 10.1002/(sici)1097-0215(19970220)74:1<69::aid-ijc12>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 36.Solito S, Falisi E, Diaz-Montero CM, Doni A, Pinton L, Rosato A, Francescato S, Basso G, Zanovello P, Onicescu G, et al. A human promyelocytic-like population is responsible for the immune suppression mediated by myeloid-derived suppressor cells. Blood. 2011;118:2254–65. doi: 10.1182/blood-2010-12-325753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Young MR, Wright MA, Matthews JP, Malik I, Prechel M. Suppression of T cell proliferation by tumor-induced granulocyte-macrophage progenitor cells producing transforming growth factor-beta and nitric oxide. J Immunol. 1996;156:1916–22. [PubMed] [Google Scholar]
- 38.Otsuji M, Kimura Y, Aoe T, Okamoto Y, Saito T. Oxidative stress by tumor-derived macrophages suppresses the expression of CD3 zeta chain of T-cell receptor complex and antigen-specific T-cell responses. Proc Natl Acad Sci USA. 1996;93:13119–24. doi: 10.1073/pnas.93.23.13119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu Q, Zhang C, Sun A, Zheng Y, Wang L, Cao X. Tumor-educated CD11bhighIalow regulatory dendritic cells suppress T cell response through arginase I. J Immunol. 2009;182:6207–16. doi: 10.4049/jimmunol.0803926. [DOI] [PubMed] [Google Scholar]
- 40.Kusmartsev SA, Li Y, Chen SH. Gr-1+ myeloid cells derived from tumor-bearing mice inhibit primary T cell activation induced through CD3/CD28 costimulation. J Immunol. 2000;165:779–85. doi: 10.4049/jimmunol.165.2.779. [DOI] [PubMed] [Google Scholar]
- 41.Dolcetti L, Peranzoni E, Ugel S, Marigo I, Fernandez Gomez A, Mesa C, Geilich M, Winkels G, Traggiai E, Casati A, et al. Hierarchy of immunosuppressive strength among myeloid-derived suppressor cell subsets is determined by GM-CSF. Eur J Immunol. 2010;40:22–35. doi: 10.1002/eji.200939903. [DOI] [PubMed] [Google Scholar]
- 42.Matuschek A, Ulbrich M, Timm S, Schneider M, Thomas Germer C, Ulrichs K, Otto C. Analysis of parathyroid graft rejection suggests alloantigen-specific production of nitric oxide by iNOS-positive intragraft macrophages. Transpl Immunol. 2009;21:183–91. doi: 10.1016/j.trim.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 43.Ischiropoulos H, Zhu L, Beckman JS. Peroxynitrite formation from macrophage-derived nitric oxide. Arch Biochem Biophys. 1992;298:446–51. doi: 10.1016/0003-9861(92)90433-w. [DOI] [PubMed] [Google Scholar]
- 44.Nagaraj S, Gupta K, Pisarev V, Kinarsky L, Sherman S, Kang L, Herber DL, Schneck J, Gabrilovich DI. Altered recognition of antigen is a mechanism of CD8+ T cell tolerance in cancer. Nat Med. 2007;13:828–35. doi: 10.1038/nm1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Serbina NV, Pamer EG. Monocyte emigration from bone marrow during bacterial infection requires signals mediated by chemokine receptor CCR2. Nat Immunol. 2006;7:311–7. doi: 10.1038/ni1309. [DOI] [PubMed] [Google Scholar]
- 46.Konieczny BT, Dai Z, Elwood ET, Saleem S, Linsley PS, Baddoura FK, Larsen CP, Pearson TC, Lakkis FG. IFN-gamma is critical for long-term allograft survival induced by blocking the CD28 and CD40 ligand T cell costimulation pathways. J Immunol. 1998;160:2059–64. [PubMed] [Google Scholar]
- 47.Heslan JM, Renaudin K, Thebault P, Josien R, Cuturi MC, Chiffoleau E. New evidence for a role of allograft accommodation in long-term tolerance. Transplantation. 2006;82:1185–93. doi: 10.1097/01.tp.0000236573.01428.f3. [DOI] [PubMed] [Google Scholar]
- 48.Heslan JM, Beriou G, Le Luduec JB, Guillonneau C, Anegon I, Soulillou JP, Cuturi MC, Chiffoleau E. Accumulation of T cells with potent regulatory properties and restricted Vbeta7-TCR rearrangements in tolerated allografts. Transplantation. 2005;80:1476–84. doi: 10.1097/01.tp.0000185198.07663.ba. [DOI] [PubMed] [Google Scholar]
- 49.Sawitzki B, Kingsley CI, Oliveira V, Karim M, Herber M, Wood KJ. IFN-gamma production by alloantigen-reactive regulatory T cells is important for their regulatory function in vivo. J Exp Med. 2005;201:1925–35. doi: 10.1084/jem.20050419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yin B, Ma G, Yen CY, Zhou Z, Wang GX, Divino CM, Casares S, Chen SH, Yang WC, Pan PY. Myeloid-derived suppressor cells prevent type 1 diabetes in murine models. J Immunol. 2010;185:5828–34. doi: 10.4049/jimmunol.0903636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sander LE, Sackett SD, Dierssen U, Beraza N, Linke RP, Muller M, Blander JM, Tacke F, Trautwein C. Hepatic acute-phase proteins control innate immune responses during infection by promoting myeloid-derived suppressor cell function. J Exp Med. 2010;207:1453–64. doi: 10.1084/jem.20091474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tu S, Bhagat G, Cui G, Takaishi S, Kurt-Jones EA, Rickman B, Betz KS, Penz-Oesterreicher M, Bjorkdahl O, Fox JG, et al. Overexpression of interleukin-1beta induces gastric inflammation and cancer and mobilizes myeloid-derived suppressor cells in mice. Cancer Cell. 2008;14:408–19. doi: 10.1016/j.ccr.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bronte V, Serafini P, De Santo C, Marigo I, Tosello V, Mazzoni A, Segal DM, Staib C, Lowel M, Sutter G, et al. IL-4-induced arginase 1 suppresses alloreactive T cells in tumor-bearing mice. J Immunol. 2003;170:270–8. doi: 10.4049/jimmunol.170.1.270. [DOI] [PubMed] [Google Scholar]
- 54.Kusmartsev S, Gabrilovich DI. Role of immature myeloid cells in mechanisms of immune evasion in cancer. Cancer Immunol Immunother. 2006;55:237–45. doi: 10.1007/s00262-005-0048-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sinha P, Clements VK, Ostrand-Rosenberg S. Interleukin-13-regulated M2 macrophages in combination with myeloid suppressor cells block immune surveillance against metastasis. Cancer Res. 2005;65:11743–51. doi: 10.1158/0008-5472.CAN-05-0045. [DOI] [PubMed] [Google Scholar]
- 56.Sinha P, Clements VK, Ostrand-Rosenberg S. Reduction of myeloid-derived suppressor cells and induction of M1 macrophages facilitate the rejection of established metastatic disease. J Immunol. 2005;174:636–45. doi: 10.4049/jimmunol.174.2.636. [DOI] [PubMed] [Google Scholar]
- 57.Terabe M, Matsui S, Park JM, Mamura M, Noben-Trauth N, Donaldson DD, Chen W, Wahl SM, Ledbetter S, Pratt B, et al. Transforming growth factor-beta production and myeloid cells are an effector mechanism through which CD1d-restricted T cells block cytotoxic T lymphocyte-mediated tumor immuno-surveillance: abrogation prevents tumor recurrence. J Exp Med. 2003;198:1741–52. doi: 10.1084/jem.20022227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gabrilovich D, Ishida T, Oyama T, Ran S, Kravtsov V, Nadaf S, Carbone DP. Vascular endothelial growth factor inhibits the development of dendritic cells and dramatically affects the differentiation of multiple hematopoietic lineages in vivo. Blood. 1998;92:4150–66. [PubMed] [Google Scholar]
- 59.Bunt SK, Yang L, Sinha P, Clements VK, Leips J, Ostrand-Rosenberg S. Reduced inflammation in the tumor microenvironment delays the accumulation of myeloid-derived suppressor cells and limits tumor progression. Cancer Res. 2007;67:10019–26. doi: 10.1158/0008-5472.CAN-07-2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Serafini P, Carbley R, Noonan KA, Tan G, Bronte V, Borrello I. High-dose granulocyte-macrophage colony-stimulating factor-producing vaccines impair the immune response through the recruitment of myeloid suppressor cells. Cancer Res. 2004;64:6337–43. doi: 10.1158/0008-5472.CAN-04-0757. [DOI] [PubMed] [Google Scholar]
- 61.Sinha P, Clements VK, Bunt SK, Albelda SM, Ostrand-Rosenberg S. Cross-talk between myeloid-derived suppressor cells and macrophages subverts tumor immunity toward a type 2 response. J Immunol. 2007;179:977–83. doi: 10.4049/jimmunol.179.2.977. [DOI] [PubMed] [Google Scholar]
- 62.Sinha P, Clements VK, Fulton AM, Ostrand-Rosenberg S. Prostaglandin E2 promotes tumor progression by inducing myeloid-derived suppressor cells. Cancer Res. 2007;67:4507–13. doi: 10.1158/0008-5472.CAN-06-4174. [DOI] [PubMed] [Google Scholar]
- 63.Pan PY, Wang GX, Yin B, Ozao J, Ku T, Divino CM, Chen SH. Reversion of immune tolerance in advanced malignancy: modulation of myeloid-derived suppressor cell development by blockade of stem-cell factor function. Blood. 2008;111:219–28. doi: 10.1182/blood-2007-04-086835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sinha P, Okoro C, Foell D, Freeze HH, Ostrand-Rosenberg S, Srikrishna G. Proinflammatory S100 proteins regulate the accumulation of myeloid-derived suppressor cells. J Immunol. 2008;181:4666–75. doi: 10.4049/jimmunol.181.7.4666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bunt SK, Sinha P, Clements VK, Leips J, Ostrand-Rosenberg S. Inflammation induces myeloid-derived suppressor cells that facilitate tumor progression. J Immunol. 2006;176:284–90. doi: 10.4049/jimmunol.176.1.284. [DOI] [PubMed] [Google Scholar]
- 66.Song X, Krelin Y, Dvorkin T, Bjorkdahl O, Segal S, Dinarello CA, Voronov E, Apte RN. CD11b+/Gr-1+ immature myeloid cells mediate suppression of T cells in mice bearing tumors of IL-1beta-secreting cells. J Immunol. 2005;175:8200–8. doi: 10.4049/jimmunol.175.12.8200. [DOI] [PubMed] [Google Scholar]
- 67.Voronov E, Shouval DS, Krelin Y, Cagnano E, Benharroch D, Iwakura Y, Dinarello CA, Apte RN. IL-1 is required for tumor invasiveness and angiogenesis. Proc Nat Acad Sci USA. 2003;100:2645–50. doi: 10.1073/pnas.0437939100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Delano MJ, Scumpia PO, Weinstein JS, Coco D, Nagaraj S, Kelly-Scumpia KM, O’Malley KA, Wynn JL, Antonenko S, Al-Quran SZ, et al. MyD88-dependent expansion of an immature GR-1(+)CD11b(+) population induces T cell suppression and Th2 polarization in sepsis. J Exp Med. 2007;204:1463–74. doi: 10.1084/jem.20062602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rutschman R, Lang R, Hesse M, Ihle JN, Wynn TA, Murray PJ. Cutting edge: Stat6-dependent substrate depletion regulates nitric oxide production. J Immunol. 2001;166:2173–7. doi: 10.4049/jimmunol.166.4.2173. [DOI] [PubMed] [Google Scholar]
- 70.Highfill SL, Rodriguez PC, Zhou Q, Goetz CA, Koehn BH, Veenstra R, Taylor PA, Panoskaltsis-Mortari A, Serody JS, Munn DH, et al. Bone marrow myeloid-derived suppressor cells (MDSCs) inhibit graft-versus-host disease (GVHD) via an arginase-1-dependent mechanism that is up-regulated by inter-leukin-13. Blood. 2010;116:5738–47. doi: 10.1182/blood-2010-06-287839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Davidson C, Verma ND, Robinson CM, Plain KM, Tran GT, Hodgkinson SJ, Hall BM. IL-13 prolongs allograft survival: association with inhibition of macrophage cytokine activation. Transpl Immunol. 2007;17:178–86. doi: 10.1016/j.trim.2006.09.035. [DOI] [PubMed] [Google Scholar]
- 72.Turnquist HR, Zhao Z, Rosborough BR, Liu Q, Castellaneta A, Isse K, Wang Z, Lang M, Stolz DB, Zheng XX, et al. IL-33 expands suppressive CD11b+ Gr-1 int and regulatory T cells, including ST2L+ Foxp3+ cells, and mediates regulatory T cell-dependent promotion of cardiac allograft survival. J Immunol. 2011;187:4598–610. doi: 10.4049/jimmunol.1100519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Marigo I, Bosio E, Solito S, Mesa C, Fernandez A, Dolcetti L, Ugel S, Sonda N, Bicciato S, Falisi E, et al. Tumor-induced tolerance and immune suppression depend on the C/EBPbeta transcription factor. Immunity. 2010;32:790–802. doi: 10.1016/j.immuni.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 74.Alleva DG, Burger CJ, Elgert KD. Tumor growth increases Ia-macrophage synthesis of tumor necrosis factor-alpha and prostaglandin E2: changes in macrophage suppressor activity. J Leukoc Biol. 1993;53:550–8. doi: 10.1002/jlb.53.5.550. [DOI] [PubMed] [Google Scholar]
- 75.Taketo MM. Cyclooxygenase-2 inhibitors in tumorigenesis (Part II) J Natl Cancer Inst. 1998;90:1609–20. doi: 10.1093/jnci/90.21.1609. [DOI] [PubMed] [Google Scholar]
- 76.Taketo MM. Cyclooxygenase-2 inhibitors in tumorigenesis (part I) J Natl Cancer Inst. 1998;90:1529–36. doi: 10.1093/jnci/90.20.1529. [DOI] [PubMed] [Google Scholar]
- 77.Ochoa AC, Zea AH, Hernandez C, Rodriguez PC. Arginase, prostaglandins, and myeloid-derived suppressor cells in renal cell carcinoma. Clin Cancer Res. 2007;13:721s–6s. doi: 10.1158/1078-0432.CCR-06-2197. [DOI] [PubMed] [Google Scholar]
- 78.Eruslanov E, Daurkin I, Ortiz J, Vieweg J, Kusmartsev S. Pivotal advance: tumor-mediated induction of myeloid-derived suppressor cells and M2-polarized macrophages by altering intracellular PGE catabolism in myeloid cells. J Leukoc Biol. 2010;88:839–48. doi: 10.1189/jlb.1209821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rodriguez PC, Hernandez CP, Quiceno D, Dubinett SM, Zabaleta J, Ochoa JB, Gilbert J, Ochoa AC. Arginase I in myeloid suppressor cells is induced by COX-2 in lung carcinoma. J Exp Med. 2005;202:931–9. doi: 10.1084/jem.20050715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fujimoto Y, Iwagaki H, Ozaki M, Ogino T, Murata H, Sun DS, Sadamori H, Takahashi HK, Tanaka N, Yagi T. Involvement of prostaglandin receptors (EPR2-4) in in vivo immunosuppression of PGE2 in rat skin transplant model. Int Immunopharmacol. 2005;5:1131–9. doi: 10.1016/j.intimp.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 81.Obermajer N, Muthuswamy R, Lesnock J, Edwards RP, Kalinski P. Positive feedback between PGE2 and COX2 redirects the differentiation of human dendritic cells toward stable myeloid-derived suppressor cells. Blood. 2011;118:5498–505. doi: 10.1182/blood-2011-07-365825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fujita M, Kohanbash G, Fellows-Mayle W, Hamilton RL, Komohara Y, Decker SA, Ohlfest JR, Okada H. COX-2 blockade suppresses gliomagenesis by inhibiting myeloid-derived suppressor cells. Cancer Res. 2011;71:2664–74. doi: 10.1158/0008-5472.CAN-10-3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Veltman JD, Lambers ME, van Nimwegen M, Hendriks RW, Hoogsteden HC, Aerts JG, Hegmans JP. COX-2 inhibition improves immunotherapy and is associated with decreased numbers of myeloid-derived suppressor cells in mesothelioma. Celecoxib influences MDSC function. BMC cancer. 2010;10:464. doi: 10.1186/1471-2407-10-464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Talmadge JE, Hood KC, Zobel LC, Shafer LR, Coles M, Toth B. Chemoprevention by cyclooxygenase-2 inhibition reduces immature myeloid suppressor cell expansion. Int Immunopharmacol. 2007;7:140–51. doi: 10.1016/j.intimp.2006.09.021. [DOI] [PubMed] [Google Scholar]
- 85.Gabrilovich D. Mechanisms and functional significance of tumour-induced dendritic-cell defects. Nat Rev Immunol. 2004;4:941–52. doi: 10.1038/nri1498. [DOI] [PubMed] [Google Scholar]
- 86.Melani C, Chiodoni C, Forni G, Colombo MP. Myeloid cell expansion elicited by the progression of spontaneous mammary carcinomas in c-erbB-2 transgenic BALB/c mice suppresses immune reactivity. Blood. 2003;102:2138–45. doi: 10.1182/blood-2003-01-0190. [DOI] [PubMed] [Google Scholar]
- 87.Huang Y, Chen X, Dikov MM, Novitskiy SV, Mosse CA, Yang L, Carbone DP. Distinct roles of VEGFR-1 and VEGFR-2 in the aberrant hematopoiesis associated with elevated levels of VEGF. Blood. 2007;110:624–31. doi: 10.1182/blood-2007-01-065714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Larrivee B, Pollet I, Karsan A. Activation of vascular endothelial growth factor receptor-2 in bone marrow leads to accumulation of myeloid cells: role of granulocyte-macrophage colony-stimulating factor. J Immunol. 2005;175:3015–24. doi: 10.4049/jimmunol.175.5.3015. [DOI] [PubMed] [Google Scholar]
- 89.Corzo CA, Condamine T, Lu L, Cotter MJ, Youn JI, Cheng P, Cho HI, Celis E, Quiceno DG, Padhya T, et al. HIF-1alpha regulates function and differentiation of myeloid-derived suppressor cells in the tumor microenvironment. J Exp Med. 2010;207:2439–53. doi: 10.1084/jem.20100587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sigrist S, Mechine-Neuville A, Mandes K, Calenda V, Braun S, Legeay G, Bellocq JP, Pinget M, Kessler L. Influence of VEGF on the viability of encapsulated pancreatic rat islets after transplantation in diabetic mice. Cell Transpl. 2003;12:627–35. doi: 10.3727/000000003108747109. [DOI] [PubMed] [Google Scholar]
- 91.Lee BW, Lee M, Chae HY, Lee S, Kang JG, Kim CS, Lee SJ, Yoo HJ, Ihm SH. Effect of hypoxia-inducible VEGF gene expression on revascularization and graft function in mouse islet transplantation. Transpl Int. 2011;24:307–14. doi: 10.1111/j.1432-2277.2010.01194.x. [DOI] [PubMed] [Google Scholar]
- 92.Ozao-Choy J, Ma G, Kao J, Wang GX, Meseck M, Sung M, Schwartz M, Divino CM, Pan PY, Chen SH. The novel role of tyrosine kinase inhibitor in the reversal of immune suppression and modulation of tumor microenvironment for immune-based cancer therapies. Cancer Res. 2009;69:2514–22. doi: 10.1158/0008-5472.CAN-08-4709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhou Z, French DL, Ma G, Eisenstein S, Chen Y, Divino CM, Keller G, Chen SH, Pan PY. Development and function of myeloid-derived suppressor cells generated from mouse embryonic and hematopoietic stem cells. Stem Cells. 2010;28:620–32. doi: 10.1002/stem.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ma G, Pan PY, Eisenstein S, Divino CM, Lowell CA, Takai T, Chen SH. Paired immunoglobin-like receptor-B regulates the suppressive function and fate of myeloid-derived suppressor cells. Immunity. 2011;34:385–95. doi: 10.1016/j.immuni.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bronte V, Serafini P, Apolloni E, Zanovello P. Tumor-induced immune dysfunctions caused by myeloid suppressor cells. J Immunother. 2001;24:431–46. doi: 10.1097/00002371-200111000-00001. [DOI] [PubMed] [Google Scholar]
- 96.Lechner MG, Liebertz DJ, Epstein AL. Characterization of cytokine-induced myeloid-derived suppressor cells from normal human peripheral blood mononuclear cells. J Immunol. 2010;185:2273–84. doi: 10.4049/jimmunol.1000901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.MacDonald KP, Rowe V, Clouston AD, Welply JK, Kuns RD, Ferrara JL, Thomas R, Hill GR. Cytokine expanded myeloid precursors function as regulatory antigen-presenting cells and promote tolerance through IL-10-producing regulatory T cells. J Immunol. 2005;174:1841–50. doi: 10.4049/jimmunol.174.4.1841. [DOI] [PubMed] [Google Scholar]
- 98.Nefedova Y, Huang M, Kusmartsev S, Bhattacharya R, Cheng P, Salup R, Jove R, Gabrilovich D. Hyperactivation of STAT3 is involved in abnormal differentiation of dendritic cells in cancer. J Immunol. 2004;172:464–74. doi: 10.4049/jimmunol.172.1.464. [DOI] [PubMed] [Google Scholar]
- 99.Guruvayoorappan C. Tumor versus tumor-associated macrophages: how hot is the link? Integr Cancer Ther. 2008;7:90–5. doi: 10.1177/1534735408319060. [DOI] [PubMed] [Google Scholar]
- 100.Mantovani A, Sica A, Allavena P, Garlanda C, Locati M. Tumor-associated macrophages and the related myeloid-derived suppressor cells as a paradigm of the diversity of macrophage activation. Hum Immunol. 2009;70:325–30. doi: 10.1016/j.humimm.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 101.Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23:549–55. doi: 10.1016/s1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- 102.Sica A, Larghi P, Mancino A, Rubino L, Porta C, Totaro MG, Rimoldi M, Biswas SK, Allavena P, Mantovani A. Macrophage polarization in tumour progression. Semin Cancer Biol. 2008;18:349–55. doi: 10.1016/j.semcancer.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 103.Sinha P, Clements VK, Miller S, Ostrand-Rosenberg S. Tumor immunity: a balancing act between T cell activation, macrophage activation and tumor-induced immune suppression. Cancer Immunol Immunother. 2005;54:1137–42. doi: 10.1007/s00262-005-0703-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kodumudi KN, Woan K, Gilvary DL, Sahakian E, Wei S, Djeu JY. A novel chemoimmunomodulating property of docetaxel: suppression of myeloid-derived suppressor cells in tumor bearers. Clin Cancer Res. 2010;16:4583–94. doi: 10.1158/1078-0432.CCR-10-0733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Fridlender ZG, Sun J, Kim S, Kapoor V, Cheng G, Ling L, Worthen GS, Albelda SM. Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer Cell. 2009;16:183–94. doi: 10.1016/j.ccr.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Dutta P, Hullett DA, Roenneburg DA, Torrealba JR, Sollinger HW, Harn DA, Burlingham WJ. Lacto-N-fucopentaose III, a pentasaccharide, prolongs heart transplant survival. Transplantation. 2010;90:1071–8. doi: 10.1097/TP.0b013e3181f8f296. [DOI] [PubMed] [Google Scholar]
- 107.Atochina O, Da’dara AA, Walker M, Harn DA. The immunomodulatory glycan LNFPIII initiates alternative activation of murine macrophages in vivo. Immunology. 2008;125:111–21. doi: 10.1111/j.1365-2567.2008.02826.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mazzoni A, Bronte V, Visintin A, Spitzer JH, Apolloni E, Serafini P, Zanovello P, Segal DM. Myeloid suppressor lines inhibit T cell responses by an NO-dependent mechanism. J Immunol. 2002;168:689–95. doi: 10.4049/jimmunol.168.2.689. [DOI] [PubMed] [Google Scholar]
- 109.Kusmartsev S, Gabrilovich DI. Immature myeloid cells and cancer-associated immune suppression. Cancer Immunol Immunother. 2002;51:293–8. doi: 10.1007/s00262-002-0280-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Brem-Exner BG, Sattler C, Hutchinson JA, Koehl GE, Kronenberg K, Farkas S, Inoue S, Blank C, Knechtle SJ, Schlitt HJ, et al. Macrophages driven to a novel state of activation have anti-inflammatory properties in mice. J Immunol. 2008;180:335–49. doi: 10.4049/jimmunol.180.1.335. [DOI] [PubMed] [Google Scholar]
- 111.Eljaafari A, Li YP, Miossec P. IFN-gamma, as secreted during an alloresponse, induces differentiation of monocytes into tolerogenic dendritic cells, resulting in FoxP3+ regulatory T cell promotion. J Immunol. 2009;183:2932–45. doi: 10.4049/jimmunol.0804352. [DOI] [PubMed] [Google Scholar]
- 112.Hutchinson JA, Riquelme P, Sawitzki B, Tomiuk S, Miqueu P, Zuhayra M, Oberg HH, Pascher A, Lutzen U, Janssen U, et al. Cutting edge: immunological consequences and trafficking of human regulatory macrophages administered to renal transplant recipients. J Immunol. 2011;187:2072–8. doi: 10.4049/jimmunol.1100762. [DOI] [PubMed] [Google Scholar]
- 113.Leuschner F, Dutta P, Gorbatov R, Novobrantseva TI, Donahoe JS, Courties G, Lee KM, Kim JI, Markmann JF, Marinelli B, et al. Therapeutic siRNA silencing in inflammatory monocytes in mice. Nat Biotechnol. 2011;29:1005–10. doi: 10.1038/nbt.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]