Significance
Replication of many positive-strand RNA viruses is cis-preferential: i.e., viral replicase proteins replicate genomic RNA molecules that have served as translation templates for their own synthesis, but not the other molecules in the same cell. Here, we show that tobacco mosaic virus replicase cotranslationally binds the 5′ untranslated region of genomic RNA and that this binding inhibits further translation and leads to genomic RNA replication. Intriguingly, full-length replicase protein could not bind genomic RNA posttranslationally due to autoinhibition by the C-terminal domain. These results reveal an elegant viral strategy to enable cis-preferential replication and phase switching from translation to replication at once.
Abstract
Genomic RNA of positive-strand RNA viruses replicate via complementary (i.e., negative-strand) RNA in membrane-bound replication complexes. Before replication complex formation, virus-encoded replication proteins specifically recognize genomic RNA molecules and recruit them to sites of replication. Moreover, in many of these viruses, selection of replication templates by the replication proteins occurs preferentially in cis. This property is advantageous to the viruses in several aspects of viral replication and evolution, but the underlying molecular mechanisms have not been characterized. Here, we used an in vitro translation system to show that a 126-kDa replication protein of tobacco mosaic virus (TMV), a positive-strand RNA virus, binds a 5′-terminal ∼70-nucleotide region of TMV RNA cotranslationally, but not posttranslationally. TMV mutants that carried nucleotide changes in the 5′-terminal region and showed a defect in the binding were unable to synthesize negative-strand RNA, indicating that this binding is essential for template selection. A C-terminally truncated 126-kDa protein, but not the full-length 126-kDa protein, was able to posttranslationally bind TMV RNA in vitro, suggesting that binding of the 126-kDa protein to the 70-nucleotide region occurs during translation and before synthesis of the C-terminal inhibitory domain. We also show that binding of the 126-kDa protein prevents further translation of the bound TMV RNA. These data provide a mechanistic explanation of how the 126-kDa protein selects replication templates in cis and how fatal collision between translating ribosomes and negative-strand RNA-synthesizing polymerases on the genomic RNA is avoided.
Virions of positive-strand RNA viruses contain genomic RNA of messenger sense. After infection, genomic RNA is released from the virions into the cytoplasm and translated to produce viral proteins, including viral RNA-dependent RNA polymerases and other replication-related proteins. These proteins are collectively called “replication proteins.” In eukaryotic positive-strand RNA viruses, replication proteins recruit genomic RNA to the cytoplasmic face of intracellular membranes to form replication complexes (1, 2). Negative-strand RNAs that are complementary to genomic RNAs are synthesized in the replication complexes, and then, using the negative-strand RNAs as templates, genomic RNA is copied and released into the cytoplasm. The recognition of template RNAs and their recruitment to the replication complexes are key processes in selective amplification of genomic RNA by positive-strand RNA viruses. In several positive-strand RNA viruses, cis-acting elements for replication-template selection have been identified, and, for some of them, it was demonstrated that replication proteins directly bind to these elements (3).
Replication of tobacco mosaic virus (TMV), poliovirus, and many other positive-strand RNA viruses is cis-preferential: i.e., replication proteins recognize their own translation templates for replication (4–13). Because viral RNA replication is error-prone, it is important for viruses to selectively eliminate defective genomes. Template selection in cis is apparently advantageous in this regard because the genomes that encode replication proteins of lower performance are amplified less efficiently. Despite its importance in viral replication as well as evolution, little is known about how replication proteins select a template RNA in cis although it was proposed that requirement of nascent or newly synthesized replication proteins for replication and restricted diffusion or integrity of the proteins underlie the phenomenon (6).
The genomic RNAs of positive-strand RNA viruses serve as templates for both translation and negative-strand RNA synthesis. During negative-strand RNA synthesis, viral RNA polymerases move along genomic RNA templates in a 3′-to-5′ direction. On the other hand, ribosomes synthesize viral proteins moving along the genomic RNA templates in a 5′-to-3′ direction. If these reactions take place on a single genomic RNA molecule at the same time, RNA polymerases and ribosomes collide, which results in the collapse of both reactions because these molecules cannot reverse direction or detach from the template RNA (14). Thus, positive-strand RNA viruses must clear ribosomes from the genomic RNA strands before negative-strand RNA synthesis occurs (15, 16).
TMV belongs to the alpha-like virus superfamily of positive-strand RNA viruses. Its genome is a 5′-capped monopartite RNA and encodes at least four proteins, including the 5′ terminal 126-kDa protein, its translational read-through product of 183 kDa, a 30-kDa cell-to-cell movement protein, and a 17.5-kDa coat protein (17). The 126-kDa and 183-kDa proteins are replication proteins (18). The 126-kDa protein harbors a methyltransferase-like domain that is involved in RNA 5′ capping in its N-terminal region and a helicase-like domain in its C-terminal region. A region between these two domains is called the intervening region, or IR. The read-through part of the 183-kDa protein contains a polymerase-like domain (19). A deletion derivative of TMV RNA, named TMV126 RNA, that encodes the 126-kDa protein but not the 183-kDa protein can replicate when the 183-kDa protein is supplied in trans from a helper virus. However, TMV126 mutants that do not encode functional 126-kDa protein cannot replicate even if the wild-type 126-kDa and 183-kDa proteins are supplied in trans (8). This and other observations indicate that the 126-kDa protein functions primarily in cis (20, 21). The 5′ untranslated region (UTR) of TMV genomic RNA called Ω is ∼70 nucleotides (nt) in length, contains 12 CAA repeats, and is reported to have unusual tertiary structure with non-Watson–Crick base pairing (22, 23). The 5′ UTR of TMV RNA is a well-known translation enhancer (24, 25) and is essential for efficient virus multiplication (26). However, the role of the 5′ UTR in replication has been unclear, mainly due to the lack of experimental systems to separately evaluate translation of viral RNA and negative- and positive-stand RNA synthesis.
To dissect the process that precedes the formation of the tobamovirus RNA replication complex on membranes, we previously developed an in vitro translation-replication system (27). Using an evacuolated tobacco protoplast extract (BYL) from which membranes were removed by centrifugation (membrane-depleted BYL, or mdBYL), we demonstrated that the replication proteins of tomato mosaic virus (ToMV), a close relative of TMV, bind ToMV RNA to form a ribonucleoprotein complex named premembrane-targeting complex (PMTC) in a translation-coupled manner (28). The PMTC is inactive in RNA synthesis but forms an active replication complex capable of synthesizing negative-strand and positive-strand RNA when it is mixed with membranes prepared from BYL. PMTC-like ribonucleoprotein (core-PMTC) is formed when a ToMV derivative that expresses the 126-kDa protein, but not the 183-kDa protein, is translated in mdBYL, which can form a replication complex when the 183-kDa protein and membranes are posttranslationally supplied (28). In the current study, we characterized tobamovirus PMTC and obtained results that provide insight into how the genomic RNA of TMV is selected as a template for replication preferentially in cis as well as how collisions between replication proteins and ribosomes are avoided.
Results
Purification of PMTC.
In our previous study, sucrose gradient centrifugation was used to purify ToMV PMTC (28); however, this method provides only a small amount of PMTC with sucrose contamination. To facilitate more extensive analysis of tobamovirus PMTC, we used size-exclusion chromatography (SEC) to obtain a larger amount of PMTC. In addition, because TMV RNA was more efficiently translated and replicated than ToMV RNA in BYL, we used TMV instead of ToMV in subsequent experiments.
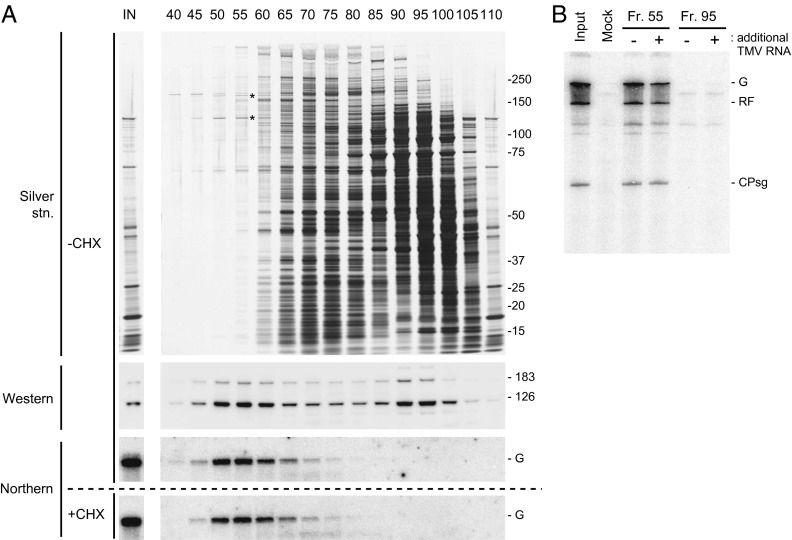
TMV RNA was translated in mdBYL, and the reaction mixture was directly fractionated by SEC using a Sephracryl S-500 column (Fig. 1A). EDTA was added to the mixture before loading onto the column to dissociate ribosomes from mRNA. TMV RNA and the replication proteins eluted together around fraction no. 55, preceding ribosomes (eluted in fractions no. 60–85). The replication proteins also eluted around fraction no. 95, in which TMV RNA was not detected (Fig. 1A). When an aliquot of fraction no. 55 was mixed with creatine phosphate, creatine kinase, ATP, and BYL membranes (30,000 × g pellet of BYL) followed by further addition of ribonucleoside triphosphates, TMV-related RNAs were synthesized (Fig. 1B), indicating that fractions around no. 55 contained PMTC (hereafter referred to as the PMTC fraction). The 126-kDa and 183-kDa proteins were the major protein components of fraction no. 55 (Fig. 1A). The replication proteins in fraction no. 95 were incapable of supporting the replication of exogenously added TMV RNA, even when BYL membranes were supplemented (Fig. 1B), confirming the previous result that PMTC formation is coupled with translation (28). When TMV RNA was incubated in mdBYL with a translation inhibitor, cycloheximide (CHX), and subjected to SEC using a Sephacryl S-500 column, TMV RNA was fractionated in a similar pattern (i.e., peaked at fraction no. 55) (Fig. 1A, Bottom).
Fig. 1.
Purification of PMTC by SEC. (A) Elution profile. TMV RNA was translated in mdBYL, and the translation mixture was fractionated by SEC using a Sephacryl S-500 column. Protein and RNA from indicated fractions were analyzed by SDS-polyacrylamide gel electrophoresis (SDS/PAGE) followed by silver staining (Top), Western blotting using anti–126-kDa protein antibodies (second panel from Top), and Northern hybridization using a 32P-labeled RNA probe that was complementary to TMV RNA (third panel from Top). (Bottom) The elution profile of TMV RNA for a control sample, in which the translation reaction in mdBYL was inhibited by CHX. Positions of size markers (kDa) are shown on the right, and the 126-kDa and 183-kDa proteins are indicated by asterisks on the silver-stained gel. Positions of the 126-kDa and 183-kDa proteins on the Western blot and TMV genomic RNA (G) on the Northern blots are also indicated. (B) TMV RNA replication tests using fraction nos. 55 and 95. Each fraction was mixed with a P30BYL membrane suspension (for lanes marked with +, purified TMV RNA was also added), incubated at 15 °C for 1 h, and further incubated with [α-32P]CTP and other ribonucleoside triphosphates. RNA was purified, separated using 8 M urea-2.4% PAGE, and 32P signals were detected using an image analyzer (BAS2500). Positions of the genomic (G) and replicative form (RF) RNAs and coat protein subgenomic RNA (CPsg) are indicated on the right.
Nuclease Resistance of the 5′ UTR of TMV RNA in PMTC.
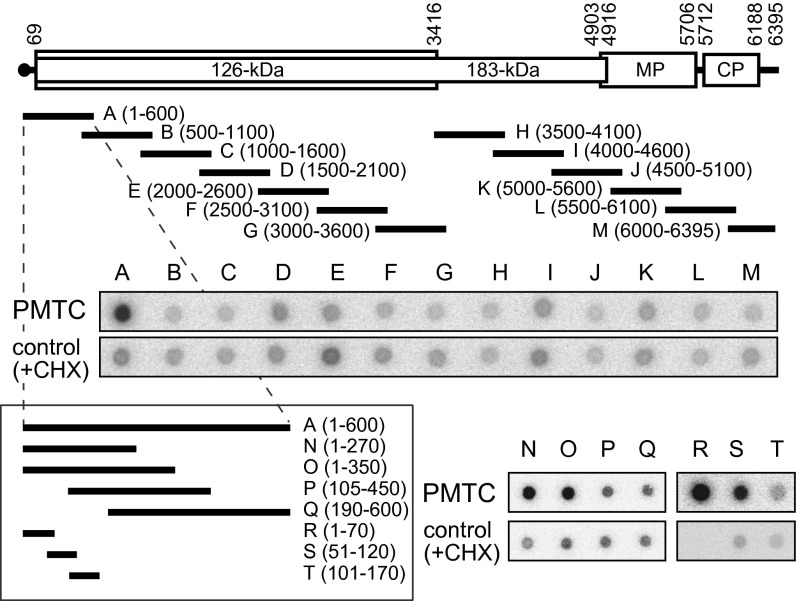
We hypothesized that replication proteins bind to a specific region of TMV RNA for selection of a replication template and that the bound region is protected from nuclease digestion. To test this possibility, the PMTC fraction was treated with micrococcal nuclease (MNase). RNA purified from the reaction mixture after MNase treatment was labeled with 32P at the 5′ terminus and hybridized to membrane-blotted 13-arrayed TMV double-stranded (ds)-cDNA fragments (∼600 nt each with a 100-nt overlap; fragments A–M) that covered the entire length of TMV RNA (Fig. 2). As a control, we used MNase-treated and 32P-labeled TMV RNA that had been incubated in mdBYL with CHX and purified by SEC for hybridization to the TMV cDNA fragments. Compared with the control, a stronger hybridization signal was observed for fragment A, which represented the 5′-terminal 600-nt region of TMV RNA (Fig. 2). By using TMV ds-cDNA fragments N–Q and synthetic single-stranded DNA fragments R–T that were complementary to TMV RNA, the MNase-resistant region in PMTC was further mapped approximately to nucleotides 1–100, which were covered by fragments N, O, R, and S, but not by fragments P, Q, or T (Fig. 2). We note that the initiation codon for the replication proteins was located at nucleotides 69–71.
Fig. 2.
Mapping of the MNase-resistant region of TMV RNA in the PMTC. Schematic diagram of the TMV genome and the positions of cDNA fragments A–T. PCR-amplified double-stranded cDNA fragments A–Q and synthetic single-stranded DNA fragments R–T complementary to TMV RNA were blotted onto membranes and hybridized with RNA fragments recovered after MNase digestion of the PMTC fraction and 5′-32P-labeled. In the “control” panels, 32P-labeled probe RNA was prepared in the same way except that the reaction in mdBYL was performed in the presence of CHX, and was used for hybridization to the DNA fragments.
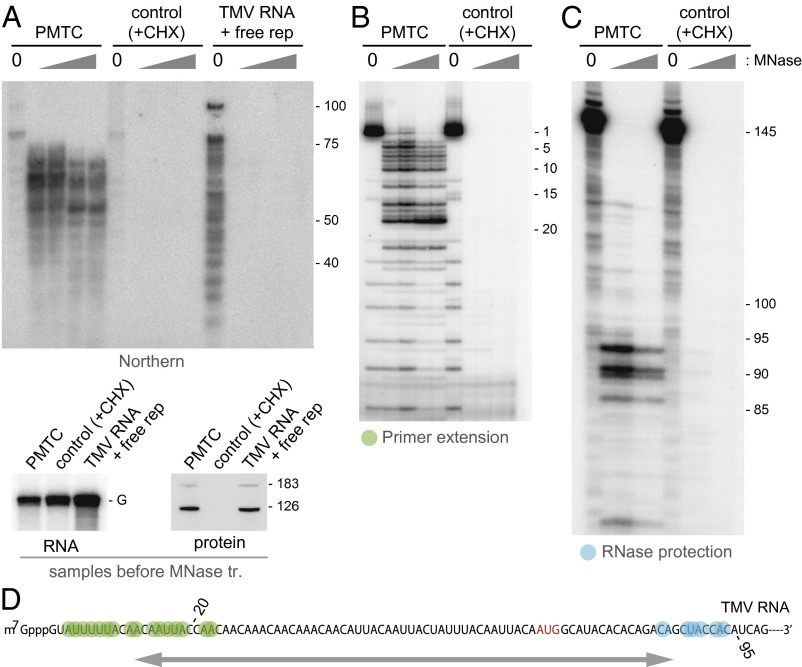
To further characterize the protected region at nucleotide-level resolution, we analyzed MNase-treated PMTC RNA by Northern blot hybridization using a 32P-labeled oligonucleotide probe that was complementary to nucleotides 1–70 of TMV RNA. The protected fragments were rather heterogeneous in size, ranging up to ∼70 nt (Fig. 3A). In the control sample in which translation in mdBYL was inhibited by CHX, no signal was detected. Hybridization signals were also not detected for MNase-treated TMV RNA that had been incubated with SEC fraction no. 95, which contained TMV RNA-free replication proteins (Fig. 3A), indicating that MNase resistance correlated with translation of TMV RNA. Further analysis with primer extension (Fig. 3B) and RNase protection (Fig. 3C) assays showed that the 5′ and 3′ termini of the MNase-resistant fragment varied, ranging from nucleotides 3–22 and from nucleotides 87–94, respectively (Fig. 3D).
Fig. 3.
Characterization of TMV RNA fragments generated by MNase treatment of the PMTC. (A) (Upper) Northern hybridization analysis of MNase-resistant RNA fragments. The PMTC fraction, the control SEC fraction, and TMV RNA incubated with fraction no. 95 containing TMV RNA-free replication proteins were treated with 0, 0.05, 0.1, 0.5, or 1 U/μL MNase. RNA was purified from the reaction mixtures, separated by 8 M urea-10% (19:1 bis) PAGE, and analyzed by Northern blot hybridization using a 32P-labeled oligonucleotide probe complementary to nucleotides 1–70 of TMV RNA. Positions of size markers are shown on the right with nucleotide length. (Lower) The presence of TMV RNA and the replication proteins in the three samples before MNase treatment. The positions for TMV genomic RNA (G) and the 183-kDa and 126-kDa proteins are indicated. (B) Primer extension analysis. The PMTC fraction and the control SEC fraction were treated with 0, 0.05, 0.1, 0.5, or 1 U/μL MNase. RNA was purified from the reaction mixtures and subjected to primer extension using a 5′-32P-labeled oligonucleotide that was complementary to the nucleotides 61–80 region of TMV RNA. Primer extension products were analyzed by 8 M urea-8% (19:1 bis) PAGE. The band positions are shown on the right with the nucleotide positions that refer to distance from the 5′ terminus. (C) RNase protection analysis. The same set of RNA samples as in B were subjected to RNase protection using a 32P-labeled RNA probe complementary to the nucleotides 31–145 region of TMV RNA. Protected RNA was analyzed by 8 M urea-8% (19:1 bis) PAGE. The band positions are shown on the right with the nucleotide positions that refer to distance from the 5′ terminus. (D) The region of TMV RNA that shows resistance to MNase in the PMTC. Nucleotides marked in green represent the 5′ terminus of the protected fragment revealed by primer extension, and those marked in blue represent the 3′ terminus revealed by RNase protection. The initiation codon for the 126-kDa protein is shown in red. Numbers indicate nucleotide positions with respect to the 5′ terminus. The protected region is roughly indicated by an arrow.
Importance of the 5′ UTR Sequence of TMV RNA for PMTC Formation and Negative-Strand RNA Synthesis.
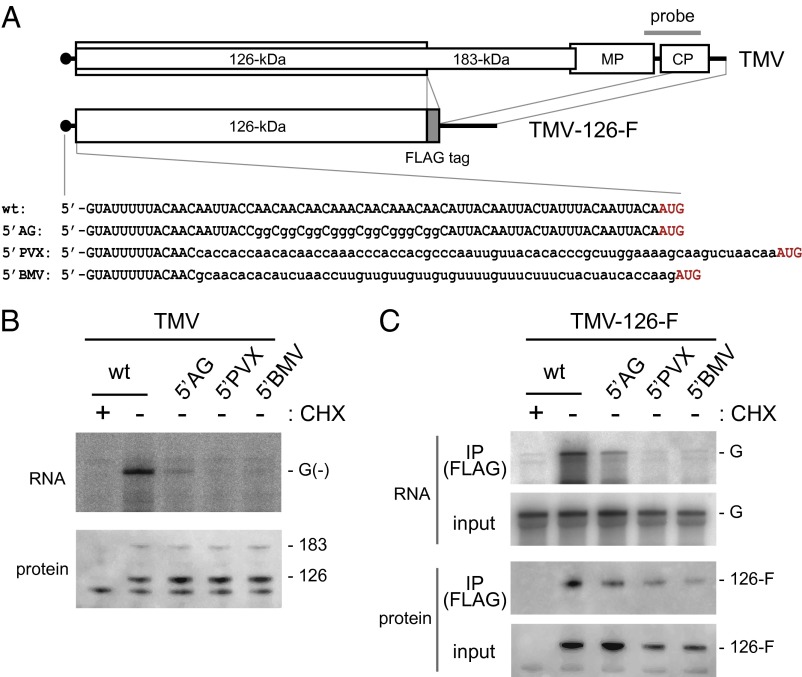
The role of the 5′ UTR of TMV RNA in viral RNA replication was investigated. We introduced mutations in the 5′ UTR that showed MNase resistance in PMTC and examined whether they formed PMTC and synthesized negative-strand RNA in an in vitro translation-replication system. In the mutant TMV-5′AG, all of the A residues in nucleotides 21–42 were replaced by G residues (Fig. 4A). In the mutants TMV-5′PVX and TMV-5′BMV, the nucleotides 14–68 region of TMV RNA was replaced by the nucleotides 16–84 region of potato virus X (PVX) RNA and the nucleotides 47–103 region of brome mosaic virus (BMV) RNA2, respectively (Fig. 4A). These mutant TMV RNAs were subjected to translation and replication reactions in BYL. For TMV-5′AG, TMV-5′PVX, and TMV-5′BMV RNAs, the accumulation of negative-strand RNA was much lower than that for wild-type TMV RNA whereas the 126-kDa and 183-kDa replication proteins accumulated to moderate levels (Fig. 4B). Thus, the 5′ UTR of TMV RNA functions as a cis-acting element required for negative-strand RNA synthesis.
Fig. 4.
Effects of mutation of the 5′ UTR of TMV RNA on RNA replication and PMTC formation. (A) Schematic diagram of TMV RNA derivatives. The position of the probe (nucleotides 5,500–6,100) used for RNase protection and Northern hybridization is shown above the TMV genome. Nucleotide sequences of the 5′ UTRs of the TMV derivatives are shown in Lower. The initiation codon for the 126-kDa protein is shown in red. (B) In vitro translation and replication. Wild-type TMV and 5′ UTR-modified TMV RNAs were subjected to translation and replication reactions using BYL (CHX +, mock translation control). RNA was purified from the reaction mixtures and analyzed by RNase protection to detect negative-strand RNA (Upper). Production of the replication proteins was examined by Western blotting using anti-ToMV replication protein antibodies (Lower). Positions for protected RNA [G(–)] and the 126-kDa and 183-kDa proteins are indicated on the right. (C) Binding of the 126-kDa protein to genomic RNA. TMV-126F (wild type) and its 5′ UTR-modified RNA derivatives were translated in mdBYL (CHX +, mock translation control) followed by immunoprecipitation with anti-FLAG antibody-conjugated beads and elution with 3× FLAG peptide [IP (FLAG)]. RNA and protein were prepared from the input material and immunopurified fractions and detected by Northern hybridization using a 32P-labeled RNA probe complementary to the genomic RNA (Upper), as well as Western blotting using anti-126-kDa protein antibody (Lower). The positions of genomic RNA (G) and the FLAG-tagged 126-kDa protein are shown on the right.
To examine whether a 5′ UTR mutation affects PMTC formation, we prepared a TMV-126-F RNA that encoded a FLAG-tagged 126-kDa protein and its derivatives with the aforementioned 5′ UTR mutations (Fig. 4A). These RNAs were translated in mdBYL, the 126-kDa protein was immunoprecipitated using anti-FLAG antibody, and coprecipitation of TMV derivative RNA, which represents formation of core-PMTC (28), was examined. TMV-5′AG-126-F, TMV-5′PVX-126-F, and TMV-5′BMV-126-F RNAs all coprecipitated with the 126-kDa protein with lower efficiency than that of TMV-126-F (Fig. 4C). The mutations did not drastically affect the production of the 126-kDa protein (Fig. 4C). These results indicate that mutations of the 5′ UTR of TMV RNA affect negative-strand RNA synthesis due to the inability of replication proteins to bind genomic RNA.
TMV Replication Protein Binds to the 5′ UTR of TMV RNA.
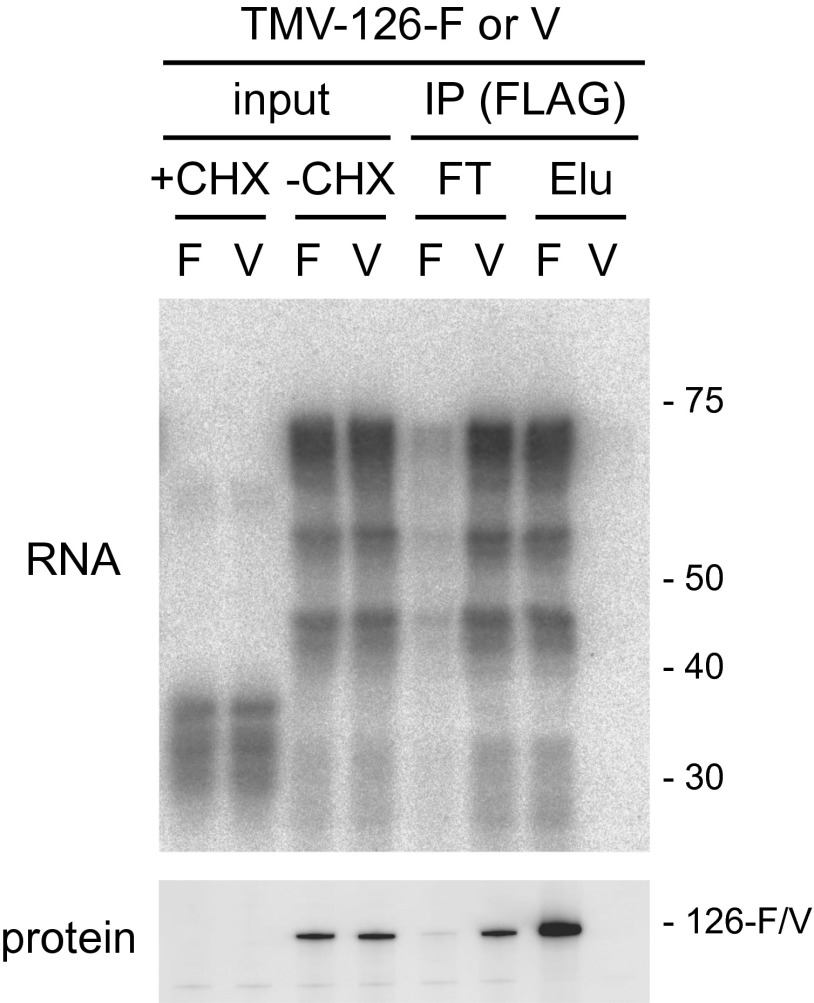
The results described above showed that the 126-kDa protein is involved in the protection of the 5′ UTR from MNase digestion and that the 126-kDa protein is associated with genomic RNA, suggesting that the 126-kDa protein binds to the 5′ UTR. To confirm this possibility, a TMV-126-F RNA, and as a control TMV-126-V RNA, in which the 126-kDa protein was C-terminally tagged with the V5 epitope, were translated in mdBYL followed by digestion with MNase. Then, the 126-kDa protein was immunoprecipitated with an anti-FLAG antibody. As expected, the 70-nt MNase-resistant fragment copurified with the 126-kDa protein that was tagged with FLAG, but not with the protein tagged with V5, indicating that the protected RNA fragment was associated with the 126-kDa protein (Fig. 5).
Fig. 5.
Association of the 126-kDa protein with the MNase-resistant RNA fragments. TMV-126-F RNA (encodes FLAG-tagged 126-kDa protein) and TMV-126-V RNA (encodes V5-tagged 126-kDa protein) were translated in mdBYL with or without CHX and treated with MNase (1 U/μL). The reaction mixtures without CHX were subjected to immunoprecipitation using anti-FLAG antibody-conjugated agarose [IP (FLAG)]. RNA was purified from the samples before immunoprecipitation (input), from flow-through fractions after immunoprecipitation (FT), and from the eluates from the beads with 3× FLAG peptide (Elu) and analyzed as in Fig. 3A except that a 32P-labeled oligonucleotide probe complementary to nucleotides 11–80 of TMV RNA was used (Upper). The 126-kDa protein was detected by Western blotting using anti–126-kDa protein antibody (Lower). The elution fractions of RNA and protein samples were concentrated 4.3-fold and 75-fold, respectively, compared with the other fractions. Positions of RNA size markers (Upper, in nt) and the FLAG- or V5-tagged 126-kDa protein (Lower) are indicated on the right.
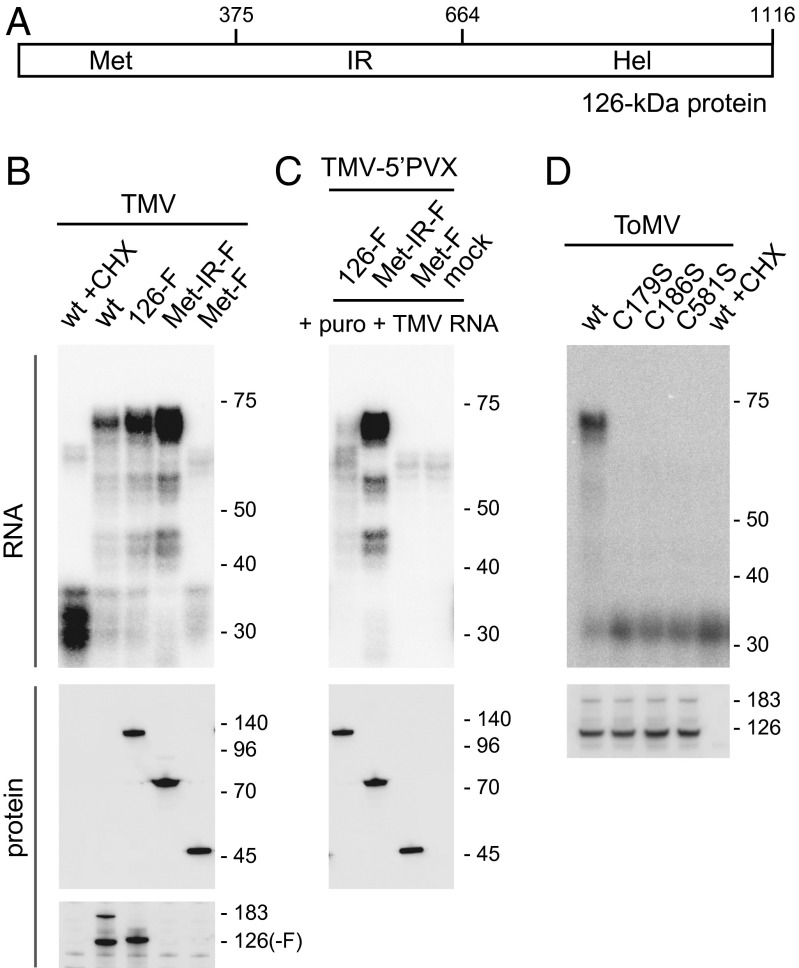
We next investigated which region in the 126-kDa protein was involved in 5′ UTR protection. Wild-type TMV RNA, TMV-126-F RNA, and TMV-126-F-derivative RNAs lacking regions corresponding to the helicase-like domain only (TMV-Met-IR-F) or helicase-like domain and IR (TMV-Met-F) (Fig. 6A) were translated in mdBYL and treated with MNase. TMV-126-F and TMV-Met-IR-F showed similar RNA protection patterns to that of wild-type TMV RNA (Fig. 6B). When the same molar amount of RNA was used, the intensity of the protected bands for TMV-Met-IR-F was stronger than that for wild-type TMV. The 70-nt protected RNA band was not detected with TMV-Met-F RNA (Fig. 6B). We confirmed that the protein products accumulated to similar levels by Western blotting with anti-FLAG antibody (Fig. 6B).
Fig. 6.
Mapping of the region of the TMV 126-kDa protein required for 5′ UTR protection in the PMTC. (A) Schematic diagram of the 126-kDa protein of TMV. Numbers represent amino acid residues from the N terminus. (B) Effect of C-terminal deletions of the 126-kDa protein on the ability to form the PMTC. Indicated TMV derivative RNAs were translated with or without CHX in mdBYL and treated with 1 U/μL MNase. RNA was purified from the reaction mixtures and analyzed as in Fig. 5. The 126-kDa protein and its derivatives were detected by Western blotting using anti-FLAG (Middle) and anti-126-kDa protein (Bottom) antibodies. Positions of RNA size markers (Top, in nt), protein size markers (Middle, in kDa), and the 126-kDa and 183-kDa proteins (Bottom) are indicated on the right. (C) Posttranslational binding of the Met-IR fragment to the 5′ UTR of exogenously added TMV RNA. Indicated TMV-5′ PVX derivative RNAs were translated in mdBYL or mock translated. After translation termination with puromycin, TMV RNA was mixed and incubated, and then treated with 1 U/μL MNase. RNA and protein were analyzed, and the data were presented as in B. (D) Effect of C-to-S mutations of ToMV 126-kDa protein on 5′ UTR binding. Indicated ToMV derivative RNAs were translated with or without CHX in mdBYL and treated with 1 U/μL MNase. RNA was purified from the reaction mixtures and analyzed as in Fig. 3A, except that a 32P-labeled oligonucleotide probe complementary to nucleotides 11–80 of ToMV RNA was used (Upper). Production of the replication proteins was examined by Western blotting using anti-ToMV replication protein antibody (Lower). Positions of RNA size markers (Upper, in nt) and the 126-kDa and 183-kDa proteins (Lower) are indicated on the right.
One possible explanation for the fact that the 126-kDa protein bound the 5′ UTR cotranslationally but not posttranslationally (Fig. 3A) is that only premature polypeptides are competent for binding. We tested the ability of full-length and truncated 126-kDa proteins to bind the 5′ UTR posttranslationally. TMV-126-F, TMV-Met-IR-F, and TMV-Met-F RNA derivatives, in which a part of the 5′ UTR was replaced by that of PVX, were constructed (called TMV-5′PVX-126-F, TMV-5′PVX-Met-IR-F, and TMV-5′PVX-Met-F, respectively). All of these RNAs were translated in mdBYL to obtain FLAG-tagged full-length and truncated 126-kDa proteins that were not bound to template RNA. After adding puromycin to terminate translation, wild-type TMV RNA was added to each reaction mixture as a binding target. The mixtures were further incubated and treated with MNase, and protected RNA was analyzed by Northern hybridization to detect the 5′ UTR sequence of the target TMV RNA. Notably, an ∼70-nt protected RNA band with a similar laddering pattern as that seen for TMV-Met-IR-F translation (Fig. 6B) was observed when target RNA was mixed with the TMV-5′PVX-Met-IR-F–translated mixture whereas the protected band was barely detected when target RNA was added to the TMV-5′PVX-126-F– or Met-F–translated mixtures (Fig. 6C). These results suggest that the Met-IR fragment of the 126-kDa protein has the ability to bind the 5′ UTR, but that the binding activity is masked by the helicase-like domain in the full-length 126-kDa protein.
Previously, we found that ToMV mutants with C179S, C186S, or C581S substitutions in the 126-kDa protein are defective in PMTC formation (29). We therefore examined whether MNase-resistant fragments were detectable with these mutants. Wild-type ToMV RNA and the associated mutant RNAs were translated in mdBYL and treated with MNase, after which RNA was extracted and analyzed by Northern blot hybridization using a probe for the ToMV 5′ UTR sequence. The MNase-resistant RNA band was observed for wild-type ToMV but not for the mutants (Fig. 6D). Thus, the ability of the replication proteins to cotranslationally bind the 5′ UTR is tightly linked to the ability to form the PMTC.
Reduced Translational Efficiency of TMV RNA in the PMTC.
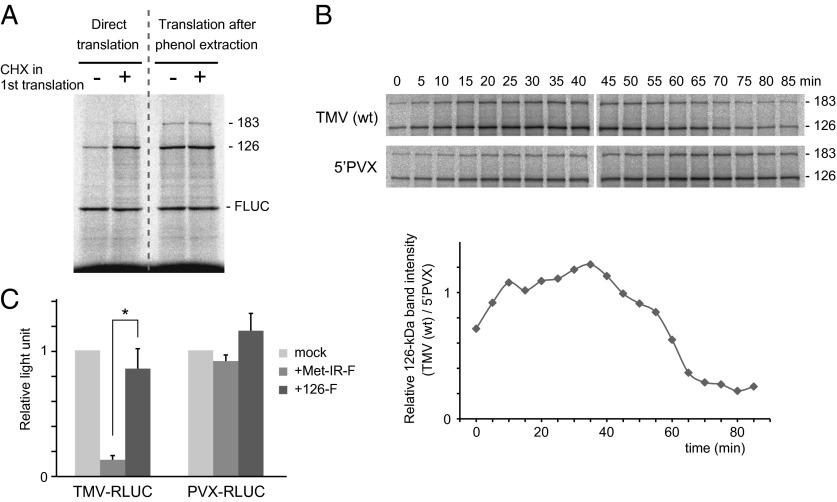
Upon translation initiation, a small (40S) ribosomal subunit binds to the 5′ UTR of an mRNA and migrates in the 5′-to-3′ direction to find the initiation codon, where a large (60S) ribosomal subunit joins to initiate translation. Strong secondary structures and/or protein binding to the 5′ UTR prevent efficient translation initiation. Given that the replication proteins bind to the 5′ UTR of TMV RNA in PMTC, we examined whether TMV RNA in the PMTC was competent for translation. After first translation in mdBYL followed by SEC fractionation, the PMTC-containing fraction was obtained. An aliquot of this fraction was used for second translation with mdBYL in the presence of [35S]-methionine to detect only newly synthesized replication proteins. Firefly luciferase (FLUC) mRNA was added to the second translation mixture as an internal control. As a non-PMTC–forming control, the SEC fraction of the mock-translated sample (TMV RNA incubated in mdBYL with CHX in the first translation) was also subjected to second translation. The FLUC-normalized amount of newly synthesized 126-kDa protein from PMTC was 13.9 ± 2.1% of the non-PMTC-forming control (mean ± SE, n = 4) (Fig. 7A, direct translation). To verify the intactness and amount of TMV RNA in the PMTC fraction, RNA in the PMTC and control fractions (supplemented with FLUC mRNA) was deproteinized by phenol extraction, purified, and used for second translation. The relative amount of newly synthesized 126-kDa protein from the deproteinized PMTC RNA was 82.8 ± 2.8% of the control (mean ± SE, n = 4) (Fig. 7A, translation after phenol extraction), indicating that intactness and amount of TMV RNA in the PMTC fraction were comparable with the control. A paired t test showed that the production of the 126-kDa protein by direct PMTC translation is significantly lower than that by translation of deproteinized RNA from the PMTC fraction (P = 0.00063). These results suggest that TMV RNA in PMTC is less competent for translation than free TMV RNA, and that this inefficient translation is due to the binding of protein factors to RNA. We suppose that the binding of TMV replication proteins to the 5′ UTR inhibits further translation. Consistent with this possibility, translation of wild-type TMV RNA, which formed PMTC, declined after 60 min in mdBYL, compared with that of TMV-5′PVX RNA, which did not form PMTC (Fig. 7B).
Fig. 7.
Effects of PMTC formation on TMV RNA translation. (A) Translation of purified PMTC in mdBYL. TMV RNA was translated with or without CHX in mdBYL, and the reaction mixtures were fractionated by SEC. The PMTC fraction and the control SEC fraction were each mixed with FLUC mRNA and were mixed with fresh mdBYL translation mixture containing [35S]-methionine and incubated (direct translation). On the other hand, an aliquot of each FLUC-supplemented fraction was deproteinized by phenol extraction, and purified RNA were translated in mdBYL in the presence of [35S]-methionine (translation after phenol extraction). Proteins were analyzed by SDS/PAGE and autography. The positions of the 126-kDa, 183-kDa, and FLUC proteins are shown on the right. (B) Time course of replication protein production from wild-type TMV and TMV-5′PVX RNAs in mdBYL. Translation reactions were performed with FLUC mRNA as an internal control but without exogenously added methionine. At time points indicated above the gels, [35S]-methionine was added, and, after a 5-min incubation, excessive (final, 1 mM) non–isotope-labeled methionine was added, and incubation was continued for a total of 90 min. Proteins were analyzed by SDS/PAGE and autoradiography. The positions of the 126-kDa and 183-kDa proteins are shown on the right. The graph shows the ratio of the FLUC-normalized 126-kDa protein band intensity for TMV (wt) to that for 5′PVX at each time point. (C) Effect of the Met-IR fragment binding on translation of reporter mRNA with viral 5′ UTRs. TMV-5′PVX-Met-IR-F or TMV-5′PVX-126-F RNA were translated in mdBYL for 30 min (for a negative control reaction, no mRNA was added). Then, Renilla luciferase mRNA that have 5′ UTR sequences from TMV RNA (TMV-RLUC) or that from TMV-5′PVX RNA (PVX-RLUC) were added to each mixture and incubated for an additional 60 min, and luciferase activity was measured. Boxes and error bars represent means and SEs of data obtained in three independent experiments. An asterisk indicates a significant difference by t test (P < 0.05).
To further confirm the inhibition of translation by 5′ UTR-bound TMV replication proteins, we tested whether translation of Renilla luciferase (RLUC) mRNA with TMV 5′ UTR (TMV-RLUC mRNA) is inhibited by the Met-IR polypeptide in trans. Remarkably, production of RLUC from TMV-RLUC mRNA was strongly inhibited by the addition of Met-IR (Fig. 7C). Such inhibition was not observed when RLUC mRNA with a fragment of PVX 5′ UTR (PVX-RLUC mRNA) was used, or when the full-length 126-kDa protein was added to translation reaction mixtures for TMV-RLUC or PVX-RLUC mRNA (Fig. 7C).
Discussion
Cotranslational Binding of TMV Replication Proteins to the 5′ UTR Is Key to the Selection of a Template for Negative-Strand RNA Synthesis.
In this study, we found that, when TMV RNA is translated in mdBYL, the 126-kDa replication protein binds to an ∼70-nt region of genomic RNA, rendering it resistant to MNase digestion. The 70-nt region was mapped to nucleotides 3–94 of TMV RNA. Because the initiation codon for the replication proteins is at nucleotides 69–71, most of the 70-nt region is located in the 5′ UTR. Most protein domains that bind single-stranded RNA in a sequence-specific manner recognize RNA elements of several nucleotides (30). Thus, one replication protein molecule may bind one or a few units of the CAA repeat, and the 70-nt region may be bound and protected by multiple replication protein molecules. Importantly, mutations in the 70-nt region of TMV RNA, and those that cause C179S, C186S, or C581S amino acid substitutions in ToMV replication proteins, affected not only binding of the replication proteins to the 70-nt region but also negative-strand RNA synthesis in BYL. These results suggest that the binding is a key event in tobamovirus RNA replication.
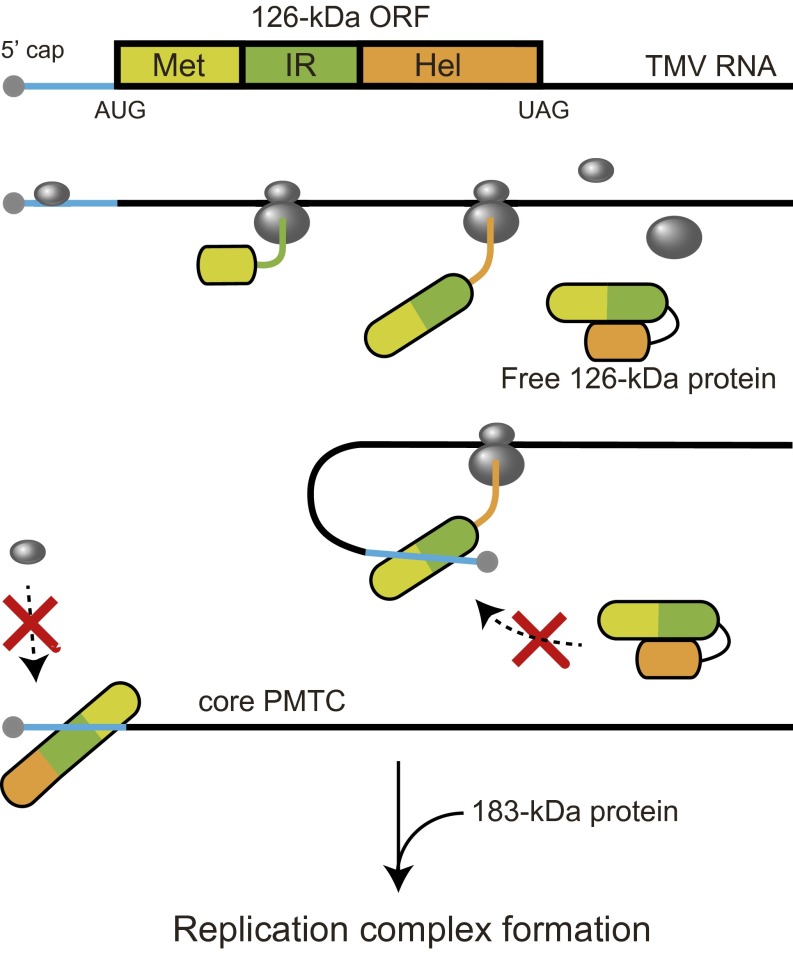
Binding of the full-length 126-kDa replication protein to the 5′ UTR of TMV RNA occurred only cotranslationally and not posttranslationally. By contrast, the Met-IR fragment of the replication protein, which lacked the helicase-like domain, bound the 5′ UTR posttranslationally and in trans. We postulate that the Met-IR region folds into a 5′ UTR binding-competent conformation before translation completes, and that a fraction of the premature translation product binds the 5′ UTR. This binding is maintained after the helicase-like domain is synthesized. However, if the helicase-like domain is synthesized before the Met-IR region binds the 5′ UTR, the helicase-like domain might bind to the Met-IR region and sterically inhibit its binding to the 5′ UTR or change the conformation of the Met-IR region to make it incompetent for 5′ UTR binding (Fig. 8). Consistent with these possibilities, the helicase-like domain of TMV was able to bind the IR region in a yeast two-hybrid system (31).
Fig. 8.
A model for cotranslational 5′ UTR binding by the TMV 126-kDa protein. See Discussion for details.
Cotranslational binding of the replication proteins to replication templates suggests that the former tends to bind the 5′ UTR in cis because the translation templates are always near premature translation products. In fact, tobamovirus replication proteins, like those of many other positive-strand RNA viruses, preferentially select template RNAs in cis (8, 20, 21). This property benefits TMV in several ways. First, it facilitates accurate template selection. Second, it secures efficient replication at early stages of infection when the levels of genomic RNA and replication proteins are low. Third, it allows rapid selection on the 126-kDa protein gene. TMV genomes carrying a more-adaptive 126-kDa protein gene will be replicated more efficiently than those carrying a less-adaptive one when they coexist in a cell: i.e., the functionality of the protein is directly linked to the fitness of its cognate genome. Fourth, inhibition of the posttranslational binding of the 126-kDa protein to the 5′ UTR by the helicase-like domain probably also secures successful postreplication events, including virion assembly and viral spread to neighboring cells, especially during the later stages of cellular infection, when higher amounts of free replication proteins and viral RNA accumulate. Degradation of the replication protein of turnip yellow mosaic virus by the ubiquitin-proteasome–mediated system (32), and nuclear transport of potyvirus replication proteins, which catalyze RNA replication in the cytoplasm (33), would confer similar advantages. Because tobamovirus replication proteins that do not participate in RNA replication act as an RNA silencing suppressor (34–36), tobamoviruses might use an autoinhibition strategy rather than degradation/sequestration strategies to maintain a pool of replication proteins for RNA silencing suppression.
Previously, Osman et al. prepared ToMV replication proteins that were solubilized from membranes of infected plants and showed that they bind to the 3′ tRNA-like structure of ToMV RNA and initiate negative-strand RNA synthesis (37). They also demonstrated that an Escherichia coli-expressed fragment of the ToMV replication protein, corresponding to amino acid residues 1–654 (Met-IR used in this study corresponds to amino acid residues 1–664 of the TMV replication protein), specifically binds to the 3′ tRNA-like structure of ToMV RNA in trans (38). After the binding of PMTC to membranes, replication proteins may gain the ability to recognize the 3′ tRNA-like structure to initiate negative-strand RNA synthesis. For many positive-strand RNA viruses, including TMV as found here, cis elements apart from the 3′-terminal region of genomic RNA play important roles in template selection (3). In some cases, the 3′-terminal region is recruited spatially close to the cis elements via RNA–RNA or RNA–protein interactions (39). As the 3′ terminus of genomic RNA must be recognized by replication proteins for correct initiation of negative-strand RNA synthesis, why do these viruses use additional non-3′ cis elements for template selection? For strict recognition, replication proteins need to bind replication templates strongly, but such tight binding might make it difficult for replication proteins to change state toward negative-strand initiation and to leave the 3′ termini of the replication templates for negative-strand elongation. Thus, it is plausible that the binding of replication proteins to the 3′ terminus of genomic RNA perhaps cannot be so strong that the processes of negative-strand synthesis initiation and elongation are affected. In fact, tobamovirus RNA replication is tolerant of replacement of the 3′-terminal sequence with other tobamovirus species or even a virus in a different family (40, 41). Thus, to secure sufficient accuracy in template selection, additional cis elements might be required.
Switching from Translation to RNA Replication in TMV.
The genomic RNA of positive-strand RNA viruses serves as a template for both translation and replication. To avoid fatal collision between protein-synthesizing ribosomes and negative-strand RNA-synthesizing RNA polymerases, ribosomes must be eliminated from genomic RNA molecules before negative-strand RNA synthesis starts. In poliovirus, with genomic RNA that has a 5′-terminal covalent linkage to a viral protein called VPg, the binding of viral RNA polymerase to an RNA element in the 5′ UTR is essential for negative-strand RNA synthesis, and this binding inhibits internal ribosome entry site-dependent translation (15). For turnip crinkle virus and barley yellow dwarf virus, which have uncapped RNA genomes, binding of their replication proteins to genomic RNA or traveling of the replication proteins during negative-strand synthesis disrupt higher RNA structures that are necessary for cap-independent translation (42, 43).
In this study, we found that TMV replication proteins inhibit translation of TMV RNA by binding to the 5′ UTR of the RNA. Because the 5′ UTR of a capped mRNA is the initial binding site for the 40S ribosomal subunit, the binding of the 126-kDa protein to the 5′ UTR may inhibit the entry of ribosomes to the 5′ UTR and/or scanning for the initiation codon. As a result of inhibiting translation initiation, ribosomes will be cleared from the genomic RNA as soon as ongoing translation completes. This model provides one more possible strategy to avoid a fatal collision between ribosomes and RNA polymerases in positive-strand RNA viruses. Lastly, we note that the TMV 5′ UTR, a general translation enhancer (24, 25), acts as a translation silencer in this context.
Materials and Methods
Plasmids.
Full-length cDNA of TMV-OM (44) RNA was synthesized by reverse transcription-PCR (RT-PCR) and inserted between the EcoRI and ThaI sites of pBR322 (nucleotides 2,519–4,359) with an SP6 promoter and linker sequences to obtain the plasmid pSPOM. The plasmid was linearized with AgeI, which cleaves DNA at ACCGGT, and used for transcription by SP6 RNA polymerase to produce infectious RNA. The complete nucleotide sequence for pSPOM was deposited in GenBank (accession no. AB861437). To construct the plasmids pSPOM-126-F and pSPOM-126-V, the nucleotides 3,419–5,813 region of the TMV-OM sequence in pSPOM was replaced by the sequences 5′-TCAATTACAGGGAGGCGCCGGAGGTGATTATAAGGATGATGATGATAAGAACTGGTCACATCCTCAATTTGAAAAGTGAGGTAAC-3′ and 5′TCAATTACAGGGAGGCGCCGGAGGTGGTAAGCCTATCCCTAACCCTCTCCTCGGTCTCGATTCTACGTGAGGTAAC-3′, respectively. In the plasmid pSPOM-5′AG, the A residues in the nucleotides 21–42 TMV-OM region of pSPOM were replaced by G residues. To construct the plasmids pSPOM-5′PVX and pSPOM-5′BMV, the nucleotides 14–68 TMV-OM region of pSPOM was replaced by the sequences 5′-CACCACCAACACAACCAAACCCACCACGCCCAATTGTTACACACCCGCTTGGAAAAGCAAGTCTAACAA-3′ and 5′-GCAACACACATCTAACCTTGTTGTTGTTGTGTTTTGTTTCTTTCTACTATCACCAAG-3′, respectively (Fig. 4A). In the pSPOM derivatives pSPOM-Met-IR-F and pSPOM-Met-F, regions encoding the 126-kDa protein amino acid residues 1–664 and 1–375, respectively, were fused to a FLAG tag-coding sequence and linked to nucleotide 5,814 of TMV-OM. ToMV-based constructs were made as described previously (29). pTMV-RLUC was created by inserting a fragment containing the SP6 promoter, 5′ UTR, and first 10 codons for the 126-kDa protein from pSPOM (the resultant transcript contains nucleotides 1–98 of TMV-OM RNA) into the vector pMI27 (45) at a site immediately upstream of the initiation codon of RLUC ORF. pPVX-RLUC was constructed following the same strategy, except that a fragment containing the SP6 promoter and 5′ UTR from pSPOM-5′PVX was inserted.
In Vitro Translation and Replication of Tobamovirus RNA.
Preparation of BYL and mdBYL and in vitro translation and replication of tobamovirus RNA were performed as described previously (27, 46). RNA for translation and replication was synthesized by using an SP6-Scribe Standard RNA IVT Kit (CELLSCRIPT) or a T7-Scribe Standard RNA IVT Kit (CELLSCRIPT) and capped by using a ScriptCap m7G Capping System kit (CELLSCRIPT). Alternatively, TMV-OM RNA prepared from purified virions was used for translation and replication.
Purification of PMTC.
TMV-OM RNA (100 μg) prepared from purified virions was translated in a 5-mL mdBYL reaction mixture at 25 °C for 1 h with or without 100 μg/mL CHX. The reaction mixture was mixed with 18 μL of 0.5 M EDTA and centrifuged at 10,000 × g at 4 °C for 10 min, and the supernatant (4.8 mL) was directly applied to a Sephacryl S-500 16/60 column (GE Healthcare). Chromatography was performed at 4 °C at a flow rate of 0.5 mL/min with 30 mM Hepes-KOH (pH 7.4), 80 mM KOAc, 2 mM DTT, and 5% glycerol. The eluate was collected (1 mL per fraction) and fraction nos. 47–58 were pooled as the PMTC fraction or the control SEC fraction unless otherwise stated.
Protein and RNA Analyses.
FLAG-tagged proteins were bound to ANTI-FLAG M2 Affinity Gel (A2220; Sigma-Aldrich) and eluted using 3× FLAG peptide (F4799; Sigma-Aldrich) as described previously (28). RNase protection and primer extension experiments were performed using an RNase Proptection Assay III kit (Ambion) and a PrimeScript First Strand cDNA Synthesis kit (Takara), respectively. Antibodies to ToMV replication proteins were produced in rabbits using an E. coli-expressed polypeptide corresponding to amino acid residues 666–1,116 as antigen. Anti-FLAG (DYKDDDDK) antibody (mouse monoclonal from clone 1E6) was purchased from Wako Pure Chemical Industries. 35S-labeled protein bands were detected using a BAS-2500 imager, and their intensities were measured by Multi Gauge version 3.0 software (Fujifilm). RLUC activity was measured using the luminometer model TD-20/20 (Promega).
Acknowledgments
We thank Drs. Manabu Yoshikawa, Masaki Nishikiori, Taichiro Iki, and Etsuko Katoh for help and discussion; Drs. Tetsuo Meshi and Shinya Tsuda for the TMV-OM RNA sequence; and Dr. Shu Miao for technical assistance. This work was supported in part by Japan Society for the Promotion of Science (JSPS) KAKENHI Grant 24380029 and a grant from the Program for Promotion of Basic Research Activities for Innovative Biosciences (to M.I.). S.M. was supported by the Precursory Research for Embryonic Science and Technology program of the Japan Science and Technology Agency and by JSPS Research Fellowship for Young Scientists 25-10559.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The sequence reported in this paper has been deposited in the GenBank database [accession no. AB861437 (pSPOM)].
References
- 1.den Boon JA, Ahlquist P. Organelle-like membrane compartmentalization of positive-strand RNA virus replication factories. Annu Rev Microbiol. 2010;64(1):241–256. doi: 10.1146/annurev.micro.112408.134012. [DOI] [PubMed] [Google Scholar]
- 2.den Boon JA, Diaz A, Ahlquist P. Cytoplasmic viral replication complexes. Cell Host Microbe. 2010;8(1):77–85. doi: 10.1016/j.chom.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu Y, Wimmer E, Paul AV. Cis-acting RNA elements in human and animal plus-strand RNA viruses. Biochim Biophys Acta. 2009;1789(9-10):495–517. doi: 10.1016/j.bbagrm.2009.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weiland JJ, Dreher TW. Cis-preferential replication of the turnip yellow mosaic virus RNA genome. Proc Natl Acad Sci USA. 1993;90(13):6095–6099. doi: 10.1073/pnas.90.13.6095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Van Bokhoven H, et al. Cis- and trans-acting elements in cowpea mosaic virus RNA replication. Virology. 1993;195(2):377–386. doi: 10.1006/viro.1993.1387. [DOI] [PubMed] [Google Scholar]
- 6.Novak JE, Kirkegaard K. Coupling between genome translation and replication in an RNA virus. Genes Dev. 1994;8(14):1726–1737. doi: 10.1101/gad.8.14.1726. [DOI] [PubMed] [Google Scholar]
- 7.van Rossum CM, Garcia ML, Bol JF. Accumulation of alfalfa mosaic virus RNAs 1 and 2 requires the encoded proteins in cis. J Virol. 1996;70(8):5100–5105. doi: 10.1128/jvi.70.8.5100-5105.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lewandowski DJ, Dawson WO. Functions of the 126- and 183-kDa proteins of tobacco mosaic virus. Virology. 2000;271(1):90–98. doi: 10.1006/viro.2000.0313. [DOI] [PubMed] [Google Scholar]
- 9.Liang Y, Gillam S. Rubella virus RNA replication is cis-preferential and synthesis of negative- and positive-strand RNAs is regulated by the processing of nonstructural protein. Virology. 2001;282(2):307–319. doi: 10.1006/viro.2001.0862. [DOI] [PubMed] [Google Scholar]
- 10.Okamoto K, et al. cis-Preferential requirement of a -1 frameshift product p88 for the replication of Red clover necrotic mosaic virus RNA1. Virology. 2008;375(1):205–212. doi: 10.1016/j.virol.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yi G, Kao C. cis- and trans-acting functions of brome mosaic virus protein 1a in genomic RNA1 replication. J Virol. 2008;82(6):3045–3053. doi: 10.1128/JVI.02390-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang J, Yeh H-H, Falk BW. cis preferential replication of Lettuce infectious yellows virus (LIYV) RNA 1: The initial step in the asynchronous replication of the LIYV genomic RNAs. Virology. 2009;386(1):217–223. doi: 10.1016/j.virol.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 13.Choi SK, Yoon J-Y, Canto T, Palukaitis P. Replication of cucumber mosaic virus RNA 1 in cis requires functional helicase-like motifs of the 1a protein. Virus Res. 2011;158(1-2):271–276. doi: 10.1016/j.virusres.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 14.Barton DJ, Morasco BJ, Flanegan JB. Translating ribosomes inhibit poliovirus negative-strand RNA synthesis. J Virol. 1999;73(12):10104–10112. doi: 10.1128/jvi.73.12.10104-10112.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gamarnik AV, Andino R. Switch from translation to RNA replication in a positive-stranded RNA virus. Genes Dev. 1998;12(15):2293–2304. doi: 10.1101/gad.12.15.2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Daijogo S, Semler BL. Mechanistic intersections between picornavirus translation and RNA replication. Adv Virus Res. 2011;80:1–24. doi: 10.1016/B978-0-12-385987-7.00001-4. [DOI] [PubMed] [Google Scholar]
- 17.Goelet P, et al. Nucleotide sequence of tobacco mosaic virus RNA. Proc Natl Acad Sci USA. 1982;79(19):5818–5822. doi: 10.1073/pnas.79.19.5818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ishikawa M, Okada Y. Replication of tobamovirus RNA. Proc Jpn Acad, Ser B. 2004;80(5):215–224. [Google Scholar]
- 19.Buck KW. Comparison of the replication of positive-stranded RNA viruses of plants and animals. Adv Virus Res. 1996;47:159–251. doi: 10.1016/S0065-3527(08)60736-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yamafuji R, Watanabe Y, Meshi T, Okada Y. Replication of TMV-L and Lta1 RNAs and their recombinants in TMV-resistant Tm-1 tomato protoplasts. Virology. 1991;183(1):99–105. doi: 10.1016/0042-6822(91)90122-r. [DOI] [PubMed] [Google Scholar]
- 21.Buck KW. Replication of tobacco mosaic virus RNA. Philos Trans R Soc Lond B Biol Sci. 1999;354(1383):613–627. doi: 10.1098/rstb.1999.0413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Agalarov SC, Sogorin EA, Shirokikh NE, Spirin AS. Insight into the structural organization of the omega leader of TMV RNA: The role of various regions of the sequence in the formation of a compact structure of the omega RNA. Biochem Biophys Res Commun. 2011;404(1):250–253. doi: 10.1016/j.bbrc.2010.11.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shirokikh NE, Agalarov SCh, Spirin AS. Chemical and enzymatic probing of spatial structure of the omega leader of tobacco mosaic virus RNA. Biochemistry (Mosc) 2010;75(4):405–411. doi: 10.1134/s0006297910040024. [DOI] [PubMed] [Google Scholar]
- 24.Gallie DR, Sleat DE, Watts JW, Turner PC, Wilson TMA. The 5′-leader sequence of tobacco mosaic virus RNA enhances the expression of foreign gene transcripts in vitro and in vivo. Nucleic Acids Res. 1987;15(8):3257–3273. doi: 10.1093/nar/15.8.3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sleat DE, et al. Characterisation of the 5′-leader sequence of tobacco mosaic virus RNA as a general enhancer of translation in vitro. Gene. 1987;60(2-3):217–225. doi: 10.1016/0378-1119(87)90230-7. [DOI] [PubMed] [Google Scholar]
- 26.Takamatsu N, et al. Deletion analysis of the 5′ untranslated leader sequence of tobacco mosaic virus RNA. J Virol. 1991;65(3):1619–1622. doi: 10.1128/jvi.65.3.1619-1622.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Komoda K, Naito S, Ishikawa M. Replication of plant RNA virus genomes in a cell-free extract of evacuolated plant protoplasts. Proc Natl Acad Sci USA. 2004;101(7):1863–1867. doi: 10.1073/pnas.0307131101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Komoda K, Mawatari N, Hagiwara-Komoda Y, Naito S, Ishikawa M. Identification of a ribonucleoprotein intermediate of tomato mosaic virus RNA replication complex formation. J Virol. 2007;81(6):2584–2591. doi: 10.1128/JVI.01921-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nishikiori M, Meshi T, Ishikawa M. Guanylylation-competent replication proteins of Tomato mosaic virus are disulfide-linked. Virology. 2012;434(1):118–128. doi: 10.1016/j.virol.2012.09.011. [DOI] [PubMed] [Google Scholar]
- 30.Auweter SD, Oberstrass FC, Allain FH-T. Sequence-specific binding of single-stranded RNA: Is there a code for recognition? Nucleic Acids Res. 2006;34(17):4943–4959. doi: 10.1093/nar/gkl620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goregaoker SP, Lewandowski DJ, Culver JN. Identification and functional analysis of an interaction between domains of the 126/183-kDa replicase-associated proteins of tobacco mosaic virus. Virology. 2001;282(2):320–328. doi: 10.1006/viro.2001.0831. [DOI] [PubMed] [Google Scholar]
- 32.Camborde L, et al. The ubiquitin-proteasome system regulates the accumulation of Turnip yellow mosaic virus RNA-dependent RNA polymerase during viral infection. Plant Cell. 2010;22(9):3142–3152. doi: 10.1105/tpc.109.072090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li XH, Carrington JC. Complementation of tobacco etch potyvirus mutants by active RNA polymerase expressed in transgenic cells. Proc Natl Acad Sci USA. 1995;92(2):457–461. doi: 10.1073/pnas.92.2.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kubota K, Tsuda S, Tamai A, Meshi T. Tomato mosaic virus replication protein suppresses virus-targeted posttranscriptional gene silencing. J Virol. 2003;77(20):11016–11026. doi: 10.1128/JVI.77.20.11016-11026.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ding XS, et al. The Tobacco mosaic virus 126-kDa protein associated with virus replication and movement suppresses RNA silencing. Mol Plant Microbe Interact. 2004;17(6):583–592. doi: 10.1094/MPMI.2004.17.6.583. [DOI] [PubMed] [Google Scholar]
- 36.Hagiwara-Komoda Y, et al. Overexpression of a host factor TOM1 inhibits tomato mosaic virus propagation and suppression of RNA silencing. Virology. 2008;376(1):132–139. doi: 10.1016/j.virol.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 37.Osman TAM, Hemenway CL, Buck KW. Role of the 3′ tRNA-like structure in tobacco mosaic virus minus-strand RNA synthesis by the viral RNA-dependent RNA polymerase In vitro. J Virol. 2000;74(24):11671–11680. doi: 10.1128/jvi.74.24.11671-11680.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Osman TAM, Buck KW. Identification of a region of the tobacco mosaic virus 126- and 183-kilodalton replication proteins which binds specifically to the viral 3′-terminal tRNA-like structure. J Virol. 2003;77(16):8669–8675. doi: 10.1128/JVI.77.16.8669-8675.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Filomatori CV, et al. A 5′ RNA element promotes dengue virus RNA synthesis on a circular genome. Genes Dev. 2006;20(16):2238–2249. doi: 10.1101/gad.1444206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ishikawa M, Meshi T, Watanabe Y, Okada Y. Replication of chimeric tobacco mosaic viruses which carry heterologous combinations of replicase genes and 3′ noncoding regions. Virology. 1988;164(1):290–293. doi: 10.1016/0042-6822(88)90648-4. [DOI] [PubMed] [Google Scholar]
- 41.Ishikawa M, Kroner P, Ahlquist P, Meshi T. Biological activities of hybrid RNAs generated by 3′-end exchanges between tobacco mosaic and brome mosaic viruses. J Virol. 1991;65(7):3451–3459. doi: 10.1128/jvi.65.7.3451-3459.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yuan X, Shi K, Meskauskas A, Simon AE. The 3′ end of Turnip crinkle virus contains a highly interactive structure including a translational enhancer that is disrupted by binding to the RNA-dependent RNA polymerase. RNA. 2009;15(10):1849–1864. doi: 10.1261/rna.1708709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Barry JK, Miller WA. A -1 ribosomal frameshift element that requires base pairing across four kilobases suggests a mechanism of regulating ribosome and replicase traffic on a viral RNA. Proc Natl Acad Sci USA. 2002;99(17):11133–11138. doi: 10.1073/pnas.162223099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nozu Y, Okada Y. Amino acid sequence of a common Japanese strain of tobacco mosaic virus. J Mol Biol. 1968;35(3):643–646. doi: 10.1016/s0022-2836(68)80021-x. [DOI] [PubMed] [Google Scholar]
- 45.Chiba Y, et al. S-adenosyl-L-methionine is an effector in the posttranscriptional autoregulation of the cystathionine γ-synthase gene in Arabidopsis. Proc Natl Acad Sci USA. 2003;100(18):10225–10230. doi: 10.1073/pnas.1831512100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ishibashi K, Komoda K, Ishikawa M (2006) In vitro translation and replication of tobamovirus RNA in a cell-free extract of evacuolated tobacco BY-2 protoplasts. Tobacco BY-2 Cells: From Cellular Dynamics to Omics, Biotechnology in Agriculture and Forestry, eds Nagata T, Matsuoka K, Inzé D (Springer, Berlin), Vol 58, pp 183–194.