Significance
This paper makes two contributions to research on the link between the social environment and health. Using data from a birth cohort study, we show that, among African American boys, those who grow up in highly disadvantaged environments have shorter telomeres (at age 9) than boys who grow up in highly advantaged environments. We also find that the association between the social environment and telomere length (TL) is moderated by genetic variation within the serotonin and dopamine pathways. Boys with the highest genetic sensitivity scores had the shortest TL when exposed to disadvantaged environments and the longest TL when exposed to advantaged environments. To our knowledge, this report is the first to document a gene–social environment interaction for TL, a biomarker of stress exposure.
Keywords: gene–environment, adversity, senescence
Abstract
Disadvantaged social environments are associated with adverse health outcomes. This has been attributed, in part, to chronic stress. Telomere length (TL) has been used as a biomarker of chronic stress: TL is shorter in adults in a variety of contexts, including disadvantaged social standing and depression. We use data from 40, 9-y-old boys participating in the Fragile Families and Child Wellbeing Study to extend this observation to African American children. We report that exposure to disadvantaged environments is associated with reduced TL by age 9 y. We document significant associations between low income, low maternal education, unstable family structure, and harsh parenting and TL. These effects were moderated by genetic variants in serotonergic and dopaminergic pathways. Consistent with the differential susceptibility hypothesis, subjects with the highest genetic sensitivity scores had the shortest TL when exposed to disadvantaged social environments and the longest TL when exposed to advantaged environments.
A large body of research has documented a positive association between exposure to disadvantaged social environments and morbidity and mortality (1, 2). A key mechanism for explaining this association is chronic stress, which is believed to degrade physiological functioning (3–6), thus “weathering” the individual and making him or her less resistant to disease (7, 8). We examined (i) whether the association between exposure to a disadvantaged environment and stress biomarkers was evident in childhood and (ii) whether the association was more pronounced for children carrying specific genetic variants. To measure the effect of stress on children’s physiological state, we used telomere length (TL) as a biomarker. A recent line of research suggests that accelerated shortening of the telomere, the (TTAGGG)n sequence repeat at the end of each chromosome, is a good biomarker of exposure to lifetime stress (3, 6, 8–11). To measure the social environment, we used an index based on four dimensions: economic conditions, parenting quality, family structure/stability, and maternal depression.
Our analysis extends the existing literature on social environments and TL in several ways. First, we focus on children, whereas prior research has focused primarily on adults, with few exceptions (12). Second, we examine a sample of African American boys whereas prior research has used mostly samples of whites. Third, we examine for the first time, to our knowledge, whether the association between the social environment and TL is moderated by variations in selected genes. Although recent research suggests that some people are genetically more sensitive than others to their social environments (13–15), research on TL has not taken account of this potential moderating factor. Finally, we use a saliva DNA source of TL, which we show to be highly correlated with blood leukocyte TL (see Figs. S2 and S3). Our study validates other work showing that multiple tissues, including saliva, can be used to measure TL (16, 17).
Disadvantaged Social Environments and Telomere Length
During DNA replication, chromosome ends shorten with each cycle of chromosomal replication and cellular division. The repetitive telomere sequence is sacrificed to protect genetic information near the ends of chromosomes. In addition, the presence of the telomere prevents fusion of adjoining chromosomal ends. Over time, due to each cell division, the telomere ends become shorter, and so the telomere has been referred to as a “mitotic clock” (11, 18–20). Associated with progressive telomere shortening, the cell activates signaling events that produce replicative senescence. Salient to this report, stress appears to augment replicative TL shortening, perhaps by promoting nonenzymatic hydrolysis of telomere base pairs (11, 18). Stress-related telomere shortening could evoke physiological weathering in a way similar to aging (8, 18, 21, 22). Research suggests several possible behavioral mediators of the negative association between stress and TL, including smoking, mental illness (particularly depression), caregiver stress, and obesity (23–25). Considering the strong association between social deprivation and these mediators, it is not surprising that some measures of social standing and social deprivation have also been found to be associated with TL (8, 9, 17, 26).
The TL literature has focused almost exclusively on adults, although several studies have used retrospective reports to measure childhood conditions (10), and one prospective study has examined the association between childhood conditions between ages 5 and 10 and TL (27) and a second study determined that the duration of exposure to institutional care between 22 and 54 mo was negatively associated with telomere length (12). A second limitation of current TL literature is that most studies are based on samples of middle-class whites; research on minority children and children from low-income families is sparse. Finally, existing research on TL has not examined the role of genetic sensitivity in modifying the association between the social environment and TL. A growing body of research shows that specific gene variants moderate the association between the social environment and children’s health and well-being (13–15). Of particular interest are genes found in the serotonergic and dopaminergic pathways. We hypothesized that the association between children’s social environment and TL would be moderated by specific gene variants.
Results
Our analysis is based on 40 African American boys (age 9) who participated in the Fragile Families and Child Wellbeing Study (FFCWS). We focused on African American children because this group is understudied, and we focused on boys because prior research indicates that boys may be more sensitive than girls to negative family conditions (14). As described in Materials and Methods, our sample was chosen to represent extreme environments, based on the child’s family’s economic conditions, parenting quality, family structure/stability, and maternal depression (see Materials and Methods for more details about these measures). Half of the children in the sample were raised in very disadvantaged environments, and the other half were raised in advantaged environments. Table 1 reports descriptive statistics for the variables used to measure children’s environments along with other variables used in the models. Body mass index (BMI) did not differ between harsh and nourishing environments. Due to concerns of outliers and influential data points in a sample of 40, we use robust ordinary least squares (OLS) regression (using Cook’s D and Huber weighting), which is 95% as efficient as traditional OLS (28).
Table 1.
Descriptive statistics for children by harsh and nurturing environment (n = 40)
| Dependent variables | Minimum | Maximum | Harsh environment | Nurturing environment |
| Telomere length (kb) | 5 | 22.5 | 9.6 (4.1) | 10.3 (2.5) |
| ln(TL) | 1.6 | 3.1 | 2.1 (0.3) | 2.3 (0.2) |
| Independent variables | ||||
| Average poverty ratio* | 0.12 | 11.4 | 0.6 (0.4) | 3.5 (2.3) |
| Harsh parenting index* | 0 | 13.8 | 6.8 (3.7) | 3.4 (2.4) |
| Family structure changes* | ||||
| Two-parent | 0 | 1 | 0 | 0.6 |
| Single mother | 0 | 1 | 0.55 | 0.05 |
| One transition | 0 | 1 | 0.2 | 0.3 |
| Multiple transitions | 0 | 1 | 0.25 | 0.05 |
| Mother’s age at birth* | 17 | 42 | 20.9 (2.5) | 27.3 (6.3) |
| Mother’s education* | ||||
| Less than high school | 0 | 1 | 0.35 | 0 |
| High school | 0 | 1 | 0.55 | 0.45 |
| At least some college | 0 | 1 | 0.1 | 0.55 |
| Age 9 standardized BMI (z-score) | −1.3 | 2.3 | 0.8 (0.8) | 0.4 (1.1) |
Numbers in parentheses indicate mean ± SD.
Indicates a significant difference between harsh and nurturing environment.
Social Predictors of Telomere Length.
Model 1 (M1) of Table 2 shows that living in a disadvantaged environment was associated with a 19% shorter TL (P = 0.02). The next set of models (M2–M5) show the associations between boys’ TL and each of the environmental measures separately. Model 2 (M2) shows that a doubling of the family income/needs ratio was associated with a 5% increase in the boys’ TL (P = 0.03). To provide more context for this estimate, in the larger Fragile Families sample the mean income/needs ratio is 0.7 for children at the 25th percentile and 2.7 for children at the 75th percentile, which is equivalent to an 8% difference in TL under this model. As a robustness test for economic conditions, we also examined the association between a boy’s TL and his mother’s education (see M5 in Table 2). Compared with children of mothers with less than a high school education, having a mother with a high school degree is associated with a 32% increase in a child’s TL (P = 0.006), and having a mother with at least some postsecondary education is associated with a 35% increase in TL (P = 0.005). In sum, family economic status was a significant predictor of a boy’s TL measured in middle childhood. Model 3 shows that a low score on the parenting quality index was associated with a 3% decrease in a boy’s TL. Finally, model 4 shows that being exposed to multiple changes in family structure was associated with a 40% decline in a boy’s TL (P = 0.010).
Table 2.
Robust regression estimates of social predictors of log telomere length
| Measure | ln(TL) | ||||
| Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | |
| Harsh environment | −0.19* | ||||
| Average income/needs ratio | 0.05* | ||||
| Harsh parenting index | −0.03 | ||||
| Family structure changes | |||||
| Two-parent | |||||
| Single mother | −0.21 | ||||
| One transition | −0.12 | ||||
| Multiple transitions | −0.40* | ||||
| Mother’s education | |||||
| Less than high school | |||||
| High school | 0.32** | ||||
| At least some college | 0.35** | ||||
*P < 0.05, **P < 0.001, one-tailed.
Gene–Environment Interactions with Telomere Length.
The models in Table 3 present estimates of the main and interaction effects of dopamine and serotonin pathway genes and the environmental factor for a boy’s TL, controlling for age of the mother and age 9 standardized BMI. Because there are a variety of ways to code genetic effects, and no set way of combining multiple genetic markers, we use two common methods of coding and summing genetic markers. A key component of this coding is using the gene–environment interaction literature of these genes to designate a “sensitizing” or reactive allele. This sensitizing allele is often the allele typically thought to confer risk, but in this case that definition would apply only to those in the harsh environment, whereas those with the same allele would see an amplification of the positive effect in the nurturing environment. Our serotonergic sensitivity score sums the sensitizing alleles or homozygous genotypes composed of sensitizing alleles for four serotonin markers from two genes (5-HTT: 5-HTTLPR and STin2; TPH2: rs4570625 and rs1386494). Our dopamine sensitivity score similarly sums the sensitizing alleles or homozygous genotypes of sensitizing alleles for four dopamine markers (DAT1, rs40184; DRD4, third exon VNTR; DRD2, Taq1a, rs1800497; COMT, Val158Met, rs4680). The distribution of specific genotypes did not differ significantly between subjects in harsh and nourishing environments (Table S1).
Table 3.
Robust regression estimates of gene–environment interactions of log telomere length
| Measure | Model 1 | Model 2 | Model 3 | Model 4 |
| Harsh environment | −0.02 | −0.03 | −0.10 | −0.18 |
| Serotonin | ||||
| A. Count of homozygous sensitizing allele genotypes | 0.01 | |||
| B. Count of sensitizing alleles | 0.03 | |||
| Dopamine | ||||
| A. Count of homozygous sensitizing allele genotypes | 0.06 | |||
| B. Count of sensitizing alleles | −0.02 | |||
| Interactions | ||||
| Serotonin A × Harsh environment | −0.22* | |||
| Serotonin B × Harsh environment | −0.21* | |||
| Dopamine A × Harsh environment | −0.18† | |||
| Dopamine B × Harsh environment | −0.07 |
Serotonin A and dopamine A refer to a genetic sensitivity score (0–4) equal to the number of homozygous genotypes composed of sensitizing alleles of serotonergic or dopaminergic pathway genes, respectively. Serotonin B and dopamine B refer to a genetic sensitivity score (0–8) equal to the number of sensitizing alleles of serotonergic or dopaminergic pathway genes, respectively. All models control for age of mother and age 9 BMI. All gene scores are mean centered. †P < 0.10, *P < 0.05, one-tailed.
Model 1 and model 2 of Table 3 suggest a significant interaction between the social environmental and the serotonin sensitivity score (M1: b = −0.22, P value = 0.016; M2: b = −0.21, P value = 0.049) such that, although the environmental measure has a negative association with TL, it is the most negative for those with variants most associated with higher levels of environmental sensitivity. M3–M4 shows the interactions for the dopamine pathway genetic sensitivity scores. Although less definitive than in the case of the serotonergic pathway, the negative association between the social environment and TL is stronger for boys with dopaminergic pathway genetic variants most associated with higher levels environmental sensitivity (M3: b = −0.18, P value = 0.098; M4: b = −0.07, P value = 0.18).
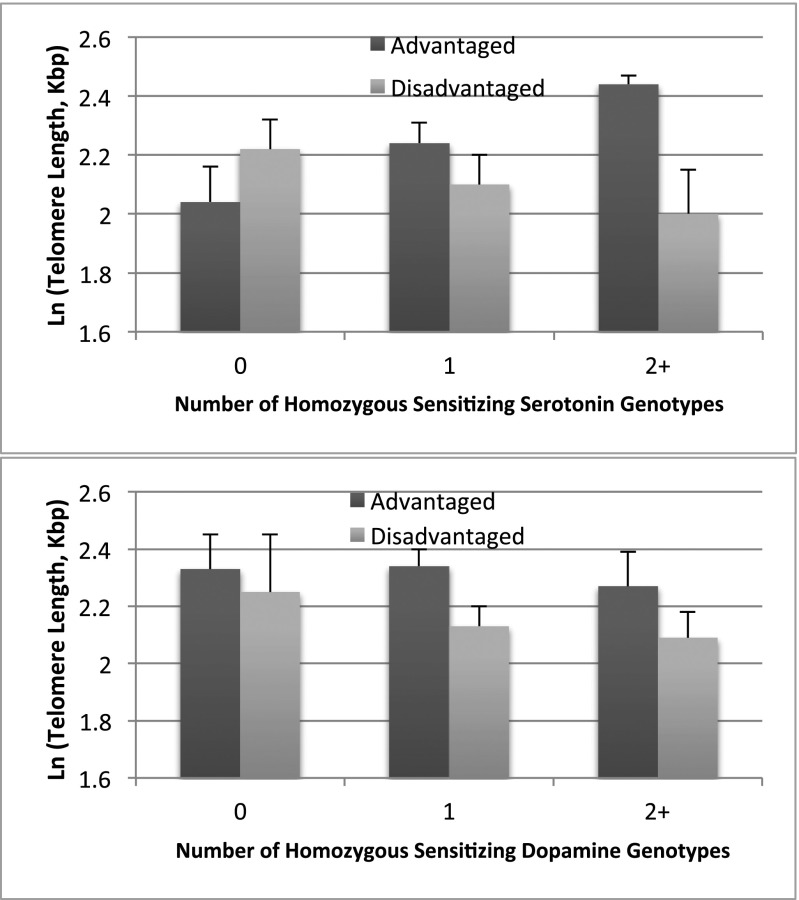
Fig. 1, Upper shows that, among children with 0 sensitizing serotonin genotypes, there is little difference in TL between children in advantaged and disadvantaged environments. However, for children with higher levels of genetic sensitivity (i.e., those with 2+ sensitizing genotypes), we see a large difference. In fact, consistent with the differential susceptibility hypothesis, the point estimates suggest that the most sensitive group has the longer TL in the advantaged environment and the shorter TL in the disadvantaged environment. This finding implies a genetically encoded differential sensitivity to the environment using TL as the biomarker (13–15). Fig. 1, Lower shows a similar (but not as strong) relationship with dopamine pathway genotypes. Namely, for boys with 0 sensitizing dopamine genotypes, there is only a small, insignificant difference in TL by environment, whereas for boys with 1 and 2+ sensitizing dopamine genotypes, there is a large and significant difference by environment, with boys with sensitizing genotypes having the longer TL in advantaged environments and the shorter TL in disadvantaged environments.
Fig. 1.
ln(telomere length) by environment type and serotonin pathway (Upper) and dopamine pathway (Lower) homozygous genotype counts. Although the possible range of homozygous genotypes was 0–4, due to the small number of minor allele frequencies the range is top-coded at 2+. For the serotonin pathway genotypes, the environment effect is borderline for 0 genotypes (P = 0.09), not significant for 1 genotype (P = 0.32), and significant for 2+ genotypes (P = 0.02). For the dopamine pathway genotypes, the environment difference is not significant for 0 genotypes (P = 0.63), significant for 1 genotype (P = 0.05), and borderline for 2+ genotypes (P = 0.08).
Discussion
Our analysis makes a number of methodological and substantive contributions. We demonstrate the utility of using saliva DNA to measure the children’s telomere length (SI Text). This is important in that most TL research uses blood, which is more difficult to collect than saliva. That saliva DNA is a valid indicator of TL makes it easier and less expensive for researchers to collect samples of TL at multiple points in time and thereby to examine how changes in the social environment are associated with changes in TL (8, 9, 17, 26). A second methodological contribution is the use of prospective data to measure children’s environmental exposures in real time. Only one study that we know of used real-time measures of children’s environments and that study examined ages 5–10 (27). Finally, our analysis is, to our knowledge, the first to examine TL in a sample of African American children.
Our analysis provides evidence that exposure to disadvantaged environments in childhood is associated with shorter TL. Decomposing the environmental factor into its different components also provided insight. Here we were able to replicate the positive association between family socioeconomic status (SES) and TL (8, 9, 17, 26) for a sample of children. In addition, we were able to document significant associations between family structure/stability and TL and harsh parenting and TL.
Due to sample size limitations, we were not able to examine the independent effect of each of these measures. Nevertheless, we tested the measured variables pairwise and found that the SES measures were the strongest predictors of TL. Although the other variables maintained the same pattern of association, the coefficients declined dramatically with the inclusion of poverty ratio and mother’s education.
Most importantly, to our knowledge, this report is the first to examine a gene–social environment interaction for a biomarker, TL. Using two genetic sensitivity scores (related to serotonin and dopamine pathways), we found that African American boys with the highest scores on the serotonin and dopamine pathway genetic sensitivity had shorter TL than their peers when exposed to disadvantaged social environments and longer TL when exposed to advantaged environments. A similar pattern was found for the dopamine pathway genetic sensitivity score.
Our findings on gene–social environment interactions raise intriguing questions about the mechanisms involved. We suggest that an individual’s genetic architecture moderates the magnitude and direction of the physiological response to exogenous stressors.
Materials and Methods
Sample.
Our data were taken from two studies: the FFCWS and a benchmark study designed to provide information on the effect of the sample collection process and tissue source for TL. FFCWS follows a stratified, multistage, probability sample of children born in large US cities (200,000+) between September 1998 and September 2000, with an oversample of children born to unmarried parents (three-quarters unwed, one-quarter wed) (29). Because of the large oversample of nonmarital births and because of the urban location of the study, the children in the sample come from a wide range of social environments. This feature of the data afforded us greater power to detect the effects of social environment than an equally sized sample of all births would have provided. Baseline interviews with mothers and fathers were conducted within 48 h of the child’s birth, and subsequent interviews were conducted when the focal child was 1, 3, 5, and 9 y old. Saliva DNA samples were taken at the age 9 follow-up, using the Oragene DNA sample collection kit (DNA Genotek Inc.). DNA was extracted following the manufacturer’s protocol and stored at −80° until use. We used data from all five waves and restricted the analysis to children with genetic information. From this pool we selected subsamples of children from the most extreme environments in the FFCWS. Initially, we identified 40 families based on a three-step process. In the first step, the sample was constrained to boys meeting the following conditions: (i) boys provided saliva at age 9 (wave 5) in-home interview, (ii) whose mother self-identified her race as black or African American, (iii) for whom no data were missing on the criterion variables described below, and (iv) who were male.
Next, we arrayed the subsample on an index of advantage–disadvantage from birth to age 9 based on an equally weighted combination of (i) family economic conditions, (ii) parenting practices, and (iii) family structure/stability. Finally, we took children with the 20 highest scores in the disadvantaged index whose mothers had experienced at least one depressive episode and the children with the 20 lowest scores on the index whose mothers had never experienced depression. Thus, boys who scored highest on this index (n = 20) lived in homes with high levels of poverty, high levels of family instability, harsh parenting, and maternal depression. Boys who scored lowest (n = 20) lived in affluent, stable families and were not exposed to either harsh parenting or maternal depression. We then assayed the children for TL.
Telomere Measurement.
Telomere length was measured using our modification of a quantitative real-time PCR assay that incorporates an oligomer standard to permit measurement of absolute (in kilobases per chromosome) rather than relative telomere length (30). Briefly, this method adapts the approach of Cawthon (31) in which relative telomere length is determined by quantitative PCR by determining the ratio of telomere copy repeats to a single-copy reference gene (the gene is 36B4). To determine absolute telomere length, an 84mer oligomer standard TTAGGG is used to construct a standard curve. A separate standard curve for the single-copy gene incorporates a 79mer containing the reference gene 36B4. This enables calculation of total telomere length per diploid genome, whereas the 36B4 product gives the number of diploid genomes. Length per chromosome is given by dividing telomere length per genome by 92, the number of telomeres per diploid genome. Samples are measured in triplicate and the results are averaged. Distribution of samples in the 96-well plates was randomized, and each plate contains at least 10 repeats from prior runs to detect and limit potential batch effects. To mitigate batch effects, reference DNA from a tel− cell line and the same line after stable integration of tel were included in each plate. Reference DNA was harvested at a single time, aliquoted, and frozen. Telomere length was normalized by this reference to assure plate-to-plate consistency. The coefficient of variation of a standard (DNA from a 40-y-old female) was 10% across ∼100, 96-well plates.
The average TL for our sample was 9.9 kb long, with a SD of 3.3 kb. Fig. S1, Upper indicates that the TL data are positively skewed (even with outliers removed). The best transformation of the data are the natural log (other transformations not shown). Fig. S1, Lower shows the distribution and Q-Q plot of log-transformed TL. Once transformed, both distributions track the normal distribution fairly well. Due to the close approximation to normality, we used log telomere length (lnTL) for the remainder of the analyses.
Most prior reports of telomere length have used DNA derived from circulating blood cells. To evaluate TL in DNA derived from blood cells and saliva, we compared TL in DNA from both origins from 16 volunteers. Telomere length was significantly greater in saliva than in blood leukocyte-derived DNA [6.5 kb ± 1.8 SD (saliva) vs. 4.2 kb ± 1.2 SD (peripheral mononuclear blood cells) P < 0.001] (Fig. S2). However, saliva and leukocyte DNA lengths were highly and significantly correlated (R = 0.72, P = 0.002) (Fig. S3).
Social Environment Measures.
In addition to comparing boys exposed to the best and worst environments, we also examined the association between TL and each of the individual components used to construct the disadvantage/advantage index. To measure the economic conditions of the household, we used the average income/needs ratio across all five waves of the study. This measure adjusts total household income by household size and is a better indicator of economic resources than income alone. To measure the mother’s completed education, we used four categories: (i) less than high school, (ii) high school graduate, and (iii) at least some college. To measure family structure/stability, we used four categories: (i) stable two-parent families, (ii) stable single-mother families, (iii) families with one partnership change, and (iv) families with multiple partnership changes. To measure harsh parenting, we used a count of how often mothers engaged in harsh psychological behaviors (e.g., yelling, threatening) and harsh physical behaviors (hitting, slapping). These items were taken from the Conflict Tactics Scale (32). To measure maternal depression, we used the composite international diagnostic interview (CIDI) short form (33). We measured mother’s self-reported age at birth. Father’s age is highly correlated with mother’s age (0.8) and was missing from multiple of the single-mother births; therefore, only mother’s age was used. BMI was assessed in the home by an interviewer and was age-standardized to age 9 to account for slight differences in age at interview.
Genes and Their Variants.
Serotonergic.
Our measure of the serotoninergic pathway comes from four genetic variants each of two genes. First, we use two length variants within the most studied gene of this system, the serotonin transporter gene (5-HTT). This gene codes for the protein that recycles the serotonin from the synapses. The two well-examined variants of the serotonin transporter gene are (i) a functional polymorphism (5-HTTLPR) in the 5′ regulatory region and (ii) a 17-bp variable number tandem repeat (VNTR) in the second intron region (called STin2 VNTR). For the 5-HTTLPR polymorphism, the most common alleles are the short (S) 14-repeat and long (L) 16-repeat of a 23-bp incomplete repeat, but other less common repeats are also found in various populations. Compared with the L allele, the S allele of the 5-HTTLPR polymorphism has been shown to be associated with reduced transcription (34). For the STin2 polymorphism, the two most common alleles are the 10 and 12 repeat, and compared with the 10-repeat allele, the 12-repeat allele is also associated with reduced transcription (35). We also used variants of the tryptophan hydroxylase 2 gene (TPH2), which codes tyrosine hydroxylase, the rate-limiting enzyme in the biosynthesis of serotonin (36). Alleles of TPH2 have been associated with depression, bipolar disorder, and other mental health problems (37, 38). We used variants at two loci related to the TPH2 gene. The first variant is rs4570625, a G/A SNP that appears associated with reduced transcription rates. Similarly, the second variant, rs1386494, a T/C SNP, is also associated with reduced transcription rates (39).
Due to the novelty of the differential susceptibility model, there is little guidance in how to code the reactivity of a genetic variant or polymorphism. To date, most studies have taken genetic markers that were formerly classified as “risky” and reclassified them as reactive or “sensitizing” (13, 15). Normally, these risky (now sensitizing) polymorphisms are the variants associated with lower transcriptional efficiency (15), and this is generally the case in our study. In combining the serotonergic system variants, we create two serotonin pathway genetic sensitivity scores by (i) summing the number of homozygous genotypes composed of sensitizing alleles (5-HTTLPR-S, STin2-12, TPH2a-G, TPH2b-T) and (ii) summing the number of sensitizing alleles. This results in a possible score of 0–4 for the first measure and 0–8 for the second measure.
Dopaminergic.
For the dopaminergic pathway, we used one measure each for four different genes along the dopaminergic pathway. DAT1 (SLC6A3, 5p15.3) is the gene that codes the dopamine transporter protein that removes dopamine from the synapstic cleft (40). The DAT1 variant (rs40184, intron 14) is a SNP, and the C allele is associated with reduced transcription of the DAT1 gene (41). Both DRD2 (Taq1a, 11q23) and DRD4 (11p15.5) code for postsynaptic dopamine receptors (42). We measured the DRD2 taq1a variant (rs1800497), which may affect expression of the receptor. For DRD4, we used the 48-bp VNTR in the third exon. We coded 6–10 repeats as “long” or 7R alleles (which make up 80% of long alleles) and call the short allele “4R” because it constitutes 85% of the short (2R-5R) alleles. Finally, catechol-O-methyltransferase (COMT, 22q11.21) codes for a major enzyme involved in the inactivation of dopamine in the synaptic cleft, and the Met allele of the Val158 Met polymorphism (rs4680) is known to decrease COMT activity (43).
Similar to the serotonergic scores, we created two dopamine pathway genetic sensitivity scores by (i) summing the number of homozygous genotypes composed of sensitizing alleles (the C allele for DAT1, 7R for DRD4, T allele for DRD2, and Met allele for COMT) and (ii) summing the number of sensitizing alleles. This results in a possible score of 0–4 for the first measure and 0–8 for the second measure.
Genotypes for HTTLPR, STin2, and DRD4 were obtained by PCR followed by gel or capillary electrophoresis, and other variants were determined by real-time PCR using primer systems supplied by Life Technology.
Supplementary Material
Acknowledgments
We thank the attendees at two meetings where this paper was presented—the 2013 Annual Meeting of the Population Association and the Fragile Families Working Group seminar series—for helpful comments. Elizabeth Conroy participated in development of the telomere assay and ran all of the samples. Funding for this study was provided by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (R01 HD076592) and the Penn State Clinical and Translational Science Institute (TL1 TR00012503). In addition, this work was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development through Grants R01HD36916, R01HD39135, and R01HD40421 and by a consortium of private foundations of the Fragile Families and Child Wellbeing Study.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1404293111/-/DCSupplemental.
References
- 1.Adler NE, Ostrove JM. Socioeconomic status and health: What we know and what we don’t. Ann N Y Acad Sci. 1999;896:3–15. doi: 10.1111/j.1749-6632.1999.tb08101.x. [DOI] [PubMed] [Google Scholar]
- 2.Shonkoff JPGA, Garner AS. Committee on Psychosocial Aspects of Child and Family Health Committee on Early Childhood, Adoption, and Dependent Care Section on Developmental and Behavioral Pediatrics The lifelong effects of early childhood adversity and toxic stress. Pediatrics. 2012;129(1):e232–e246. doi: 10.1542/peds.2011-2663. [DOI] [PubMed] [Google Scholar]
- 3.Epel ES, et al. Accelerated telomere shortening in response to life stress. Proc Natl Acad Sci USA. 2004;101(49):17312–17315. doi: 10.1073/pnas.0407162101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McEwen BS. Allostasis and allostatic load: Implications for neuropsychopharmacology. Neuropsychopharmacology. 2000;22(2):108–124. doi: 10.1016/S0893-133X(99)00129-3. [DOI] [PubMed] [Google Scholar]
- 5.Seeman T, Epel E, Gruenewald T, Karlamangla A, McEwen BS. Socio-economic differentials in peripheral biology: Cumulative allostatic load. Ann N Y Acad Sci. 2010;1186:223–239. doi: 10.1111/j.1749-6632.2009.05341.x. [DOI] [PubMed] [Google Scholar]
- 6.Shalev I. Early life stress and telomere length: Investigating the connection and possible mechanisms: A critical survey of the evidence base, research methodology and basic biology. Bioessays. 2012;34(11):943–952. doi: 10.1002/bies.201200084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Geronimus AT. The weathering hypothesis and the health of African-American women and infants: Evidence and speculations. Ethn Dis. 1992;2(3):207–221. [PubMed] [Google Scholar]
- 8.Geronimus AT, et al. Do US black women experience stress-related accelerated biological aging?: A novel theory and first population-based test of black-white differences in telomere length. Hum Nat. 2010;21(1):19–38. doi: 10.1007/s12110-010-9078-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carroll JE, Diez-Roux AV, Adler NE, Seeman TE. Socioeconomic factors and leukocyte telomere length in a multi-ethnic sample: Findings from the multi-ethnic study of atherosclerosis (MESA) Brain Behav Immun. 2013;28:108–114. doi: 10.1016/j.bbi.2012.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Price LH, Kao HT, Burgers DE, Carpenter LL, Tyrka AR. Telomeres and early-life stress: An overview. Biol Psychiatry. 2013;73(1):15–23. doi: 10.1016/j.biopsych.2012.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shalev I, et al. Stress and telomere biology: A lifespan perspective. Psychoneuroendocrinology. 2013;38(9):1835–1842. doi: 10.1016/j.psyneuen.2013.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Drury SS, et al. Telomere length and early severe social deprivation: Linking early adversity and cellular aging. Mol Psychiatry. 2012;17(7):719–727. doi: 10.1038/mp.2011.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Belsky J, Bakermans-Kranenburg MJ, van IJzendoorn MH. For better and for worse: Differential susceptibility to environmental influences. Curr Dir Psychol Sci. 2007;16(6):300–304. [Google Scholar]
- 14.Boyce WT, Ellis BJ. Biological sensitivity to context: I. An evolutionary-developmental theory of the origins and functions of stress reactivity. Dev Psychopathol. 2005;17(2):271–301. doi: 10.1017/s0954579405050145. [DOI] [PubMed] [Google Scholar]
- 15.Mitchell C, et al. Genetic differential sensitivity to social environments: Implications for research. Am J Public Health. 2013;103(Suppl 1):S102–S110. doi: 10.2105/AJPH.2013.301382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Daniali L, et al. Telomeres shorten at equivalent rates in somatic tissues of adults. Nat Commun. 2013;4:1597. doi: 10.1038/ncomms2602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Theall KP, Brett ZH, Shirtcliff EA, Dunn EC, Drury SS. Neighborhood disorder and telomeres: Connecting children’s exposure to community level stress and cellular response. Soc Sci Med. 2013;85:50–58. doi: 10.1016/j.socscimed.2013.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Armanios M, Blackburn EH. The telomere syndromes. Nat Rev Genet. 2012;13(10):693–704. doi: 10.1038/nrg3246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blackburn EH. Telomeres: Structure and synthesis. J Biol Chem. 1990;265(11):5919–5921. [PubMed] [Google Scholar]
- 20.Heidinger BJ, et al. Telomere length in early life predicts lifespan. Proc Natl Acad Sci USA. 2012;109(5):1743–1748. doi: 10.1073/pnas.1113306109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harley CB, Futcher AB, Greider CW. Telomeres shorten during ageing of human fibroblasts. Nature. 1990;345(6274):458–460. doi: 10.1038/345458a0. [DOI] [PubMed] [Google Scholar]
- 22.Hayflick L, Moorhead PS. The serial cultivation of human diploid cell strains. Exp Cell Res. 1961;25:585–621. doi: 10.1016/0014-4827(61)90192-6. [DOI] [PubMed] [Google Scholar]
- 23.Valdes AM, et al. Obesity, cigarette smoking, and telomere length in women. Lancet. 2005;366(9486):662–664. doi: 10.1016/S0140-6736(05)66630-5. [DOI] [PubMed] [Google Scholar]
- 24.Damjanovic AK, et al. Accelerated telomere erosion is associated with a declining immune function of caregivers of Alzheimer’s disease patients. J Immunol. 2007;179(6):4249–4254. doi: 10.4049/jimmunol.179.6.4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Simon NM, et al. Telomere shortening and mood disorders: Preliminary support for a chronic stress model of accelerated aging. Biol Psychiatry. 2006;60(5):432–435. doi: 10.1016/j.biopsych.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 26.Needham BL, et al. Socioeconomic status, health behavior, and leukocyte telomere length in the National Health and Nutrition Examination Survey, 1999–2002. Soc Sci Med. 2013;85:1–8. doi: 10.1016/j.socscimed.2013.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shalev I, et al. Exposure to violence during childhood is associated with telomere erosion from 5 to 10 years of age: A longitudinal study. Mol Psychiatry. 2013;18(5):576–581. doi: 10.1038/mp.2012.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hamilton LC. srd1: How robust is robust regression? Stata Tech Bull. 1992;1(2):21–26. [Google Scholar]
- 29.Reichman NE, Teitler JO, Garfinkel I, McLanahan SS. Fragile families: Sample and design. Child Youth Serv Rev. 2001;23(4-5):303–326. [Google Scholar]
- 30.O’Callaghan NJ, Fenech M. A quantitative PCR method for measuring absolute telomere length. Biol Proced Online. 2011;13:3. doi: 10.1186/1480-9222-13-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cawthon RM. Telomere measurement by quantitative PCR. Nucleic Acids Res. 2002;30(10):e47. doi: 10.1093/nar/30.10.e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Straus MA, Hamby SL, BoneyMcCoy S, Sugarman DB. The revised Conflict Tactics Scales (CTS2): Development and preliminary psychometric data. J Fam Issues. 1996;17(3):283–316. [Google Scholar]
- 33.Kessler RC, Andrews G, Mroczek D, Üstün TB, Wittchen H-U. The World Health Organization Composite International Diagnostic Interview Short Form (CIDI-SF) Int J Methods Psychiatr Res. 1998;7(4):171–185. [Google Scholar]
- 34.Heils A, et al. Allelic variation of human serotonin transporter gene expression. J Neurochem. 1996;66(6):2621–2624. doi: 10.1046/j.1471-4159.1996.66062621.x. [DOI] [PubMed] [Google Scholar]
- 35.Hranilovic D, et al. Serotonin transporter promoter and intron 2 polymorphisms: Relationship between allelic variants and gene expression. Biol Psychiatry. 2004;55(11):1090–1094. doi: 10.1016/j.biopsych.2004.01.029. [DOI] [PubMed] [Google Scholar]
- 36.Walther DJ, et al. Synthesis of serotonin by a second tryptophan hydroxylase isoform. Science. 2003;299(5603):76. doi: 10.1126/science.1078197. [DOI] [PubMed] [Google Scholar]
- 37.Mössner R, et al. Transmission disequilibrium of polymorphic variants in the tryptophan hydroxylase-2 gene in children and adolescents with obsessive-compulsive disorder. Int J Neuropsychopharmacol. 2006;9(4):437–442. doi: 10.1017/S1461145705005997. [DOI] [PubMed] [Google Scholar]
- 38.Zhou ZF, et al. Haplotype-based linkage of tryptophan hydroxylase 2 to suicide attempt, major depression, and cerebrospinal fluid 5-hydroxyindoleacetic acid in 4 populations. Arch Gen Psychiatry. 2005;62(10):1109–1118. doi: 10.1001/archpsyc.62.10.1109. [DOI] [PubMed] [Google Scholar]
- 39.Porcelli S, Fabbri C, Drago A, Gibiino S, De Ronchi D, Serretti A. Genetics and antidepressant: Where we are. Clinical Neuropsychiatry. 2011;8(2):99–150. [Google Scholar]
- 40.Bannon MJ, Whitty CJ. Neurokinin receptor gene expression in substantia nigra: Localization, regulation, and potential physiological significance. Can J Physiol Pharmacol. 1995;73(7):866–870. doi: 10.1139/y95-119. [DOI] [PubMed] [Google Scholar]
- 41.Heinz A, et al. Genotype influences in vivo dopamine transporter availability in human striatum. Neuropsychopharmacology. 2000;22(2):133–139. doi: 10.1016/S0893-133X(99)00099-8. [DOI] [PubMed] [Google Scholar]
- 42.Noble EP, Blum K, Ritchie T, Montgomery A, Sheridan PJ. Allelic association of the D2 dopamine receptor gene with receptor-binding characteristics in alcoholism. Arch Gen Psychiatry. 1991;48(7):648–654. doi: 10.1001/archpsyc.1991.01810310066012. [DOI] [PubMed] [Google Scholar]
- 43.Lachman HM, et al. Human catechol-O-methyltransferase pharmacogenetics: Description of a functional polymorphism and its potential application to neuropsychiatric disorders. Pharmacogenetics. 1996;6(3):243–250. doi: 10.1097/00008571-199606000-00007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.