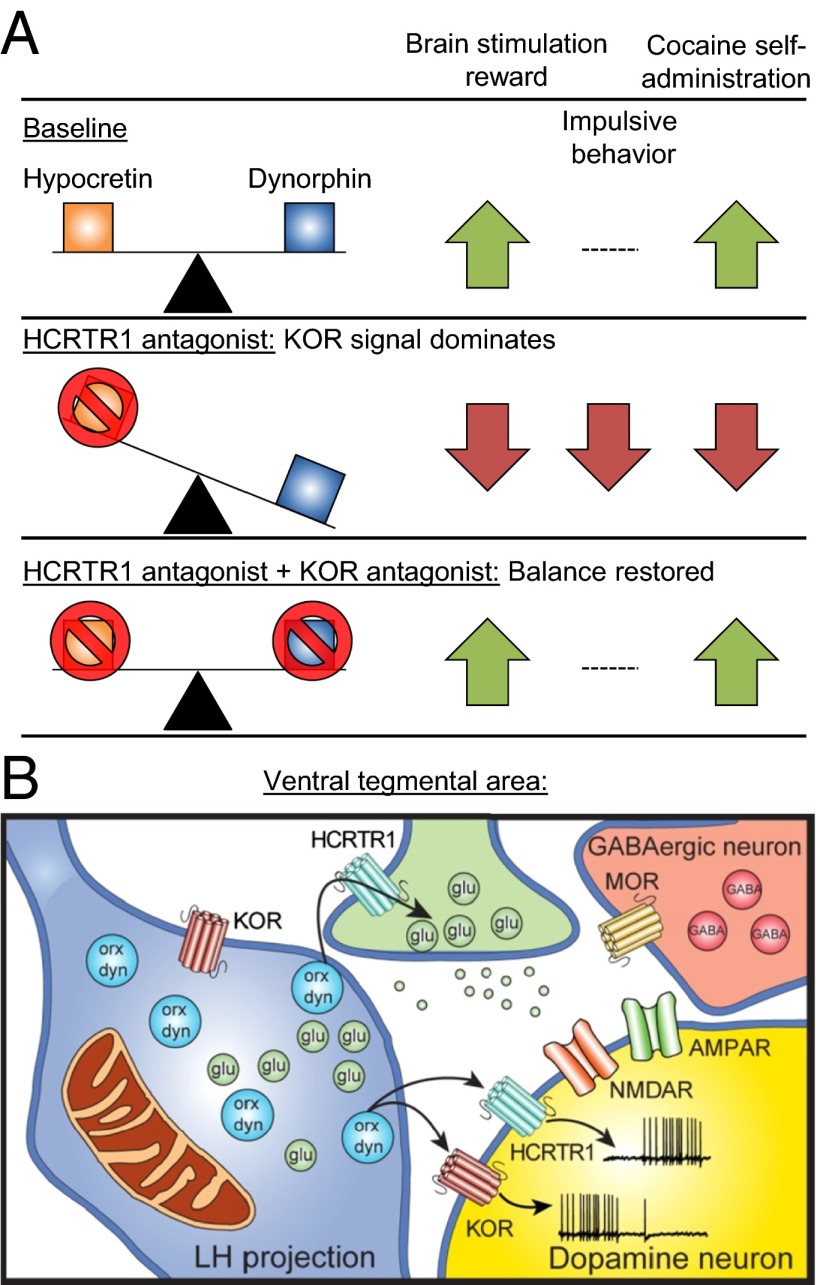
In PNAS, Muschamp et al. (1) report that hypocretin and dynorphin are coexpressed in the same synaptic vesicles of hypothalamic neurons. Their data from behavioral, pharmacological, and electrophysiological studies suggest that hypocretin and dynorphin are coreleased, and that they play opposing roles in cocaine self-administration, brain stimulation reward, and impulsivity. The authors’ data also suggest that the critical brain site for these opposing effects of coreleased hypocretin and dynorphin is the ventral tegmental area (VTA), the cell body region of the mesolimbic dopamine reward system (2). Fig. 1 provides a summary of the main findings from the study.
Fig. 1.
(A) Summary of the behavioral findings. Selective blockade of HCRTR1 reveals KOR signaling, which decreases brain stimulation reward, decreases impulsive behavior, and decreases cocaine self-administration. However, concurrent blockade of both HCRTR1 and KOR reverses these effects, returning the behavior in all three tests to the baseline condition. (B) Summary of the electrophysiological findings in the VTA. Lateral hypothalamus (LH) projections to the VTA contain hypocretin and dynorphin in the same vesicles. Dopamine neurons in the VTA express both HCRTR1 and KORs; activation of KORs inhibits and activation of HCRTR1 activates the dopamine cells. Saturation of both receptors produces no net effect on dopamine cells. Hypocretin may act together with excitatory glutamate input to overcome the inhibitory effect of dynophin and GABA transmission onto dopamine cells. dyn, dynorphin; glu, glutamate; MOR, mu-opioid receptor; orx, hypocretin.
In 1979, Avram Goldstein et al. discovered the third endogenous opioid peptide, termed dynorphin (3). Dynorphin is widely distributed in the brain and its physiological and behavioral effects are a result of selective activation of kappa-opioid receptors (KORs) (4). Activation of KORs induces stress-like negative psychological and physiological states, and blockade of KORs decreases stress-induced responses (5, 6). The dynorphin/KOR system also plays a complicated role in cocaine reward (5, 7). During initial cocaine self-administration activation of KORs decreases drug intake, during extended cocaine access blockade of these receptors decreases drug intake, and after extinction of cocaine self-administration activation of KORs reinstates drug seeking, while their blockade decreases stress-induced reinstatement of cocaine seeking (5, 7, 8).
In 1998, two independent groups discovered hypocretin/orexin (9, 10). Hypocretin neurons are located in the tuberal hypothalamus and have extensive projections throughout the neuraxis, including the VTA (11). Hypocretin binds to G protein-coupled hypocretin 1 and 2 receptors (HCRTR1/2) (10), and the peptide plays an important role in promoting arousal (9, 10), maintaining sleep homeostasis (12), and—most relevant to the present study—the rewarding effects of cocaine and other rewards via its action on VTA dopamine neurons (13, 14).
In 2001, Chou et al. (15) reported that hypocretin and dynorphin colocalize in the same hypothalamic neurons (15). In 2006, Li and van den Pol (16) extended these findings and provided electrophysiological data indicating that inhibitory dynorphin and excitatory hypocretin are simultaneously coreleased after stimulation of hypothalamic hypocretin neurons. The functional significance of hypocretin/dynorphin corelease is unknown, because despite the above-mentioned anatomical and physiological findings, investigators typically study in isolation the role of hypocretin and dynorphin in stress responses, reward processes, and motivated behavior. Muschamp et al. (1) have elegantly addressed this important gap in the current literature. They used neuroanatomical, electrophysiological, neuropharmacological, and behavioral procedures to characterize the role of hypocretin and dynorphin corelease on electrical brain stimulation reward (BSR), intravenous cocaine self-administration, cocaine-induced impulsivity, and VTA dopamine synaptic physiology.
At the neuroanatomical level, Muschamp et al. (1) used an immunofluorescence assay and replicated the previous finding (15) that hypocretin and dynorphin are coexpressed in the lateral, perifonical, and dorsomedial nuclei of the mouse hypothalamus. Using electron microscopy, they then examined the subcellular localization of hypocretin and dynorphin and observed colocalization of hypocretin and dynorphin in the same synaptic vesicles, which were distributed along both axons and dendrites. This finding suggests that neuronal activity could lead to corelease of these seemingly functionally opposing peptides, and subsequent studies tested this idea.
At the behavioral level, the authors first explored the role of the interaction between the dynorphin and hypocertin in BSR, where mice lever-press for rewarding lateral hypothalamus electrical stimulation. Once the mice were self-stimulating at a reliable rate, they received systemic or VTA injections of the HCRTR1 antagonist SB334867. Both manipulations caused a dose-dependent elevation of BSR threshold, which indicates a reduced efficacy for the simulation to produce reward. Critically, the effect of SB334867 on BSR was reversed by systemic or intra-VTA injections of nor-binaltorphimine (norBNI), a long-lasting KOR antagonist. In a second experiment, the authors examined the role of hypocretin and dynorphin cotransmission in impulsivity using the five-choice serial reaction task (5-CSRTT) in rats. Systemic blockade of HCRTR1 with SB334867 decreased spontaneous premature responses (the operational measure of impulsivity in the task). Once again, blockade of both HCRTR1 and KOR restored the behavior of the rat to the baseline condition. A relationship between the HCRTR1 system and cocaine was then demonstrated, whereby systemic blockade of HCRTR1 reversed cocaine-induced impulsivity.
In the third behavioral experiment, the authors followed up on the relationship between hypocretin and cocaine-induced impulsivity to assess whether hypocretin and dynorphin cotransmission plays a role in the rewarding effects of cocaine. Using the drug self-administration procedure, they found that HCRTR1 knockout mice self-administered less cocaine than wild-type mice. Surprisingly, they found that systemic injections of the KOR antagonist norBNI, at a dose that decreased cocaine self-administration in wild-type mice, partially restored the reduced cocaine intake in the knockout mice. These data represent one of the first demonstrations that a behavioral effect in the mouse induced by deleting one type of receptor can be ameliorated by pharmacological blockade of a different receptor. Finally, the authors reported that in rats the VTA injections of SB334867 decreased cocaine self-administration and that this effect was reversed by VTA injections of norBNI.
At the synaptic level, the authors assessed the effect of hypocretin and dynorphin on cell firing of VTA dopamine neurons of mice using slice electrophysiological recording. When hypocretin and dynorphin were administered separately, the firing rate of VTA dopamine neurons was increased and decreased, respectively. More importantly, most cells (∼65%) were dual-responsive to hypocretin and dynorphin, and coadministration of both peptides caused no net change in firing rate in these neurons.
Based on the results described above and other findings described in the paper, Muschamp et al. (1) reached three major conclusions: (i) Hypocretin and dynorphin are copackaged in the same synaptic vesicles of hypothalamic neurons, and therefore likely act as cotransmitters; (ii) under certain conditions, one of the main functions of hypocretin signaling is to counteract the effects of activation of the KORs by dynorphin; (iii) the opposing roles of hypocretin and dynorphin in drug and nondrug rewards are because of their effects on VTA dopamine neurons, the cell body region of the mesolimbic dopamine reward system.
This elegant multidisciplinary study of Muschamp et al. (1) raises several questions for future research and also has some more general implications for the study of the role of neuropeptides in normal and pathological behaviors. Perhaps the most important question is under what physiological conditions the net impact on behavior and physiology of one peptide dominates over the other peptide. This question was not addressed in the present study in which the conclusions were primarily based on classic ex vivo electrophysiological recording and pharmacological receptor blockade.
Another question relates to the anatomical specificity of the putative cotransmission of hypocretin and dynorphin. The authors studied a potential role of cotransmission of the peptides on VTA-mediated behaviors. Thus, a question for future research is whether the opposite actions of the two peptides also occur in other brain areas innervated by hypothalamic hypocretin neurons, such as the nucleus accumbens shell (11) or paraventricular thalamus (17). Interestingly, hypocretin neurons themselves seem to be more sensitive to KOR activation than HCRTR1 activation (16).
Another fascinating question derived from this study is whether the balance of the coreleased peptides is changed by experience, such as exposure to stress or drugs of abuse. For example, after a significant event do the hypocretin hypothalamic neurons change their peptide expression profile? Or, do the postsynaptic neurons change their receptor balance (HCRTR1 vs. KOR) to fine-tune their responsiveness to the coreleased peptides? Such data are critically important from the perspective of future medication development for psychiatric disorders, as suggested by the authors.
Finally, from a “big picture” perspective, perhaps the largest contribution of the paper for future research in neuroscience and psychiatry is not the particular findings reported in it, but the realization that to fully understand the physiological and behavioral role of a given peptide, it is critical to take into account the entire peptide and neurotransmitter profile of the neuronal population under study. This is a daunting prospect, but it holds the potential to significantly increase the likelihood of effective psychiatric medications, as well as giving us a greater appreciation of the complexity of the central nervous system.
Acknowledgments
The authors are supported by the National Institute on Drug Abuse, Intramural Research Program. N.J.M. received support from Early Career Fellowship 1053308 by the National Health and Medical Research Council.
Footnotes
The authors declare no conflict of interest.
See companion article on page E1648.
References
- 1.Muschamp JW, et al. Hypocretin (orexin) facilitates reward by attenuating the antireward effects of its cotransmitter dynorphin in ventral tegmental area. Proc Natl Acad Sci USA. 2014;111:E1648–E1655. doi: 10.1073/pnas.1315542111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wise RA. Dopamine, learning and motivation. Nat Rev Neurosci. 2004;5(6):483–494. doi: 10.1038/nrn1406. [DOI] [PubMed] [Google Scholar]
- 3.Goldstein A, Tachibana S, Lowney LI, Hunkapiller M, Hood L. Dynorphin-(1-13), an extraordinarily potent opioid peptide. Proc Natl Acad Sci USA. 1979;76(12):6666–6670. doi: 10.1073/pnas.76.12.6666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chavkin C, Goldstein A. Demonstration of a specific dynorphin receptor in guinea pig ileum myenteric plexus. Nature. 1981;291(5816):591–593. doi: 10.1038/291591a0. [DOI] [PubMed] [Google Scholar]
- 5.Bruchas MR, Land BB, Chavkin C. The dynorphin/kappa opioid system as a modulator of stress-induced and pro-addictive behaviors. Brain Res. 2010;1314:44–55. doi: 10.1016/j.brainres.2009.08.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Van’t Veer A, Carlezon WA., Jr Role of kappa-opioid receptors in stress and anxiety-related behavior. Psychopharmacology (Berl) 2013;229(3):435–452. doi: 10.1007/s00213-013-3195-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wee S, Koob GF. The role of the dynorphin-kappa opioid system in the reinforcing effects of drugs of abuse. Psychopharmacology (Berl) 2010;210(2):121–135. doi: 10.1007/s00213-010-1825-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bruijnzeel AW. Kappa-opioid receptor signaling and brain reward function. Brain Res Brain Res Rev. 2009;62(1):127–146. doi: 10.1016/j.brainresrev.2009.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Lecea L, et al. The hypocretins: Hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci USA. 1998;95(1):322–327. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sakurai T, et al. Orexins and orexin receptors: A family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92(4):573–585. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- 11.Peyron C, et al. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci. 1998;18(23):9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sutcliffe JG, de Lecea L. The hypocretins: Excitatory neuromodulatory peptides for multiple homeostatic systems, including sleep and feeding. J Neurosci Res. 2000;62(2):161–168. doi: 10.1002/1097-4547(20001015)62:2<161::AID-JNR1>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 13.Aston-Jones G, et al. Lateral hypothalamic orexin/hypocretin neurons: A role in reward-seeking and addiction. Brain Res. 2010;1314:74–90. doi: 10.1016/j.brainres.2009.09.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Borgland SL, et al. Orexin A/hypocretin-1 selectively promotes motivation for positive reinforcers. J Neurosci. 2009;29(36):11215–11225. doi: 10.1523/JNEUROSCI.6096-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chou TC, et al. Orexin (hypocretin) neurons contain dynorphin. J Neurosci. 2001;21(19):RC168. doi: 10.1523/JNEUROSCI.21-19-j0003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li Y, van den Pol AN. Differential target-dependent actions of coexpressed inhibitory dynorphin and excitatory hypocretin/orexin neuropeptides. J Neurosci. 2006;26(50):13037–13047. doi: 10.1523/JNEUROSCI.3380-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kirouac GJ, Parsons MP, Li S. Orexin (hypocretin) innervation of the paraventricular nucleus of the thalamus. Brain Res. 2005;1059(2):179–188. doi: 10.1016/j.brainres.2005.08.035. [DOI] [PubMed] [Google Scholar]