Abstract
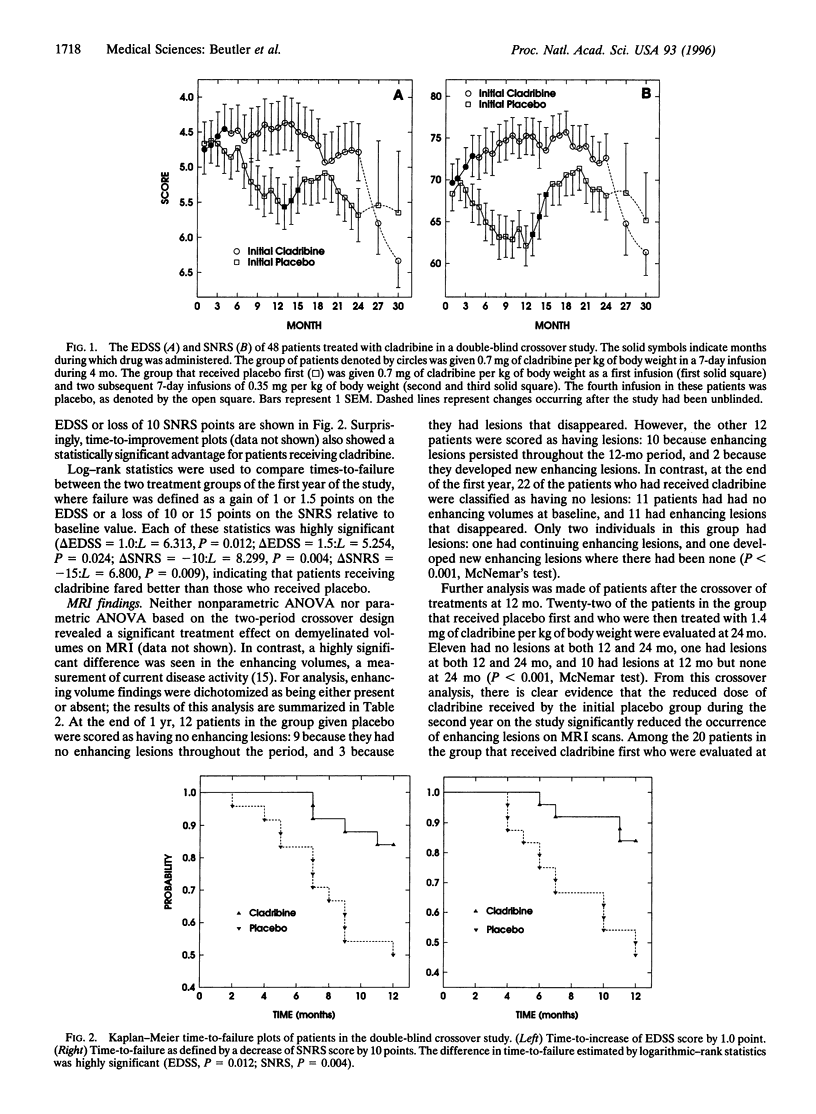
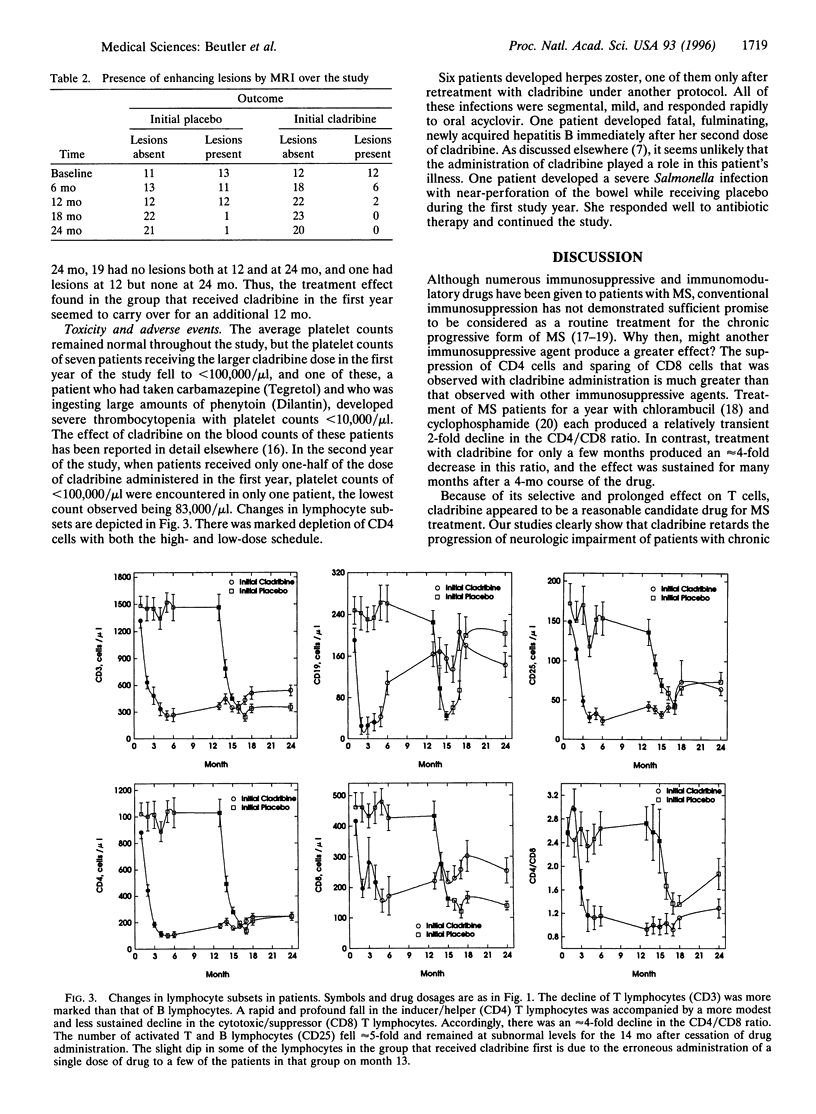
A 2-year, placebo-controlled, double-blind, crossover study was started in 1992 to evaluate cladribine, an immunosuppressive drug, in the treatment of chronic progressive multiple sclerosis. In the first year patients were given cladribine 0.10 mg/kg per day for 7 days as four monthly courses for a total of 2.8 mg/kg or placebo. During the second year patients treated with placebo during the first year were given i.v. infusions of 0.10 mg, 0.05 mg, and 0.05 mg of cladribine per kg of body weight per day for 7 consecutive days in three successive monthly courses, for a total dose of 1.4 mg/kg. Patients who had been treated previously with cladribine were crossed over to placebo. Analysis of the results revealed a favorable influence on the neurological performance scores, both in the Kurtze extended disability status and the Scripps neurological rating scale, and on MRI findings in patients treated with cladribine. In the first year the most striking finding was that while clinical deterioration continued in the placebo-treated patients, the condition of patients who received cladribine stabilized or even improved slightly. Toxicity and therapeutic response were dose-related.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beutler E. Cladribine (2-chlorodeoxyadenosine) Lancet. 1992 Oct 17;340(8825):952–956. doi: 10.1016/0140-6736(92)92826-2. [DOI] [PubMed] [Google Scholar]
- Beutler E., Koziol J. A., McMillan R., Sipe J. C., Romine J. S., Carrera C. J. Marrow suppression produced by repeated doses of cladribine. Acta Haematol. 1994;91(1):10–15. doi: 10.1159/000204236. [DOI] [PubMed] [Google Scholar]
- Beutler E. New chemotherapeutic agent: 2-chlorodeoxyadenosine. Semin Hematol. 1994 Jan;31(1):40–45. [PubMed] [Google Scholar]
- Carson D. A., Wasson D. B., Beutler E. Antileukemic and immunosuppressive activity of 2-chloro-2'-deoxyadenosine. Proc Natl Acad Sci U S A. 1984 Apr;81(7):2232–2236. doi: 10.1073/pnas.81.7.2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson D. A., Wasson D. B., Kaye J., Ullman B., Martin D. W., Jr, Robins R. K., Montgomery J. A. Deoxycytidine kinase-mediated toxicity of deoxyadenosine analogs toward malignant human lymphoblasts in vitro and toward murine L1210 leukemia in vivo. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6865–6869. doi: 10.1073/pnas.77.11.6865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson D. A., Wasson D. B., Taetle R., Yu A. Specific toxicity of 2-chlorodeoxyadenosine toward resting and proliferating human lymphocytes. Blood. 1983 Oct;62(4):737–743. [PubMed] [Google Scholar]
- Chiappelli F., Myers L. W., Ellison G. W., Liao D., Fahey J. L. Preferential reductions in lymphocyte sub-populations induced by monthly pulses of chlorambucil: studies in patients with chronic progressive multiple sclerosis. Int J Immunopharmacol. 1991;13(5):455–461. doi: 10.1016/0192-0561(91)90064-e. [DOI] [PubMed] [Google Scholar]
- Ford H. C. Multiple sclerosis: a survey of alternative hypotheses concerning aetiology, pathogenesis and predisposing factors. Med Hypotheses. 1987 Oct;24(2):201–207. doi: 10.1016/0306-9877(87)90105-8. [DOI] [PubMed] [Google Scholar]
- Goodkin D. E., Bailly R. C., Teetzen M. L., Hertsgaard D., Beatty W. W. The efficacy of azathioprine in relapsing-remitting multiple sclerosis. Neurology. 1991 Jan;41(1):20–25. doi: 10.1212/wnl.41.1.20. [DOI] [PubMed] [Google Scholar]
- Goodkin D. E., Cookfair D., Wende K., Bourdette D., Pullicino P., Scherokman B., Whitham R. Inter- and intrarater scoring agreement using grades 1.0 to 3.5 of the Kurtzke Expanded Disability Status Scale (EDSS). Multiple Sclerosis Collaborative Research Group. Neurology. 1992 Apr;42(4):859–863. doi: 10.1212/wnl.42.4.859. [DOI] [PubMed] [Google Scholar]
- Grieb P., Ryba M., Stelmasiak Z., Nowicki J., Solski J., Jakubowska B. Cladribine treatment of multiple sclerosis. Lancet. 1994 Aug 20;344(8921):538–538. [PubMed] [Google Scholar]
- Khoury S. J., Guttmann C. R., Orav E. J., Hohol M. J., Ahn S. S., Hsu L., Kikinis R., Mackin G. A., Jolesz F. A., Weiner H. L. Longitudinal MRI in multiple sclerosis: correlation between disability and lesion burden. Neurology. 1994 Nov;44(11):2120–2124. doi: 10.1212/wnl.44.11.2120. [DOI] [PubMed] [Google Scholar]
- Koziol J. A., Maxwell D. A. A distribution-free test for paired growth curve analyses with application to an animal tumour immunotherapy experiment. Stat Med. 1982 Jan-Mar;1(1):83–89. doi: 10.1002/sim.4780010111. [DOI] [PubMed] [Google Scholar]
- Kurtzke J. F. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology. 1983 Nov;33(11):1444–1452. doi: 10.1212/wnl.33.11.1444. [DOI] [PubMed] [Google Scholar]
- Liliemark J., Albertioni F., Hassan M., Juliusson G. On the bioavailability of oral and subcutaneous 2-chloro-2'-deoxyadenosine in humans: alternative routes of administration. J Clin Oncol. 1992 Oct;10(10):1514–1518. doi: 10.1200/JCO.1992.10.10.1514. [DOI] [PubMed] [Google Scholar]
- Miller D. H., Barkhof F., Berry I., Kappos L., Scotti G., Thompson A. J. Magnetic resonance imaging in monitoring the treatment of multiple sclerosis: concerted action guidelines. J Neurol Neurosurg Psychiatry. 1991 Aug;54(8):683–688. doi: 10.1136/jnnp.54.8.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moody D. J., Fahey J. L., Grable E., Ellison G. W., Myers L. W. Administration of monthly pulses of cyclophosphamide in multiple sclerosis patients. Delayed recovery of several immune parameters following discontinuation of long-term cyclophosphamide treatment. J Neuroimmunol. 1987 Mar;14(2):175–182. doi: 10.1016/0165-5728(87)90051-8. [DOI] [PubMed] [Google Scholar]
- Poser C. M., Paty D. W., Scheinberg L., McDonald W. I., Davis F. A., Ebers G. C., Johnson K. P., Sibley W. A., Silberberg D. H., Tourtellotte W. W. New diagnostic criteria for multiple sclerosis: guidelines for research protocols. Ann Neurol. 1983 Mar;13(3):227–231. doi: 10.1002/ana.410130302. [DOI] [PubMed] [Google Scholar]
- Sipe J. C., Knobler R. L., Braheny S. L., Rice G. P., Panitch H. S., Oldstone M. B. A neurologic rating scale (NRS) for use in multiple sclerosis. Neurology. 1984 Oct;34(10):1368–1372. doi: 10.1212/wnl.34.10.1368. [DOI] [PubMed] [Google Scholar]
- Sipe J. C., Romine J. S., Koziol J. A., McMillan R., Zyroff J., Beutler E. Cladribine in treatment of chronic progressive multiple sclerosis. Lancet. 1994 Jul 2;344(8914):9–13. doi: 10.1016/s0140-6736(94)91046-4. [DOI] [PubMed] [Google Scholar]
- Weiner H. L., Hafler D. A. Immunotherapy of multiple sclerosis. Ann Neurol. 1988 Mar;23(3):211–222. doi: 10.1002/ana.410230302. [DOI] [PubMed] [Google Scholar]



