Significance
Hypocretin (orexin) and dynorphin are neuromodulators that play an important role in regulating affect and motivation. Orexin is critical for reward and is implicated in drug seeking, whereas dynorphin mediates negative mood and is implicated in depressive-like states. Considering these opposing effects, reports that both peptides are expressed in the same neurons and are coreleased are counterintuitive. Here, we demonstrate that orexin and dynorphin are coexpressed within the same synaptic vesicles and that this colocalization has a profound influence on reward, drug taking, and impulsive-like behavior. The fact that orexin occludes the depressive-like antireward effects of dynorphin significantly changes how we view the functional role of orexin in the brain.
Keywords: addiction, kappa-opioid receptor, mood, neurotransmission, stress
Abstract
Hypocretin (orexin) and dynorphin are neuropeptides with opposing actions on motivated behavior. Orexin is implicated in states of arousal and reward, whereas dynorphin is implicated in depressive-like states. We show that, despite their opposing actions, these peptides are packaged in the same synaptic vesicles within the hypothalamus. Disruption of orexin function blunts the rewarding effects of lateral hypothalamic (LH) stimulation, eliminates cocaine-induced impulsivity, and reduces cocaine self-administration. Concomitant disruption of dynorphin function reverses these behavioral changes. We also show that orexin and dynorphin have opposing actions on excitability of ventral tegmental area (VTA) dopamine neurons, a prominent target of orexin-containing neurons, and that intra-VTA orexin antagonism causes decreases in cocaine self-administration and LH self-stimulation that are reversed by dynorphin antagonism. Our findings identify a unique cellular process by which orexin can occlude the reward threshold-elevating effects of coreleased dynorphin and thereby act in a permissive fashion to facilitate reward.
Orexin promotes arousal (1) and has been implicated in the rewarding effects of food (2, 3), sexual behavior (4), and drugs of abuse (5, 6). It is produced primarily within the hypothalamus (7), and acts at orexin 1 receptor (OX1R) and OX2R (also known as Hcrt-R1 and Hcrt-R2), which are expressed in many brain areas, including the ventral tegmental area (VTA) of the midbrain (8). Dynorphin, in contrast, is expressed widely, promotes depressive-like behaviors, and plays a key role in mediating the aversive effects of stress (9, 10). Activation of kappa-opioid receptor (KORs), the receptors at which dynorphin acts (11), can attenuate the rewarding effects of drugs of abuse (12, 13) via actions that are mediated, at least in part, within midbrain dopamine (DA) systems (14, 15). Despite their seemingly opposing effects on motivation, there is evidence that these peptides may act in tandem; for example, both orexin and dynorphin are released during electrical stimulation of the hypothalamus (16). Like DA neurons, orexin and dynorphin neurons increase their activity in response to arousing stimuli like rewards and stressors (17). The functional effects of this pattern of neuropeptide coexpression on brain reward systems, and, in turn, on motivated behavior, are poorly understood because orexin and dynorphin are not traditionally studied together. Given their opposing effects on behavior and neuronal physiology when studied alone, it can be hypothesized that dominance in the effects of one peptide over the other could cause widely divergent behavioral phenotypes in reward sensitivity. For example, dominant orexin signaling may enhance reward sensitivity and reward seeking, whereas dominant dynorphin signaling may result in decreased reward sensitivity and anergia. Because these states have major relevance to psychiatric illnesses like addiction and depression, where reward processing is disordered, we sought to examine how these peptides, alone and in combination, affect motivated behaviors and the VTA DA circuit that regulates them. To do this, we used EM to characterize peptide colocalization at a microstructural level, as well as behavioral techniques that assess the sensitivity of brain reward circuitry, impulse control, and drug taking, after pharmacological or genetic manipulation of the orexin–dynorphin system. Additionally, we used electrophysiology to determine how the concomitant presence of orexin and dynorphin, alone or in combination with antagonists at their receptors, affects the excitability of VTA DA neurons.
Results
Orexin and Dynorphin Are Cotransmitters.
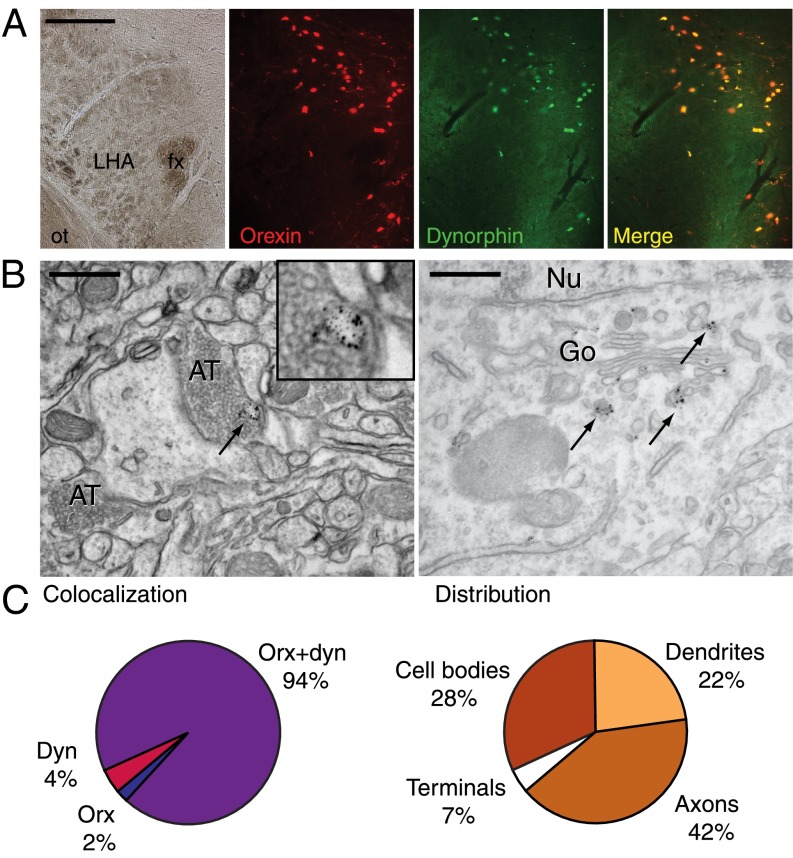
We confirmed coexpression of orexin and dynorphin within the same neurons of the mouse lateral, perifornical, and dorsomedial hypothalamus using fluorescence microscopy (18) (Fig. 1A). The existence of neurons that express multiple transmitters has been described in other brain circuits and may represent the neural basis for filtering mechanisms by which release of coexpressed neurotransmitters occurs at differential firing rates (19). Using EM, we found, however, that orexin and dynorphin are colocalized within the same synaptic vesicles. Most instances of copackaging were observed in unmyelinated, varicose axonal processes, where immunolabeling was found in or near vesicles. Within neuronal cell bodies, significant labeling was associated with the Golgi complex, whereas none was found in adjacent nuclei (Fig. 1 B and C). Dendrites also contained vesicle-associated labeling for both peptides, suggesting potential dendritic release of these transmitters. A small number of microstructural profiles captured axon terminals with labeling for both peptides located in large (∼100 nm) vesicles located outside the release zone of asymmetrical synapses (Fig. 1 B and C), providing support for the conclusion that orexin and dynorphin function as cotransmitters and that, under normal conditions, they are released together rather than differentially as a function of cell firing frequency.
Fig. 1.
Orexin and dynorphin are cotransmitters in neurons of the hypothalamus. (A, Far Left) Bright-field photomicrograph shows the area of hypothalamus examined for orexin (red) and dynorphin (green) immunoreactivity. (A, Far Right) Merged two-channel image (yellow) confirms orexin and dynorphin within the same neurons. (Scale bar: 250 μm.) (B) Immunogold labeling of alternate sections revealed close association of orexin (large particles) and dynorphin (small particles) in several cellular compartments. Representative electron micrographs show immunolabeling for orexin and dynorphin in vesicles within axon terminals (Left, Inset) and those associated with the Golgi complex (Right). Black arrows indicate immunolabeling. (Scale bar: 500 nm. Magnification: Inset, 67,300×.) (C) Extent of orexin-dynorphin double labeling and its distribution. Most instances revealed orexin and dynorphin in close association in the same organelle (e.g., vesicle) within axons and cell bodies. A total of 971,384 μm2 of hypothalamic tissue from two mice was examined, and n = 139 instances of immunolabeling were quantified. AT, axon terminal; Dyn, dynorphin; fx, fornix; Go, Golgi complex; LHA, lateral hypothalamic area; Nu, nucleus; Orx, orexin; ot, optic tract.
Reward Threshold-Elevating Effects of Orexin Blockade Are Reversed by Dynorphin Blockade.
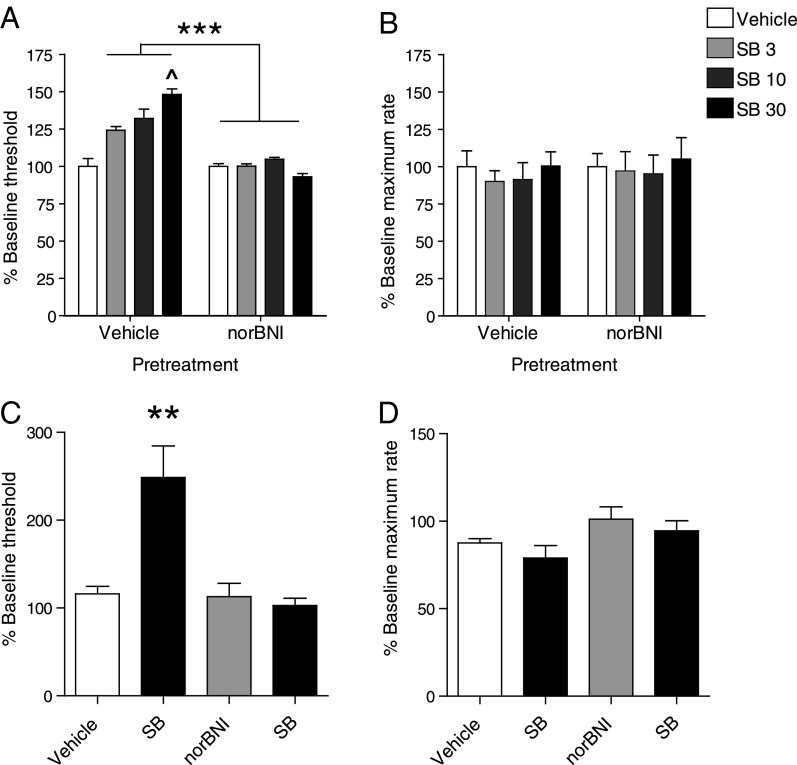
To explore the functional significance of this unique pattern of transmitter expression, we examined whether disruptions in orexin and dynorphin signaling can affect complex behaviors that reflect normal and aberrant motivation. In C57BL/6 mice trained to perform intracranial self-stimulation (ICSS) reinforced with lateral hypothalamic (LH) stimulation (20), blockade of OX1Rs by N-(2-methyl-6-benzoxazolyl)-N″-1,5-naphthyridin-4-yl urea (SB334867) during the light phase caused dose-dependent increases in reward thresholds (Fig. 2A; one-way repeated measures ANOVA for Dose: F3,12 = 4.44, P < 0.02). Increases in ICSS thresholds reflect treatment-induced reductions in the rewarding impact of the stimulation, a depressive-like sign indicative of decreased sensitivity to reward (20). This effect was not due to sedation or other nonspecific behavioral impairments, because ICSS response rates were unaffected (Fig. 2B; one-way repeated measures ANOVA for Dose: F3,24 = 0.33, P > 0.80).
Fig. 2.
Reward threshold-elevating effects of orexin blockade are reversed by dynorphin blockade. (A) Blockade of orexin signaling at OX1R by SB334867 (0–30 mg/kg, i.p.) elevates reward thresholds in the ICSS test, indicating decreased reward. This effect is blocked by pretreatment with the KOR antagonist norBNI (10 mg/kg, i.p.). ***P < 0.001, between groups; ^P < 0.05, difference from baseline. (B) Drug manipulations had no effect on performance capabilities in the ICSS test, because maximum rates of responding were unaffected (n = 5). Intra-VTA delivery of SB334867 (SB; 3.2 ng per 0.5 μL) and norBNI (2.5 μg per 1 μL) (C) produces more pronounced effects on ICSS than systemic delivery of these drugs, as shown in A, in the absence of any motor effects (D) (n = 4). **P < 0.01. Electrode placements are shown in Fig. S1 A and B.
Elevations in reward thresholds caused by SB334867 were prevented by pretreatment with nor-binaltorphimine (norBNI) [two-way repeated measures ANOVA for Drug (between subjects factor) × Dose SB (within subjects factor) interaction: F3,24 = 3.98, P < 0.01], which produces long-lasting blockade of dynorphin actions at KORs (10). These data suggest that the loss of orexin signaling reveals latent antireward effects of coreleased dynorphin. Administration of norBNI alone did not decrease reward thresholds. Although this effect may be related to the unique pharmacodynamics of norBNI and other prototypical KOR antagonists (10), it may also indicate that there is redundancy in processes that modulate the activity of brain reward circuits or that phasic increases in orexin tone alone (unopposed by coreleased dynorphin) are insufficient to convey a reward signal from the stimulation site in the lateral hypothalamus. These findings may at first seem inconsistent with the work of others who examined SB334867 on ICSS threshold during the dark phase (21). However, there is considerable evidence that decreases in orexin function can have consequences that depend on whether animals are tested during their light or dark phase. For example, food and water retain their rewarding effects in orexin KO mice when testing is performed during the dark phase but not during the light phase (22), the time at which we performed all of our behavioral testing.
To localize the effects of systemic SB334867 and norBNI administration on ICSS, a separate cohort of mice were implanted with LH stimulating electrodes and VTA guide cannulae. Microinfusion of SB334867 into VTA caused marked increases in reward thresholds, indicating decreased reward sensitivity. Although intra-VTA norBNI alone had no effect on ICSS thresholds, it blocked the threshold-elevating effects of subsequent SB334867 infusion (Fig. 2C; one-way repeated measures ANOVA for Drug: F3,9 = 10.98, P < 0.01). Although intracranial drug infusions tended to produce modest reductions in maximum rates of responding compared with systemic drug injections, these effects did not reach statistical significance (Fig. 2D; one-way repeated measures ANOVA for Drug: F3,9 = 1.03, P = 0.112).
Impulsivity Regulated by Orexin and Dynorphin Transmission.
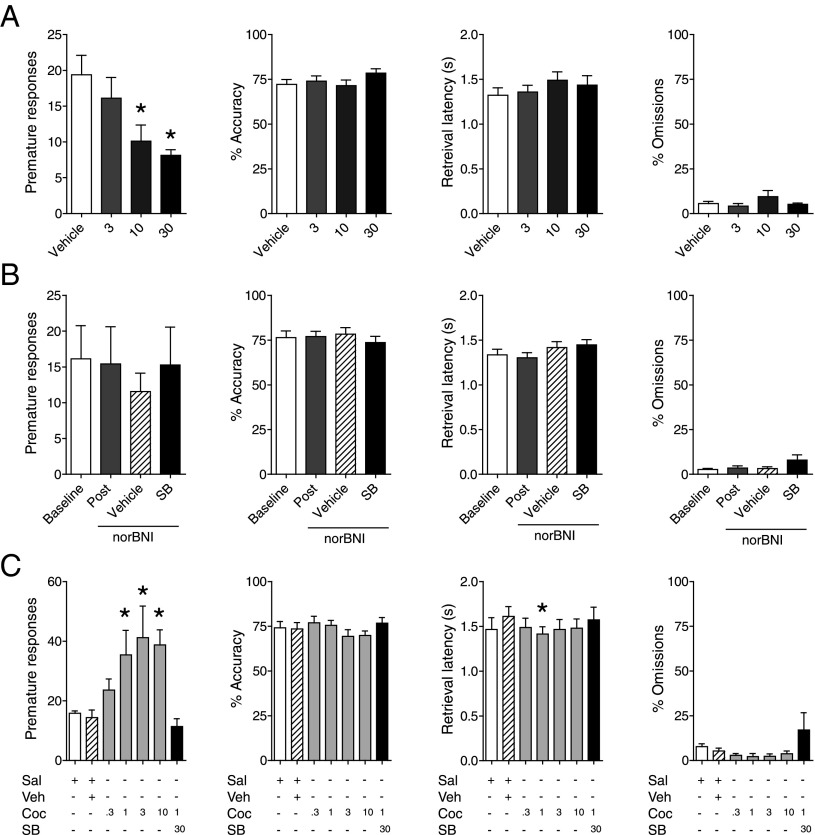
Impulsivity is characterized by deficits in the suppression of reward-seeking behaviors, with high levels of impulsivity being a common feature of many psychiatric illnesses (23). Drugs of abuse, including cocaine, can also trigger increases in impulsivity, which is hypothesized to drive the development of addiction (24). Considering the key role for coreleased orexin and dynorphin in controlling sensitivity to the rewarding effect of LH stimulation in the ICSS test, we hypothesized that interactions between these two neuropeptides may influence baseline impulsivity and cocaine-induced deficits in this behavior. Impulsivity can be quantified in rodents by measuring premature responses in the 5-choice serial reaction time task (5-CSRTT) (25), an animal model analogous to the continuous performance test used to study attention in humans. Premature responding in this test tends to be low under normal conditions and is exacerbated by drugs that elevate DA transmission (26). We used the 5-CSRTT to examine the contribution of the orexin–dynorphin system to spontaneous and cocaine-induced impulsive behavior. When administered alone, SB334867 further reduced the already low number of spontaneous premature responses (Fig. 3A; F3,21 = 4.89, P < 0.01). These reductions occurred in the absence of effects on response accuracy (F3,21 = 1.45, P = 0.25), pellet retrieval latency (F3,21 = 0.91, P = 0.44), or the number of stimulus trials completed (F3,21 = 1.46, P = 0.25), indicating that they were not due to degraded vigilance or motor capabilities. Administration of norBNI, however, reversed the effects of SB334867 on premature responding (Fig. 3B; F3,18 = 0.45, P = 0.71), suggesting that unopposed dynorphin transmission is critical in mediating these antiimpulsive effects. Given alone or in combination with SB334867, norBNI produced no effects on measures of response accuracy (F3,18 = 0.66, P > 0.58), latency (F3,18 = 3.09, P > 0.06), or the number of stimulus trials completed (F3,18 = 2.38, P > 0.10). Pretreatment with SB334867 also prevented the twofold increase in premature responding induced by cocaine (Fig. 3C; F6,24 = 5.84, P < 0.01). These data provide evidence that orexin neurotransmission can regulate impulsive behavior under both baseline and cocaine-stimulated conditions in a dynorphin-sensitive manner.
Fig. 3.
Impulsivity behavior regulated by balance of orexin and dynorphin. (A) SB334867 attenuates premature responses in the 5-CSRTT rat model of motor impulsivity. Measures of accuracy, latency to retrieve the food pellet, and number of trials omitted were unaffected (n = 8). *P < 0.05, difference from vehicle and 3-mg/kg groups. (B) Pretreatment with norBNI (10 mg/kg, i.p.) attenuates the effect of SB334867 (10 mg/kg, i.p.) on premature responding, whereas other measures remain unaffected. Post, Veh, and SB measures were made after norBNI injection (indicated by underlining) (n = 7). (C) Pretreatment with SB334867 blocks cocaine-induced increases in impulsive behavior. As in B, other measures of task performance were unaffected (n = 5). *P < 0.05. All data in A–C are represented as mean ± SEM. Coc, cocaine; Post, post-norBNI pretreatment; Sal, saline vehicle for cocaine; Veh, dimethyl sulfoxide vehicle for SB334867.
Dynorphin Mediates Reduced Cocaine Self-Administration in OX1R-Null Mice.
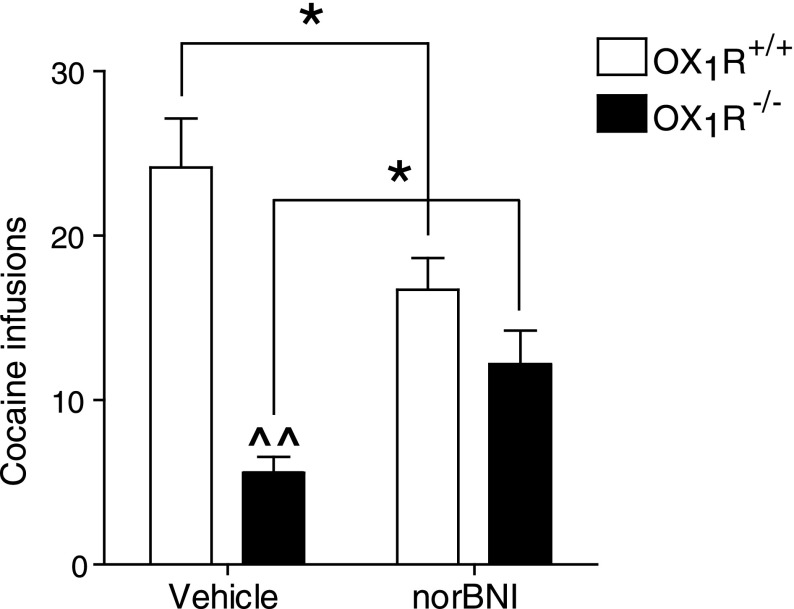
Vulnerability to addiction is markedly increased in impulsive individuals, and cocaine-induced increases in impulsivity are hypothesized to contribute to the emergence of addiction (23, 27). Moreover, orexin transmission and dynorphin transmission have been independently implicated in regulating the rewarding effects of cocaine and other drugs of abuse (28–32). We hypothesized that interactions between orexin and dynorphin transmission may directly control drug taking. To explore this possibility, we examined i.v. cocaine self-administration in genetically modified mice lacking OX1Rs (OX1R−/−). Mice of this genotype exhibit significantly lower cocaine self-administration across a broad range of doses (0.1–1 mg/kg per infusion) but demonstrate unaltered responding for food rewards under the same reinforcement schedules (33), suggesting that reductions in cocaine taking are not secondary to deficits in behavioral performance. Moreover, OX1R−/− mice show normal rates of cocaine self-administration during the approximately three initial sessions of cocaine access but then rapidly show decreases in cocaine taking (33). We confirmed this phenotype at a dose of 0.3 mg/kg per infusion, indicating that signaling via OX1Rs plays a critical role in establishing and maintaining cocaine self-administration behavior [Fig. 4; two-way repeated measures ANOVA, Genotype (between subjects factor) × Drug Treatment (within subjects factor): F1,12 = 12.91, P < 0.01]. Just as pretreatment with norBNI restored normal ICSS and impulsive-like behavior in mice given SB334867, it also partially restored cocaine self-administration in OX1R−/− mice, providing a unique example in which a behavioral deficit produced by genetic ablation in the function of one neurotransmitter system is rescued by blockade of another. These findings suggest that, in OX1R−/− mice, the unopposed actions of dynorphin attenuate the rewarding properties of cocaine, and thereby decrease self-administration of the drug. Interestingly, in OX1R+/+ (control) mice, norBNI unexpectedly reduced cocaine self-administration. One possible explanation for this effect is that dynorphin released by nonorexin neurons, such as the so-called “direct” striatonigral medium spiny neurons, has the opposite effects on cocaine intake and may actually facilitate the rewarding effects of cocaine. The existence of two populations of KORs with opposite roles in cocaine reward would also explain why norBNI only partially reversed the deficits in cocaine-taking behavior detected in the OX1R KO mice. Alternatively, KOR antagonism reduces the aversive or stressful effects of cocaine withdrawal (34), which contribute to drug intake patterns (17). Regardless, these data suggest that the depressive-like effects of dynorphin prevail in the absence of intact orexin signaling, producing reductions in the rewarding effects of cocaine, whereas the effects of orexin facilitate the rewarding effects of cocaine, extending their duration in the absence of dynorphin signaling.
Fig. 4.
Reduced cocaine self-administration in OX1R KO mice is restored by KOR blockade. Impairment of orexin signaling at OX1R by genetic deletion of this receptor reduces i.v. self-administration of cocaine (0.3 mg/kg per infusion). This deficit is partially rescued by pretreatment with norBNI (10 mg/kg, i.p.) (n = 7). *P < 0.05 between groups; ^^P < 0.01 within groups.
Orexin and Dynorphin Can Exert Balanced Opposing Effects on Excitability of VTA DA Neurons.
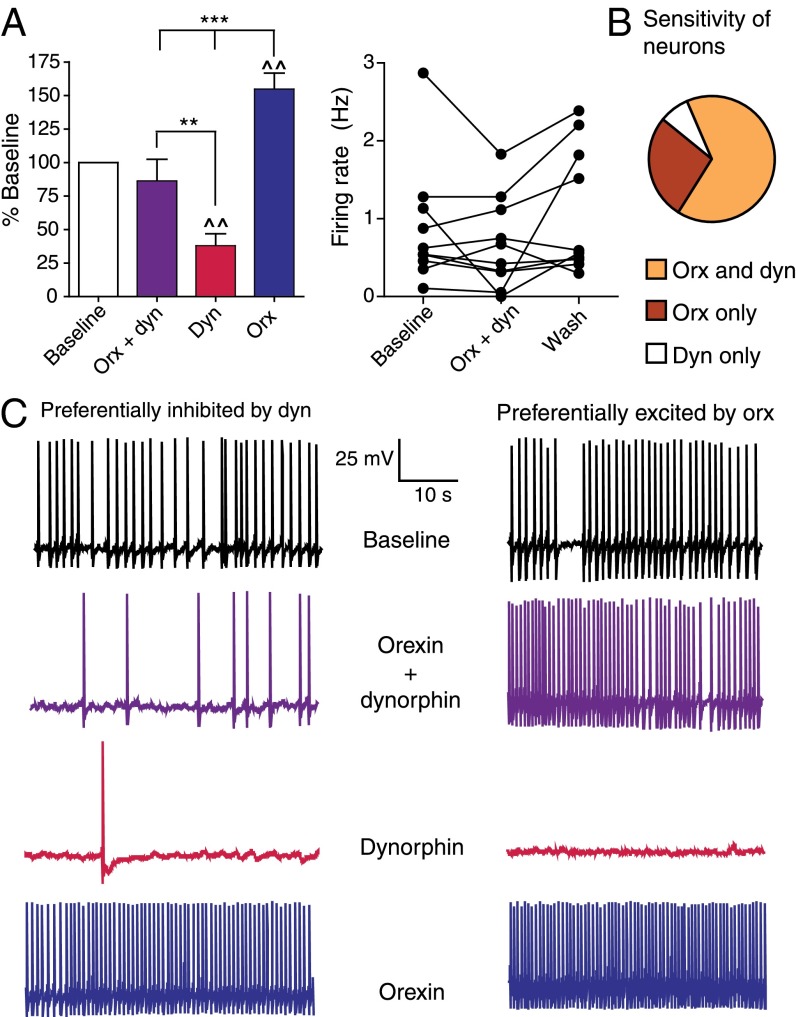
Brain structures that receive input from hypothalamic orexin and dynorphin neurons are potentially exposed to both peptides, and thus subject to their opposing effects on neuronal excitability (35, 36). The extent to which the effects of one peptide prevail over those of the other likely depends upon numerous factors, including the relative abundance of each peptide, longevity in the extracellular space, and receptor expression in different populations of target neurons, as well as interactions between the receptors and their intracellular signaling mechanisms in postsynaptic cells. Impulsivity and cocaine reward are regulated, at least in part, by DA neurons in the VTA (26), a prominent target of hypothalamic orexin-containing cells (37). Moreover, infusion of orexin into the VTA enhances drug seeking (6). To assess the relative contributions of each peptide on the activity of VTA neurons, we made electrophysiological recordings from DA cells in C57BL/6 mouse brain slices exposed to orexin and dynorphin applied singly or together. As anticipated, when applied separately, orexin was uniformly excitatory, whereas dynorphin produced only inhibitory effects (Fig. 5 A and B; F2,50 = 18.95, P ≤ 0.01). In the population of DA neurons recorded, most responded to saturating concentrations of both peptides, although a small minority were selectively responsive only to orexin or dynorphin (Fig. 5B). Remarkably, when both peptides were applied to the dual-responsive neurons (n = 10), there was no net effect on firing rate (Fig. 5A), suggesting that the opposing effects of each peptide at saturating concentrations effectively cancel one another out upon corelease. Four of the 10 neurons showed preferential inhibition by dynorphin despite the presence of orexin, whereas one cell was preferentially excited (>1.5-fold change) by orexin despite the presence of dynorphin (Fig. 5 A and C). Overall, although more cells were responsive to orexin than dynorphin, those cells that were responsive to both peptides had no net change in firing rate when orexin and dynorphin were coapplied, suggesting that the opposing influences of each peptide were balanced within the set of VTA DA neurons studied.
Fig. 5.
Orexin and dynorphin exert balanced but opposing effects on VTA DA neurons. (A, Left) Coapplied orexin and dynorphin result in no net change in the firing rate of VTA DA neurons (n = 10). Applied singly, dynorphin was inhibitory and orexin was excitatory. Values are presented as mean ± SEM. **P < 0.01; ***P < 0.001; ^^P < 0.01 difference from baseline. (A, Right) Effect of coapplied orexin and dynorphin on the firing rate of individual VTA DA neurons (●). (B) Summary of neuropeptide effects on neurons recorded (n = 26). Most cells tested were responsive to both peptides (65.4%), whereas the remaining cells were selectively responsive to either orexin (26.9%) or dynorphin (7.7%). (C) Representative traces show spontaneous firing of two different VTA DA neurons in midbrain slices after coapplication of orexin A and dynorphin A (1–17) and subsequent applications of either peptide alone. A number of dual-responsive cells showed preferential responsiveness to either peptide during coapplication. (Left) Pictured is a neuron in which the inhibitory effects of dynorphin occlude the excitatory effects of orexin. (Right) Conversely, some neurons were excited by orexin during coapplication with dynorphin (column of traces). Despite these effects, A shows no net change in excitability of the population of neurons recorded.
To elucidate further the potential orexin–dynorphin interactions within VTA DA neurons that were sensitive to both orexin and dynorphin, we attempted, alternatively, to augment the inhibitory effects of bath-applied dynorphin by treatment with SB334867 (Fig. S2A; F5,25 = 2.13, P < 0.01) or to enhance the excitatory effects of bath-applied orexin by treatment with norBNI (Fig. S2B; F3,27 = 5.48, P < 0.01). In both experiments, OX1R and KOR blockade failed to produce these effects, suggesting that SB334867 and norBNI are not exerting effects via nonspecific actions. More importantly, these data suggest that the tone of each peptide in vitro is insufficient to be influenced by application of small-molecule antagonists like SB334867 and norBNI. This finding is consistent with previous work indicating that exocytosis of large, peptide-containing vesicles typically occurs only at high sustained firing frequencies not normally present in slice preparations (38).
To verify that norBNI did not influence behavior through “off-target” actions directly at OX1Rs, we next examined the effects of this antagonist on OX1R signaling. Specifically, we used a fluorometric imaging plate reader (FLIPR) assay to determine the ability of bath-applied orexin A, SB334867, or norBNI to induce intracellular calcium transients in cultured CHO cells expressing human OX1Rs. Although orexin A produced the expected increases in intracellular calcium (EC50 = 0.01 μM) and SB334867 dose-dependently attenuated this effect (EC50 = 0.035 μM), norBNI failed to produce any effects on either baseline or orexin A-evoked increases in intracellular calcium. This suggests that the effects of norBNI on VTA DA neuronal physiology are solely via proposed KOR signaling mechanisms and the drug has no direct effect at OX1R (39) (Fig. S3 A–C).
Orexin–Dynorphin Interactions in VTA Regulate Cocaine Self-Administration.
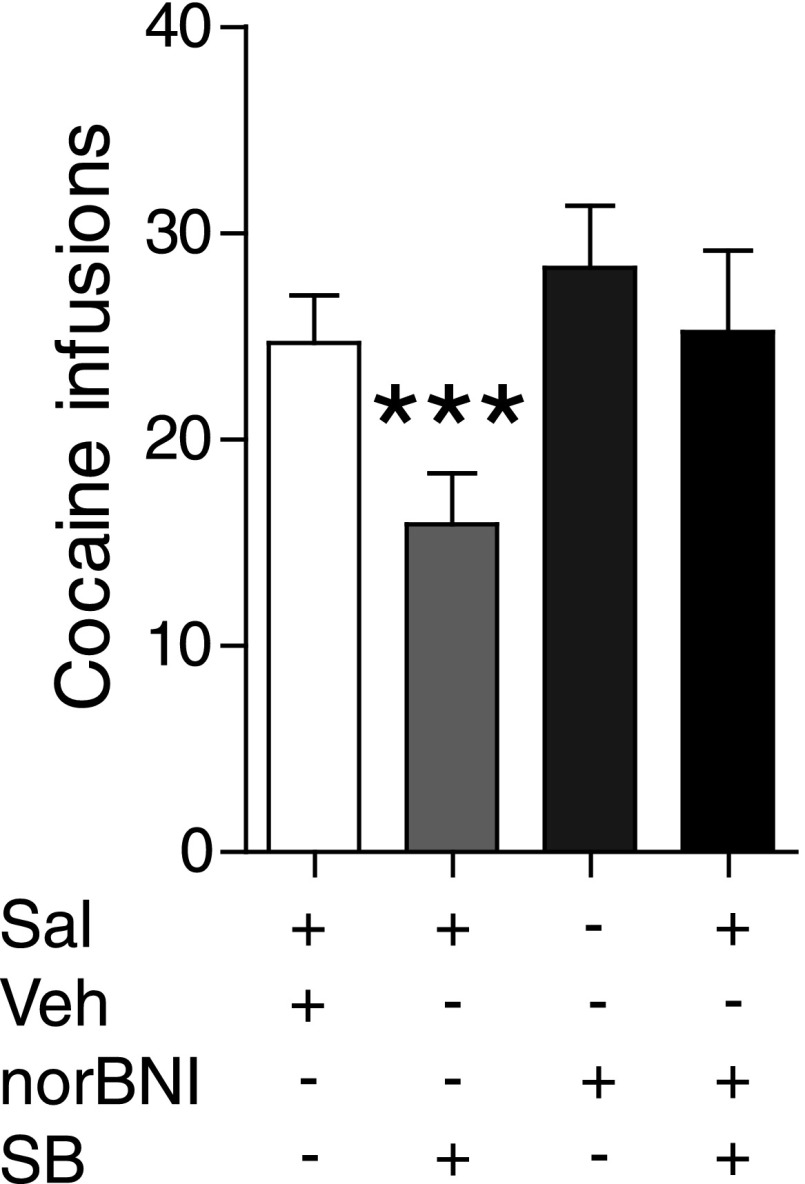
Our electrophysiology studies demonstrate that dynamic interactions between orexin and dynorphin regulate VTA DA activity, and that VTA neurons likely serve as a key substrate for the effects of the orexin–dynorphin system on motivated behaviors. To test this hypothesis directly, we examined the effects of intra-VTA infusion of SB334867 on i.v. cocaine self-administration in rats. Compared with intra-VTA vehicle infusion, intra-VTA SB334867 caused a marked reduction of cocaine intake that was blocked by norBNI (Fig. 6; one-way ANOVA: F3,24 = 11.56, P < 0.01), suggesting that unopposed dynorphin actions within this brain area attenuate cocaine reward. These results appear to be at variance with those that have demonstrated the absence of intra-VTA SB334867 on cocaine self-administration in low-effort fixed ratio 1 (FR1) schedules of reinforcement (40). However, several reports have shown that as task demands increase, SB334867 is more effective in reducing drug taking (2, 33). Because rats in this experiment were performing a higher effort FR5 schedule, the present findings are consistent with this literature. These data provide direct evidence that the opposing nature of orexin and dynorphin on VTA DA neuronal physiology may exert significant effects on reward-driven behaviors.
Fig. 6.
Orexin–dynorphin interactions in VTA mediate drug taking. Cocaine self-administration is reduced by intra-VTA SB334867 (3 μg per side), whereas this effect is reversed by pretreatment with norBNI (10 mg/kg, i.p.) (n = 9). ***P < 0.001.
Discussion
We report that orexin and dynorphin, neuropeptides that can produce opposite effects on motivation, are found in the same synaptic vesicles. The finding that these neuropeptides are copackaged and presumably coreleased under the same physiological conditions (16) has far-reaching implications because it raises the possibility that this process also occurs in systems traditionally conceptualized as depending primarily on individual transmitters. We also demonstrate that orexin, signaling via OX1Rs, attenuates key functional and behavioral effects of its cotransmitter dynorphin. Orexin-dynorphin neurons express increased levels of the immediate early gene c-Fos in response to rewards and reward-predictive cues (4, 6, 22), indicating high levels of neuronal activation that favor neuropeptide release. We then provide evidence that corelease of orexin can occlude effects of dynorphin on motivated behavior via its actions on DA neurons in the VTA. Blockade of orexin can produce dynorphin- or KOR agonist-like effects on ICSS and cocaine-related behaviors that are reversed with KOR antagonism (13, 41, 42). Previous studies of each of these peptides in isolation support these conclusions: Direct infusion of orexin into the VTA reinstates drug seeking (6), whereas intra-VTA infusion of KOR agonists produces depressive-like effects, such as dysphoria (43). We hypothesize that orexin normally acts together with excitatory reward-responsive inputs to the VTA [e.g., glutamate from prefrontal cortex and other structures (44, 45)] to overcome the inhibitory influence of dynorphin and local GABA transmission on DA neurons, enhancing forebrain DA release associated with reward and motivated behavior.
It is important to emphasize that although boosting orexin transmission appears to be able to offset the depressive effects of KOR activation, KOR antagonism does not produce a purely reciprocal effect (elevated reward function). We hypothesize that this may be due, in part, to the different pharmacodynamic and pharmacokinetic profiles of SB334867 and norBNI. The former drug shows classic activity and a t1/2 of ∼24 min (46), whereas a single injection of the latter produces functional antagonism of KORs that persists for weeks (10). In addition, prototypical KOR antagonists like norBNI are “biased agonists” that may simultaneously activate other signaling pathways, such as that of c-Jun kinase (39), thereby producing acute effects or compensatory adaptations that are sufficient to offset higher levels of orexin tone. Definitive conclusions about whether these effects are reciprocal await the development of short-acting KOR antagonists that do not act upon other intracellular signaling pathways; such compounds are not currently available (10). Further, the present experiments focus on the VTA and cannot rule out the possibility that the effects of orexin and dynorphin may not be dichotomous in other structures or that the VTA is the only structure in which orexin–dynorphin interactions influence behavior. For instance, there is evidence that orexin is involved in the stress response and may participate alongside dynorphin to engender negative affective states that accompany drug withdrawal (40, 47). Clearly, additional work is necessary to determine the circumstances, anatomical loci, and mechanisms that appear to permit concerted vs. opposing actions of orexin and dynorphin in different behavioral paradigms.
Our data also support the view that the action of both peptides is modulatory, because disruption of either OX1Rs or KORs reduced but did not abolish the behaviors tested. For instance, ICSS behavior persisted even at high doses of SB334867, demonstrating that orexin alone is not sufficient to account for the rewarding effects of LH stimulation. One possibility is that although orexin may not maintain ICSS behavior, it mitigates its disruption by offsetting the actions of dynorphin. Differential expression of orexin and dynorphin by the same population of hypothalamic neurons may be a mechanism by which the excitability of DA neurons in the VTA can be regulated by external stimuli, as well as by experience or disease. As one example, orexin mRNA levels are decreased following a type of chronic social stress that results in a depressive-like phenotype in mice (48) and rats (49). This depressive-like phenotype could be due, at least in part, to reductions in orexin expression that render the actions of dynorphin unopposed. These findings have important implications for interpreting data involving orexin and dynorphin in isolation, because adaptations in one system may be counterbalanced by adaptations in the other. They may also add flexibility in the design of therapeutic strategies to treat disorders ranging from narcolepsy to mood and impulse-control disorders. For example, a unique approach to treating conditions caused by dysregulation of orexin might be to manipulate KOR function, and, conversely, disorders characterized by altered dynorphin function might be offset by manipulations of orexin systems.
Materials and Methods
Animals.
The adult male C57BL/6J mice (8 wk of age; Jackson Laboratory) used in EM experiments were group-housed (three to five per cage); those used for ICSS were housed singly following surgery. Adult male (350 g) Sprague–Dawley rats (Charles River Laboratories) were used in the 5-CSRTT and cocaine self-administration experiments and were housed in groups of four. The adult male C57BL/6J mice (postnatal days 19–21) used for the electrophysiology experiments were group-housed (three to five per cage). The OX1−/− mice and their OX1+/+ littermates (6 wk of age) used for the self-administration studies were obtained from Jackson Laboratory and were backcrossed more than seven generations to C57BL/6 mice. These mice were group-housed (two per cage). All animals were housed under temperature-controlled conditions on a 12-h light/dark cycle, and behavioral testing occurred 4–5 h into the light cycle; food and water were available ad libitum unless otherwise indicated. Procedures were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (50) and were approved by the Institutional Animal Care and Use Committees of McLean Hospital, the University of British Columbia, and Scripps Florida.
Immunohistochemistry and Microscopy.
Fluorescence and silver-enhanced gold immunolabeling for orexin A or prodynorphin was carried out on alternate mouse brain sections according to previously reported procedures (51), and processed following standard EM protocols. Nonoverlapping regions of immunolabeled tissue were then randomly selected and photographed for quantification of particles using ImageJ software (National Institutes of Health) (SI Materials and Methods, Immunohistochemistry and Microscopy).
Electrophysiology.
Patch pipettes (3–5 MΩ) were filled with 143 mM potassium gluconate, 10 mM Hepes, 0.2 mM EGTA, 2 mM MgATP, 0.3 mM NaGTP (with a pH of 7.2), and 270–280 mM mOsmol. Data were acquired at 20 kHz and filtered at 2 kHz using pClamp 10.0 software (Molecular Devices). After acquiring a whole-cell configuration, cells were voltage-clamped at −70 mV and a series of voltage steps (250 ms, from −60 to −130 mV in 10-mV steps) were applied to detect hyperpolarizing (Ih) currents. Ih was determined as the change in current between ∼30 ms and 248 ms after the voltage step was applied. Intrinsic activity of VTA DA neurons was measured in current-clamp mode. Experiments began when a stable baseline firing rate was achieved; substrates were then applied for 5 min and subsequently washed out with artificial cerebrospinal fluid. The last 3 min of each 5-min segment were used for data analysis. Dynorphin A (1–17; 200 nM) and orexin A (100 nM) were obtained from American Peptide and dissolved in distilled water. These concentrations were previously found to exert saturating effects on VTA cell activity (5, 52). Thiorphan (1 μM) and bestatin (10 μM) were obtained from Sigma–Aldrich, dissolved in distilled water, and applied together with dynorphin A (SI Materials and Methods, Electrophysiology).
FLIPR Assay.
OX1R activity was assessed by measuring intracellular calcium levels by FLIPR assay as described previously (53) (SI Materials and Methods, Fluorometric Imaging Plate Reader Assay).
ICSS.
Mice were implanted with monopolar stimulating electrodes or cannulae (PlasticsOne) under ketamine/xylazine (80 and 10 mg/kg, respectively, i.p.; Sigma) directed stereotactically to the LH (18) and/or contralateral VTA [from bregma: anteroposterior (AP), −3.2 mm; mediolateral (ML), −0.5 mm; dorsoventral (DV), −4.7 mm from dura]. After a 7-d recovery period, mice were trained to respond for brain stimulation as described previously (18). The lowest frequency that supported responding (threshold) was computed using least-squares line-of-best-fit analysis. When mice fulfilled stability criteria for ICSS thresholds (±10% over 5 consecutive days), effects of drug treatments were measured. SB334867 (Scripps Florida) or DMSO vehicle was given on alternate days using a Hamilton syringe (0.1 mL/kg i.p.), and thresholds were immediately quantified in 15-min test sessions. The norBNI (10 mg/kg i.p.; Sigma) was given in saline (10 mL/kg) 48 h before the start of ICSS testing.
The 5-CSRTT.
Rats were food-restricted (to 85% of free-feeding weight) and trained in computer-controlled operant chambers housed inside ventilated, sound-attenuating cabinets (Med Associates), and 5-CSRTT procedures carried out as described (25). SB334867 (in DMSO, 0.1 mL/kg) and/or cocaine (in saline, 1 mL/kg; Sigma) was given by i.p. injection 10 min before testing, as in other experiments, and norBNI was given at least 48 h before the start of testing (SI Materials and Methods, The 5-Choice Serial Reaction Time Task).
I.V. Cocaine Self-Administration.
Rats and mice were anesthetized with an isoflurane (1–3% vol/vol) oxygen vapor mixture and surgically prepared with Silastic (VWR Scientific) catheters in the jugular vein according to established procedures (54). Immediately following catheter implantation in the rats, bilateral stainless-steel guide cannulae (23 gauge, 17 mm in length) were implanted in the VTA (from bregma: AP, 5.3 mm; ML, ±0.7 mm; DV, −7.5 mm from dura). Testing with SB334867 or norBNI during 60-min daily sessions was performed after stable cocaine intake was achieved (<20% variation in responding for 3 consecutive days; SI Materials and Methods, I.V. Cocaine Self-Administration).
Statistics.
Data are expressed as mean ± SEM. For ICSS experiments, two-way repeated measures ANOVA was used to compare means between SB334867-treated and norBNI + SB334867-treated conditions. One-way repeated measures ANOVA with Newman–Keuls post hoc tests was used to compare means within SB334867 and norBNI + SB334867 conditions. One-way repeated measures ANOVA and Newman–Keuls tests were also used to compare means in all 5CSRTT experiments. Two-way repeated measures ANOVA was used to compare means between treatment groups in cocaine self-administration experiments with OX1R KO mice. One-way repeated measures ANOVA and Newman–Keuls tests were used to compare mean responses to orexin and dynorphin by VTA DA neurons. One-way repeated measures ANOVA and Newman–Keuls tests were also used to compare means of cocaine intake in rats treated with SB334867 and norBNI. Differences were considered significant if P < 0.05.
Supplementary Material
Acknowledgments
We thank Dr. Garrett Fitzmaurice for helpful comments on the manuscript and Miranda S. Gallo and Melissa Chen for assistance in data collection. This work was supported by National Institutes of Health Grants F32-DA026250 and K99-DA031767 (to J.W.M.), F32-DA024932 and K99-DA031222 (to J.A.H.), R01-DA023915 (to. P.J.K.), and R01-MH063266 (to W.A.C.) and by a Natural Sciences and Engineering Research Council discovery grant (to S.L.B.).
Footnotes
Conflict of interest statement: W.A.C. holds a patent (US Patent 6,528,518; Assignee: McLean Hospital) related to the use of kappa-opioid antagonists for the treatment of depressive disorders. All other authors declare no competing financial interests.
This article is a PNAS Direct Submission.
See Commentary on page 5765.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1315542111/-/DCSupplemental.
References
- 1.Adamantidis AR, Zhang F, Aravanis AM, Deisseroth K, de Lecea L. Neural substrates of awakening probed with optogenetic control of hypocretin neurons. Nature. 2007;450(7168):420–424. doi: 10.1038/nature06310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Borgland SL, et al. Orexin A/hypocretin-1 selectively promotes motivation for positive reinforcers. J Neurosci. 2009;29(36):11215–11225. doi: 10.1523/JNEUROSCI.6096-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sharf R, et al. Orexin signaling via the orexin 1 receptor mediates operant responding for food reinforcement. Biol Psychiatry. 2010;67(8):753–760. doi: 10.1016/j.biopsych.2009.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Muschamp JW, Dominguez JM, Sato SM, Shen RY, Hull EM. A role for hypocretin (orexin) in male sexual behavior. J Neurosci. 2007;27(11):2837–2845. doi: 10.1523/JNEUROSCI.4121-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borgland SL, Taha SA, Sarti F, Fields HL, Bonci A. Orexin A in the VTA is critical for the induction of synaptic plasticity and behavioral sensitization to cocaine. Neuron. 2006;49(4):589–601. doi: 10.1016/j.neuron.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 6.Harris GC, Wimmer M, Aston-Jones G. A role for lateral hypothalamic orexin neurons in reward seeking. Nature. 2005;437(7058):556–559. doi: 10.1038/nature04071. [DOI] [PubMed] [Google Scholar]
- 7.Peyron C, et al. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci. 1998;18(23):9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marcus JN, et al. Differential expression of orexin receptors 1 and 2 in the rat brain. J Comp Neurol. 2001;435(1):6–25. doi: 10.1002/cne.1190. [DOI] [PubMed] [Google Scholar]
- 9.Bruchas MR, Land BB, Chavkin C. The dynorphin/kappa opioid system as a modulator of stress-induced and pro-addictive behaviors. Brain Res. 2010;1314:44–55. doi: 10.1016/j.brainres.2009.08.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carroll FI, Carlezon WA., Jr Development of κ opioid receptor antagonists. J Med Chem. 2013;56(6):2178–2195. doi: 10.1021/jm301783x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chavkin C, James IF, Goldstein A. Dynorphin is a specific endogenous ligand of the kappa opioid receptor. Science. 1982;215(4531):413–415. doi: 10.1126/science.6120570. [DOI] [PubMed] [Google Scholar]
- 12.Bruijnzeel AW. kappa-Opioid receptor signaling and brain reward function. Brain Res Brain Res Rev. 2009;62(1):127–146. doi: 10.1016/j.brainresrev.2009.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wee S, Koob GF. The role of the dynorphin-kappa opioid system in the reinforcing effects of drugs of abuse. Psychopharmacology (Berl) 2010;210(2):121–135. doi: 10.1007/s00213-010-1825-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shippenberg TS, Zapata A, Chefer VI. Dynorphin and the pathophysiology of drug addiction. Pharmacol Ther. 2007;116(2):306–321. doi: 10.1016/j.pharmthera.2007.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang Y, Butelman ER, Schlussman SD, Ho A, Kreek MJ. Effect of the endogenous kappa opioid agonist dynorphin A(1-17) on cocaine-evoked increases in striatal dopamine levels and cocaine-induced place preference in C57BL/6J mice. Psychopharmacology (Berl) 2004;172(4):422–429. doi: 10.1007/s00213-003-1688-3. [DOI] [PubMed] [Google Scholar]
- 16.Li Y, van den Pol AN. Differential target-dependent actions of coexpressed inhibitory dynorphin and excitatory hypocretin/orexin neuropeptides. J Neurosci. 2006;26(50):13037–13047. doi: 10.1523/JNEUROSCI.3380-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koob GF, Le Moal M. Addiction and the brain antireward system. Annu Rev Psychol. 2008;59:29–53. doi: 10.1146/annurev.psych.59.103006.093548. [DOI] [PubMed] [Google Scholar]
- 18.Chou TC, et al. Orexin (hypocretin) neurons contain dynorphin. J Neurosci. 2001;21(19):RC168. doi: 10.1523/JNEUROSCI.21-19-j0003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bamford NS, et al. Heterosynaptic dopamine neurotransmission selects sets of corticostriatal terminals. Neuron. 2004;42(4):653–663. doi: 10.1016/s0896-6273(04)00265-x. [DOI] [PubMed] [Google Scholar]
- 20.Carlezon WA, Jr, Chartoff EH. Intracranial self-stimulation (ICSS) in rodents to study the neurobiology of motivation. Nat Protoc. 2007;2(11):2987–2995. doi: 10.1038/nprot.2007.441. [DOI] [PubMed] [Google Scholar]
- 21.Riday TT, et al. Orexin-1 receptor antagonism does not reduce the rewarding potency of cocaine in Swiss-Webster mice. Brain Res. 2012;1431:53–61. doi: 10.1016/j.brainres.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McGregor R, Wu MF, Barber G, Ramanathan L, Siegel JM. Highly specific role of hypocretin (orexin) neurons: Differential activation as a function of diurnal phase, operant reinforcement versus operant avoidance and light level. J Neurosci. 2011;31(43):15455–15467. doi: 10.1523/JNEUROSCI.4017-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Wit H. Impulsivity as a determinant and consequence of drug use: A review of underlying processes. Addict Biol. 2009;14(1):22–31. doi: 10.1111/j.1369-1600.2008.00129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Winstanley CA, Olausson P, Taylor JR, Jentsch JD. Insight into the relationship between impulsivity and substance abuse from studies using animal models. Alcohol Clin Exp Res. 2010;34(8):1306–1318. doi: 10.1111/j.1530-0277.2010.01215.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bari A, Dalley JW, Robbins TW. The application of the 5-choice serial reaction time task for the assessment of visual attentional processes and impulse control in rats. Nat Protoc. 2008;3(5):759–767. doi: 10.1038/nprot.2008.41. [DOI] [PubMed] [Google Scholar]
- 26.Robbins TW. The 5-choice serial reaction time task: Behavioural pharmacology and functional neurochemistry. Psychopharmacology (Berl) 2002;163(3-4):362–380. doi: 10.1007/s00213-002-1154-7. [DOI] [PubMed] [Google Scholar]
- 27.Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35(1):217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smith RJ, See RE, Aston-Jones G. Orexin/hypocretin signaling at the orexin 1 receptor regulates cue-elicited cocaine-seeking. Eur J Neurosci. 2009;30(3):493–503. doi: 10.1111/j.1460-9568.2009.06844.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boutrel B, et al. Role for hypocretin in mediating stress-induced reinstatement of cocaine-seeking behavior. Proc Natl Acad Sci USA. 2005;102(52):19168–19173. doi: 10.1073/pnas.0507480102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Narita M, et al. Direct involvement of orexinergic systems in the activation of the mesolimbic dopamine pathway and related behaviors induced by morphine. J Neurosci. 2006;26(2):398–405. doi: 10.1523/JNEUROSCI.2761-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hollander JA, Lu Q, Cameron MD, Kamenecka TM, Kenny PJ. Insular hypocretin transmission regulates nicotine reward. Proc Natl Acad Sci USA. 2008;105(49):19480–19485. doi: 10.1073/pnas.0808023105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lawrence AJ, Cowen MS, Yang HJ, Chen F, Oldfield B. The orexin system regulates alcohol-seeking in rats. Br J Pharmacol. 2006;148(6):752–759. doi: 10.1038/sj.bjp.0706789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hollander JA, Pham D, Fowler CD, Kenny PJ. Hypocretin-1 receptors regulate the reinforcing and reward-enhancing effects of cocaine: Pharmacological and behavioral genetics evidence. Front Behav Neurosci. 2012;6:47. doi: 10.3389/fnbeh.2012.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Potter DN, Damez-Werno D, Carlezon WA, Jr, Cohen BM, Chartoff EH. Repeated exposure to the κ-opioid receptor agonist salvinorin A modulates extracellular signal-regulated kinase and reward sensitivity. Biol Psychiatry. 2011;70(8):744–753. doi: 10.1016/j.biopsych.2011.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Korotkova TM, Sergeeva OA, Eriksson KS, Haas HL, Brown RE. Excitation of ventral tegmental area dopaminergic and nondopaminergic neurons by orexins/hypocretins. J Neurosci. 2003;23(1):7–11. doi: 10.1523/JNEUROSCI.23-01-00007.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Margolis EB, Hjelmstad GO, Bonci A, Fields HL. Kappa-opioid agonists directly inhibit midbrain dopaminergic neurons. J Neurosci. 2003;23(31):9981–9986. doi: 10.1523/JNEUROSCI.23-31-09981.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fadel J, Deutch AY. Anatomical substrates of orexin-dopamine interactions: Lateral hypothalamic projections to the ventral tegmental area. Neuroscience. 2002;111(2):379–387. doi: 10.1016/s0306-4522(02)00017-9. [DOI] [PubMed] [Google Scholar]
- 38.Torrealba F, Carrasco MA. A review on electron microscopy and neurotransmitter systems. Brain Res Brain Res Rev. 2004;47(1-3):5–17. doi: 10.1016/j.brainresrev.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 39.Bruchas MR, Chavkin C. Kinase cascades and ligand-directed signaling at the kappa opioid receptor. Psychopharmacology (Berl) 2010;210(2):137–147. doi: 10.1007/s00213-010-1806-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sharf R, Sarhan M, Dileone RJ. Role of orexin/hypocretin in dependence and addiction. Brain Res. 2010;1314:130–138. doi: 10.1016/j.brainres.2009.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Todtenkopf MS, Marcus JF, Portoghese PS, Carlezon WA., Jr Effects of kappa-opioid receptor ligands on intracranial self-stimulation in rats. Psychopharmacology (Berl) 2004;172(4):463–470. doi: 10.1007/s00213-003-1680-y. [DOI] [PubMed] [Google Scholar]
- 42.Tomasiewicz HC, Todtenkopf MS, Chartoff EH, Cohen BM, Carlezon WA., Jr The kappa-opioid agonist U69,593 blocks cocaine-induced enhancement of brain stimulation reward. Biol Psychiatry. 2008;64(11):982–988. doi: 10.1016/j.biopsych.2008.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bals-Kubik R, Ableitner A, Herz A, Shippenberg TS. Neuroanatomical sites mediating the motivational effects of opioids as mapped by the conditioned place preference paradigm in rats. J Pharmacol Exp Ther. 1993;264(1):489–495. [PubMed] [Google Scholar]
- 44.Moorman DE, Aston-Jones G. Orexin/hypocretin modulates response of ventral tegmental dopamine neurons to prefrontal activation: Diurnal influences. J Neurosci. 2010;30(46):15585–15599. doi: 10.1523/JNEUROSCI.2871-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mahler SV, Smith RJ, Aston-Jones G. Interactions between VTA orexin and glutamate in cue-induced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl) 2013;226(4):687–698. doi: 10.1007/s00213-012-2681-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Porter RA, et al. 1,3-Biarylureas as selective non-peptide antagonists of the orexin-1 receptor. Bioorg Med Chem Lett. 2001;11(14):1907–1910. doi: 10.1016/s0960-894x(01)00343-2. [DOI] [PubMed] [Google Scholar]
- 47.Koob GF. A role for brain stress systems in addiction. Neuron. 2008;59(1):11–34. doi: 10.1016/j.neuron.2008.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lutter M, et al. Orexin signaling mediates the antidepressant-like effect of calorie restriction. J Neurosci. 2008;28(12):3071–3075. doi: 10.1523/JNEUROSCI.5584-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nocjar C, Zhang J, Feng P, Panksepp J. The social defeat animal model of depression shows diminished levels of orexin in mesocortical regions of the dopamine system, and of dynorphin and orexin in the hypothalamus. Neuroscience. 2012;218:138–153. doi: 10.1016/j.neuroscience.2012.05.033. [DOI] [PubMed] [Google Scholar]
- 50.Committee on Care and Use of Laboratory Animals . Guide for the Care and Use of Laboratory Animals. Bethesda: Natl Inst Health; 1985. DHHS Publ No (NIH) 85-23. [Google Scholar]
- 51.Yi H, Leunissen J, Shi G, Gutekunst C, Hersch S. A novel procedure for pre-embedding double immunogold-silver labeling at the ultrastructural level. J Histochem Cytochem. 2001;49(3):279–284. doi: 10.1177/002215540104900301. [DOI] [PubMed] [Google Scholar]
- 52.Ford CP, Beckstead MJ, Williams JT. Kappa opioid inhibition of somatodendritic dopamine inhibitory postsynaptic currents. J Neurophysiol. 2007;97(1):883–891. doi: 10.1152/jn.00963.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Smart D, et al. Characterization of recombinant human orexin receptor pharmacology in a Chinese hamster ovary cell-line using FLIPR. Br J Pharmacol. 1999;128(1):1–3. doi: 10.1038/sj.bjp.0702780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fowler CD, Lu Q, Johnson PM, Marks MJ, Kenny PJ. Habenular α5 nicotinic receptor subunit signalling controls nicotine intake. Nature. 2011;471(7340):597–601. doi: 10.1038/nature09797. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.