Abstract
Amyotrophic lateral sclerosis (ALS) is one of the most complex motor neuron diseases. Even though scientific discoveries are accelerating with an unprecedented pace, to date more than 30 clinical trials have ended with failure and staggering frustration. There are too many compounds that increase life span in mice, but too little evidence that they will improve human condition. Increasing the chances of success for future clinical trials requires advancement of preclinical tests. Recent developments, which enable the visualization of diseased motor neurons, have the potential to bring novel insight. As we change our focus from mice to motor neurons, it is possible to foster a new vision that translates into effective and long-term treatment strategies in ALS and related motor neuron disorders (MND).
ALS and related MND are considered ‘orphan’ diseases, yet the devastating nature of the disease and the cost associated with patient care have kept drug companies interested in identifying compounds that improve patient health and quality of life. However, despite all sincere efforts, drug discovery for MND has been the source of deep frustration.
Identification of the G93A mutation in the gene encoding super oxide dismutase (SOD1) as one of the causes of ALS marked an important milestone for ALS genetics 20 years ago [1]. A genetic cause for approximately 10–20% of familial ALS (fALS) was identified. Generation of mouse models that overexpress the mutant form of the human SOD1 gene, such as the hSOD1G93A mice [2], has been revolutionary for preclinical studies [3]. Extension of life span in mouse models of ALS has been accepted as one of the major criteria before moving into clinical trials [4]. During early ages of drug discovery, only compounds that significantly improved longevity in hSOD1G93A and other mouse models of ALS were considered for clinical trials.
To date, numerous compounds have enhanced the life span of hSOD1G93A mice and have improved their overall health. However, only riluzole has received US Food and Drug Administration (FDA) approval, although it extends patient survival only by 3–4 months without improving their quality of life [5,6]. Since riluzole, the drug discovery field has faced constant failures over the years. By contrast, the molecular, genetic and cellular basis of the disease is beginning to emerge [7–13]. In addition, novel in vitro technologies are being developed for high-throughput prescreening of compounds before moving into clinical trials [14,15]. However, none of these developments has yet translated into success in clinical trials. Induced pluripotent stem cell (iPSC)-derived motor neurons generated from mouse models of the disease and cells isolated from patients offer great advantages for their ability to mimic many aspects of diseased spinal motor neurons in culture [14,16]. The application of these cells in drug discovery efforts has been recently reviewed [17].
Here, we focus our attention on in vivo models, propose a shift in critical thinking from mouse survival to neuron biology, and discuss the importance of revealing the upper motor neuron survival requirements before moving into clinical trials. Now is the time to assess the limitations of the past, and there are questions that await answers: (i) are clinical trials really failing? (ii) Is the extension of life span in mice a dependable readout for potential success in clinical trials? and (iii) can preclinical screening be improved?
Are clinical trials really failing?
Clinical trials have not yet resulted in favorable outcomes, but they are far from being a ‘failure’. Immense effort has been put into their methodological design and outcome measures, and they have been constantly improving with new considerations [18,19]. Although methodologies are of high quality, the limited number of patients and the unknown factors that cause pathology in patients add to the complexity. In addition, the absence of direct translation from mice to humans raises a valid concern: ‘are these really the correct compounds to be used in clinical trials?’
The heterogeneous and complex nature of the disease is well known [20,21], and the mechanisms underlying motor neuron vulnerability are beginning to emerge. For example, neuroinflammation, glutamate-mediated excitotoxicity, defects in protein folding, mitochondrial dysfunction and oxidative stress have been identified as prominent drug targets for ALS/MND [22]. Therefore, compounds targeting these distinct pathways have been tested in clinical trials. Celecoxib, minocycline, thalidomide and lenalidomide target neuroinflammation [23–26], riluzole and ceftriaxone act mainly upon glutamate-mediated excitotoxicity [27,28], arimoclomol targets protein folding [29,30] and ederavone and AEOL-10150 are antioxidants against superoxide-mediated damage [31–33]. Owing to the absence of early detection markers and proper biomarkers for the disease, numerous patients who are at different stages and who develop the disease potentially because of different underlying causes are included in the same study. Thus, it is hard to interpret the result of such studies. In addition, the field suffers from the lack of presumed negative clinical data, which is different from the null result (i.e. data that do not affect the outcome).
In a clinical trial, the patients who develop the disease mainly because of defects in a particular pathway would display greatest benefit from the compounds that selectively target that pathway. Interestingly, in almost all clinical trials, a subset of the patient population showed improved condition. However, their numbers have been mostly limited, because none of the compounds displayed an overarching affect on most patients. It is possible that each clinical trial has been successful within only a select subset of the patient population. It is also important to remember that ALS is a multifactorial disease, and it might be unrealistic to imagine that one compound will have a broad spectrum of efficacy on pathologies that are widespread and, at times unrelated. Therefore, we suggest that the future of clinical trials should include combinatorial studies, and that patients who display improvement in their condition should not be considered as ‘outliers’, because they might indeed represent the target population, especially for the compounds tested.
The centralization of patient data and the forming of centers that include many different institutions and universities have been revolutionary [34]. Clinical trials have evolved into multicenter methods, joining patient information and data from several centers into one clinical trial to meet the challenges and logistics of patient population [e.g. NEALS consortium, http://www.alsconsortium.org; and Network for Excellence in Neuroscience Clinical Trials (NeuroNEXT), http://www.ninds.nih.gov/news_and_events/proceedings/20101217-NEXT.htm]. This not only increases the patient number for each study, but also allows further investigation of potential ways of stratifying them into proper groups (e.g. site of onset, rate of progression, age of onset, sex, genetic background and mutation information).
Grouping patients based on the molecular and cellular basis of their disease pathology is a real challenge, and one that is not currently possible. However, there are important developments towards this goal. The identification and characterization of early detection markers in ALS, and the establishment of dependable biomarkers for disease progression, is an active area of research [35]. Such studies either use DNA and gene expression profiles of patients who responded positively in a select set of clinical trials, or perform proteomics studies on blood, plasma and cerebrospinal fluid (CSF), obtained from patients and control subjects. With the identification of biomarkers and early detection markers, both the selection and inclusion criterion for clinical trials will be improved. Although these developments would advance the outcome measures, because most of the drug targets are determined based on the information obtained from mouse models of the disease, one of the most important question remains: ‘are these really the correct compounds to be used in clinical trials?’
Is the extension of life span in mouse a dependable readout for potential success in clinical trials?
Since the generation of the hSOD1G93A transgenic ALS mouse model, which recapitulates many aspects of the disease pathology observed in patients with ALS, including progressive degeneration of both spinal (SMN) [2] and corticospinal motor neurons (CSMN) [36,37] and increased astrogliosis [38], the model has become one of the major in vivo tools for compound screening. However, failure after failure in more than 30 clinical trials started questioning the validity of life-span extension in mice as a readout for success in clinical trials [4,8,39]. It is possible that poor lab-to-lab reproducibility also contributed to ineffective translation from animal models to clinical efficacy. Although important information was gained from using disease models, the drug industry started to lose interest in their use as a tool to inform the potential efficacy of compounds for patient survival. Are the mouse models to be blamed for the lack of straightforward translation? We believe that it is not the mouse to be blamed, but our unrealistic expectation.
Numerous clinical trials were initiated using the animal models for preclinical screening (Table 1). The compounds tested targeted pathways such as mitochondrial dysfunction, oxidative stress, protein folding, autophagy, apoptosis, neuroinflammation and glutamate excitotoxicity. Here, we review these studies, focusing particularly on their neuroprotective effects on SMN and their overall effects on the life expectancy of the mouse models.
TABLE 1.
Summary of preclinical drug trials investigating SMN neuroprotection and their clinical translation
| Drug | Life span (days) | Refs | Neuroprotection (SMN number) | Refs | Clinical trial | Refs |
|---|---|---|---|---|---|---|
| Neurotrophins | ||||||
| IGF-1 | +37 | [40] | 78% increase | [40] | Slowed disease progression | [44] |
| +29 | [41] | No change | [41] | Safe, but no improvement | [45] | |
| Phase III, no difference | [46] | |||||
| VEGF | +38 | [53] | 125% increase | [53] | Phase I/II completed (NCT00800501b) | |
| Phase II completed (NCT00748501b) | ||||||
| BDNF | n.s.a | [52] | No change | [52] | Phase III, (subcutaneous), no improvement | [48] |
| n.s. | [51] | Phase III, (intrathecal), no improvement | [50] | |||
| Mitochondria | ||||||
| Creatine | +26 | [54] | No change | [54] | Safe, but no improvement | [56] |
| No change | [55] | Safe, but no improvement | [57] | |||
| Antioxidant | ||||||
| Ederavone | n.s. | [32] | Significant increase | [32] | Phase II, safe, may delay disease progression | [61] |
| Phase III, completed (NCT00424463b) | ||||||
| AEOL 10150 | +27 | [31] | 53% increase | [31] | Phase I, well-tolerated | [62] |
| +15 | [33] | 38% increase | [33] | |||
| Protein folding | ||||||
| Arimoclomol | +28 | [30] | 74% increase | [30] | Phase IIa, well-tolerated and safe | [63] |
| +10 | [29] | 96% increase | [29] | Phase II/III, recruiting participants (NCT00706147b) | ||
| Autophagy | ||||||
| Lithium | +38 | [65] | No change | [65] | Delayed disease progression | [65] |
| −8 | [67] | No change in α-SMN | [67] | Not well tolerated, no improvement of disease | [68] | |
| Safe, but no improvement (NCT00818389b) | [69] | |||||
| Rapamycin | −19 | [70] | 34% decrease | [70] | ||
| Apoptosis | ||||||
| TCH346 | n.s. | [72] | No change | [72] | Phase II/III, no improvement | [73] |
| PBA | +27 | [74] | 122% increase | [74] | Completed (NCT00107770b) | |
| +17 | [33] | Significant increase | [33] | |||
| +16 | [91] | |||||
| zVAD-fmk | +27 | [75] | No change | [75] | ||
| Inflammation | ||||||
| Celecoxib | +28 | [23] | Significant increase | [23] | Safe, but no improvement | [76] |
| Thalidomide | +21 | [24] | Significant increase | [24] | Phase II, no improvement (NCT00140452b) | [78] |
| Lenalidomide | +24 | [24] | Significant increase | [24] | ||
| +15 | [77] | 33% increase | [77] | |||
| Minocycline | +21 | [26] | Increase | [26] | Phase III, harmful effect on ALS patients (NCT00047723b) | [80] |
| +9 | [79] | No change | [79] | |||
| +21d | [25] | No changec,d | [25] | |||
| Glutamate | ||||||
| Riluzole | +13 | [27] | No change | [55] | Slowed disease progression and improved survival | [5] |
| +13 | [92] | Well tolerated and increased survival | [6] | |||
| +9 | [91] | |||||
| Ceftriaxone | +10 | [28] | Significant increase | [28] | Phase III completed (NCT00349622b) | |
| ZK 187638 | +17 | [81] | 27% increase | [81] | ||
n.s., not significant.
ClinicalTrials.gov identifier.
Study used slowly-progressing hSOD1G37R mice with later end-stage.
Study reported number of SMN axons instead of cell bodies.
Neurotrophins
Neurons depend on neurotrophins and growth factors for survival. Of these, insulin-like growth factor (IGF)-1, vascular endothelial growth factor (VEGF) and brain-derived neurotropic factor (BDNF) are the most studied for their potential role as ‘drugs’ for MNDs.
IGF-1 is one of the most extensively studied growth factors for MNDs. It was delivered to SMN via adeno-associated virus (AAV) retrograde transduction from the leg muscle at the time of disease symptom onset in hSOD1G93A mice, providing a 78% increase in SMN numbers at 110 days, but failed to show difference at end-stage [40]. IGF-1 treatment delayed disease onset by 31 days, extended the life span by 22 days, attenuated astrogliosis [41] and improved motor function, as measured by grip strength and rotarod performance [40]. Remarkably, IGF-1 prevents glutamate-induced motor neuron death in rat spinal cord cultures [42], and enhances axon outgrowth of CSMN selectively in vitro [43]. These encouraging findings led to the initiation of an initial double-blind, placebo-controlled, randomized study of 266 patients. The recombinant human IGF-1 (rhIGF-1) slowed the progression of functional impairment and the decline in health-related quality of life in patients with ALS [44]. However, another randomized double-blind study of 183 patients failed to show any significant difference between the treatment groups, as assessed by the Appel ALS rating scale [45]. Finally, the Phase III randomized, double-blind, placebo-controlled study of 330 patients, after 2 years of treatment with subcutaneous rhIGF-1 injection, failed to yield any difference between treatment groups in their manual muscle testing score or tracheostomy-free survival and rate of change in the revised ALS functional rating scale (ALSFRS-R) [46]. These conflicting findings in different clinical trials initiated debates on the delivery method of IGF-1, its half-life and stability in patients and the selection criteria for patient inclusion in studies.
An initial double-blind, placebo-controlled Phase I/II study of recombinant human BDNF delivered via daily subcutaneous injections was encouraging [47]. BDNF was not only well tolerated, but also seemed to improve forced vital capacity, walking speed and even survival. However, a randomized, double-blind, placebo controlled parallel-group phase III study with 1135 patients failed to show any improvement in either the primary end points (change from baseline forced vital capacity and survival at 9 months) or the secondary end points (incidence of selected respiratory events, ALSFRS, Sickness Impact Profile physical dimension score, syllable repetition, walking speed and Ashworth spasticity score) [48]. More recently, intrathecal infusion of recombinant methionyl human BDNF (r-metHuBDNF) has been shown to be safe and well tolerated in a phase I/II trial [49]; however, it lacked clinical efficacy in a multicenter phase III trial [50]. Transplantation of human neural progenitor cells engineered to express BDNF using adenoviral vectors to hSOD1G93A mice had no significant effect on motor performance or life span of the mice [51]. Intramuscular injections of naked DNA encoding BDNF into hSOD1G93A mice also failed to improve motor function, life span, weight loss or the number of motor neurons in the lumbar spinal cord [52].
VEGF delivery using lentiviral vectors in hSOD1G93A mice led to as high as a 125% increase in the numbers of lumbar SMN at 115 days [53]. VEGF treatment also significantly increased the life span and slowed down motor performance defects, as detected by gait analysis. Clinical studies investigating the safety and tolerability of VEGF in patients with ALS (NCT00800501) and a VEGF activator SB-509 (NCT00748501) are now complete, but lack detailed information.
The potential problem with the administration of growth factors is the lack of control over the stability and half-life of proteins in human CSF and/or plasma. Therefore, finding the perfect route of administration that enables constant and sustainable levels of growth factors is key for success.
Mitochondrial dysfunction
Mitochondrial function is one of the major pathways that is affected in diseased motor neurons. Creatine treatment was reported to offer neuroprotection for SMN in hSOD1G93A mice, with a significant increase in life span and enhanced rotarod performance [54], but failed to improve SMN numbers in another study [55]. A double-blind, placebo-controlled, sequential clinical trial testing the effects of creatine monohydrate on survival and disease progression in 175 patients with ALS reported its safety, but did not improve survival or functional measurements of isometric arm strength, forced vital capacity, functional status and quality of life [56]. Another randomized double-blind, placebo-controlled trial of 104 patients with ALS also showed that creatine monohydrate was well tolerated, but did not improve maximum voluntary isometric contraction, grip strength, ALSFRS-R or motor unit number estimates [57].
Dexpramipexole, a mitochondrion-targeted antioxidant, increased the life span of hSOD1G93A mice by 7 days without any obvious motor function benefits [58]. A two-part, multicenter, double-blind Phase II study of dexpramipexole in patients with ALS showed that it was safe and well tolerated [59]. Post hoc analysis of data suggested a reduction in ALSFRS-R decline [60], although the difference was not significant. A Phase III study (NCT01622088) has recently been terminated because it failed to meet its primary efficacy end point.
Antioxidants
Treatment with ederavone (MCI-186), a free-radical scavenger, provided significant neuroprotection in hSOD1G93A mice, without significantly extending their life span [32]. Ederavone also significantly enhanced motor function as assessed by rotarod and grip strength tests, and led to a reduction in abnormal SOD1 deposits in the spinal cord. A phase II clinical study, using 20 subjects, reported safety and delayed progression of disease, as measured by ALSFRS-R [61]. A Phase III study looking at the safety and efficacy of ederavone in patients with ALS has also been completed (NCT00424463).
AEOL 10150 (manganese porphyrin) treatment of hSOD1G93A mice starting at disease onset led to an 53% increase in SMN number [31], whereas starting the treatment after the appearance of motor dysfunction led to a 38% increase [33]. Additionally, life span increased with slow disease progression and enhanced motor function, as evidenced by the lack of complete hind-limb paralysis and significantly improved rotarod performance [31,33]. A phase I clinical trial of AEOL 10150 also proved subject tolerance [62].
Protein folding
Treatment of hSOD1G93A mice from an early age with arimoclomol, a co-inducer of heat shock proteins, led to a 74% increase in SMN numbers, significantly increased life span by 22%, and delayed disease onset by 23 days [30]. Late-stage treatment of hSOD1G93A mice starting at 90 days also increased SMN numbers by 96%, improved muscle force, but did not increase life span [29]. A phase IIa clinical study of arimoclomol in 44 patients showed that doses up to 300 mg/day were safe and well tolerated [63]. A phase II/III randomized, placebo-controlled trial of arimoclomol in SOD1-positive familial ALS is currently recruiting patients (NCT00706147) [64].
Autophagy
A pilot study of lithium carbonate, an inducer of autophagy, showed an impressive level of neuroprotection in hSOD1G93A mice [65]. Additionally, lithium increased life span by 36%, delayed disease onset and improved rotarod performance, grip strength and stride length significantly. Clinical testing of lithium on a small group of patients (total of 44) slowed disease progression [65,66]. However, another study using hSOD1G93A mice on both C57 and 129 Sv backgrounds found no neuroprotective effect of lithium on SMN numbers. Moreover, both life span and disease onset were decreased, and motor performance worsened [67]. Similarly, a multicenter, single-blind, randomized, dose-finding trial of 171 patients found that lithium was not well tolerated, and did not offer any improvement in survival or quality of life [68]. Another double-blind, placebo-controlled clinical trial of 84 patients (NCT00818389) did not raise concerns for safety, but was stopped after 6 months because criterion for futility was met with no difference in mean decline in the ALSFRS score [69].
Rapamycin, a more specific inducer of autophagy compared with lithium, has also been tested in animal models. In hSOD1G93A mice, rapamycin treatment led to a 34% decrease in SMN number, with a significantly decreased life span, earlier disease onset and shorter disease duration [70]. Interestingly, rapamycin treatment of a TDP43 fronto temporal lobe degeneration mouse model significantly enhanced rotarod performance without an effect on SMN numbers [71].
Apoptosis
GAPDH ligand TCH346 (CGP 3466B), an inhibitor of apoptosis, did not offer neuroprotection in hSOD1G93A mice, and had no effect on disease onset, progression or life-span [72]. Similar to preclinical findings, a randomized, double-blind, placebo-controlled clinical trial of 591 patients with ALS yielded no significant differences in the mean rate of decline of ALSFRS-R, survival, pulmonary function or manual muscle testing [73].
Treatment with the histone deacetylase inhibitor sodium phenylbutyrate (PBA) led to a 122% increase in SMN number in hSOD1G93A mice, extended life span significantly and improved motor performance, as measured by rotarod and stride-length analysis [33,74]. However, treatment with zVAD-fmk, a broad caspase inhibitor, had no significant effect on lumbar SMN numbers, but led to significantly higher numbers of cervical SMN, delayed disease onset and prolonged survival [75].
Inflammation
Upon identification of non-neuronal cells as contributors to disease initiation and progression, another area of research has developed that targets non-neuronal cells to improve motor neuron health. Celecoxib, a cyclooxygenase 2 (COX-2) inhibitor, reduces production of prostaglandin E2. When administered to hSOD1G93A mice starting at postnatal day P28, celexoib provided significant neuroprotection of both small and large SMN at 15 weeks and end-stage, increased life span of the mice by 28 days, significantly delayed decline of motor activity and prevented weight loss [23]. A double-blind, placebo-controlled clinical trial using 300 subjects revealed that celexoib was safe and well tolerated, although it did not have a beneficial effect on the rate of change in upper extremity motor function as measured by maximum voluntary isometric contraction strength, vital capacity, motor unit number estimates, ALSFRS-R or survival [76].
Thalidomide and lenalidomide, inhibitors of tumor necrosis factor alpha (TNF-α) and other cytokines, led to significant increases in the life span and number of SMN in hSOD1G93A mice, attenuated weight loss and enhanced motor performance on rotarod testing [24,77]. A phase II study using thalidomide for treatment of ALS (NCT00140452) revealed no improvement in the ALSFRS or pulmonary function, but did highlight several adverse effects [78].
Minocycline, which inhibits microglial activation, provided SMN neuroprotection in both hSOD1G93A [26] and hSOD1G37R [25] mice, but offered no neuroprotection when administered after disease onset [79]. Minocycline also delayed decline in rotarod performance, disease onset and mortality, but had no effect on astrogliosis [25,26] or even increased microgliosis [79]. A multicenter, randomized, placebo-controlled phase III trial using 427 patients revealed that minocycline had harmful effects on patients with faster deterioration in ALSFRS-R, forced vital capacity and manual muscle testing scores, as well as greater mortality during the 9-month treatment phase, with no improvement in quality-of-life scores [80].
Glutamate excitotoxicity
Glutamate is removed by astrocytes from the synapses, and failure of proper glutamate removal results in glutamate excitotoxicity, a major pathway that leads to motor neuron degeneration. β-Lactam antibiotics, such as ceftriaxone, increased expression of the glutamate transporter GLT1 (EAAT2), offering neuroprotection from glutamate excitotoxicity. In hSOD1G93A mice, ceftriaxone treatment led to a significant increase in SMN numbers and increased the life span of the mice by 10 days, as well as resulting in a reduction of hypercellular gliosis and a significantly delayed loss of muscle strength and body weight [28]. A phase III clinical trial of ceftriaxone in patients with ALS has been completed (NCT00349622). AMPA antagonist ZK 187638 treatment of hSOD1G93A mice led to a 27% increase in the number of lumbar SMN, extended life span, and significant delayed weight loss and motor function impairment [81].
Riluzole has been the only compound to receive FDA approval and has been extensively reviewed elsewhere [82,83]. Interestingly, it was found to be beneficial for patients with ALS in clinical trials [5,6] before investigation in animal models. Riluzole treatment delayed disease onset by 12 days, preserved motor function and extended life span by 2 weeks in hSOD1G93A mice, and proved beneficial in other mouse models of MND, such as progressive motor neuronopathy, spinal muscular atrophy and the wobbler mouse [27,84–86]. Analysis of SMN in hSOD1G93A mice interestingly revealed no change in the numbers of medium- or large-sized lumbar SMN between treatment groups [55].
The review of the past and current studies using compounds that selectively act on a distinct pathway, has revealed differences between clinical trials, generated more questions on the selection of compounds, their route of administration and the validity of mouse models, and has also generated concern about the translation of preclinical results to human condition. However, the inclusion of SMN survival data has been promising towards the goal of improving preclinical screening.
Can preclinical screening be improved?
Mice and humans differ greatly, and there is mounting evidence to suggest that the use of mice as a model system, especially for immune diseases [87], is not appropriate. Therefore, increased life span in mice might not translate into increased survival in patients, and readouts based on mouse survival might be misleading. In contrast to their differences at the organismal and systems level, their cells, especially neurons, have much in common. A motor neuron in a mouse is similar to a motor neuron in a patient. Their development, maturation, mechanisms of their cellular function and intricate details of their neuronal properties are almost identical [88]. Such striking similarities at a cellular level prompt us to propose that focusing on the motor neuron requirements for survival could reveal information that can translate toward improvement of the human condition.
Focusing on the health of SMN in preclinical screening
The importance of a paradigm shift from mouse to motor neurons has recently been recognized. The ALS/MND consensus meeting agreed upon the need for neuroprotection analysis on SMN as a preclinical test and recommended including the ‘assessment of the motor neurons present throughout the lumbar region of the spinal cord’ [4]. Therefore, more recent preclinical studies include the analysis of SMN survival and health using histological staining of the spinal cord sections isolated from mouse models at different stages of disease. The presence and number of SMN cell bodies were quantitatively assayed to determine the efficacy of the tested compounds on SMN survival. Inclusion of motor neuron survival at a cellular level is an improvement toward making the right decision before clinical trials.
Inclusion of upper motor neuron health in preclinical screening
In ALS, it is the motor neuron circuitry that degenerates, which involves the motor neurons, located in the motor cortex, brainstem and the spinal cord [2,36,37]. Humans are especially dependent on their cortex for the initiation and modulation of voluntary movement. CSMN, which have a unique executive function with their ability to collect, integrate, translate and transfer the input of the cerebral cortex to spinal cord targets, act as the ‘spokesperson’ of the cerebral cortex for motor neuron circuitry. This unique ability makes them an important target for the health and stability of motor function in patients. For example, CSMN death and defects in corticospinal tract lead to paralysis in patients, but only a minor inability to control precise aspects of voluntary movement in mice. These behavioral dissimilarities result from differences between descending paths and other neuronal connectivity patterns [89]. Therefore, using mice as a model system for MND comes with challenges.
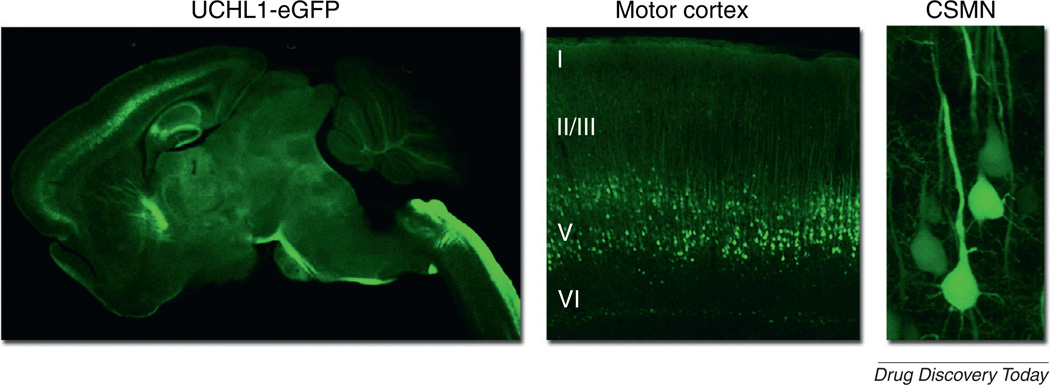
However, because of the importance of CSMN for motor function in patients with ALS, we need to focus on the survival requirement of this neuron population. A novel reporter mouse, the UCHL1-eGFP mouse, was recently generated in which CSMN were selectively labeled by eGFP expression under the control of ubiquitin C-terminal hydrolase-L1 (UCHL1) promoter (Fig. 1) [37]. Even though eGFP expression is specific to CSMN in the motor cortex at all ages, in the spinal cord, eGFP expression becomes restricted to small-diameter α- and γ SMN, which are resistant to degeneration in ALS [90]. Crossbreeding UCHL1-eGFP and hSOD1G93A mice generated hSOD1G93A-UeGFP mice, which mimic the previously reported reduction in the number of CSMN with disease progression in the hSOD1G93A mice [36].
FIGURE 1.
eGFP expression under the control of ubiquitin C-terminal hydrolase-L1 (UCHL1) promoter selectively labels corticospinal motor neurons (CSMN) in UCHL1-eGFP mice [37]. CSMN are large pyramidal neurons that are located in layer V of the motor cortex. They are subcerebral projection neurons and the corticospinal tract passes through the pons and enters the spinal cord at the pyramidal decussation, traveling at the dorsal funiculus of the spinal cord until it reaches spinal targets.
The hSOD1G93A-UeGFP reporter line now enables detailed cellular investigation of CSMN health throughout the disease, as well as direct investigation of neuroprotection provided by compound treatment in animal models of ALS/MND [37]. Compounds can be administrated either orally via food or drink, or can be delivered by injection. Owing to selective and long-term eGFP expression in CSMN, neuroprotection offered by administered compounds can be studied with respect to disease (Fig. 2), and SMN survival can be assessed using conventional methods, such as Nissl or choline acetyl transferase (ChAT) staining. Such studies at a cellular level can also be coupled with behavioral analysis that test motor function, such as the rotarod, grip strength and gait analysis (Fig. 3).
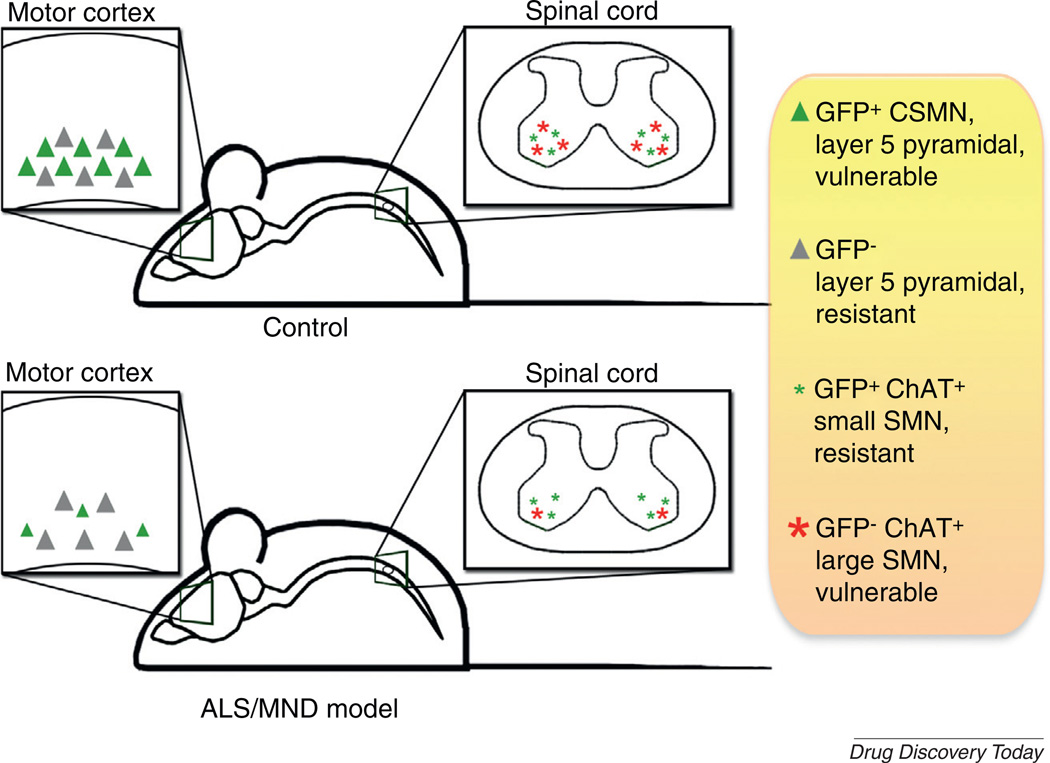
FIGURE 2.
The ubiquitin C-terminal hydrolase-L1 (UCHL1)-eGFP reporter mouse model provides an invaluable tool to study motor neuron degeneration in animal models of amyotrophic lateral sclerosis and related motor neuron disorders (ALS/MND). Upon proper mating strategies, ALS/MND mouse models with eGFP+ corticospinal motor neurons (CSMN) can be generated. In these transgenic disease models, vulnerable CSMN are genetically labeled in the motor cortex (green triangle) and can be visually identified among others that are not genetically labeled (gray triangle). In the spinal cord of UCHL1-eGFP mice, the eGFP expression becomes restricted to small-diameter spinal motor neurons (SMN) at P30 and genetically labels SMN that are mostly resistant to degeneration (green stars). Abbreviation: ChAT, Choline acetyl transferase.
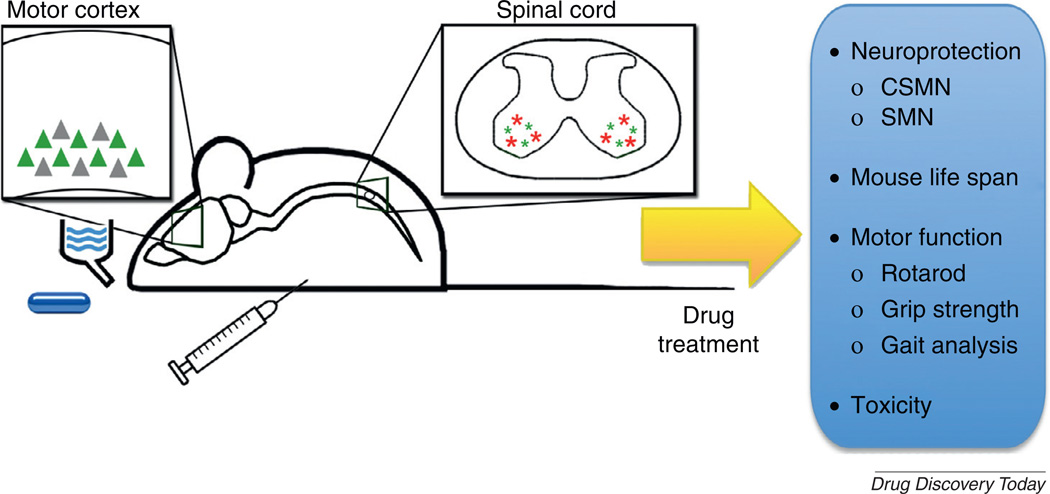
FIGURE 3.
The ubiquitin C-terminal hydrolase-L1 (UCHL1)-eGFP reporter mouse enables investigation of neuroprotection provided to vulnerable and diseased corticospinal motor neurons (CSMN) by compound and/or drug treatment. The compounds can be administered orally by drinking water or food. Alternatively, they can be directly delivered by intraperitoneal injection. CSMN survival can be monitored and quantitatively analyzed at different stages of the disease, together with other measures for improved motor function (e.g. Grip test or gait analysis). Spinal motor neuron (SMN) neuroprotection can still be studied using conventional methods such as Nissl histology or ChAT immunofluorescence, with the added benefit of visualizing and assessing the numbers of degeneration-resistant SMN separately, distinguished by eGFP expression.
Concluding remarks and prospects
Although it seems as though all clinical trials have failed in ALS and that the future is bleak, there is light at the end of the tunnel. Numerous developments and improvements in critical thinking, analysis and understanding suggest that the near future will witness unprecedented achievements in the field of ALS. First, it has been realized that it is not only the mice, but also the motor neurons in the mouse models that are important for translating biological findings toward clinical trials. This has been a major paradigm shift in thinking, but it came with many challenges. How can one analyze neurons within the complex and heterogeneous structure of the cortex and spinal cord?
SMN were easy to identify based on their size and location in the spinal cord, and most recent preclinical assays have studied their health and stability. However, CSMN were never considered or included in any of the preclinical screens. We hope that this lack of information on CSMN pathology will change in the future. In an effort to reveal the true potential of compounds, their efficacy on the survival of both CSMN and SMN need to be studied. The tools are now available to investigate the biology of CSMN with respect to disease and compound administration. These novel developments will help build improved preclinical screening platforms for compound selection, increasing the probability of success in clinical trials.
Developments in different areas are emerging together towards one goal: building effective drug-treatment strategies for ALS/MND. Improvement of preclinical tests by focusing on the survival needs of both cortical and spinal motor neurons, determining early detection as well as biomarkers for ALS, and improving the design and outcome measures for clinical trials will cumulatively contribute to the identification of compounds that will improve patient health and quality of life. Although the past has not revealed success, it has paved the way for multiple success stories beginning to emerge.
Acknowledgements
This work is supported by grants from the Les Turner ALS Foundation and the Wenske Foundation (PHO). BG was supported by an NIH postdoctoral MAD (Mechanisms of Aging and Dementia) training grant (NIH 5T32AG020506-09).
References
- 1.Rosen DR, et al. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature. 1993;362:59–62. doi: 10.1038/362059a0. [DOI] [PubMed] [Google Scholar]
- 2.Gurney ME, et al. Motor neuron degeneration in mice that express a human Cu, Zn superoxide dismutase mutation. Science. 1994;264:1772–1775. doi: 10.1126/science.8209258. [DOI] [PubMed] [Google Scholar]
- 3.Gurney ME. The use of transgenic mouse models of amyotrophic lateral sclerosis in preclinical drug studies. J. Neurol. Sci. 1997;152(Suppl. 1):S67–S73. doi: 10.1016/s0022-510x(97)00247-5. [DOI] [PubMed] [Google Scholar]
- 4.Ludolph AC, et al. Guidelines for preclinical animal research in ALS/MND: a consensus meeting. Amyotroph. Lateral Scler. 2010;11:38–45. doi: 10.3109/17482960903545334. [DOI] [PubMed] [Google Scholar]
- 5.Bensimon G, et al. A controlled trial of riluzole in amyotrophic lateral sclerosis. ALS/Riluzole Study Group. N. Engl. J. Med. 1994;330:585–591. doi: 10.1056/NEJM199403033300901. [DOI] [PubMed] [Google Scholar]
- 6.Lacomblez L, et al. Dose-ranging study of riluzole in amyotrophic lateral sclerosis. Amyotrophic Lateral Sclerosis/Riluzole Study Group II. Lancet. 1996;347:1425–1431. doi: 10.1016/s0140-6736(96)91680-3. [DOI] [PubMed] [Google Scholar]
- 7.Barber SC, Shaw PJ. Oxidative stress in ALS: key role in motor neuron injury and therapeutic target. Free Radic. Biol. Med. 2010;48:629–641. doi: 10.1016/j.freeradbiomed.2009.11.018. [DOI] [PubMed] [Google Scholar]
- 8.Benatar M. Lost in translation: treatment trials in the SOD1 mouse and in human ALS. Neurobiol. Dis. 2007;26:1–13. doi: 10.1016/j.nbd.2006.12.015. [DOI] [PubMed] [Google Scholar]
- 9.Bosco DA, Landers JE. Genetic determinants of amyotrophic lateral sclerosis as therapeutic targets. CNS Neurol. Disord. Drug Targets. 2010;9:779–790. doi: 10.2174/187152710793237494. [DOI] [PubMed] [Google Scholar]
- 10.Habib AA, Mitsumoto H. Emerging drugs for amyotrophic lateral sclerosis. Expert Opin. Emerg. Drugs. 2011;16:537–558. doi: 10.1517/14728214.2011.604312. [DOI] [PubMed] [Google Scholar]
- 11.Kuzma-Kozakiewicz M, Kwiecinski H. New therapeutic targets for amyotrophic lateral sclerosis. Expert Opin. Ther. Targets. 2011;15:127–143. doi: 10.1517/14728222.2011.542152. [DOI] [PubMed] [Google Scholar]
- 12.Pawlyk AC, et al. Current nervous system related drug targets for the treatment of amyotrophic lateral sclerosis. Curr. Pharm. Des. 2010;16:2053–2073. doi: 10.2174/138161210791293024. [DOI] [PubMed] [Google Scholar]
- 13.Zinman L, Cudkowicz M. Emerging targets and treatments in amyotrophic lateral sclerosis. Lancet Neurol. 2011;10:481–490. doi: 10.1016/S1474-4422(11)70024-2. [DOI] [PubMed] [Google Scholar]
- 14.Son EY, et al. Conversion of mouse and human fibroblasts into functional spinal motor neurons. Cell Stem Cell. 2011;9:205–218. doi: 10.1016/j.stem.2011.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Benmohamed R, et al. Identification of compounds protective against G93A SOD1 toxicity for the treatment of amyotrophic lateral sclerosis. Amyotroph. Lat. Scler. 2011;12:87–96. doi: 10.3109/17482968.2010.522586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Egawa N, et al. Drug screening for ALS using patient-specific induced pluripotent stem cells. Sci. Transl. Med. 2012;4:145ra104. doi: 10.1126/scitranslmed.3004052. [DOI] [PubMed] [Google Scholar]
- 17.Sandoe J, Eggan K. Opportunities and challenges of pluripotent stem cell neurodegenerative disease models. Nat. Neurosci. 2013;16:780–789. doi: 10.1038/nn.3425. [DOI] [PubMed] [Google Scholar]
- 18.Berry JD, Cudkowicz ME. New considerations in the design of clinical trials for amyotrophic lateral sclerosis. Clin. Investig. 2011;1:1375–1389. doi: 10.4155/cli.11.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cudkowicz ME, et al. Toward more efficient clinical trials for amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. 2010;11:259–265. doi: 10.3109/17482960903358865. [DOI] [PubMed] [Google Scholar]
- 20.Boillee S, et al. ALS: a disease of motor neurons and their nonneuronal neighbors. Neuron. 2006;52:39–59. doi: 10.1016/j.neuron.2006.09.018. [DOI] [PubMed] [Google Scholar]
- 21.Robberecht W, Philips T. The changing scene of amyotrophic lateral sclerosis. Nat. Rev. Neurosci. 2013;14:248–264. doi: 10.1038/nrn3430. [DOI] [PubMed] [Google Scholar]
- 22.Cozzolino M, et al. Amyotrophic lateral sclerosis: new insights into underlying molecular mechanisms and opportunities for therapeutic intervention. Antioxid. Redox Signal. 2012;17:1277–1330. doi: 10.1089/ars.2011.4328. [DOI] [PubMed] [Google Scholar]
- 23.Drachman DB, et al. Cyclooxygenase 2 inhibition protects motor neurons and prolongs survival in a transgenic mouse model of ALS. Ann. Neurol. 2002;52:771–778. doi: 10.1002/ana.10374. [DOI] [PubMed] [Google Scholar]
- 24.Kiaei M, et al. Thalidomide and lenalidomide extend survival in a transgenic mouse model of amyotrophic lateral sclerosis. J. Neurosci. 2006;26:2467–2473. doi: 10.1523/JNEUROSCI.5253-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kriz J, et al. Minocycline slows disease progression in a mouse model of amyotrophic lateral sclerosis. Neurobiol. Dis. 2002;10:268–278. doi: 10.1006/nbdi.2002.0487. [DOI] [PubMed] [Google Scholar]
- 26.Van Den Bosch L, et al. Minocycline delays disease onset and mortality in a transgenic model of ALS. Neuroreport. 2002;13:1067–1070. doi: 10.1097/00001756-200206120-00018. [DOI] [PubMed] [Google Scholar]
- 27.Gurney ME, et al. Riluzole preserves motor function in a transgenic model of familial amyotrophic lateral sclerosis. Neurology. 1998;50:62–66. doi: 10.1212/wnl.50.1.62. [DOI] [PubMed] [Google Scholar]
- 28.Rothstein JD, et al. Beta-lactam antibiotics offer neuroprotection by increasing glutamate transporter expression. Nature. 2005;433:73–77. doi: 10.1038/nature03180. [DOI] [PubMed] [Google Scholar]
- 29.Kalmar B, et al. Late stage treatment with arimoclomol delays disease progression and prevents protein aggregation in the SOD1 mouse model of ALS. J. Neurochem. 2008;107:339–350. doi: 10.1111/j.1471-4159.2008.05595.x. [DOI] [PubMed] [Google Scholar]
- 30.Kieran D, et al. Treatment with arimoclomol, a coinducer of heat shock proteins, delays disease progression in ALS mice. Nat. Med. 2004;10:402–405. doi: 10.1038/nm1021. [DOI] [PubMed] [Google Scholar]
- 31.Crow JP, et al. Manganese porphyrin given at symptom onset markedly extends survival of ALS mice. Ann. Neurol. 2005;58:258–265. doi: 10.1002/ana.20552. [DOI] [PubMed] [Google Scholar]
- 32.Ito H, et al. Treatment with edaravone, initiated at symptom onset, slows motor decline and decreases SOD1 deposition in ALS mice. Exp. Neurol. 2008;213:448–455. doi: 10.1016/j.expneurol.2008.07.017. [DOI] [PubMed] [Google Scholar]
- 33.Petri S, et al. Additive neuroprotective effects of a histone deacetylase inhibitor and a catalytic antioxidant in a transgenic mouse model of amyotrophic lateral sclerosis. Neurobiol. Dis. 2006;22:40–49. doi: 10.1016/j.nbd.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 34.Otto M, et al. Roadmap and standard operating procedures for biobanking and discovery of neurochemical markers in ALS. Amyotroph. Lateral Scler. 2012;13:1–10. doi: 10.3109/17482968.2011.627589. [DOI] [PubMed] [Google Scholar]
- 35.Bowser R, et al. Biomarkers in amyotrophic lateral sclerosis: opportunities and limitations. Nat. Rev. Neurol. 2011;7:631–638. doi: 10.1038/nrneurol.2011.151. [DOI] [PubMed] [Google Scholar]
- 36.Ozdinler PH, et al. Corticospinal motor neurons and related subcerebral projection neurons undergo early and specific neurodegeneration in hSOD1G93A transgenic ALS mice. J. Neurosci. 2011;31:4166–4177. doi: 10.1523/JNEUROSCI.4184-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yasvoina MV, et al. eGFP expression under UCHL1 promoter genetically labels corticospinal motor neurons and a subpopulation of degeneration-resistant spinal motor neurons in an ALS mouse model. J. Neurosci. 2013;33:7890–7904. doi: 10.1523/JNEUROSCI.2787-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Levine JB, et al. Astrocytes interact intimately with degenerating motor neurons in mouse amyotrophic lateral sclerosis (ALS) Glia. 1999;28:215–224. [PubMed] [Google Scholar]
- 39.Gordon PH, Meininger V. How can we improve clinical trials in amyotrophic lateral sclerosis? Nat. Rev. Neurol. 2011;7:650–654. doi: 10.1038/nrneurol.2011.147. [DOI] [PubMed] [Google Scholar]
- 40.Kaspar BK, et al. Retrograde viral delivery of IGF-1 prolongs survival in a mouse ALS model. Science. 2003;301:839–842. doi: 10.1126/science.1086137. [DOI] [PubMed] [Google Scholar]
- 41.Kaspar BK, et al. Synergy of insulin-like growth factor-1 and exercise in amyotrophic lateral sclerosis. Ann. Neurol. 2005;57:649–655. doi: 10.1002/ana.20451. [DOI] [PubMed] [Google Scholar]
- 42.Vincent AM, et al. IGF-I prevents glutamate-induced motor neuron programmed cell death. Neurobiol. Dis. 2004;16:407–416. doi: 10.1016/j.nbd.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 43.Ozdinler PH, Macklis JD. IGF-I specifically enhances axon outgrowth of corticospinal motor neurons. Nat. Neurosci. 2006;9:1371–1381. doi: 10.1038/nn1789. [DOI] [PubMed] [Google Scholar]
- 44.Lai EC, et al. Effect of recombinant human insulin-like growth factor-I on progression of ALS. A placebo-controlled study. The North America ALS/IGF-I Study Group. Neurology. 1997;49:1621–1630. doi: 10.1212/wnl.49.6.1621. [DOI] [PubMed] [Google Scholar]
- 45.Borasio GD, et al. A placebo-controlled trial of insulin-like growth factor-I in amyotrophic lateral sclerosis. European ALS/IGF-I Study Group. Neurology. 1998;51:583–586. doi: 10.1212/wnl.51.2.583. [DOI] [PubMed] [Google Scholar]
- 46.Sorenson EJ, et al. Subcutaneous IGF-1 is not beneficial in 2-year ALS trial. Neurology. 2008;71:1770–1775. doi: 10.1212/01.wnl.0000335970.78664.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bradley WG, et al. A Phase I/II study of recombinant human brain-derived neurotrophic factor in patients with amytrophic lateral sclerosis. Ann. Neurol. 1995;38:971. [Google Scholar]
- 48.the BDNF Study Group. A controlled trial of recombinant methionyl human BDNF in ALS (Phase III) Neurology. 1999;52:1427–1433. doi: 10.1212/wnl.52.7.1427. [DOI] [PubMed] [Google Scholar]
- 49.Ochs G, et al. A phase I/II trial of recombinant methionyl human brain derived neurotrophic factor administered by intrathecal infusion to patients with amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. Other Motor Neuron Disord. 2000;1:201–206. doi: 10.1080/14660820050515197. [DOI] [PubMed] [Google Scholar]
- 50.Kalra S, et al. A prospective, randomized, placebo-controlled evaluation of corticoneuronal response to intrathecal BDNF therapy in ALS using magnetic resonance spectroscopy: feasibility and results. Amyotroph. Lateral Scler. Other Motor Neuron Disord. 2003;4:22–26. doi: 10.1080/14660820310006689. [DOI] [PubMed] [Google Scholar]
- 51.Park S, et al. Growth factor-expressing human neural progenitor cell grafts protect motor neurons but do not ameliorate motor performance and survival in ALS mice. Exp. Mol. Med. 2009;41:487–500. doi: 10.3858/emm.2009.41.7.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Calvo AC, et al. Lack of a synergistic effect of a non-viral ALS gene therapy based on BDNF and a TTC fusion molecule. Orphanet. J. Rare Dis. 2011;6:10. doi: 10.1186/1750-1172-6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Azzouz M, et al. VEGF delivery with retrogradely transported lentivector prolongs survival in a mouse ALS model. Nature. 2004;429:413–417. doi: 10.1038/nature02544. [DOI] [PubMed] [Google Scholar]
- 54.Klivenyi P, et al. Neuroprotective effects of creatine in a transgenic animal model of amyotrophic lateral sclerosis. Nat. Med. 1999;5:347–350. doi: 10.1038/6568. [DOI] [PubMed] [Google Scholar]
- 55.Snow RJ, et al. Creatine supplementation and riluzole treatment provide similar beneficial effects in copper, zinc superoxide dismutase (G93A) transgenic mice. Neuroscience. 2003;119:661–667. doi: 10.1016/s0306-4522(03)00212-4. [DOI] [PubMed] [Google Scholar]
- 56.Groeneveld GJ, et al. A randomized sequential trial of creatine in amyotrophic lateral sclerosis. Ann. Neurol. 2003;53:437–445. doi: 10.1002/ana.10554. [DOI] [PubMed] [Google Scholar]
- 57.Shefner JM, et al. A clinical trial of creatine in ALS. Neurology. 2004;63:1656–1661. doi: 10.1212/01.wnl.0000142992.81995.f0. [DOI] [PubMed] [Google Scholar]
- 58.Danzeisen R, et al. Targeted antioxidative and neuroprotective properties of the dopamine agonist pramipexole and its nondopaminergic enantiomer SND919CL2x [(+)2-amino-4,5,6,7-tetrahydro-6-Lpropylamino-benzathiazole dihydrochloride] J. Pharmacol. Exp. Ther. 2006;316:189–199. doi: 10.1124/jpet.105.092312. [DOI] [PubMed] [Google Scholar]
- 59.Cudkowicz M, et al. The effects of dexpramipexole (KNS-760704) in individuals with amyotrophic lateral sclerosis. Nat. Med. 2011;17:1652–1656. doi: 10.1038/nm.2579. [DOI] [PubMed] [Google Scholar]
- 60.Rudnicki SA, et al. Dexpramipexole effects on functional decline and survival in subjects with amyotrophic lateral sclerosis in a Phase II study: subgroup analysis of demographic and clinical characteristics. Amyotroph. Lateral Scler. Frontotemporal Degener. 2013;14:44–51. doi: 10.3109/17482968.2012.723723. [DOI] [PubMed] [Google Scholar]
- 61.Yoshino H, Kimura A. Investigation of the therapeutic effects of edaravone, a free radical scavenger, on amyotrophic lateral sclerosis (Phase II study) Amyotroph. Lateral Scler. 2006;7:241–245. doi: 10.1080/17482960600881870. [DOI] [PubMed] [Google Scholar]
- 62.Orrell RW. AEOL-10150 (Aeolus) Curr. Opin. Investig. Drugs. 2006;7:70–80. [PubMed] [Google Scholar]
- 63.Cudkowicz ME, et al. Arimoclomol at dosages up to 300 mg/day is well tolerated and safe in amyotrophic lateral sclerosis. Muscle Nerve. 2008;38:837–844. doi: 10.1002/mus.21059. [DOI] [PubMed] [Google Scholar]
- 64.Lanka V, et al. Arimoclomol: a potential therapy under development for ALS. Expert Opin. Investig. Drugs. 2009;18:1907–1918. doi: 10.1517/13543780903357486. [DOI] [PubMed] [Google Scholar]
- 65.Fornai F, et al. Lithium delays progression of amyotrophic lateral sclerosis. Proc. Natl. Acad. Sci. U. S. A. 2008;105:2052–2057. doi: 10.1073/pnas.0708022105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fornai F, et al. Lithium in ALS: from the bench to the bedside. Amyotroph. Lateral Scler. 2008;9:123–124. doi: 10.1080/17482960802028197. [DOI] [PubMed] [Google Scholar]
- 67.Pizzasegola C, et al. Treatment with lithium carbonate does not improve disease progression in two different strains of SOD1 mutant mice. Amyotroph. Lateral Scler. 2009;10:221–228. doi: 10.1080/17482960902803440. [DOI] [PubMed] [Google Scholar]
- 68.Chio A, et al. Lithium carbonate in amyotrophic lateral sclerosis: lack of efficacy in a dose-finding trial. Neurology. 2010;75:619–625. doi: 10.1212/WNL.0b013e3181ed9e7c. [DOI] [PubMed] [Google Scholar]
- 69.Aggarwal SP, et al. Safety and efficacy of lithium in combination with riluzole for treatment of amyotrophic lateral sclerosis: a randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2010;9:481–488. doi: 10.1016/S1474-4422(10)70068-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang X, et al. Rapamycin treatment augments motor neuron degeneration in SOD1(G93A) mouse model of amyotrophic lateral sclerosis. Autophagy. 2011;7:412–425. doi: 10.4161/auto.7.4.14541. [DOI] [PubMed] [Google Scholar]
- 71.Wang IF, et al. Autophagy activators rescue and alleviate pathogenesis of a mouse model with proteinopathies of the TAR DNA-binding protein 43. Proc. Natl. Acad. Sci. U. S. A. 2012;109:15024–15029. doi: 10.1073/pnas.1206362109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Groeneveld GJ, et al. CGP 3466B has no effect on disease course of (G93A) mSOD1 transgenic mice. Amyotroph. Lateral Scler. Other Motor Neuron Disord. 2004;5:220–225. doi: 10.1080/14660820410019530. [DOI] [PubMed] [Google Scholar]
- 73.Miller R, et al. Phase II/III randomized trial of TCH346 in patients with ALS. Neurology. 2007;69:776–784. doi: 10.1212/01.wnl.0000269676.07319.09. [DOI] [PubMed] [Google Scholar]
- 74.Ryu H, et al. Sodium phenylbutyrate prolongs survival and regulates expression of anti-apoptotic genes in transgenic amyotrophic lateral sclerosis mice. J. Neurochem. 2005;93:1087–1098. doi: 10.1111/j.1471-4159.2005.03077.x. [DOI] [PubMed] [Google Scholar]
- 75.Li M, et al. Functional role of caspase-1 and caspase-3 in an ALS transgenic mouse model. Science. 2000;288:335–339. doi: 10.1126/science.288.5464.335. [DOI] [PubMed] [Google Scholar]
- 76.Cudkowicz ME, et al. Trial of celecoxib in amyotrophic lateral sclerosis. Ann. Neurol. 2006;60:22–31. doi: 10.1002/ana.20903. [DOI] [PubMed] [Google Scholar]
- 77.Neymotin A, et al. Lenalidomide (Revlimid) administration at symptom onset is neuroprotective in a mouse model of amyotrophic lateral sclerosis. Exp. Neurol. 2009;220:191–197. doi: 10.1016/j.expneurol.2009.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Stommel EW, et al. Efficacy of thalidomide for the treatment of amyotrophic lateral sclerosis: a phase II open label clinical trial. Amyotroph. Lateral Scler. 2009;10:393–404. doi: 10.3109/17482960802709416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Keller AF, et al. Treatment with minocycline after disease onset alters astrocyte reactivity and increases microgliosis in SOD1 mutant mice. Exp. Neurol. 2011;228:69–79. doi: 10.1016/j.expneurol.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 80.Gordon PH, et al. Efficacy of minocycline in patients with amyotrophic lateral sclerosis: a phase III randomised trial. Lancet Neurol. 2007;6:1045–1053. doi: 10.1016/S1474-4422(07)70270-3. [DOI] [PubMed] [Google Scholar]
- 81.Tortarolo M, et al. Glutamate AMPA receptors change in motor neurons of SOD1G93A transgenic mice and their inhibition by a noncompetitive antagonist ameliorates the progression of amytrophic lateral sclerosis-like disease. J. Neurosci. Res. 2006;83:134–146. doi: 10.1002/jnr.20715. [DOI] [PubMed] [Google Scholar]
- 82.Bellingham MC. A review of the neural mechanisms of action and clinical efficiency of riluzole in treating amyotrophic lateral sclerosis: what have we learned in the last decade? CNS Neurosci. Ther. 2011;17:4–31. doi: 10.1111/j.1755-5949.2009.00116.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Scott S, et al. Design, power, and interpretation of studies in the standard murine model of ALS. Amyotroph. Lateral Scler. 2008;9:4–15. doi: 10.1080/17482960701856300. [DOI] [PubMed] [Google Scholar]
- 84.Haddad H, et al. Riluzole attenuates spinal muscular atrophy disease progression in a mouse model. Muscle Nerve. 2003;28:432–437. doi: 10.1002/mus.10455. [DOI] [PubMed] [Google Scholar]
- 85.Ishiyama T, et al. Riluzole slows the progression of neuromuscular dysfunction in the wobbler mouse motor neuron disease. Brain Res. 2004;1019:226–236. doi: 10.1016/j.brainres.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 86.Kennel P, et al. Riluzole prolongs survival and delays muscle strength deterioration in mice with progressive motor neuronopathy (pmn) J. Neurol. Sci. 2000;180:55–61. doi: 10.1016/s0022-510x(00)00423-8. [DOI] [PubMed] [Google Scholar]
- 87.Seok J, et al. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc. Natl. Acad. Sci. U. S. A. 2013;110:3507–3512. doi: 10.1073/pnas.1222878110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Molyneaux BJ, et al. Molecular development of corticospinal motor neuron circuitry. Novartis Found. Symp. 2007;288:3–15. doi: 10.1016/j.expneurol.2006.02.074. discussion 15–20, 96–18. [DOI] [PubMed] [Google Scholar]
- 89.Lemon RN. Descending pathways in motor control. Annu. Rev. Neurosci. 2008;31:195–218. doi: 10.1146/annurev.neuro.31.060407.125547. [DOI] [PubMed] [Google Scholar]
- 90.Kawamura Y, et al. Morphometric comparison of the vulnerability of peripheral motor and sensory neurons in amyotrophic lateral sclerosis. J. Neuropathol. Exp. Neurol. 1981;40:667–675. doi: 10.1097/00005072-198111000-00008. [DOI] [PubMed] [Google Scholar]
- 91.Del Signore SJ, et al. Combined riluzole and sodium phenylbutyrate therapy in transgenic amyotrophic lateral sclerosis mice. Amyotroph. Lateral Scler. 2009;10:85–94. doi: 10.1080/17482960802226148. [DOI] [PubMed] [Google Scholar]
- 92.Waibel S, et al. Rasagiline alone and in combination with riluzole prolongs survival in an ALS mouse model. J. Neurol. 2004;251:1080–1084. doi: 10.1007/s00415-004-0481-5. [DOI] [PubMed] [Google Scholar]