Significance
Gambling games are associated with a distorted psychological processing of random sequences (the gambler’s fallacy) and unrewarded outcomes that fall close to a jackpot (near misses). Problem gamblers appear more susceptible to these effects. Here, we show that these two gambling distortions are disrupted in patients with brain injury affecting the insula compared with patients with damage to the ventromedial prefrontal cortex or amygdala. In a roulette task (red/black predictions), comparison groups chose either color less after longer runs of that color outcome. On a slot machine task, comparison groups rated higher motivation following near misses relative to full misses. Our results generate a clinical hypothesis that, in disordered gambling, these cognitions may be underpinned by excessive recruitment of insula circuitry.
Keywords: neuropsychology, emotion, insular cortex
Abstract
Gambling is a naturalistic example of risky decision-making. During gambling, players typically display an array of cognitive biases that create a distorted expectancy of winning. This study investigated brain regions underpinning gambling-related cognitive distortions, contrasting patients with focal brain lesions to the ventromedial prefrontal cortex (vmPFC), insula, or amygdala (“target patients”) against healthy comparison participants and lesion comparison patients (i.e., with lesions that spare the target regions). A slot machine task was used to deliver near-miss outcomes (i.e., nonwins that fall spatially close to a jackpot), and a roulette game was used to examine the gambler’s fallacy (color decisions following outcome runs). Comparison groups displayed a heightened motivation to play following near misses (compared with full misses), and manifested a classic gambler’s fallacy effect. Both effects were also observed in patients with vmPFC and amygdala damage, but were absent in patients with insula damage. Our findings indicate that the distorted cognitive processing of near-miss outcomes and event sequences may be ordinarily supported by the recruitment of the insula. Interventions to reduce insula reactivity could show promise in the treatment of disordered gambling.
Gambling is a widespread activity with a lifetime prevalence of 78% in the United States (1) and a past-year prevalence of 73% in the United Kingdom (2). The widespread recognition that “the house always wins,” reflecting the negative expected value of gambling, makes gambling an enduring puzzle for psychological and economic models of choice behavior. Cognitive approaches to gambling explain this nonnormative behavior with reference to a number of cognitive distortions and irrational beliefs that occur during gambling play, which cause the gambler to overestimate his likelihood of winning (3, 4). The illusion of control refers to how superficial features of a game, such as a choice or instrumental response, promote erroneous perceptions of skill over outcomes that are determined only by chance (5). Near-miss outcomes (nonwins that fall close to the jackpot) increase motivations to play, plausibly by fueling beliefs about skill acquisition (6). The gambler’s fallacy is a bias in the processing of randomness, whereby recent consecutive outcomes are considered less likely to repeat, and conversely, outcomes that have not occurred in the recent history are perceived as “due” (7).
These distortions are reliably observed in field studies, e.g., casino environments (8), and are not confined to gambling; illusory control and the gambler’s fallacy are observed in stock traders (9), and near misses influence decision-making in occupational settings (10). In the laboratory, these distortions can be elicited with gambling games, allowing the comparison of these biases between different clinical groups. The overall level of distorted thinking is elevated in people with gambling problems (11, 12), and these cognitions can be targeted effectively in psychological therapy for disordered gambling (13).
The neurobiological basis of these gambling-related distortions has received little attention to date. Functional imaging studies of pathological gambling have focused largely on abnormalities in appetitive processing, reinforcement learning, and executive functions (14–17). These studies identify dysregulation across an extended brain network (sometimes termed the brain reward system) that includes the ventromedial prefrontal cortex (vmPFC), striatum, amygdala, and insula. However, in these experiments, the direction of signal abnormality (i.e., hyperactivity vs. hypoactivity) is not consistent, and the precise neural signatures are highly task-dependent (18). A previous functional MRI (fMRI) study of a simplified slot machine found that near misses recruited overlapping neural circuitry to the jackpot wins in the ventral striatum and insula, and that insula responses increased with higher levels of trait-related susceptibility to gambling distortions (6, 19). Other neuroimaging work has indicated sensitivity of insula and medial prefrontal cortex to sequences of consecutive outcomes, and subsequent updating of choice strategy (20–22).
The aim of the present study was to investigate brain regions underlying gambling distortions by studying patients with focal brain injury. Unlike functional neuroimaging, this neuropsychological approach allows causal inferences to be made concerning the necessary role of candidate brain regions in psychological processes (23). We identified cases with focal brain damage affecting the vmPFC, the insula, or the amygdala; injury to these regions impairs real-life decision-making and emotional behavior (24–26). Furthermore, neuropsychological testing in pathological gamblers has identified a profile of impaired risky choice that is highly reminiscent of vmPFC damage in particular (27, 28). Given the exaggeration of gambling-related cognitive distortions in problem gamblers, an intuitive prediction might be that the lesion groups would show an enhanced sensitivity to near-miss outcomes, illusory control, and gambler’s fallacy. However, the alternative prediction is also plausible: given that these nonnormative gambling biases also occur in healthy participants, in whom they are underpinned by the recruitment of reward-related neural circuitry (6, 19, 20), the lesion groups might be immunized against these gambling distortions.
Results
Patients with lesions affecting the vmPFC (n = 17; Fig. S1), insula (n = 8; Fig. 1), and amygdala (n = 6; Fig. S2) completed two gambling tasks. A slot machine task (Fig. 2A) measured the sensitivity to near-miss outcomes, and included a manipulation of personal choice that provides a measure of the illusion of control. A roulette task (Fig. 2B) measured the susceptibility to the gambler’s fallacy. With the inclusion of multiple lesion subgroups of fairly small sizes, the first stage of analysis collapsed the three groups into a pooled “target group” (n = 31) for comparison against healthy participants (n = 16) and a lesion comparison group (n = 13; Fig. S3) that comprised a mixture of patients with posterior, lateral temporal, and superior frontal cortex damage. This approach maximizes power to detect an effect in regions that are anatomically interconnected, and likely to operate as a functional circuit (26). At a second stage of analysis, the target group was separated into the constituent subgroups to directly compare the effects of vmPFC, insula, and amygdala damage, given evidence for differential functional specializations of these regions (29–31). In both analyses, the groups did not differ significantly in terms of age, years of education, or sex distribution (Table S1). When asked about their gambling involvement in real life, most participants reported “none” or “occasional” involvement, with only two cases (one with a lesion affecting vmPFC and one lesion comparison patient) reporting “regular” gambling. Two lesion patients reported that their gambling had increased following their brain injury; both were males in the vmPFC lesion group, and one of these was the only participant identified as a probable pathological gambler on a screening instrument, the South Oaks Gambling Screen (32) (threshold ≥5; this participant scored 6). There were no group differences on the Gambling Related Cognitions Scale (33), a trait-related measure of their beliefs about gambling and susceptibility to gambling biases.
Fig. 1.
Lesion overlap in the insula lesion group. On the coronal slices, the radiological convention (i.e., right shown at left) is applied. Warmer colors represent greater lesion overlap across patients. Seven of the eight patients had lesions that were mappable from MRI scans; one further patient had a CT scan that was not of sufficient quality for lesion mapping, but was clinically inspected for verification of lesion location in the insula. In the right-sided cases, there is maximal lesion overlap in the insula and secondary somatosensory cortex area in all four patients (red). In the left-sided cases, the overlap in the three mappable cases (yellow) is in the insula, but is relatively small because damage in one patient was focused on the anterior insula.
Fig. 2.
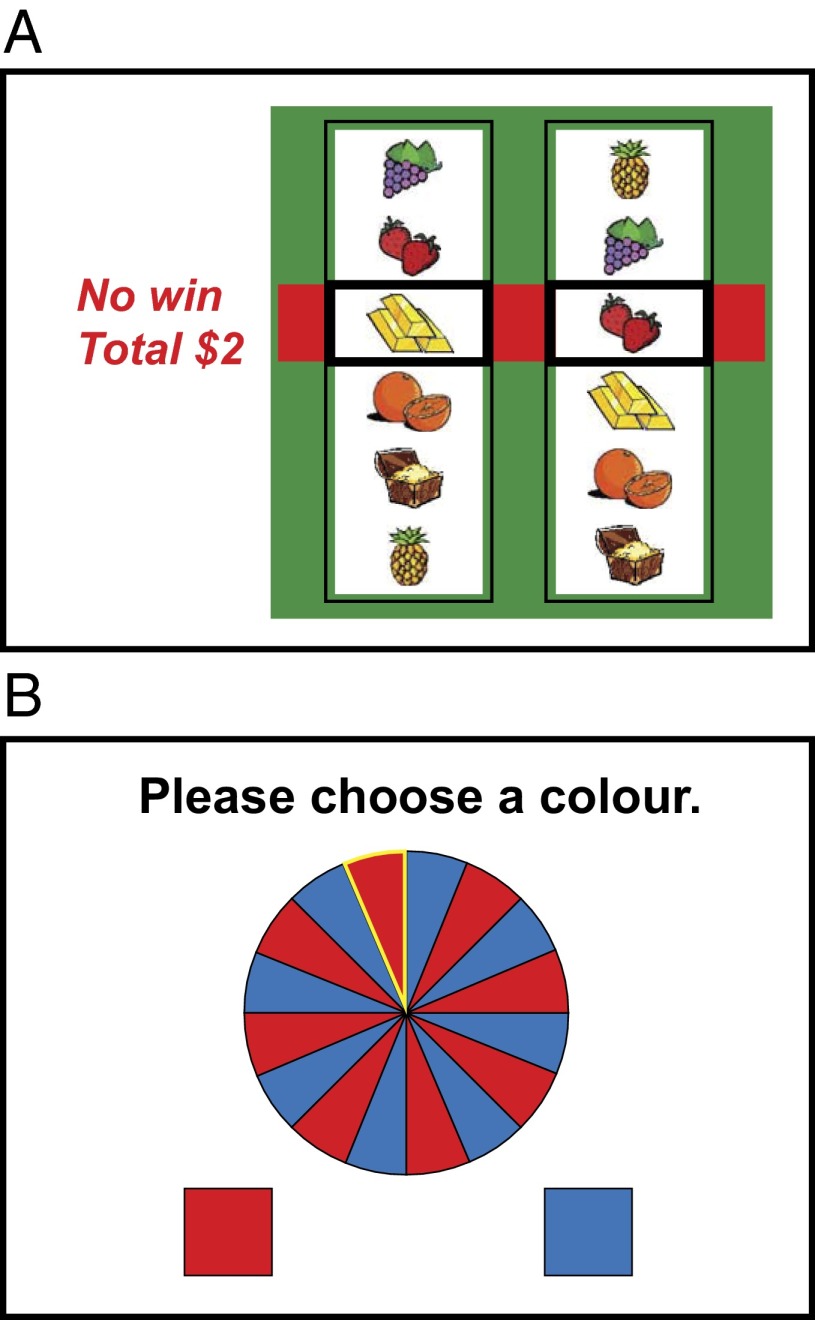
Graphical illustration of the two gambling simulations. (A) The slot machine task was used to measure the effects of near-miss outcomes (shown) on postoutcome ratings. (B) The roulette task involved red/blue color choices to measure the gambler’s fallacy.
Slot Machine Task.
A simulated two-reel slot machine was used to deliver wins, as well as near-miss and full-miss nonwinning outcomes. On half the trials, the participant was asked to select a “play icon” from six alternatives on the left reel; on the remaining trials, the play icon was selected automatically by the computer. Following this icon selection, the right hand reel spun and slowed to a standstill. If the right reel aligned with the selected icon on the left reel, the participant received a hypothetical win (“win $1!”). All other outcomes were designated nonwins (“no win”). On-screen Likert scales were presented following icon selection (“please rate your chances of winning”) and following the outcome message (“how pleased are you with that result?”; “how much do you want to continue the game?”).
On the ratings of “chances of winning,” all groups manifested a higher expectancy of winning when selecting the play icon themselves, compared with when the play icon was selected automatically [main effect: F(1,47) = 7.03, P = 0.011]. This influence of personal choice is consistent with an illusion of control. The effect did not vary significantly across groups [main effect of group, F(2,47) = 0.49, P = 0.613; group × choice interaction, F(2,47) = 1.00, P = 0.377; Table S2].
Comparing the subjective ratings following winning outcomes against all nonwin outcomes, the wins were rated as more pleasant [F(1,45) = 90.3; P < 0.001] and there was a significant group × outcome interaction [F(2,45) = 4.32; P = 0.019]. Win responsivity was blunted in the lesion comparison group (∆ = 45.5, SD = 56.8) compared with the target group (∆ = 108.3, SD = 53.9, P = 0.005) and the healthy participants (∆ = 87.5, SD = 54.2, P = 0.071), who did not differ (P = 0.247; Fig. S4). Pleasantness ratings did not vary as a function of personal choice [F(1,45) = 1.66, P = 0.204]. Wins also increased the motivation to continue [F(1,43) = 11.4, P = 0.002], and this effect did not vary across groups [F(2,43) = 0.64, P = 0.532] or as a function of personal choice [F(1,43) = 0.68, P = 0.413].
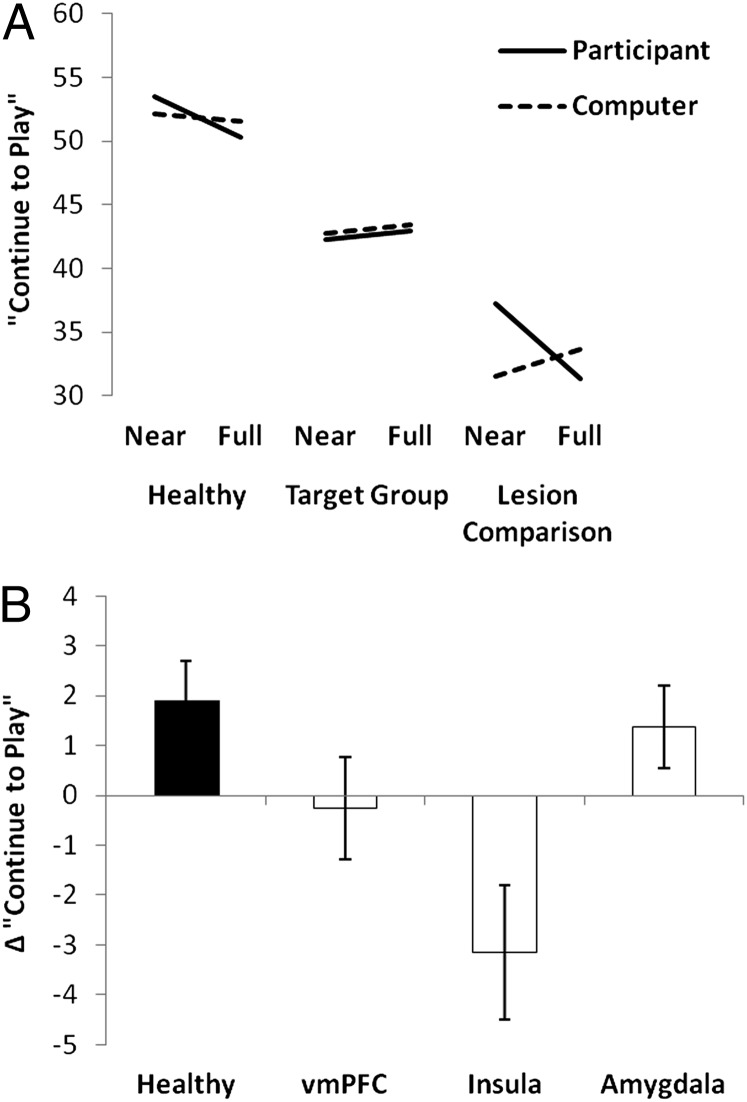
The next set of tests compared the near misses against the full misses, which are both nonwin outcomes that are objectively equivalent. On the motivation rating, a statistically reliable outcome × control interaction was seen [F(1,43) = 6.03, P = 0.018; Fig. 3A]. Consistent with past observations with this task, the interaction was driven by the participant-chosen trials, on which near misses tended to increase the motivation to continue playing (mean = 45.2, SD = 29.2) compared with full-misses (mean = 43.6, SD = 29.9), although this effect was marginally significant [t45 = 1.69, P = 0.10]. On trials without personal control, near misses and full misses did not differ [t45 = 0.76, P = 0.453]. In addition, there was a significant outcome × group interaction [F(2,43) = 3.69, P = 0.033] such that the motivational effect of near misses was attenuated in the target group (∆ = −0.70, SD = 3.51) compared with healthy participants (∆ = 1.91, SD = 3.01; P = 0.019) and the lesion comparison group (∆ = 1.92, SD = 2.81; P = 0.068). There were no significant effects in the equivalent model for pleasantness ratings.
Fig. 3.
Motivational ratings of near-miss outcomes on the slot machine task, displayed separately for participant-chosen and computer-chosen trials. (A) Target group, lesion comparison group, and healthy participants. (B) Change scores (Δ) for the motivational ratings after near misses (minus full misses) for the target subgroups. Error bars indicate SEM.
Subdividing the target group into the vmPFC, insula, and amygdala subgroups, the overall task sensitivities were similar to the first model: the perceived chances of winning was higher on personal choice trials, and winning outcomes were rated as more pleasant and increased the motivation to continue the game. An additional effect was a further manifestation of the illusion of control: an outcome × choice interaction was observed on the pleasantness ratings following wins compared with nonwins [F(1,20) = 12.1, P = 0.002], such that participant-chosen wins were rated as more pleasant (mean = 69.9, SD = 31.5) than computer-chosen wins (mean = 61.1, SD = 33.5; t22 = 2.87, P = 0.009). This effect did not vary across groups [F(2,20) = 0.402, P = 0.674]. Critically, the motivational ratings to near misses compared with full misses revealed a further dissociation between the three lesion sites [outcome × group interaction: F(2,21) = 3.47, P = 0.050]. The insula group showed a smaller (and in fact inverted) motivational response to near misses (minus full misses: ∆ = −3.2, SD = 3.6) compared with the amygdala group (∆ = +1.4, SD = 2.1; P = 0.018; Fig. 3B). The insula group also differed at trend from the vmPFC group (∆ = −0.3, SD = 3.4; P = 0.074).
Roulette Task.
Participants played 90 successive trials on a binary-choice roulette task. The roulette wheel displayed an equal number of red and blue segments, and on each trial, the participant first guessed red or blue, and then gave a confidence rating on a 21-point visual analog scale. After these two responses, the wheel spun briefly and the outcome was displayed for that trial. Consecutive outcomes of the same color are referred to as “runs” (i.e., blue, red, red, red is an outcome run of length 3), and consecutive correct or incorrect predictions are referred to as “streaks.”
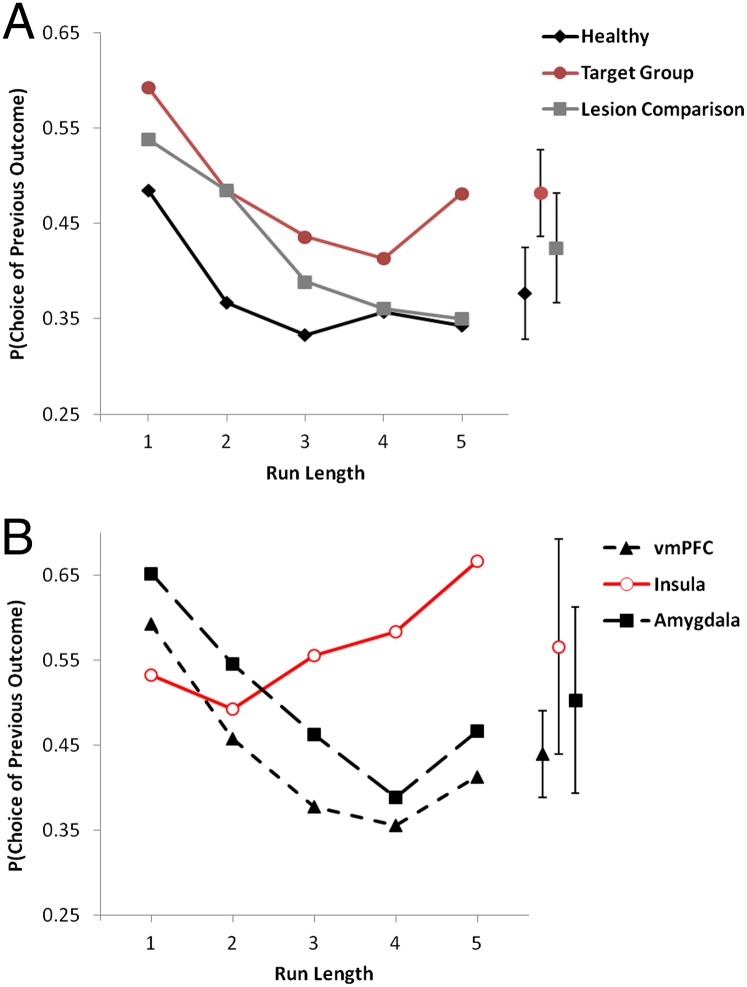
To quantify the gambler’s fallacy, we calculated the probability of choosing either color as a function of the run length of that color (7). In a model comparing the target group, lesion comparison group, and healthy participants, there was a strong main effect of run length [F(4,200) = 8.83, P < 0.001], with decreasing choice of either color after longer runs of that color. This gambler’s fallacy effect did not vary across groups [main effect of group: F(2,50) = 1.14, P = 0.327; group × run length interaction, F(8,200) = 0.56, P = 0.728; Fig. 4A].
Fig. 4.
Choice behavior on the roulette task. (A) Target group, lesion comparison group, and healthy participants. (B) Subdividing the target group to display the insula, vmPFC, and amygdala lesion groups. The ordinate presents the proportion of trials on which the participant’s color choice matched the outcome of the previous spin.
Comparing the subgroups of target patients (15 vmPFC, 6 insula, 6 amygdala), the analysis of color choice again showed the main effect of run length [F(4,96) = 3.55, P = 0.010], as well as a significant group × run length interaction [F(8,96) = 2.14, P = 0.039]. Calculating a change score based on the difference between shorter run lengths (i.e., one or two) and longer run lengths (i.e., three, four, or five), the insula group showed positive recency on average (∆ = −0.09, SD = 0.18), differing significantly from the vmPFC group (∆ = 0.14, SD = 0.16) (P = 0.005) and the amygdala group (∆ = 0.16, SD = 0.14; P = 0.012), who did not differ in expression of the typical gambler’s fallacy (P = 0.831; Fig. 4B).
We also examined confidence ratings on the roulette game as a function of winning and losing streaks. Comparing the target group against the lesion comparison group and healthy participants, subjective confidence did not vary significantly as a function of either winning streak length [F(4,184) = 0.25, P = 0.833] or losing streak length [F(4,184) = 1.61, P = 0.192]. As such, there was no discernible “hot hand” effect in our data (7) (although see also Table S3). The effects of streak length did not interact significantly with group status (all F < 1.39, P > 0.259), and there were no additional effects within the target group. Several additional metrics were derived to characterize choice behavior on the roulette task. There were no differences between groups in the overall choice of red vs. blue, or the “stickiness” of choice according to the previous choice or the previous outcome (Table S4). We computed variables reflecting win-stay and lose-shift biases; there was a significant difference between the target group, lesion comparison group, and healthy participants in the lose-shift score [F(2,50) = 3.25, P = 0.047] but not the win-stay score [F(2,50) = 0.39, P = 0.679]. Target patients were more likely to switch color choice following an unsuccessful prediction than healthy controls (P = 0.023), but this tendency did not vary significantly between the vmPFC, insula, and amygdala subgroups [F(2,24) = 0.362, P = 0.700].
Discussion
The key effect described here is that a group of patients with stable brain injury affecting the insula region show a marked attenuation of two cognitive distortions that were elicited in healthy participants and patients with lesions to other structures, and which can be widely observed during naturalistic gambling across various games. On the slot machine task, near-miss outcomes (whereby the reel stopped one position from a win) typically increase the self-reported motivation to continue with the game (6, 34); this effect was selectively absent in the insula group. On the roulette task, binary choice displayed a classic negative recency, whereby the choice of either color decreased as a function of the preceding run of that color (7); the insula group did not manifest this avoidance of recent outcomes. To our knowledge, these data provide the first evidence for the causal involvement of the insula region in some of the cognitions characteristic of gambling behavior.
The roulette task used here was a simple guessing game, tapping strategic decision-making that may be modulated—erroneously—by the recent outcome history. Here, patients with brain injury affecting the insula distributed their choices between the two color options, and showed no apparent differences in basic stickiness or self-reported confidence, but they deviated from the other groups in that they did not show a gambler’s fallacy bias. An intriguing feature is that the averaged data for the insula group displayed modest positive recency in their roulette color choice (Fig. 4B). On the slot machine task, near misses were similarly observed to be demotivating (Fig. 3B). This apparent inversion implies some systematic tendency in the insula group, but based on an alternative model of the task contingencies (and supported by different regions of the decision-making network). In a probabilistic environment, it is beneficial to select recently reinforced options, and a recent fMRI study indicated that dorsal striatal responses track reinforcement learning parameters in such a task (35). Other work highlights involvement of dorsolateral prefrontal cortex in detecting pattern violations (36) and switching responding after longer runs (37). One relevant procedural difference in the “matched pennies” task used by Xue et al. (37) is that a history bar was presented, showing participants the recent outcomes. After long runs of the same outcome, the history bar may serve as a direct cue to switch, thus lessening any reliance on participants’ internal model of the task. Further behavioral work could usefully compare strategic choice in the presence or absence of such cues for the reinforcement history.
In light of evidence that gambling cognitions are increased in disordered gambling (11, 13), these data generate a testable hypothesis that overrecruitment of insula circuitry may underlie gambling-related cognitive distortions. In fact, functional neuroimaging provides some support for this idea. In healthy participants, insula responsivity to near-miss outcomes correlated positively with their trait susceptibility to gambling distortions on a self-report scale (6, 38). With a monetary incentive delay task in treatment-seeking pathological gamblers, overactivity of anterior insula during loss anticipation was correlated with gambling severity scores (39). These effects may be mediated by the established role of the insula in the representation of bodily states, i.e., interoception (40). Certainly, gambling is an intensely exciting, visceral activity, and near misses were previously shown to induce physiological changes in skin conductance and heart rate (34, 41). One could hypothesize that the central processing of these peripheral signals is abolished by insula damage. Via a similar mechanism, insula activity has been linked to drug craving, such that smokers who suffered infarcts to the insula region quit smoking and described an abolition of the urge to smoke (42). Animal models have corroborated these effects of insula damage on drug self-administration, with lesions centered on posterior, granular insula (43, 44). Therapeutic strategies to reduce insula responsivity, such as mindfulness- or meditation-based techniques (45, 46) or GABAergic medications (47), may usefully augment cognitive therapy for psychological distortions in the treatment of problem gambling.
In addition to its interoceptive functions, the insula is increasingly thought to play a critical role in decision-making under uncertainty. fMRI studies indicate heightened insula signal following outcomes from risky decisions, and these responses vary across subjects as a function of risk-taking propensities (22, 48, 49). Anterior insula appears to represent risk predictions during choice, and risk prediction errors in response to decision outcomes (50). Such predictions about the uncertainty of the environment are relevant to the near-miss effect (51) and gambler’s fallacy (35), and arguably less relevant to the illusion of control effect that did not vary across lesion groups here. Past work in cases with insula lesions has shown increases in risk-taking and impaired discrimination between risky gains and risky losses (31, 52). By using an investment task in which most participants are loss-averse, patients with brain injury to vmPFC, insula, or amygdala achieved higher profits (26), and these effects were strongest in the insula subgroup (n = 4), who also failed to modify their investment behavior as a function of prior outcome (i.e., losses vs. wins)—an effect that resembles the abolition of the gambler’s fallacy (Table S5 also displays an analogous effect in icon selection on the slot machine task). Although the target group in the present study showed a greater lose-shift tendency on the roulette task, this effect did not vary across groups (and was numerically weakest in the insula subgroup), and thus seems unlikely to contribute to their positive recency across successive red or black outcomes.
At the current time, it is not known whether the insula involvement in decision-making and risky choice is dissociable from its interoceptive functions. Although an integrative model has been proposed that anterior insula represents predictions of internal states and decision uncertainty (53), other work highlights functional segregation within the insula, in which decision-making localizes to the anterior, agranular insula adjacent to the orbitofrontal region, and visceral representations may be located more posteriorly (54, 55). Neuropsychological studies in stroke cases lack the specificity to resolve anterior–posterior insula effects, but we note that the insula lesion overlap in the present study was located posteriorly.
In the patients with injury to the vmPFC and amygdala, the effects of near misses and the gambler’s fallacy were comparable to those of participants in the healthy and lesion comparison groups. Data from neuropsychological testing and functional neuroimaging in pathological gamblers provide much evidence for disruption of the vmPFC and orbitofrontal cortex (16, 27, 28, 56), as well as preliminary evidence for amygdala involvement in loss aversion (57) and gain expectancies (17). Nevertheless, our data do not support the involvement of these regions in either the near-miss effect or gambler’s fallacy. Concerning the lack of effect in the vmPFC lesion group, it may be pertinent that neither of our gambling tasks loaded heavily on risk-taking or representations of expected value (31, 58). Rather, the slot machine task primarily measured emotional reactivity. Past studies have also found no effects of vmPFC lesions on the responses to obtained financial gains (59), mood induction (60), or emotional images, providing attentional engagement is adequate (61). We did observe some diminution of win responsivity in the lesion comparison group on pleasantness ratings. Given that this effect was not predicted, and the heterogeneous nature of the damage in the lesion comparison group, this effect is treated with caution.
Some further observations require additional testing to fully resolve. In our insula group, damage extended into the dorsal part of the basal ganglia in some patients. Single case analysis (Table S6) indicated that these patients were most disrupted on the two distortions, but also showed that some attenuation was clearly present in the insula cases with no striatal involvement. Larger studies are needed to resolve the functional dissociations between insula and (dorsal) striatum (62). It could be reasoned that striatally mediated effects should also interfere with win processing and the personal choice manipulation (63, 64), which was not the case. Finally, the disruption of the near-miss effect and the gambler’s fallacy in the insula cases implies some linkage of these two gambling distortions, raising a broader question of how the various gambling-related cognitive distortions should be organized at a psychological or neural level. In conclusion, we provide neuropsychological evidence for the causal involvement of the insula in two gambling-related cognitive distortions, generating a testable hypothesis of insula overactivity in disordered gambling.
Materials and Methods
Participants.
Neurological patients were recruited from the patient registry in the department of neurology at the University of Iowa. All patients had focal, stable lesions that were predominantly of an adult-onset nature, and all sustained at least 1 y before testing. All lesion cases have undergone extensive screening and neuropsychological evaluation that rule out dementia and diffuse cognitive deficits, and these measures have been presented in previous studies (24, 30, 31, 52). Exclusion criteria were a history of mental retardation, learning disability, or psychiatric illness including substance abuse. Patients were selected for eligibility on the basis of neuroanatomical status obtained from MRI or CT scanning (as detailed later). For the vmPFC group (Fig. S1), the criterion for inclusion was damage in the unilateral or bilateral portions of the mesial orbital/ventromedial sector of the prefrontal cortex and/or the frontal pole. None of the patients in the vmPFC group had damage involving the amygdala or the insular cortex. Lesion etiology in the vmPFC group was hemorrhage caused by ruptured aneurysm of the anterior communicating artery or benign tumor resections, and the group including a mixture of bilateral (n = 12), right unilateral (n = 3), and left unilateral (n = 2) lesions.
In the insula lesion group (Fig. 1), the lesion involved damage to any part of the insular cortex (anterior and/or posterior) and/or the adjacent secondary somatosensory cortex. In the insula group, lesion etiology was a middle cerebral artery stroke in all cases, and all lesions were unilateral (left, n = 4; right, n = 4). In individual cases, some lesions extended medially into the edge of the basal ganglia (internal capsule and possible putamen; Table S6 shows single-case analysis), laterally into superior temporal lobe, posteriorly into the inferior parietal cortex, and anteriorly into the inferior frontal gyrus. None of the cases had damage that reached the medial temporal lobe or the medial prefrontal cortex.
In the amygdala lesion group (Fig. S2), the lesion involved selective bilateral damage to the amygdala in one case (caused by Urbach-Wiethe disease) or unilateral left-sided damage in the other five cases. In the unilateral cases, lesion etiology was surgical resection to treat pharmacoresistent epilepsy, and the damage included the amygdala but extended to adjacent regions of the hippocampus, parahippocampal gyrus, and entorhinal cortex. None of the cases had damage that reached the insula or the medial prefrontal cortex.
The lesion comparison group (Fig. S3) involved brain damage sparing the target brain regions, i.e., the lesion did not include any insula, amygdala, or mesial orbital/vmPFC. These cases had unilateral damage that was mostly caused by strokes and a few benign tumor resections.
A further 16 healthy participants were recruited through community advertising. The study was approved by the human subjects committee at the University of Iowa. Before enrollment in the study, written informed consent was acquired in accordance with the Declaration of Helsinki. Participants were testing in quiet laboratory conditions. In addition to the two gambling tasks, participants completed the South Oaks Gambling Screen (32), a self-report symptom checklist for pathological gambling, and the Gambling Related Cognitions Scale (33), a 24-item questionnaire assessing trait susceptibility to gambling cognitions.
Neuroanatomical Analysis.
Lesion location was generally confirmed by using MRI, with a 1.5-T General Electric scanner with a spin gradient sequence, in 1.5-mm contiguous T1-weighted coronal slices. MRI scanning was not available in every case (see Fig. 1 and Figs. S1–S3); for those cases, a CT scan was acquired instead with the use of a Picker 1200 or Toshiba Express SX scanner, with tilt angle optimized per subject to avoid clip-related artifact (zoom, 2.4; field of view, 51 cm; fovea, 212.5 mm; slice thickness, 2–4 mm). For all mappable lesions, the lesions of individual patients were transferred manually onto a normal reference brain by using the MAP-3 technique (65).
Slot Machine Task.
Participants completed 60 trials (after four practice trials) on a simplified two-reel slot machine task, described in detail by Clark et al. (6). The screen background color (white or black) designated two choice conditions: participant-chosen trials, in which the participant selected the “play icon” on the left reel by scrolling the reel up or down, and computer-chosen trials, in which the play icon was selected automatically. Following icon selection, the right reel spun and decelerated (mean spin time, 4.2 s) to deliver a win ($1) or a near-miss or full-miss outcome (outcome duration, 6 s). Current earnings were displayed in the intertrial interval (duration, 5 s); instructions specified that the participant was playing for “pretend money.” The outcomes and choice condition (participant-chosen, computer-chosen) occurred in a fixed pseudorandom sequence such that wins occurred on one in six trials and near misses occurred on one in three trials. On each trial, three Likert ratings were taken: following icon selection, “How do you rate your chances of winning?” (0 to +100); and following the outcome, “How pleased are you with the result?” (−100 to +100) and “How much do you want to continue to play?” (0 to +100).
Roulette Task.
This binary choice task was modified from previous work (7). The roulette wheel displayed an equal number of red and blue segments, and, on each trial, the participant first guessed red or blue, and then gave a confidence rating on a 21-point scale. Following the color choice and confidence rating, the wheel spun for 800–1,200 ms, and the outcome was presented (e.g., “blue: you win”). Participants completed three practice trials, followed by a total of 90 trials, by using a prespecified color sequence to deliver runs of one to five consecutive outcomes of the same color. This fixed sequence had an equal probability of either color, and a probability of alternation of 0.48.
Statistical Analysis.
Some patients could not be tested on all measures, and there are further exclusions on the two tasks for participants who did not vary their ratings at all (on the slot machine task), or did not vary their choice behavior sufficiently on the roulette game (>95% to red or black). Although such uniform responding is a reasonable approach that does not violate the rules of either task, these cases would be inherently insensitive to the distortions of interest here. ANOVA models used the Greenhouse–Geisser correction, with two-tailed P < 0.05. Post hoc comparisons were tested by using least significant differences, as is appropriate for three-group designs (66).
Supplementary Material
Acknowledgments
This work was supported by Medical Research Council (United Kingdom) Grant G1100554 (to L.C.), a PhD studentship from the Medical Research Council (to B.S.), National Institute of Neurological Disorders and Stroke [National Institutes of Health (NIH)] Grant P01 NS19632 (to A.B. and D.T.), and National Institute on Drug Abuse (NIH) Grants R01 DA023051 and R01 DA022549 (to A.B. and D.T.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1322295111/-/DCSupplemental.
References
- 1.Kessler RC, et al. DSM-IV pathological gambling in the National Comorbidity Survey Replication. Psychol Med. 2008;38(9):1351–1360. doi: 10.1017/S0033291708002900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wardle H, et al. British Gambling Prevalence Survey. London: National Centre for Social Research; 2010. [Google Scholar]
- 3.Clark L. Decision-making during gambling: An integration of cognitive and psychobiological approaches. Philos Trans R Soc Lond B Biol Sci. 2010;365(1538):319–330. doi: 10.1098/rstb.2009.0147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ladouceur R, Walker M. A cognitive perspective on gambling. In: Salkovskis PM, editor. Trends in Cognitive and Behavioural Therapies. Chichester, UK: Wiley; 1996. pp. 89–120. [Google Scholar]
- 5.Langer EJ. The illusion of control. J Pers Soc Psychol. 1975;32:311–328. [Google Scholar]
- 6.Clark L, Lawrence AJ, Astley-Jones F, Gray N. Gambling near-misses enhance motivation to gamble and recruit win-related brain circuitry. Neuron. 2009;61(3):481–490. doi: 10.1016/j.neuron.2008.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ayton P, Fischer I. The hot hand fallacy and the gambler’s fallacy: Two faces of subjective randomness? Mem Cognit. 2004;32(8):1369–1378. doi: 10.3758/bf03206327. [DOI] [PubMed] [Google Scholar]
- 8.Croson R, Sundali J. The gambler’s fallacy and the hot hand: Empirical data from casinos. J Risk Uncertain. 2005;30:195–209. [Google Scholar]
- 9.Fenton-O’Creevy M, Nicholson N, Soane E, Willman P. Trading on illusions: Unrealistic perceptions of control and trading performance. J Occup Organ Psychol. 2003;76:53–68. [Google Scholar]
- 10.Tinsley CH, Dillon R, Cronin M. How near-miss events amplify or attenuate risky decision making. Manage Sci. 2012;58:1596–1613. [Google Scholar]
- 11.Michalczuk R, Bowden-Jones H, Verdejo-Garcia A, Clark L. Impulsivity and cognitive distortions in pathological gamblers attending the UK National Problem Gambling Clinic: A preliminary report. Psychol Med. 2011;41(12):2625–2635. doi: 10.1017/S003329171100095X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miller NV, Currie SR. A Canadian population level analysis of the roles of irrational gambling cognitions and risky gambling practices as correlates of gambling intensity and pathological gambling. J Gambl Stud. 2008;24(3):257–274. doi: 10.1007/s10899-008-9089-5. [DOI] [PubMed] [Google Scholar]
- 13.Fortune EE, Goodie AS. Cognitive distortions as a component and treatment focus of pathological gambling: A review. Psychol Addict Behav. 2012;26(2):298–310. doi: 10.1037/a0026422. [DOI] [PubMed] [Google Scholar]
- 14.Balodis IM, et al. Diminished frontostriatal activity during processing of monetary rewards and losses in pathological gambling. Biol Psychiatry. 2012;71(8):749–757. doi: 10.1016/j.biopsych.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miedl SF, Peters J, Büchel C. Altered neural reward representations in pathological gamblers revealed by delay and probability discounting. Arch Gen Psychiatry. 2012;69(2):177–186. doi: 10.1001/archgenpsychiatry.2011.1552. [DOI] [PubMed] [Google Scholar]
- 16.Reuter J, et al. Pathological gambling is linked to reduced activation of the mesolimbic reward system. Nat Neurosci. 2005;8(2):147–148. doi: 10.1038/nn1378. [DOI] [PubMed] [Google Scholar]
- 17.van Holst RJ, Veltman DJ, Büchel C, van den Brink W, Goudriaan AE. Distorted expectancy coding in problem gambling: Is the addictive in the anticipation? Biol Psychiatry. 2012;71(8):741–748. doi: 10.1016/j.biopsych.2011.12.030. [DOI] [PubMed] [Google Scholar]
- 18.Limbrick-Oldfield EH, van Holst RJ, Clark L. Fronto-striatal dysregulation in drug addiction and pathological gambling: Consistent inconsistencies? Neuroimage Clin. 2013;2:385–393. doi: 10.1016/j.nicl.2013.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shao R, Read J, Behrens TE, Rogers RD. Shifts in reinforcement signalling while playing slot-machines as a function of prior experience and impulsivity. Translat Psychiatry. 2013;3:e235. doi: 10.1038/tp.2012.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Akitsuki Y, et al. Context-dependent cortical activation in response to financial reward and penalty: An event-related fMRI study. Neuroimage. 2003;19(4):1674–1685. doi: 10.1016/s1053-8119(03)00250-7. [DOI] [PubMed] [Google Scholar]
- 21.Elliott R, Friston KJ, Dolan RJ. Dissociable neural responses in human reward systems. J Neurosci. 2000;20(16):6159–6165. doi: 10.1523/JNEUROSCI.20-16-06159.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xue G, Lu Z, Levin IP, Bechara A. The impact of prior risk experiences on subsequent risky decision-making: the role of the insula. Neuroimage. 2010;50(2):709–716. doi: 10.1016/j.neuroimage.2009.12.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rorden C, Karnath HO. Using human brain lesions to infer function: A relic from a past era in the fMRI age? Nat Rev Neurosci. 2004;5(10):813–819. doi: 10.1038/nrn1521. [DOI] [PubMed] [Google Scholar]
- 24.Bechara A, Tranel D, Damasio H. Characterization of the decision-making deficit of patients with ventromedial prefrontal cortex lesions. Brain. 2000;123(pt 11):2189–2202. doi: 10.1093/brain/123.11.2189. [DOI] [PubMed] [Google Scholar]
- 25.Damasio A, Damasio H, Tranel D. Persistence of feelings and sentience after bilateral damage of the insula. Cereb Cortex. 2013;23(4):833–846. doi: 10.1093/cercor/bhs077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shiv B, Loewenstein G, Bechara A, Damasio H, Damasio AR. Investment behavior and the negative side of emotion. Psychol Sci. 2005;16(6):435–439. doi: 10.1111/j.0956-7976.2005.01553.x. [DOI] [PubMed] [Google Scholar]
- 27.Goudriaan AE, Oosterlaan J, de Beurs E, van den Brink W. Neurocognitive functions in pathological gambling: A comparison with alcohol dependence, Tourette syndrome and normal controls. Addiction. 2006;101(4):534–547. doi: 10.1111/j.1360-0443.2006.01380.x. [DOI] [PubMed] [Google Scholar]
- 28.Lawrence AJ, Luty J, Bogdan NA, Sahakian BJ, Clark L. Problem gamblers share deficits in impulsive decision-making with alcohol-dependent individuals. Addiction. 2009;104(6):1006–1015. doi: 10.1111/j.1360-0443.2009.02533.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arana FS, et al. Dissociable contributions of the human amygdala and orbitofrontal cortex to incentive motivation and goal selection. J Neurosci. 2003;23(29):9632–9638. doi: 10.1523/JNEUROSCI.23-29-09632.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bechara A, Damasio H, Damasio AR, Lee GP. Different contributions of the human amygdala and ventromedial prefrontal cortex to decision-making. J Neurosci. 1999;19(13):5473–5481. doi: 10.1523/JNEUROSCI.19-13-05473.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Clark L, et al. Differential effects of insular and ventromedial prefrontal cortex lesions on risky decision-making. Brain. 2008;131(pt 5):1311–1322. doi: 10.1093/brain/awn066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lesieur HR, Blume SB. The South Oaks Gambling Screen (SOGS): A new instrument for the identification of pathological gamblers. Am J Psychiatry. 1987;144(9):1184–1188. doi: 10.1176/ajp.144.9.1184. [DOI] [PubMed] [Google Scholar]
- 33.Raylu N, Oei TP. The Gambling Related Cognitions Scale (GRCS): Development, confirmatory factor validation and psychometric properties. Addiction. 2004;99(6):757–769. doi: 10.1111/j.1360-0443.2004.00753.x. [DOI] [PubMed] [Google Scholar]
- 34.Clark L, Crooks B, Clarke R, Aitken MR, Dunn BD. Physiological responses to near-miss outcomes and personal control during simulated gambling. J Gambl Stud. 2012;28(1):123–137. doi: 10.1007/s10899-011-9247-z. [DOI] [PubMed] [Google Scholar]
- 35.Jessup RK, O’Doherty JP. Human dorsal striatal activity during choice discriminates reinforcement learning behavior from the gambler’s fallacy. J Neurosci. 2011;31(17):6296–6304. doi: 10.1523/JNEUROSCI.6421-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huettel SA, Mack PB, McCarthy G. Perceiving patterns in random series: Dynamic processing of sequence in prefrontal cortex. Nat Neurosci. 2002;5(5):485–490. doi: 10.1038/nn841. [DOI] [PubMed] [Google Scholar]
- 37.Xue G, Juan CH, Chang CF, Lu ZL, Dong Q. Lateral prefrontal cortex contributes to maladaptive decisions. Proc Natl Acad Sci USA. 2012;109(12):4401–4406. doi: 10.1073/pnas.1111927109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dymond S, et al. Almost winning: Induced MEG theta power in insula and orbitofrontal cortex increases during gambling near-misses and is associated with BOLD signal and gambling severity. Neuroimage. 2014;91:210–219. doi: 10.1016/j.neuroimage.2014.01.019. [DOI] [PubMed] [Google Scholar]
- 39.Choi JS, et al. Altered brain activity during reward anticipation in pathological gambling and obsessive-compulsive disorder. PLoS ONE. 2012;7(9):e45938. doi: 10.1371/journal.pone.0045938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Craig AD. How do you feel—now? The anterior insula and human awareness. Nat Rev Neurosci. 2009;10(1):59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- 41.Dixon MJ, et al. Psychophysiological arousal signatures of near-misses in slot machine play. Int Gambl Stud. 2011;11:1–14. [Google Scholar]
- 42.Naqvi NH, Rudrauf D, Damasio H, Bechara A. Damage to the insula disrupts addiction to cigarette smoking. Science. 2007;315(5811):531–534. doi: 10.1126/science.1135926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Contreras M, et al. A role for the insular cortex in long-term memory for context-evoked drug craving in rats. Neuropsychopharmacology. 2012;37(9):2101–2108. doi: 10.1038/npp.2012.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pushparaj A, et al. Electrical stimulation of the insular region attenuates nicotine-taking and nicotine-seeking behaviors. Neuropsychopharmacology. 2013;38(4):690–698. doi: 10.1038/npp.2012.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kirk U, Downar J, Montague PR. Interoception drives increased rational decision-making in meditators playing the ultimatum game. Front Neurosci. 2011;5:49. doi: 10.3389/fnins.2011.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Paulus MP, Stewart JL. Interoception and drug addiction. Neuropharmacology. 2014;76(Pt B):342–350. doi: 10.1016/j.neuropharm.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wiebking C, et al. GABA in the insula - a predictor of the neural response to interoceptive awareness. Neuroimage. 2014;86:10–18. doi: 10.1016/j.neuroimage.2013.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kuhnen CM, Knutson B. The neural basis of financial risk taking. Neuron. 2005;47(5):763–770. doi: 10.1016/j.neuron.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 49.Paulus MP, Rogalsky C, Simmons A, Feinstein JS, Stein MB. Increased activation in the right insula during risk-taking decision making is related to harm avoidance and neuroticism. Neuroimage. 2003;19(4):1439–1448. doi: 10.1016/s1053-8119(03)00251-9. [DOI] [PubMed] [Google Scholar]
- 50.Preuschoff K, Quartz SR, Bossaerts P. Human insula activation reflects risk prediction errors as well as risk. J Neurosci. 2008;28(11):2745–2752. doi: 10.1523/JNEUROSCI.4286-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Clark L, et al. Learning and affect following near-miss outcomes in simulated gambling. J Behav Decis Making. 2013;26:442–450. [Google Scholar]
- 52.Weller JA, Levin IP, Shiv B, Bechara A. The effects of insula damage on decision-making for risky gains and losses. Soc Neurosci. 2009;4(4):347–358. doi: 10.1080/17470910902934400. [DOI] [PubMed] [Google Scholar]
- 53.Singer T, Critchley HD, Preuschoff K. A common role of insula in feelings, empathy and uncertainty. Trends Cogn Sci. 2009;13(8):334–340. doi: 10.1016/j.tics.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 54.Chang LJ, Yarkoni T, Khaw MW, Sanfey AG. Decoding the role of the insula in human cognition: Functional parcellation and large-scale reverse inference. Cereb Cortex. 2013;23(3):739–749. doi: 10.1093/cercor/bhs065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Deen B, Pitskel NB, Pelphrey KA. Three systems of insular functional connectivity identified with cluster analysis. Cereb Cortex. 2011;21(7):1498–1506. doi: 10.1093/cercor/bhq186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rømer Thomsen K, et al. Altered paralimbic interaction in behavioral addiction. Proc Natl Acad Sci USA. 2013;110(12):4744–4749. doi: 10.1073/pnas.1302374110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.De Martino B, Camerer CF, Adolphs R. Amygdala damage eliminates monetary loss aversion. Proc Natl Acad Sci USA. 2010;107(8):3788–3792. doi: 10.1073/pnas.0910230107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rushworth MF, Noonan MP, Boorman ED, Walton ME, Behrens TE. Frontal cortex and reward-guided learning and decision-making. Neuron. 2011;70(6):1054–1069. doi: 10.1016/j.neuron.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 59.Camille N, et al. The involvement of the orbitofrontal cortex in the experience of regret. Science. 2004;304(5674):1167–1170. doi: 10.1126/science.1094550. [DOI] [PubMed] [Google Scholar]
- 60.Gillihan SJ, et al. Contrasting roles for lateral and ventromedial prefrontal cortex in transient and dispositional affective experience. Soc Cogn Affect Neurosci. 2011;6(1):128–137. doi: 10.1093/scan/nsq026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Damasio AR, Tranel D, Damasio H. Individuals with sociopathic behavior caused by frontal damage fail to respond autonomically to social stimuli. Behav Brain Res. 1990;41(2):81–94. doi: 10.1016/0166-4328(90)90144-4. [DOI] [PubMed] [Google Scholar]
- 62.Palminteri S, et al. Critical roles for anterior insula and dorsal striatum in punishment-based avoidance learning. Neuron. 2012;76(5):998–1009. doi: 10.1016/j.neuron.2012.10.017. [DOI] [PubMed] [Google Scholar]
- 63.Coricelli G, et al. Regret and its avoidance: A neuroimaging study of choice behavior. Nat Neurosci. 2005;8(9):1255–1262. doi: 10.1038/nn1514. [DOI] [PubMed] [Google Scholar]
- 64.Tricomi EM, Delgado MR, Fiez JA. Modulation of caudate activity by action contingency. Neuron. 2004;41(2):281–292. doi: 10.1016/s0896-6273(03)00848-1. [DOI] [PubMed] [Google Scholar]
- 65.Frank RJ, Damasio H, Grabowski TJ. Brainvox: An interactive, multimodal visualization and analysis system for neuroanatomical imaging. Neuroimage. 1997;5(1):13–30. doi: 10.1006/nimg.1996.0250. [DOI] [PubMed] [Google Scholar]
- 66.Cardinal RN, Aitken MRF. ANOVA for the Behavioural Sciences Researcher. Mahwah, NJ: Lawrence Erlbaum; 2006. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.