Significance
The cellular mechanisms underlying the transition from compensatory to deleterious cardiac hypertrophy remain to be elucidated. In the current work, we demonstrate decreased ErbB2 interacting protein (Erbin) expression in human failing hearts and, using mice models, we show that Erbin expression is important for compensated hypertrophy. Our findings reveal a previously unidentified link between Erbin and ERK signaling in the heart and implicate Erbin in the development of cardiac hypertrophy and heart failure. Erbin is particularly interesting due to its well-established interaction with the ErBb2/Her2 receptor. Clinical studies have observed cardiotoxicity associated with Herceptin therapy, reflecting the crucial role of Her2-mediated signaling in cardiac homeostasis. In this study, we describe a cardioprotective role for Erbin, which suggests it is a potential target for cardiac gene therapy.
Abstract
ErbB2 interacting protein (Erbin) is a widely expressed protein and participates in inhibition of several intracellular signaling pathways. Its mRNA has been found to be present in relatively high levels in the heart. However, its physiological role in the heart has not been explored. In the present work, we elucidated the role of Erbin in cardiac hypertrophy. Cardiac hypertrophy was induced in mice either by isoproterenol administration or by aortic constriction. The level of Erbin was significantly decreased in both models. Erbin−/− mice rapidly develop decompensated cardiac hypertrophy, and following severe pressure overload all Erbin−/− mice died from heart failure. Down-regulation of Erbin expression was also observed in biopsies derived from human failing hearts. It is known that Erbin inhibits Ras-mediated activation of the extracellular signal-regulated kinase (ERK) by binding to Soc-2 suppressor of clear homolog (Shoc2). Our data clearly show that ERK phosphorylation is enhanced in the heart tissues of Erbin−/− mice. Furthermore, we clearly demonstrate here that Erbin associates with Shoc2 in both whole hearts and in cardiomyocytes, and that in the absence of Erbin, Raf is phosphorylated and binds Shoc2, resulting in ERK phosphorylation. In conclusion, Erbin is an inhibitor of pathological cardiac hypertrophy, and this inhibition is mediated, at least in part, by modulating ERK signaling.
Cardiovascular disease remains the number one cause of mortality in the Western world, with heart failure representing the fastest-growing subclass over the past decade (1, 2). In myocardial hypertrophy caused by exercise, pregnancy, or developmental signals, cardiac structure and function are normal. However, in response to insults such as sustained excessive workloads, pathological cardiac hypertrophy is characterized by changes in contractility, loss of myocytes with fibrosis, systolic or diastolic dysfunction, and fetal gene reactivation. Pathological cardiac hypertrophy predisposes individuals to heart failure, arrhythmia, and sudden death (1, 2). The tyrosine kinase receptor avian erythroblastic leukemia viral oncogene homolog 2 (ErbB2), also known in humans as Her2, is an important regulator in cardiac hypertrophy development and heart failure (3). ErbB2-deficient conditional mutants develop dilated cardiomyopathy (3). ErbB2 signaling in cardiomyocytes is therefore essential for the prevention of dilated cardiomyopathy. However, the mechanism by which ErbB2 exerts its cardiac effects in general, and on cardiac hypertrophy in particular, is poorly defined.
ErbB2 interacting protein (Erbin), a member of the leucine-rich repeat and PDZ domain proteins (4), was originally reported as a binding partner of Her2/neu (ErbB2) (4). Erbin is a widely expressed protein, and high levels of Erbin are present in the heart (5). Recently, Erbin has been demonstrated to form a complex with Her2 and β2-adrenergic receptor in cardiomyocytes. Also, Erbin has been shown to protect cardiomyocytes from apoptosis induced by chronic catecholamine stimulation (6). However, its physiological cardiac function and its role in cardiac hypertrophy are unknown. Erbin−/− mice have defective remyelination following axonal injury (7), but no further pathology has been described to date.
The regulatory roles assigned to Erbin have recently expanded. Erbin participates in the inhibition of a variety of cellular signaling pathways, including Ras-mediated activation of extracellular signal-regulated kinase (ERK) (8, 9), nuclear factor κB (10), and transforming growth factor β signaling pathways (11). Interestingly, these three signaling pathways have been shown to play a significant role in cardiac hypertrophy (12–15).
In the current study, we demonstrate that Erbin expression is decreased in cardiac hypertrophy induced in mice by either isoproterenol administration or aortic constriction. Erbin expression was significantly decreased in end-stage heart failure in humans. Exaggerated pathological cardiac hypertrophy was observed in Erbin−/− mice compared with wild-type (WT) mice. The levels of B-type natriuretic peptide (BNP) and atrial natriuretic peptide (ANP) were significantly increased in isoproterenol-administered Erbin−/− mice, indicating fetal gene reactivation typical of pathological cardiac hypertrophy. Moreover, aortic constriction resulted in rapidly decompensated response in Erbin−/− mice as well as heart failure and death.
Thus, our results indicate that Erbin serves as a major inhibitor of pathological cardiac hypertrophy.
Results
Erbin Is Down-Regulated in Cardiac Hypertrophy.
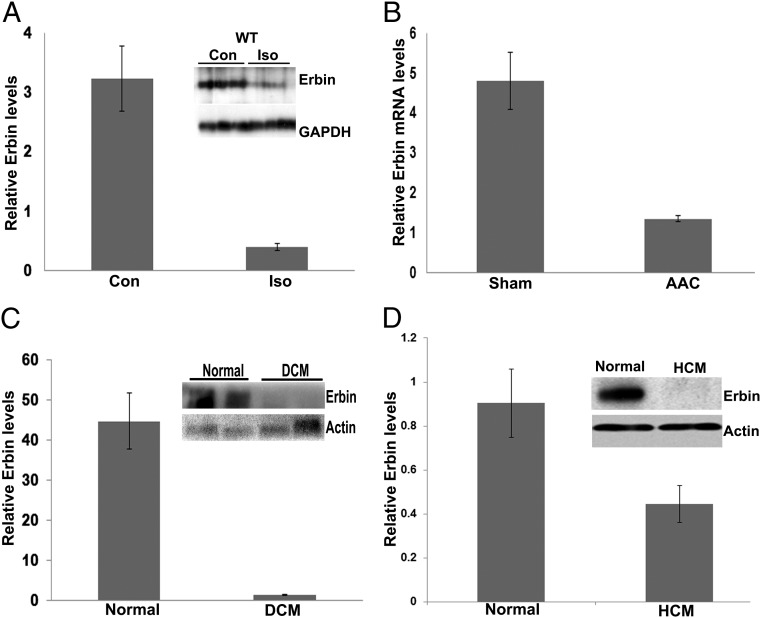
A recent study revealed Erbin as an important regulator in cardiomyocyte protection in cultured cardiomyocytes (6). Therefore, cardiac expression of Erbin in mice was assessed. Immunofluorescence revealed that it is expressed in all cardiac cell types. The highest level was detected in the endothelium (Fig. S1A). Erbin was down-regulated during cardiac hypertrophy induced by either isoproterenol administration (Fig. 1A and Fig. S1 A and C) or abdominal aortic constriction (AAC) (Fig. 1B). This down-regulation of Erbin expression was observed only in cardiomyocytes (Fig. S1A).
Fig. 1.
Erbin expression is decreased during cardiac hypertrophy and heart failure. (A) Western blot analysis of Erbin protein levels in hearts from WT mice at baseline (Con) and after treatment with isoproterenol for 7 d (Iso). Densitometry results are expressed as Erbin:GAPDH ratio. Results represent mean ± SEM (n = 3). (B) Real-time PCR analysis of Erbin expression in hearts from WT mice after mild AAC or sham surgery. Results are expressed as Erbin:β-actin ratio, and represent mean ± SEM (n = 5). (C) Western blot analysis of Erbin in human heart biopsies from normal patients (Normal) and end-stage heart failure patients (DCM; dilated cardiomyopathy). Densitometry results are expressed as Erbin:β-actin ratio. Results represent mean ± SEM (n = 6). (D) Western blot analysis of Erbin in human heart biopsies from normal patients (Normal) and end-stage heart failure patients (HCM; hypertrophic cardiomyopathy). Densitometry results are expressed as Erbin:β-actin ratio. Results represent mean ± SEM (n = 5).
Erbin was also decreased in human heart biopsies of end-stage heart failure patients, both those with dilated cardiomyopathy (Fig. 1C) and those with hypertrophic cardiomyopathy (Fig. 1D). These results suggest that the down-regulation of Erbin is associated with cardiac hypertrophy and heart failure in both mice and humans.
Characterization of Hearts of Erbin−/− Mice at Baseline.
To evaluate the cardiac role played by Erbin, heart weight (HW) and body weight (BW) of Erbin−/− mice and their normal siblings were determined in 8-wk-old mice. In WT mice, the HW:BW ratio was 6.0 ± 0.1 mg/g whereas in Erbin−/− mice the ratio was 5.9 ± 0.3 mg/g, and so there was no significant difference in the HW:BW ratio in Erbin−/− mice compared with WT mice (n = 5) (Fig. 2A and Table S1).
Fig. 2.
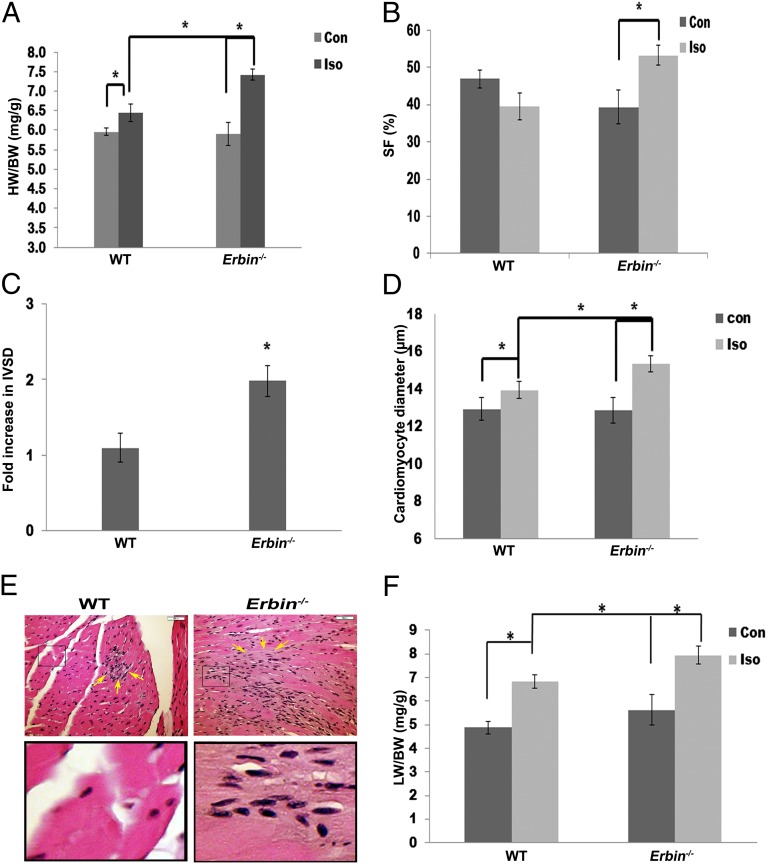
Cardiac hypertrophy is exacerbated in Erbin−/− mice. (A) HW:BW ratios in Erbin−/− mice and their normal littermates (WT) at baseline (Con) and after treatment with isoproterenol (Iso) for 7 d. Results are expressed as mean ± SEM (n = 5–7). *P < 0.05. (B and C) Echocardiography of WT and Erbin−/− mice. Shortening fraction (B) and fold increase in diastolic interventricular septal thickness (IVSD) (*P = 0.01) (C) were measured at baseline (Con) and after treatment with isoproterenol (Iso) for 7 d. Results represent mean ± SEM (n = 5–7). *P < 0.05. (D) Cardiomyocyte diameter of WT and Erbin−/− mice at baseline and after isoproterenol administration for 7 d. Results represent mean ± SEM (n = 5–7). *P < 0.05. (E) H&E staining of hearts derived from WT and Erbin−/− mice after treatment with isoproterenol for 7 d. One representative slide is shown out of six. (Scale bars, 50 μM.) (Lower) Magnification (6×) of the boxed areas (Upper). Several of the fibroblasts are marked by arrows. (F) LW:BW ratios in Erbin−/− mice and their normal littermates (WT) at baseline (Con) and after treatment with isoproterenol (Iso) for 7 d. Results represent mean ± SEM (n = 7–10). *P < 0.05.
Echocardiography of Erbin−/− and WT mice was performed. At baseline, shortening fraction (SF) of Erbin−/− mice was 39.3 ± 4.6%, whereas in WT mice it was 46.9 ± 2.4% (P = 0.01, n = 17–18; Table S2).
Thus, except for a slightly lower SF in Erbin−/− mice compared with WT mice, no significant baseline differences in phenotype were observed in Erbin−/− mice compared with WT littermates.
Cardiac Hypertrophy Is Exacerbated in Erbin−/− Mice.
Isoproterenol administration was used as a cardiac hypertrophy model. Isoproterenol induced an 8% increase in HW:BW ratio in WT mice (Fig. 2A; P = 0.04, n = 5), whereas in Erbin−/− mice a 25% increase was observed (P = 0.005, n = 7). The HW:BW ratio in isoproterenol-treated Erbin−/− mice was 15% higher than in WT mice (P = 0.003, n = 5–7). There were no significant differences in BW (Table S1).
The cardiac function of Erbin−/− mice was further examined by echocardiography (Fig. 2 B and C). Erbin−/− mice treated with isoproterenol had a 1.9 ± 0.2-fold increase in the thickness of the diastolic interventricular septum (P = 0.01, n = 5–7), whereas no change was observed in WT mice (Fig. 2C). Isoproterenol increased SF in Erbin−/− mice compared with the lower baseline (P = 0.004, n = 7), whereas no significant change was observed in WT mice (Fig. 2B and Table S3). No significant change in left ventricular end diastolic diameter (LVEDD) was observed in any of the mice groups (Table S3).
Cardiomyocyte diameter was measured by microscopic analysis of fixed LV sections (Fig. 2D). Isoproterenol induced an 8% increase in myocyte diameter in WT mice (P = 0.05, n = 5), whereas a larger increase of 19% was observed in Erbin−/− mice (P = 0.003, n = 5). Gross fibrosis was observed in the hearts of isoproterenol-treated Erbin−/− mice, whereas only focal, early signs of fibrosis were noted in isoproterenol-treated WT mice (Fig. 2E). Moreover, the ratio of lung weight (LW) to BW in isoproterenol-treated Erbin−/− mice was 14% higher than in WT mice (P = 0.002, n = 7–10) (Fig. 2F).
Thus, Erbin−/− mice have an exaggerated and rapidly decompensated response to β-adrenergic stimulation compared with WT littermates.
Cardiac Hypertrophy Biomarkers in WT and Erbin−/− Mice Treated with Isoproterenol.
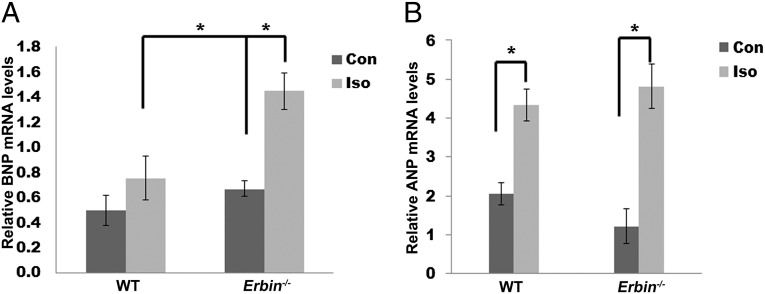
Biochemical markers of cardiac hypertrophy were measured with and without isoproterenol treatment. mRNA levels of ANP and BNP, both known pathological cardiac hypertrophy markers, were measured in heart extracts of Erbin−/− and WT mice. No significant differences in either BNP and ANP were observed between the two untreated groups (Fig. 3 A and B). The WT mice responded to isoproterenol treatment by a 1.5- (P = 0.05, n = 5–10) and a 2.2-fold increase (P = 0.002, n = 5–10) in BNP and ANP mRNA levels, respectively (Fig. 3 A and B). The induction of BNP and ANP expression in Erbin−/− mice was greater, with increases of 2.2-fold for BNP (P = 0.002, n = 5–10) and 3-fold for ANP (P = 0.000003, n = 5–10).
Fig. 3.
ANP and BNP expression in hearts of WT and Erbin−/− mice . Real-time PCR quantification of BNP (A) and ANP (B) mRNA in hearts from Erbin−/− and WT mice after treatment with isoproterenol (Iso) or saline (Con) (n = 5–10). *P < 0.05.
The significantly greater induction of hypertrophic markers in Erbin−/− mice, compared with WT littermates, further supports the morphometry and physiology results, indicating that cardiac hypertrophy is exacerbated in isoproterenol-treated Erbin−/− mice.
Erbin Inhibits Cardiac Hypertrophy Induced by Chronic Pressure Overload.
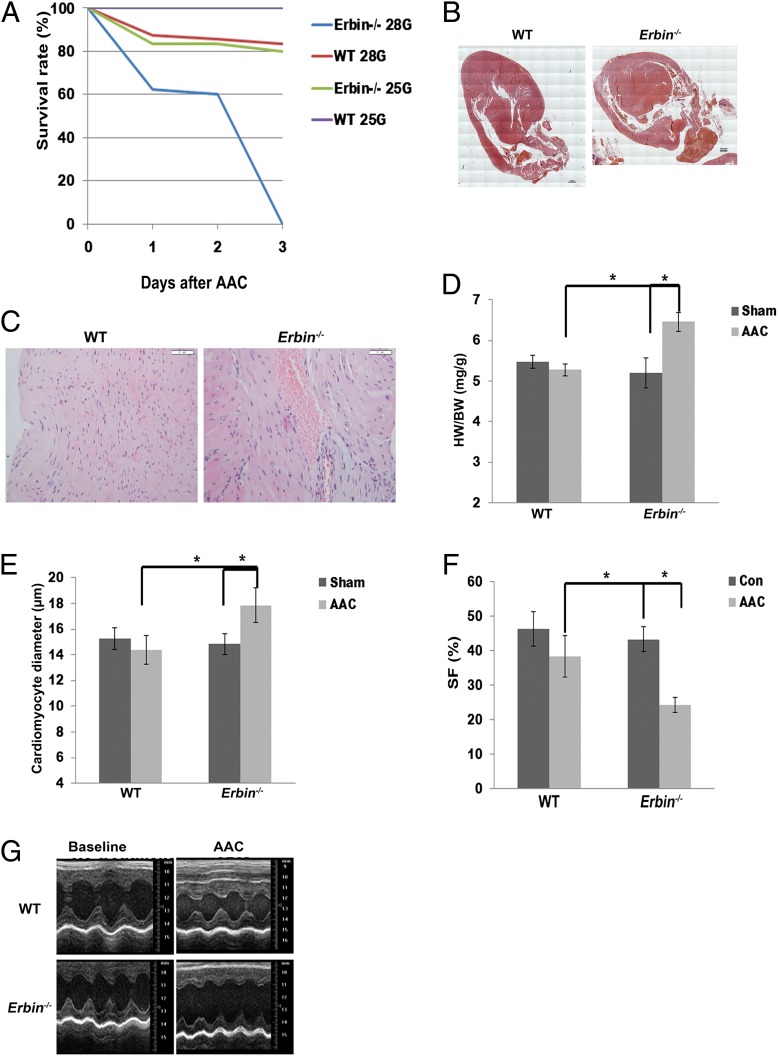
The effect of Erbin on cardiac hypertrophic response was also assessed in a model of pressure overload, aortic constriction. Erbin−/− and WT mice were subjected to severe [28-gauge (G) needle] AAC. This causes pathological cardiac hypertrophy due to increased afterload (16). Three days after surgery, all Erbin−/− mice died, whereas mortality rate in WT mice was only 37% (n = 8) (Fig. 4A). No mortality occurred following sham operation in both groups. Postmortem histological examination revealed that Erbin−/− mice had globular hearts (Fig. 4B) with hemorrhages in the myocardium (Fig. 4C), whereas WT mice exhibited cardiac hypertrophic response with intact apical structure (Fig. 4B). Due to the high mortality rate seen with severe AAC, a milder AAC (25G) was performed. The survival rate up to 10 d after surgery was lower in Erbin−/− mice (Fig. 4A), with 20% mortality, than in WT mice, all of whom survived (n = 6). Mild AAC induced a 19% increase in the HW:BW ratio of Erbin−/− mice (P = 0.004, n = 6), whereas no increase was observed in the HW:BW ratio of WT mice (Fig. 4D and Table S4). Cardiomyocyte diameter was increased in Erbin−/− mice following mild AAC (P = 0.0009, n = 6), whereas no change was observed in WT littermates (Fig. 4E). LV function was significantly decreased in Erbin−/− mice (Fig. 4 F and G and Table S5). AAC decreased SF in Erbin−/−mice by 40% (P = 0.000003, n = 6), whereas no significant change was observed in WT mice. It should be noted that no other pathology was observed in the kidneys, liver, or lungs of either Erbin−/− or WT mice, suggesting that Erbin’s effect is cardiac-specific.
Fig. 4.
Erbin inhibits cardiac hypertrophy induced by chronic pressure overload. (A) Erbin−/− and WT mice underwent either mild (25G) or severe (28G) AAC. Survival was observed for 10 d. The survival rate for the first 3 d is depicted, because no further mortality was observed in the mild AAC model up to 10 d (n = 8). No mortality was observed in sham operated mice. (B and C) H&E staining of hearts derived from WT and Erbin−/− mice 3 d after severe AAC operation. Globular hearts and very thick interventricular septi were observed in Erbin−/− mice (B). (Scale bars, 100 μM.) Hemorrhage in the myocardium could be seen in Erbin−/− mice (C). (Scale bars, 50 μM.) One representative slide is shown out of four. (D) HW:BW ratios in Erbin−/− mice and WT littermates after mild AAC or sham surgery. The results shown represent mean ± SEM (n = 6). *P < 0.05. (E) Cardiomyocyte diameter of WT and Erbin−/− mice 10 d after mild AAC or sham surgery. Results represent mean ± SEM (n = 6). *P < 0.05. (F) Echocardiography of WT and Erbin−/− mice. SF was measured at baseline (Con) and 10 d after mild AAC. Results represent mean ± SEM (n = 6). *P < 0.05. (G) Representative echocardiographic M-mode images of WT and Erbin−/− mice at baseline and 10 d after mild AAC.
Thus, these results support our findings from the β-adrenergic activation model that cardiac hypertrophy is exacerbated in Erbin−/− mice, followed by rapid decompensation and end-stage heart failure.
ERK Phosphorylation Is Augmented in Erbin−/− Mice.
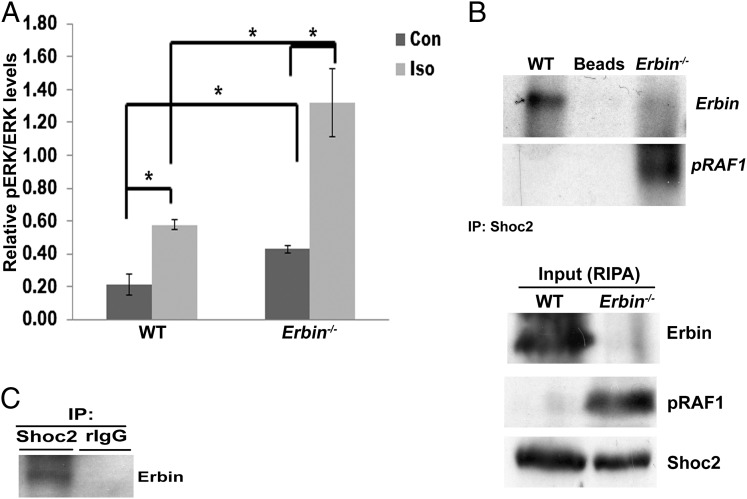
ERK and Akt signaling pathways are among the most thoroughly characterized pathways mediating cardiac hypertrophy (17, 18). Therefore, ERK and Akt phosphorylation in Erbin−/− mice was studied. There was no difference in the level of Akt phosphorylation between Erbin−/− and WT mice at baseline, and isoproterenol treatment caused a similar increase in Akt phosphorylation in the two groups (Fig. S2A). Surprisingly, phosphorylated ERK (pERK) was twofold higher in the hearts of untreated Erbin−/− mice compared with WT mice (P = 0.04, n = 8) (Fig. 5A). Isoproterenol induced a twofold increase in pERK in WT mice (P = 0.007, n = 6), whereas a threefold increase was observed in Erbin−/− mice (P = 0.02, n = 6). Thus, following isoproterenol administration, the pERK level was threefold higher in Erbin−/− mice compared with WT mice (P = 0.003).
Fig. 5.
ERK phosphorylation is enhanced in Erbin−/− hearts. (A) Western blot analysis of pERK and ERK in hearts from WT and Erbin−/− mice at baseline and after isoproterenol. Densitometry results are expressed as pERK:ERK ratio, and represent mean ± SEM (n = 3). *P < 0.05. (B) Coimmunoprecipitation of Erbin and pRaf1 in WT and Erbin−/− heart lysates using anti-Shoc2 antibody for immunoprecipitation (IP). Anti-Erbin and anti-pRaf1 antibodies were used for immunoblotting. The specificity of the Erbin band was verified by using Erbin−/− mice. One representative experiment out of three is shown. Corresponding lysates [Input, radioimmunoprecipitation assay buffer (RIPA)] were analyzed by Western blot analysis of Erbin, pRaf1, and Shoc2. (C) Coimmunoprecipitation of Erbin in neonatal rat cardiomyocytes using anti-Shoc2 antibody for immunoprecipitation and anti-Erbin antibody for immunoblotting. Nonspecific rabbit IgG-coated (rIgG) beads were used as a negative control. One representative experiment out of three is shown.
Erbin silencing by specific siRNA increased pERK levels fourfold compared with nontargeting (NT) siRNA (P = 0.007; Fig. S2B). Isoproterenol induced a twofold increase in pERK both in cells treated with NT siRNA and in those treated with Erbin siRNA, maintaining the fourfold higher pERK level (Fig. S2B). These results indicate that Erbin is involved in the inhibition of ERK phosphorylation.
Because β-adrenergic activation results in ERK phosphorylation, the function of the β-adrenergic system was evaluated by measuring peak heart rate (HR) and cAMP levels. This was carried out immediately following either isoproterenol or saline administration. Peak HR was similarly elevated in WT and Erbin−/− mice [from 441 ± 13 to 584 ± 20 beats per min (bpm) and from 431 ± 10 to 580 ± 4 bpm, respectively; n = 6]. Moreover, isoproterenol induced a similar 1.5-fold increase in cAMP levels in both groups of mice (from 0.17 ± 0.04 to 0.26 ± 0.02 pmol/mL and from 0.25 ± 0.03 to 0.4 ± 0.02 pmol/mL, respectively; n = 6). These results indicate that the β-adrenergic system is not impaired in Erbin−/− mice and that the increase in ERK phosphorylation in this system is not G protein-dependent.
Previous studies have shown that Erbin binds Soc-2 suppressor of clear homolog (Shoc2; Sur-8) (8), which acts as a scaffold protein necessary for the stability of the Ras–Raf complex. Thus, Erbin serves as an inhibitor of ERK phosphorylation by disrupting the Ras–Raf complex (19). Erbin association with Shoc2 was measured by coimmunoprecipitation in vivo. Erbin immunoprecipitated with Shoc2 in WT hearts but not in Erbin−/− hearts (Fig. 5B). Erbin and Shoc2 were also found to be associated in neonatal rat cardiomyocytes (Fig. 5C) and in untreated HEK293T cells (Fig. S2C). Because Shoc2 plays a critical role in Raf activation (20), its association with phosphorylated Raf (pRaf) was measured in Erbin−/− mice and their WT littermates (Fig. 5B). pRaf was immunoprecipitated with Shoc2 in Erbin−/− hearts but not in WT hearts. These data suggest that Erbin inhibits ERK phosphorylation by associating with Shoc2 and by the dissociation of pRaf from Shoc2.
Discussion
Erbin is a widely expressed protein, transcribed at high levels in the heart and participating in the inhibition of a variety of cellular signaling pathways (5), but its cardiac function has not been explored. Interestingly, Erbin−/− mice under basal conditions appear to have a nearly normal heart, suggesting that Erbin is not required for the baseline regulation of cardiac growth and function. In Erbin−/− mice exposed to isoproterenol, however, we noticed an exacerbated response, including an abnormal increase in HW:BW, increases in both ANP and BNP levels that are typical of pathological cardiac hypertrophy (21), asymmetric interventricular septal thickening, and cardiac fibrosis, indicating pathologic cardiac hypertrophy.
Erbin−/− mice undergoing AAC presented with a severely exaggerated hypertrophic response, end-stage heart failure, and death. Therefore, it seems that the lack of Erbin promotes an exaggerated hypertrophic response, which is rapidly decompensated. In addition, Erbin is down-regulated in end-stage heart failure patients. Although we cannot prove that this down-regulation is a cause of heart failure rather than its result, it is a reasonable working hypothesis in the context of our results in mice. Thus, this study has demonstrated for the first time, to our knowledge, a role for Erbin in cardiac hypertrophy and has suggested that it is important in the transition to decompensated hypertrophy.
The ErbB2 (Her2/neu) signaling pathway is essential for cardiac development and remodeling, and for the maintenance of cardiac viability, structure, and function (22–26). Conditional knockout mice, induced by a deletion of cardiomyocyte ErbB2, revealed adverse cardiac remodeling with features of dilated cardiomyopathy (3). The cellular mechanisms through which ErbB2 exerts its cardiac effects in general, and on cardiac hypertrophy in particular, are still unknown. However, Erbin, as an ErbB2 binding partner (4), could be considered a likely candidate.
Surprisingly, even at baseline, pERK levels were elevated in Erbin−/− mice. It was previously shown that Erbin can bind Shoc2, disrupting the Ras–Raf complex and decreasing ERK phosphorylation (8). In addition, Shoc2 knockout mice exhibit severe heart defects (27).
In the current study, we show that Erbin binds Shoc2 in the intact heart, suggesting that Erbin can indeed regulate pERK through Shoc2 during cardiac hypertrophy. ERK activation is one of a limited number of intracellular signaling pathways resulting in adaptive cardiac hypertrophy (28), but it can also mediate pathological hypertrophy. It seems that intermittent or controlled activation is essential, and that the temporal component might be critical (28); Erbin−/− mice appear to be lacking this controlled activation.
Our current work suggests a previously unknown role for Erbin in cardiac hypertrophy and that its absence results in disruption of the adaptive hypertrophic response, leading to decompensation and heart failure.
Materials and Methods
Animals and Procedures.
A detailed description of the materials and methods used in this study is found in SI Materials and Methods. These methods include mice, human biopsies, quantitative RT-PCR, histological analysis, antibodies, RNA isolation, and immunoblotting. Experiments with animals were performed in compliance with the Israeli Prevention of Cruelty to Animals Law and were approved by the Hebrew University Animal Care and Use Committee. Human left ventricular tissue was collected with a protocol approved by the Papworth (Cambridge) Hospital Tissue Bank Review Board and the Cambridgeshire Research Ethics Committee (United Kingdom). Written consent was obtained from every individual according to the Papworth Tissue Bank protocol.
Statistics.
Exact Mann–Whitney test was performed for echocardiography measurements before and after isoproterenol treatment. BNP and ANP mRNA levels, pERK, LW:BW ratio, HW:BW ratio, SF after AAC, cardiomyocyte diameter, and treatment or lack of treatment with isoproterenol or AAC were evaluated by exact Kruskal–Wallis test. Null hypothesis was rejected at the P < 0.05 level for all tests. Data are reported as mean ± SEM.
Supplementary Material
Acknowledgments
We thank Prof. Norman Grover from the Hebrew University for statistical analysis. This work was supported by the United States Binational Science Foundation (E.R.); Israeli Academy of Science (E.R.); Cooperation Program in Cancer Research of the Deutsches Krebsforschungszentrum; Israel’s Ministry of Science and Technology (E.R.); German-Israel Foundation for Scientific Research and Development (E.R.); National Research Foundation of Singapore (Hebrew University of Jerusalem–Campus for Research Excellence and Technological Enterprise) (E.R.); Israel Science Foundation (S.T.); La Ligue Nationale contre le Cancer (Label Ligue 2013; J.-P.B.); and in part by the Dean’s Fund of the Faculty of Medicine of the Hebrew University (S.T.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1320350111/-/DCSupplemental.
References
- 1.Heineke J, Molkentin JD. Regulation of cardiac hypertrophy by intracellular signalling pathways. Nat Rev Mol Cell Biol. 2006;7(8):589–600. doi: 10.1038/nrm1983. [DOI] [PubMed] [Google Scholar]
- 2.Hill JA, Olson EN. Cardiac plasticity. N Engl J Med. 2008;358(13):1370–1380. doi: 10.1056/NEJMra072139. [DOI] [PubMed] [Google Scholar]
- 3.Crone SA, et al. ErbB2 is essential in the prevention of dilated cardiomyopathy. Nat Med. 2002;8(5):459–465. doi: 10.1038/nm0502-459. [DOI] [PubMed] [Google Scholar]
- 4.Borg JP, et al. ERBIN: A basolateral PDZ protein that interacts with the mammalian ERBB2/HER2 receptor. Nat Cell Biol. 2000;2(7):407–414. doi: 10.1038/35017038. [DOI] [PubMed] [Google Scholar]
- 5.Huang YZ, Wang Q, Xiong WC, Mei L. Erbin is a protein concentrated at postsynaptic membranes that interacts with PSD-95. J Biol Chem. 2001;276(22):19318–19326. doi: 10.1074/jbc.M100494200. [DOI] [PubMed] [Google Scholar]
- 6.Shi M, et al. β2-AR-induced Her2 transactivation mediated by Erbin confers protection from apoptosis in cardiomyocytes. Int J Cardiol. 2013;167(4):1570–1577. doi: 10.1016/j.ijcard.2012.04.093. [DOI] [PubMed] [Google Scholar]
- 7.Tao Y, et al. Erbin regulates NRG1 signaling and myelination. Proc Natl Acad Sci USA. 2009;106(23):9477–9482. doi: 10.1073/pnas.0901844106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dai P, Xiong WC, Mei L. Erbin inhibits RAF activation by disrupting the sur-8-Ras-Raf complex. J Biol Chem. 2006;281(2):927–933. doi: 10.1074/jbc.M507360200. [DOI] [PubMed] [Google Scholar]
- 9.Huang YZ, Zang M, Xiong WC, Luo Z, Mei L. Erbin suppresses the MAP kinase pathway. J Biol Chem. 2003;278(2):1108–1114. doi: 10.1074/jbc.M205413200. [DOI] [PubMed] [Google Scholar]
- 10.McDonald C, et al. A role for Erbin in the regulation of Nod2-dependent NF-kappaB signaling. J Biol Chem. 2005;280(48):40301–40309. doi: 10.1074/jbc.M508538200. [DOI] [PubMed] [Google Scholar]
- 11.Dai F, et al. Erbin inhibits transforming growth factor beta signaling through a novel Smad-interacting domain. Mol Cell Biol. 2007;27(17):6183–6194. doi: 10.1128/MCB.00132-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bueno OF, et al. The MEK1-ERK1/2 signaling pathway promotes compensated cardiac hypertrophy in transgenic mice. EMBO J. 2000;19(23):6341–6350. doi: 10.1093/emboj/19.23.6341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hunter JJ, Tanaka N, Rockman HA, Ross J, Jr, Chien KR. Ventricular expression of a MLC-2v-ras fusion gene induces cardiac hypertrophy and selective diastolic dysfunction in transgenic mice. J Biol Chem. 1995;270(39):23173–23178. doi: 10.1074/jbc.270.39.23173. [DOI] [PubMed] [Google Scholar]
- 14.Zelarayan L, et al. NF-kappaB activation is required for adaptive cardiac hypertrophy. Cardiovasc Res. 2009;84(3):416–424. doi: 10.1093/cvr/cvp237. [DOI] [PubMed] [Google Scholar]
- 15.Rosenkranz S. TGF-beta1 and angiotensin networking in cardiac remodeling. Cardiovasc Res. 2004;63(3):423–432. doi: 10.1016/j.cardiores.2004.04.030. [DOI] [PubMed] [Google Scholar]
- 16.Rockman HA, et al. Segregation of atrial-specific and inducible expression of an atrial natriuretic factor transgene in an in vivo murine model of cardiac hypertrophy. Proc Natl Acad Sci USA. 1991;88(18):8277–8281. doi: 10.1073/pnas.88.18.8277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Proud CG. Ras, PI3-kinase and mTOR signaling in cardiac hypertrophy. Cardiovasc Res. 2004;63(3):403–413. doi: 10.1016/j.cardiores.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 18.Kehat I, et al. Extracellular signal-regulated kinases 1 and 2 regulate the balance between eccentric and concentric cardiac growth. Circ Res. 2011;108(2):176–183. doi: 10.1161/CIRCRESAHA.110.231514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matsunaga-Udagawa R, et al. The scaffold protein Shoc2/SUR-8 accelerates the interaction of Ras and Raf. J Biol Chem. 2010;285(10):7818–7826. doi: 10.1074/jbc.M109.053975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rodriguez-Viciana P, Oses-Prieto J, Burlingame A, Fried M, McCormick F. A phosphatase holoenzyme comprised of Shoc2/Sur8 and the catalytic subunit of PP1 functions as an M-Ras effector to modulate Raf activity. Mol Cell. 2006;22(2):217–230. doi: 10.1016/j.molcel.2006.03.027. [DOI] [PubMed] [Google Scholar]
- 21.Wilkins BJ, et al. Calcineurin/NFAT coupling participates in pathological, but not physiological, cardiac hypertrophy. Circ Res. 2004;94(1):110–118. doi: 10.1161/01.RES.0000109415.17511.18. [DOI] [PubMed] [Google Scholar]
- 22.Chan R, Hardy WR, Laing MA, Hardy SE, Muller WJ. The catalytic activity of the ErbB-2 receptor tyrosine kinase is essential for embryonic development. Mol Cell Biol. 2002;22(4):1073–1078. doi: 10.1128/MCB.22.4.1073-1078.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lemmens K, Segers VF, Demolder M, De Keulenaer GW. Role of neuregulin-1/ErbB2 signaling in endothelium-cardiomyocyte cross-talk. J Biol Chem. 2006;281(28):19469–19477. doi: 10.1074/jbc.M600399200. [DOI] [PubMed] [Google Scholar]
- 24.Rohrbach S, Niemann B, Silber RE, Holtz J. Neuregulin receptors erbB2 and erbB4 in failing human myocardium. Basic Res Cardiol. 2005;100(3):240–249. doi: 10.1007/s00395-005-0514-4. [DOI] [PubMed] [Google Scholar]
- 25.Baliga RR, et al. NRG-1-induced cardiomyocyte hypertrophy. Role of PI-3-kinase, p70(S6K), and MEK-MAPK-RSK. Am J Physiol. 1999;277(5 Pt 2):H2026–H2037. doi: 10.1152/ajpheart.1999.277.5.H2026. [DOI] [PubMed] [Google Scholar]
- 26.Zhao YY, et al. Neuregulins promote survival and growth of cardiac myocytes. Persistence of ErbB2 and ErbB4 expression in neonatal and adult ventricular myocytes. J Biol Chem. 1998;273(17):10261–10269. doi: 10.1074/jbc.273.17.10261. [DOI] [PubMed] [Google Scholar]
- 27.Yi J, et al. Endothelial SUR-8 acts in an ERK-independent pathway during atrioventricular cushion development. Dev Dyn. 2010;239(7):2005–2013. doi: 10.1002/dvdy.22343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maillet M, van Berlo JH, Molkentin JD. Molecular basis of physiological heart growth: Fundamental concepts and new players. Nat Rev Mol Cell Biol. 2013;14(1):38–48. doi: 10.1038/nrm3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.