Significance
It is commonly accepted that the plant hormone auxin mediates apical dominance. However, we have discovered that apical dominance strongly correlates with sugar availability and not apically supplied auxin. We have revealed that apical dominance is predominantly controlled by the shoot tip’s intense demand for sugars, which limits sugar availability to the axillary buds. These findings overturn a long-standing hypothesis on apical dominance and encourage us to reevaluate the relationship between hormones and sugars in this and other aspects of plant development.
Keywords: shoot branching, sink demand, decapitation, girdling, long-distance signaling
Abstract
For almost a century the plant hormone auxin has been central to theories on apical dominance, whereby the growing shoot tip suppresses the growth of the axillary buds below. According to the classic model, the auxin indole-3-acetic acid is produced in the shoot tip and transported down the stem, where it inhibits bud growth. We report here that the initiation of bud growth after shoot tip loss cannot be dependent on apical auxin supply because we observe bud release up to 24 h before changes in auxin content in the adjacent stem. After the loss of the shoot tip, sugars are rapidly redistributed over large distances and accumulate in axillary buds within a timeframe that correlates with bud release. Moreover, artificially increasing sucrose levels in plants represses the expression of BRANCHED1 (BRC1), the key transcriptional regulator responsible for maintaining bud dormancy, and results in rapid bud release. An enhancement in sugar supply is both necessary and sufficient for suppressed buds to be released from apical dominance. Our data support a theory of apical dominance whereby the shoot tip’s strong demand for sugars inhibits axillary bud outgrowth by limiting the amount of sugar translocated to those buds.
Apical dominance is the process whereby the shoot tip inhibits the outgrowth of axillary buds further down the stem to control the number of growing shoot tips and branches. In response to the loss of their shoot tips, plants have evolved rapid long-distance signaling mechanisms to release axillary buds and replenish the plant with new growing shoot tips. Since the 1930s, theories regarding apical dominance have involved the plant hormone auxin (indole-3-acetic acid, IAA), which moves down the stem from the shoot tip (1). Depletion of IAA from the stem after the loss of the shoot tip (e.g., decapitation) is commonly thought to induce the growth of new branches. This auxin depletion is central to all established apical dominance models, whether they focus on auxin transport from buds or auxin regulation of other hormones, including cytokinin and strigolactone (2–5).
The finding that apically derived auxin does not move into the axillary buds (6, 7) has resulted in a debate among researchers as to how auxin inhibits those buds. The major theories on apical dominance are not necessarily mutually exclusive. In the auxin transport canalization-based model, axillary buds are thought to remain dormant until a sufficient amount of auxin is able to flow out of the buds (2, 8–10). This bud-derived auxin gradually becomes canalized into a small number of cell files that later become the vascular tissue that supports the growth of the growing branch. The continual flow of auxin from the shoot tip is thought to maintain apical dominance by preventing auxin flow from axillary buds. In the second messenger theory, apically derived auxin inhibits axillary bud growth indirectly by inhibiting cytokinin production and/or promoting strigolactone synthesis (4, 11, 12). Unlike auxin, cytokinin and strigolactone are thought to move into the axillary bud to promote or inhibit bud growth, respectively. Consistent with this theory, axillary bud growth can be regulated by direct application of strigolactone and cytokinin to the buds (4).
At first consideration, the flow of auxin from the shoot tip down the plant seems to be an ideal system to both maintain apical dominance as well as to initiate bud growth after the loss of the shoot tip. It is widely observed that lateral bud growth can be reduced by auxin supplied to the stump of decapitated plants; however, the growth inhibition is usually incomplete, even in the model species pea (Pisum sativum) and Arabidopsis thaliana (9, 13–17). Closer investigation reveals a substantial disconnect between apical auxin supply and bud outgrowth. First, treatment of stumps of decapitated stems with auxin fails to prevent the initial bud growth, with auxin acting only on the later stages of bud outgrowth (14). Second, auxin depletion in wild-type stems caused by decapitation, stem girdling, or auxin transport inhibitors does not always promote bud outgrowth (5, 14, 15). Third, auxin is ineffective at inhibiting bud outgrowth after decapitation in some species, including Arabidopsis thaliana, and under some conditions, including high light irradiances (15). Finally, in studies in which the shoot tip and bud are separated by a long distance, bud growth after decapitation is observed before any expected or measured changes in IAA content in the stem adjacent to the bud (5, 14, 18).
Specifically, in 20-cm-tall pea plants, we have shown the zone of IAA depletion after decapitation extends only one-third of the full distance required to promote the furthest buds (14). Computational modeling of auxin transport and auxin depletion indicates that even a 0.1% drop in IAA content would not be perceived at this furthest node until well after bud growth has commenced (18). We also showed that naphthylphthalamic acid blocks auxin transport from the shoot tip and reduces auxin content in the upper zone of the stem but has no effect on early bud growth (14). Moreover, exogenous auxin could not prevent bud growth in decapitated plants for almost 24 h after treatment (14, 16). Consequently, it is unlikely that IAA levels directly or indirectly regulate the early outgrowth response of buds to decapitation.
Here, we have used digital time-lapse photography technology to accurately measure axillary bud growth in response to different physiological treatments. Although we acknowledge that auxin plays a role in apical dominance, we have been able to rule out auxin as the master regulator owing to its lack of correlation with early axillary bud growth. In 1924, the nutritive hypothesis proposed that access to plant nutrients is the major factor regulating axillary bud growth (19–21). Our study has drawn upon concepts of the nutritive hypothesis to reveal that apical dominance is maintained largely by the sugar demand of the shoot tip, which limits the amount of sugars available to the axillary buds. After shoot tip loss, sugars accumulate in axillary buds within a time frame consistent with bud growth. We show here that this decapitation response can also be mimicked by supplying exogenous sugars to intact plants. With these findings, the previously mentioned disparities between auxin content and bud outgrowth can be explained by taking into account sugar availability to the buds.
Results and Discussion
Axillary Buds Are Released by a Positive Shoot-Derived Signal.
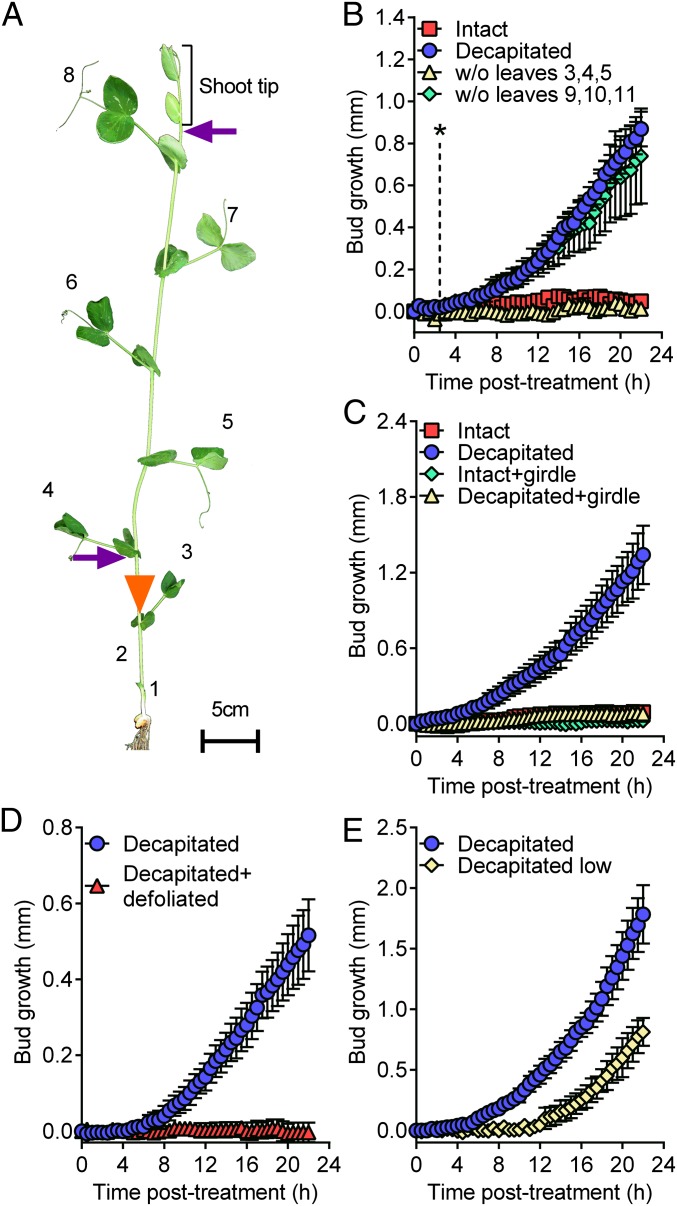
Using plants tall enough to separate the shoot tip from the basal node 2 by 20 cm, we have previously shown that measurable bud growth after decapitation, termed bud release, can be observed at this node while it remains below the zone of depleted auxin (14, 18). To test whether this effect of bud release beyond the zone of auxin depletion was specific to plant age or a particular node (14), we investigated auxin content and bud outgrowth in intact and decapitated garden pea plants taller than 150 cm, selecting node 7 for analysis (Fig. S1). Again bud outgrowth was observed before any measureable IAA depletion in the adjacent stem. The rapid release of axillary buds after decapitation may be a common feature of a wide range of plant species, because a similar rapid bud release in response to decapitation was also observed in the distantly related species, Nicotiana tabacum (Fig. S2). In pea, by closely examining bud growth using digital time-lapse photography, we were able to observe significant bud growth in buds 40 cm below the shoot tip within 2.5 h of decapitation (Fig. 1 A and B and Movie S1). A signal moving from the shoot tip to release bud inhibition in the lower nodes within 2.5 h would need to travel at 16 cm h−1 or faster. The rapid signal that releases bud inhibition therefore moves at least 1 order of magnitude faster than the speeds reported for IAA exported from the shoot tip. Rapid and long-distance bud release can also be induced at a similar rate by removing a number of young expanding leaves from the shoot tip (Fig. 1B), which have previously been reported to be crucial for maintaining apical dominance (22).
Fig. 1.
Mature leaves are the source of the rapid decapitation-induced signal. (A) Positions of leaves (numbered), girdle (5) (triangle), and sites of decapitation (B–E, upper arrow, and E, lower arrow) used in this study. (B) Decapitation as well as removal of expanding leaves (node 9–11) within the shoot tip, but not the mature (node 3–5) leaves, induced rapid bud release at node 2; n = 4. The dotted line with an asterisk indicates the time at which the growth kinetics between buds of intact and decapitated plants became significantly different according to nonoverlapping 95% confidence intervals between the two treatments after an ANOVA analysis of data from three separate experiments, n = 11–12. (C) Stem girdling below node 4 prevented rapid bud release. n = 4. (D) Defoliating plants from nodes 3 to 8 prevented the decapitation-induced rapid bud release. n = 3 or 4. (E) Decapitation of the plants low on the stem delayed bud release. n = 4. All data are mean ± SEM.
To better understand the nature of this apical dominance signal, we tested the possibility that the shoot tip, composed of expanding leaves and internodes, prevents bud release by producing a growth inhibitor that moves more rapidly than IAA. Here we used a girdling technique (5) that physically prevents the transport of compounds from the upper shoot to the buds below the girdle. As we have shown previously for longer-term bud growth (5), plants that are girdled low on the stem, and therefore below the majority of expanded leaves, failed to activate bud release (Fig. 1 A and C and Movie S1). This experiment demonstrates that the depletion of any shoot-derived inhibitor, including auxin, is not sufficient to promote rapid bud release, because these compounds will become depleted below the girdle. Moreover, the lack of transport through the girdle also prevented rapid bud release in decapitated plants (Fig. 1C and Movie S1), indicating that the shoot above the girdle must produce a signal that promotes bud release.
Photoassimilates Correlate with Bud Release.
The significant impact of girdling on bud release (Fig. 1C) is consistent with the nutritive hypothesis of apical dominance (19–21) whereby access to plant nutrients, including photoassimilates, is a major determinant of axillary bud growth. To explore the importance of shoot-derived nutrients for bud release, we removed all of the mature leaves before decapitation. Leaf removal completely abolished the decapitation-induced bud release (Fig. 1D), revealing an absolute requirement of mature leaves for the release from apical dominance. From this, we predicted that a delay or prevention of bud release would occur in plants that are decapitated lower in the stem, owing to the reduced number of leaves. The current auxin-centered apical dominance theories would predict that decapitation low on the stem would instead result in a promotion of bud release due to the increased rate at which auxin will begin to deplete at node 2 (2, 4, 5). However, as we predicted, plants decapitated in the lower third of the stem resulted in a 5-h delay in bud release compared with plants decapitated above the highest expanded leaf (Fig. 1E). This slower response to decapitation lower on the stem indicates that leaves, or possibly recently fixed photoassimilates, are crucial for bud release.
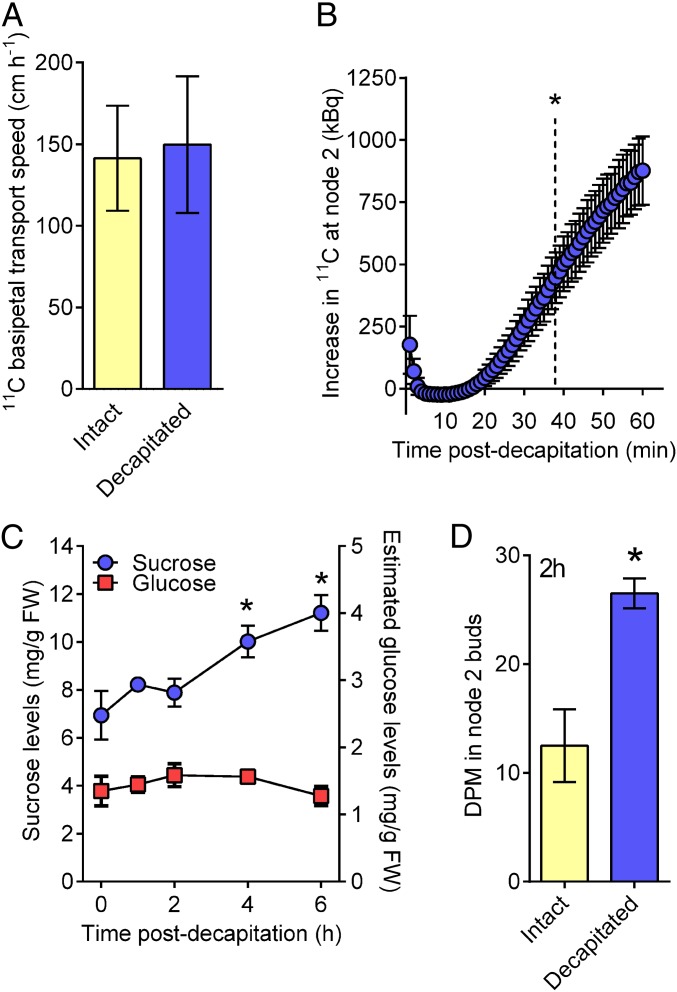
To test for involvement of photoassimilates in apical dominance, we first evaluated whether the speed of photoassimilate transport is sufficient to precede bud release. We supplied upper leaves with [11C] CO2 and then monitored the movement of the 11C-labeled photoassimilates through sensors attached to upper and lower positions on the plant stem. The speed of 11C transport in both intact and decapitated plants calculated using these sensors was ∼150 cm h−1 (Fig. 2A), which is more than 2 orders of magnitude faster than IAA and also faster than the 16 cm h−1 that we calculated as the minimum speed of a signal required to promote bud release (Fig. 1B). Although the transport speeds were equivalent in decapitated and intact plants (Fig. 2A), a greater amount of photoassimilate was transported to node 2 in decapitated plants than in intact plants (Fig. 2B). Labeled carbon photoassimilates that were fixed in the youngest fully expanded leaf at the top of the plant significantly accumulated in the node 2 region within 38 min of decapitation (Fig. 2B) and before bud growth. This is a conservative estimate of timing because all of the leaves, including those closer to node 2, would have continued to load unlabeled photoassimilates into the phloem, and after decapitation these photoassimilates would also be directed predominantly down through the stem (23).
Fig. 2.
Loss of apical dominance causes rapid carbon redistribution and sucrose accumulation in axillary buds. (A) Speed of 11C-photoassimilate flow through the phloem is rapid but unchanged after decapitation. n = 3. (B) Decapitation rapidly increased the amount of 11C radioactivity that accumulated at node 2 after 11CO2 feeding to the uppermost fully expanded leaf. n = 3. Data represent the increase in radioactivity observed in decapitated plants over intact controls ± SEM. Dotted line indicates the time at which the difference became statistically significant based on a one-sample, two-tailed t test. (C) Sucrose, but not glucose, accumulated in node 2 buds after decapitation. Twenty to twenty-four buds per replicate, n = 6. An ANOVA with a Dunnett’s multiple comparison of means test was performed comparing all samples with intact controls. (D) Decapitation after 14C-sucrose feeding to node 4 petioles enhanced 14C uptake in node 2 buds. Six buds per replicate, n = 4. Statistical significance from intact controls was determined using a two-tailed t test. All data are mean ± SEM. *Statistical difference from controls (P ≤ 0.05).
Examination of the endogenous sugar content of axillary buds by mass spectrometry revealed that total sucrose levels increased by 44% in node 2 buds within 4 h of decapitation, whereas glucose levels remained unaffected (Fig. 2C). The poor correlation between sucrose and glucose content in growing buds is consistent with sucrose being delivered to the buds (23), rather than being produced locally from starch metabolism (24). We next examined sucrose uptake using 14C-sucrose, which enables a more sensitive measure of recently acquired carbon in buds compared with total sucrose levels. By supplying 14C-sucrose to a single leaf (node 4), we identified that, within 2 h, decapitation resulted in a doubling in 14C translocated from node 4 into node 2 buds (Fig. 2D). These data indicate that after decapitation the plant increases its endogenous carbon supply to the axillary buds within the timeframe sufficient to induce bud release (Figs. 1 and 2).
Sucrose Supply Promotes Bud Release and Inhibits BRANCHED1 Expression.
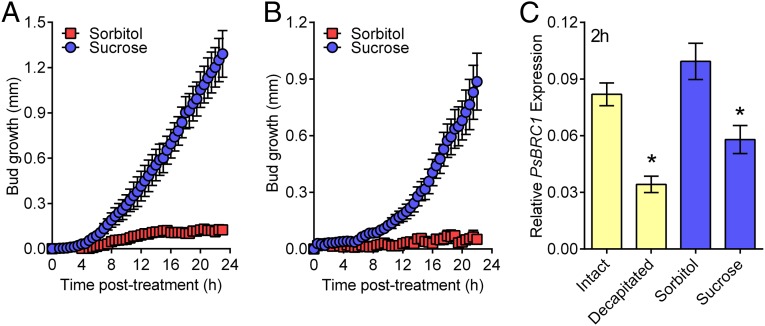
Next we tested whether sucrose, the major form of transported photosynthate (23), can activate bud release, and hence whether a relatively low sucrose supply to axillary buds may limit bud release. Intact plants supplemented with exogenous sucrose displayed rapid axillary bud release (Fig. 3A and Fig. S3), similar to decapitated plants (Fig. 1 B–E). Sucrose could also restore rapid bud release to plants that have been decapitated low on their stem (Fig. 3B). We evaluated bud growth responses in intact plants by supplying a range of sucrose concentrations typically reported for phloem sap (25–27). All concentrations caused an early growth response, including 100 mM, which is at the low end of typical sucrose levels in phloem (Fig. S3). These treatments were given as a single initial treatment but had effects on bud lengths in the longer term. At day 3 the highest dose of exogenous sucrose, 600 mM, had caused significantly more growth than other sucrose concentrations, which in turn were significantly longer than those of control plants (Fig. S3). This experiment indicates that although sucrose supply may act as a switch for the early outgrowth (up to one day), it has more of a dose-dependent energy-supply role over time.
Fig. 3.
Sucrose addition rapidly initiates bud release and suppresses the branching repressor, BRC1. (A) Sucrose feeding via the nodes 3 and 5 petioles rapidly initiated bud release of intact plants. (B) Sucrose feeding via the node 3 petiole rescued the delayed bud release of plants decapitated low on the stem; n = 4. (C) BRC1 expression in node 2 buds was inhibited within 2 h by both decapitation and sucrose supply to intact plants (P ≤ 0.05). Twenty buds were collected per replicate, n = 3. *Statistical difference from controls (P ≤ 0.05) based on a two-tailed t test. All data are mean ± SEM.
BRANCHED1 (BRC1) is a key transcription factor gene that, on the basis of its mutant branching phenotype, is required for bud inhibition. BRC1 is known to be transcriptionally regulated in axillary buds of pea by strigolactone and cytokinin (4) and is thought to inhibit bud activation by repressing the cell cycle and meristem activity (28). Like the response to decapitation, sucrose supplied to intact plants caused a substantial reduction in BRC1 expression in node 2 buds (Fig. 3C) within the 2-h timeframe in which we observe statistically significant bud growth (Fig. 1). The ability of sucrose to promote cell cycle progression directly (29) or indirectly via BRC1 (28) indicates that increased sucrose availability to axillary buds may activate bud release by promoting the cell cycle and thereby stimulating meristematic cell division in the buds.
This release of buds by exogenous sucrose in intact or decapitated plants is consistent with the increased bud growth observed in isolated rose stem segments supplied with sugars (30, 31) and in plants with genetically altered sugar signaling and/or metabolism (32). Our results indicate that enhancing sugar supply to axillary buds is sufficient for bud release (Fig. 3), and conversely, limiting sugar availability to axillary buds (Fig. 1 C–E) is part of the mechanism used by plants to maintain strong apical dominance. Consistent with this conclusion, the reduced tillering phenotype of the tin mutant in wheat has recently been linked to reduced carbohydrate availability to the axillary buds (33).
Our studies explain why auxin supplied to the decapitated stump is unable to inhibit the early growth of buds after decapitation, despite its ability to reduce their growth at a later stage (14). It would be interesting to determine whether enhanced delivery of sucrose to buds affects auxin biosynthesis, metabolism, and/or conjugation (34–36) in buds and whether this may have a role in early bud growth. However, our data reveal that it is unlikely that auxin transport plays a role during the early growth period after decapitation. Reducing auxin transport from the buds, by directly applying the auxin transport inhibitor naphthylphthalamic acid to those buds, was unable to inhibit decapitation-induced bud growth over the first 24-h period (Fig. S4), despite its ability to cause longer-term inhibition (12). These data further highlight the role of nonauxin regulation during bud release.
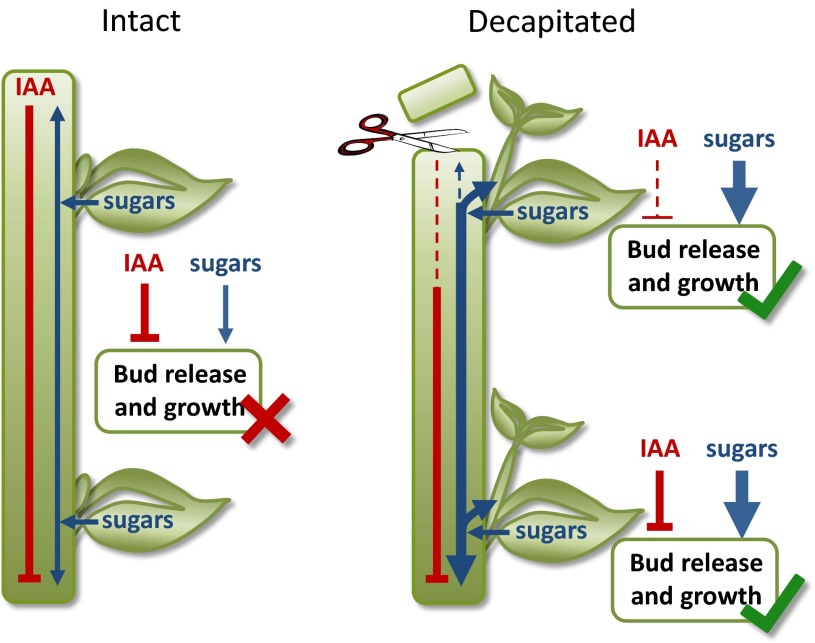
On the basis of our findings we propose a model (Fig. 4), in which the sugar demand of the shoot tip is crucial in maintaining apical dominance. Decapitation removes apical sugar demand and rapidly increases sucrose availability to axillary buds. This is sufficient to cause bud release, regardless of the auxin status of the adjacent stem. The role of auxin is prominent in the later stages of branch growth (14, 37) rather than during the initial bud release. Possessing both the rapid sucrose-based response and the longer-term auxin response could be advantageous to the plant. After decapitation the enhanced sucrose supply enables rapid bud release along the length of the stem. The buds are small, and because sucrose is now in excess, there is little cost to the plant of immediately initiating bud release. This provides an important advantage in terms of interplant competition and the relative speed at which the plant can recover from the loss of its shoot tip. However, if all buds were to continue to grow, the plant phenotype would be drastically altered from the initially apically dominant state to an overly bushy phenotype. Consequently, auxin plays a role in determining which buds will continue to grow out, by functioning with the other plant hormones, cytokinins and strigolactones, to either promote the progression of growing axillary buds into branches or to force them back into dormancy (38). Unlike the sucrose effect, this hormonal effect is substantially dependent on bud position because auxin is depleted in a basipetal gradient, and buds growing to branches will become additional sources of auxin (2) (Fig. 4). Consequently, in contrast with hormone models (8–12), another outcome of this combined sugar demand and hormone signaling model of shoot branching (Fig. 4) is the ease with which the decapitation of plants with strong apical dominance (no branches) can lead to a new architecture of basal and aerial branches (39, 40). In addition to providing rapid responses to changes in sink demand and regulating the number and position of branches, the involvement of sugars, and not simply hormones, could also prevent excessive bud outgrowth under poor growing conditions.
Fig. 4.
Apical dominance is controlled by sugar and hormone responses. Apical dominance is maintained in intact plants predominately by limiting the axillary bud’s access to sugars. After the loss of the shoot tip, sugars rapidly accumulate in axillary buds and, as the sugar content of the buds surpasses a threshold, the buds are released. In contrast, the loss of the apical supply of auxin results in a depletion of auxin in the stem. However, auxin depletion will differ spatially and temporally along the stem because auxin depletion is relatively slow and therefore the growing buds in the upper shoot will be affected before those lower on the stem. In this model, auxin is predominately involved in prioritizing the later stages of branch growth, whereas sugars are predominately responsible for the initial bud release. Line diagrams reveal mechanisms at each bud; the width of solid lines indicates abundance, with dashed lines indicating low levels.
Conclusions
The dogma of auxin-mediated apical dominance has persisted largely because auxin is typically capable of inhibiting the later stages of bud outgrowth after decapitation (14) and because it regulates the levels of other hormones known to affect shoot branching (41). However, by observing the earliest stages of bud release, we have shown that auxin depletion is not sufficient to induce bud release after decapitation (Fig. 1 C and D and Movie S1). Rather, our results demonstrate that sugars are both necessary and sufficient for axillary bud release from apical dominance (Fig. 3). Our data support a growing body of evidence that sugars function as important regulators of plant development (42–44) and indicate that limiting their availability to axillary buds is central to the maintenance of apical dominance.
Materials and Methods
Plant Material, Growth Conditions, and Treatments.
Except where described otherwise, plants used in this study were eight-leaf-expanded Pisum sativum cv Torsdag, grown in a temperature-controlled glasshouse as described previously (4). Unless otherwise stated, decapitation involved cutting through internode within 5 mm of the shoot tip (Fig. 1A). Girdling was performed as described previously (5). Sugar feeding to the petiole (45) involved rapid immersion of the cut surfaces in solutions after removal of the leaflets at node 3 (decapitated at node 3; Fig. 3B; 100 mM) or node 3 and 5 (intact plants with five expanded leaves; Fig. 3A; 400 mM; Fig. S3B; six expanded leaves; Fig. S3A). For 14C uptake studies, 0.1 µCi of 14C-labeled sucrose was supplied to the cut petiole of node 4 leaf, and node 2 buds were harvested at 2 h.
Time-Lapse Photography.
High-definition C910 webcams (Logitech; www.logitech.com) recorded continuous time-lapse images of a single axillary bud at 30-min intervals. Using multiple cameras, images of 8–10 individual buds were recorded simultaneously. Bud length in each image was calculated in the ImageJ software package (http://imagej.nih.gov/ij/) using a scale bar, which was included in every image, to calibrate measurements.
Measurement of IAA and Sucrose Level.
Sucrose was extracted from buds in a 1:1.35:1 mix of water:methanol:chloroform. Sucrose levels were determined by negative ion electrospray ultraperformance liquid chromatography (UPLC)-MS using a Waters Acquity H-series UPLC with a BEH amide column (2.1 × 50mm × 1.7 μm particles) coupled to a Waters Xevo triple quadrupole mass spectrometer. The mass spectrometer was operated in negative ion electrospray mode with a needle voltage of 2.3 kV, and selected ion monitoring (SIM) was used. The ion source temperature was 130 °C, the desolvation gas was nitrogen at 950 L h−1, the cone gas flow was 50 L h−1, and the desolvation temperature was 450 °C. SIM ions were sucrose, m/z 341.1; 13C12 sucrose (internal standard), m/z 353; and glucose, 179.1 Cone voltage was 24 V for both, and dwell time was 120 ms per channel. Glucose levels were estimated using 13C12 sucrose as an internal standard.
IAA was extracted, and IAA levels measured using the above instrument, as previously described (46).
Measurement of Carbon-11 Allocation to Lower Stems and Transport Speeds.
The positron-emitting isotope carbon-11 (11C; t1/2 = 20.4 min), as 11CO2, was generated and administered to the node 9 leaf of 10-leaf-expanded plants as a 30-s pulse in continuously streaming air in a leaf cuvette with photosynthetically active radiation 600 µmol m−2 s−1, as previously described (47). Leaf fixation, carbon export from the leaf, photoassimilate transport speed, and lower stem allocation were monitored in real time using a detector built into the leaf cuvette and two detectors shielded with collimated lead and positioned to detect radioactivity from the upper and lower stem (48). The time taken for the 11C photoassimilates in the phloem to move between the upper and lower stem detectors was used to calculate transport speeds. The effect of decapitation on 11C accumulation was determined using data collected from the detector positioned on the lower stem at node 2.
Gene Expression Analysis.
The node 2 axillary buds were harvested from 20 intact plants (five-leaf expanded) that had been fed with 400 mM sucrose or sorbitol, as well as from intact and decapitated (internode 5) plants. Total RNA was extracted as reported previously (4) and its quality determined by gel electrophoresis. Reverse transcription of 500 ng of RNA was performed using the iScript reverse transcription kit (BioRad) as per the manufacturer’s instructions. Real-time PCR was performed using Sooadvanced SYBER green supermix (BioRad) as per the manufacturer’s instructions on a CFX384 Touch real-time PCR detection system (BioRad). A melt curve analysis was included for quality assurance. Primer sequences for BRC1 and EF1α were as described in ref. 4. Actin forward primer (AGTGGTCGTACAACCGGTATTGT); Actin reverse primers (GATGGCATGGAGGAAGAGAGAAAC, GAGGATAGCATGTGGAACTGAGAA, GAGGAAGAGCATTCCCCTCGTA). The real-time data were processed in CFX Manager 2.1 software (BioRad) and then extracted and analyzed by LinRegPCR and Microsoft Excel as previously described (4). Gene expression was normalized against both a geomean of reference gene expression (Actin and EF1α) and the expression in the control samples.
Supplementary Material
Acknowledgments
We thank J. Botella, P. Brewer, E. Dun, G. Hammer, J. Hanan, M. Tanurdzic (University of Queensland), J. Fowler (Brookhaven National Laboratory), J. Lunn (Max Planck Institute for Molecular Plant Physiology), S. Tyerman (Adelaide University), S. Smith (University of Western Australia), and J. Patrick (University of Newcastle) for their comments on the manuscript and/or helpful discussions; and N. Davies (Central Science Laboratory, University of Tasmania), R. Powell, S. Kerr, K. Condon (University of Queensland), D. Glassop, and G. Bonnet (Commonwealth Scientific and Industrial Research Organization Plant Industry) for technical assistance. Funding was provided by the Australian Research Council (Grant DP110100808) and the US Department of Energy through its Office of Biological and Environmental Research (under Contract DE-AC02-98CH10886), as well as a Goldhaber Distinguished Fellowship (B.A.B.) and Australian Research Council Future Fellowship (FT100100806; C.A.B.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1322045111/-/DCSupplemental.
References
- 1.Thimann KV, Skoog F, Kerckhoff WG. On the inhibition of bud development and other functions of growth substance in Vicia faba. Proc R Soc Lond B Biol Sci. 1934;114(798):317–339. [Google Scholar]
- 2.Domagalska MA, Leyser O. Signal integration in the control of shoot branching. Nat Rev Mol Cell Biol. 2011;12(4):211–221. doi: 10.1038/nrm3088. [DOI] [PubMed] [Google Scholar]
- 3.Gomez-Roldan V, et al. Strigolactone inhibition of shoot branching. Nature. 2008;455(7210):189–194. doi: 10.1038/nature07271. [DOI] [PubMed] [Google Scholar]
- 4.Dun EA, de Saint Germain A, Rameau C, Beveridge CA. Antagonistic action of strigolactone and cytokinin in bud outgrowth control. Plant Physiol. 2012;158(1):487–498. doi: 10.1104/pp.111.186783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferguson BJ, Beveridge CA. Roles for auxin, cytokinin, and strigolactone in regulating shoot branching. Plant Physiol. 2009;149(4):1929–1944. doi: 10.1104/pp.109.135475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Booker J, Chatfield S, Leyser O. Auxin acts in xylem-associated or medullary cells to mediate apical dominance. Plant Cell. 2003;15(2):495–507. doi: 10.1105/tpc.007542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sachs T, Thimann KV. The role of auxins and cytokinins in the release of buds from dominance. Am J Bot. 1967;54(1):136–144. [Google Scholar]
- 8.Bennett T, Leyser O. Something on the side: Axillary meristems and plant development. Plant Mol Biol. 2006;60(6):843–854. doi: 10.1007/s11103-005-2763-4. [DOI] [PubMed] [Google Scholar]
- 9.Crawford S, et al. Strigolactones enhance competition between shoot branches by dampening auxin transport. Development. 2010;137(17):2905–2913. doi: 10.1242/dev.051987. [DOI] [PubMed] [Google Scholar]
- 10.Prusinkiewicz P, et al. Control of bud activation by an auxin transport switch. Proc Natl Acad Sci USA. 2009;106(41):17431–17436. doi: 10.1073/pnas.0906696106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brewer PB, Koltai H, Beveridge CA. Diverse roles of strigolactones in plant development. Mol Plant. 2013;6(1):18–28. doi: 10.1093/mp/sss130. [DOI] [PubMed] [Google Scholar]
- 12.Brewer PB, Dun EA, Ferguson BJ, Rameau C, Beveridge CA. Strigolactone acts downstream of auxin to regulate bud outgrowth in pea and Arabidopsis. Plant Physiol. 2009;150(1):482–493. doi: 10.1104/pp.108.134783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beveridge CA, Symons GM, Turnbull CGN. Auxin inhibition of decapitation-induced branching is dependent on graft-transmissible signals regulated by genes Rms1 and Rms2. Plant Physiol. 2000;123(2):689–698. doi: 10.1104/pp.123.2.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morris SE, Cox MCH, Ross JJ, Krisantini S, Beveridge CA. Auxin dynamics after decapitation are not correlated with the initial growth of axillary buds. Plant Physiol. 2005;138(3):1665–1672. doi: 10.1104/pp.104.058743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cline MG. Exogenous auxin effects on lateral bud outgrowth in decapitated shoots. Ann Bot (Lond) 1996;78(2):255–266. [Google Scholar]
- 16.Cline MG, Chatfield SP, Leyser O. NAA restores apical dominance in the axr3-1 mutant of Arabidopsis thaliana. Ann Bot (Lond) 2001;87(1):61–65. [Google Scholar]
- 17.Thimann KV. On the nature of inhibitions caused by auxin. Am J Bot. 1937;24(7):407–412. [Google Scholar]
- 18.Renton M, Hanan J, Ferguson BJ, Beveridge CA. Models of long-distance transport: how is carrier-dependent auxin transport regulated in the stem? New Phytol. 2012;194(3):704–715. doi: 10.1111/j.1469-8137.2012.04093.x. [DOI] [PubMed] [Google Scholar]
- 19.Loeb J. Regeneration from a Physico-Chemical Viewpoint. New York: McGraw-Hill; 1924. [Google Scholar]
- 20.Gregory FG, Veale JA. A reassessment of the problem of apical dominance. Symp Soc Exp Biol. 1957;11:1–20. [PubMed] [Google Scholar]
- 21.Phillips IDJ. Apical dominance. Annu Rev Plant Physiol Plant Mol Biol. 1975;26:341–367. [Google Scholar]
- 22.Snow R. An inhibitor of growth extracted from pea leaves. Nature. 1939;144(3656):906. [Google Scholar]
- 23.Ayre BG. Membrane-transport systems for sucrose in relation to whole-plant carbon partitioning. Mol Plant. 2011;4(3):377–394. doi: 10.1093/mp/ssr014. [DOI] [PubMed] [Google Scholar]
- 24.Stitt M, Zeeman SC. Starch turnover: Pathways, regulation and role in growth. Curr Opin Plant Biol. 2012;15(3):282–292. doi: 10.1016/j.pbi.2012.03.016. [DOI] [PubMed] [Google Scholar]
- 25.Nadwodnik J, Lohaus G. Subcellular concentrations of sugar alcohols and sugars in relation to phloem translocation in Plantago major, Plantago maritima, Prunus persica, and Apium graveolens. Planta. 2008;227(5):1079–1089. doi: 10.1007/s00425-007-0682-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jensen KH, Savage JA, Holbrook NM. Optimal concentration for sugar transport in plants. J R Soc Interface. 2013;10(83):20130055. doi: 10.1098/rsif.2013.0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Merchant A, et al. Phloem sap and leaf delta13C, carbohydrates, and amino acid concentrations in Eucalyptus globulus change systematically according to flooding and water deficit treatment. J Exp Bot. 2010;61(6):1785–1793. doi: 10.1093/jxb/erq045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.González-Grandío E, Poza-Carrión C, Sorzano COS, Cubas P. BRANCHED1 promotes axillary bud dormancy in response to shade in Arabidopsis. Plant Cell. 2013;25(3):834–850. doi: 10.1105/tpc.112.108480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Skylar A, Sung F, Hong FX, Chory J, Wu XL. Metabolic sugar signal promotes Arabidopsis meristematic proliferation via G2. Dev Biol. 2011;351(1):82–89. doi: 10.1016/j.ydbio.2010.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Henry C, et al. Regulation of RhSUC2, a sucrose transporter, is correlated with the light control of bud burst in Rosa sp. Plant Cell Environ. 2011;34(10):1776–1789. doi: 10.1111/j.1365-3040.2011.02374.x. [DOI] [PubMed] [Google Scholar]
- 31.Rabot A, et al. Insight into the role of sugars in bud burst under light in the rose. Plant Cell Physiol. 2012;53(6):1068–1082. doi: 10.1093/pcp/pcs051. [DOI] [PubMed] [Google Scholar]
- 32.Kelly G, et al. The pitfalls of transgenic selection and new roles of AtHXK1: A high level of AtHXK1 expression uncouples hexokinase1-dependent sugar signaling from exogenous sugar. Plant Physiol. 2012;159(1):47–51. doi: 10.1104/pp.112.196105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kebrom TH, et al. Inhibition of tiller bud outgrowth in the tin mutant of wheat is associated with precocious internode development. Plant Physiol. 2012;160(1):308–318. doi: 10.1104/pp.112.197954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lilley JLS, Gee CW, Sairanen I, Ljung K, Nemhauser JL. An endogenous carbon-sensing pathway triggers increased auxin flux and hypocotyl elongation. Plant Physiol. 2012;160(4):2261–2270. doi: 10.1104/pp.112.205575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sairanen I, et al. Soluble carbohydrates regulate auxin biosynthesis via PIF proteins in Arabidopsis. Plant Cell. 2012;24(12):4907–4916. doi: 10.1105/tpc.112.104794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Korasick DA, Enders TA, Strader LC. Auxin biosynthesis and storage forms. J Exp Bot. 2013;64(9):2541–2555. doi: 10.1093/jxb/ert080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bangerth F. Dominance among fruits sinks and the search for a correlative signal. Physiol Plant. 1989;76(4):608–614. [Google Scholar]
- 38.Stafstrom JP, Ripley BD, Devitt ML, Drake B. Dormancy-associated gene expression in pea axillary buds. Cloning and expression of PsDRM1 and PsDRM2. Planta. 1998;205(4):547–552. doi: 10.1007/s004250050354. [DOI] [PubMed] [Google Scholar]
- 39.Beveridge CA. Long-distance signalling and a mutational analysis of branching in pea. Plant Growth Regul. 2000;32(2-3):193–203. [Google Scholar]
- 40.Cline MG, Sadeski K. Is auxin the repressor signal of branch growth in apical control? Am J Bot. 2002;89(11):1764–1771. doi: 10.3732/ajb.89.11.1764. [DOI] [PubMed] [Google Scholar]
- 41.Dun EA, Brewer PB, Beveridge CA. Strigolactones: Discovery of the elusive shoot branching hormone. Trends Plant Sci. 2009;14(7):364–372. doi: 10.1016/j.tplants.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 42.Ruan YL. Signaling role of sucrose metabolism in development. Mol Plant. 2012;5(4):763–765. doi: 10.1093/mp/sss046. [DOI] [PubMed] [Google Scholar]
- 43.Tognetti JA, Pontis HG, Martínez-Noël GM. Sucrose signaling in plants: A world yet to be explored. Plant Signal Behav. 2013;8(3):e23316. doi: 10.4161/psb.23316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Eveland AL, Jackson DP. Sugars, signalling, and plant development. J Exp Bot. 2012;63(9):3367–3377. doi: 10.1093/jxb/err379. [DOI] [PubMed] [Google Scholar]
- 45.Lin YH, Lin MH, Gresshoff PM, Ferguson BJ. An efficient petiole-feeding bioassay for introducing aqueous solutions into dicotyledonous plants. Nat Protoc. 2011;6(1):36–45. doi: 10.1038/nprot.2010.171. [DOI] [PubMed] [Google Scholar]
- 46.Tivendale ND, et al. Biosynthesis of the halogenated auxin, 4-chloroindole-3-acetic acid. Plant Physiol. 2012;159(3):1055–1063. doi: 10.1104/pp.112.198457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Babst BA, Karve AA, Judt T. Radio-metabolite analysis of carbon-11 biochemical partitioning to non-structural carbohydrates for integrated metabolism and transport studies. Plant Cell Physiol. 2013;54(6):1016–1025. doi: 10.1093/pcp/pct045. [DOI] [PubMed] [Google Scholar]
- 48.Babst BA, et al. Jasmonic acid induces rapid changes in carbon transport and partitioning in Populus. New Phytol. 2005;167(1):63–72. doi: 10.1111/j.1469-8137.2005.01388.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.