Significance
Directed migration of diverse cell types is critical in biological processes ranging from development and morphogenesis to immune response, wound healing, and regeneration. However, techniques to specifically and easily direct, manipulate, and study cell migration in vitro and in vivo are currently limited. We conceived of a strategy to directly control cell migration to arbitrary user-defined locations, independent of native chemotaxis receptors. In this work, we demonstrate that genetic modification of cells with an engineered G protein-coupled receptor allows us to redirect their migration to a bioinert drug-like small molecule, clozapine-N-oxide. This technology provides a generalizable tool to systematically control cell migration in vitro and in vivo and could be a valuable module for engineering future therapeutic cellular devices.
Keywords: GPCR, cellular therapeutics, synthetic biology
Abstract
Directed migration of diverse cell types plays a critical role in biological processes ranging from development and morphogenesis to immune response, wound healing, and regeneration. However, techniques to direct, manipulate, and study cell migration in vitro and in vivo in a specific and facile manner are currently limited. We conceived of a strategy to achieve direct control over cell migration to arbitrary user-defined locations, independent of native chemotaxis receptors. Here, we show that genetic modification of cells with an engineered G protein-coupled receptor allows us to redirect their migration to a bioinert drug-like small molecule, clozapine-N-oxide (CNO). The engineered receptor and small-molecule ligand form an orthogonal pair: The receptor does not respond to native ligands, and the inert drug does not bind to native cells. CNO-responsive migration can be engineered into a variety of cell types, including neutrophils, T lymphocytes, keratinocytes, and endothelial cells. The engineered cells migrate up a gradient of the drug CNO and transmigrate through endothelial monolayers. Finally, we demonstrate that T lymphocytes modified with the engineered receptor can specifically migrate in vivo to CNO-releasing beads implanted in a live mouse. This technology provides a generalizable genetic tool to systematically perturb and control cell migration both in vitro and in vivo. In the future, this type of migration control could be a valuable module for engineering therapeutic cellular devices.
The ability of many cell types to migrate long distances within the body and specifically localize to target sites of action is critical for their proper function. For example, immune cells rapidly home to sites of infection, concentrating their powerful cytotoxic and proinflammatory activities for maximum efficacy while limiting damage to healthy tissue. In morphogenesis, cells undergo a complex stereotyped process involving migration as well as proliferation, differentiation, and programmed cell death to produce fully developed multicellular structures. In wound healing and regenerative processes, stem and progenitor cells home to injured tissues from nearby sites—as well as from distant locations including the bone marrow—to provide a stream of new cells to replenish and provide trophic support to old and damaged cells.
Cell migration is also an important factor to consider in the use of cells as therapeutic agents. The use of cells for the treatment of a growing array of diseases including cancer, autoimmunity, and chronic wounds is currently being explored (1–6). The appropriate and efficient localization of therapeutic cells to sites of disease has been identified as an important factor for successful cell-based therapy (7–17). However, preclinical studies and clinical trials to date have shown that the homing to sites of disease of many cell types commonly used as therapeutics is frequently impaired or limited, especially after ex vivo expansion of cells in culture (7, 12, 18, 19).
The ability to redirect the migration of cells to any user-specified location in the body would be a powerful enabling technology for basic research as well as for future applications, but there are currently few easily generalizable strategies to accomplish this goal. We conceived of an approach to direct cellular homing to small molecules by expressing, in motile cells, engineered G protein-coupled receptors (GPCRs) called receptors activated solely by a synthetic ligand (RASSLs) (20, 21).
RASSLs are engineered to be unresponsive to endogenous ligands but can be activated by pharmacologically inert orthogonal small molecules (Fig. 1A). Versions of these receptors exist for the three major GPCR signaling pathways (Gαs-, Gαi-, and Gαq-coupled receptors), and the design of a new arrestin-biased variant has recently been reported (21, 22). Because GPCRs control many important physiological functions, including cell migration, we hypothesized that, by expressing these engineered receptors in motile cells, we could develop a general strategy for establishing user control over cell homing (Fig. 1B). Here, we use a family of second-generation RASSLs, known as designer receptors exclusively activated by a designer drug (DREADDs), that are activated only by the small molecule clozapine-N-oxide (CNO), an inert metabolite of the FDA-approved antipsychotic drug clozapine (Fig. S1) (20). CNO is highly bioavailable in rodents and humans, lacks affinity for any known receptors, channels, and transporters, and does not cause any appreciable physiological effects when systemically administered in normal mice (20, 23, 24).
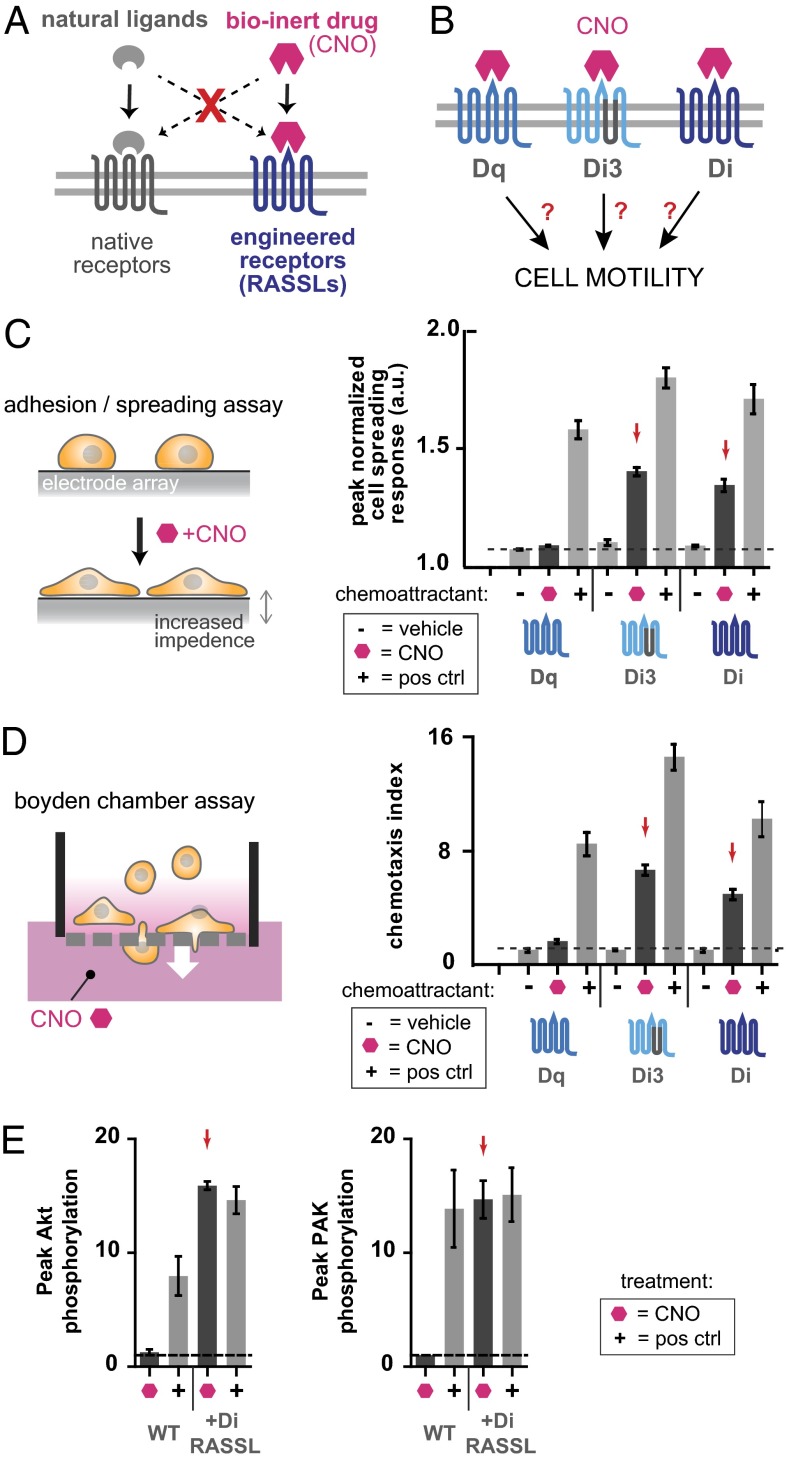
Fig. 1.
Engineered Gαi-coupled GPCRs Di3 and Di mediate cytoskeletal changes and chemotaxis of HL-60 neutrophils in response to CNO. (A) RASSLs are engineered GPCRs that interact orthogonally with a bioinert small-molecule drug. Natural ligands do not interact with the engineered receptors, and the bioinert drug that activates the engineered receptors does not interact with native receptors. (B) We tested whether certain second-generation RASSLs known as DREADDs could mediate cell motility. (C) Changes in electrical impedance that result from cell spreading in response to drug or ligand are detected by an electrode array. HL-60 neutrophils transiently transfected to express engineered GPCRs were plated on fibronectin-coated impedance assay plates and stimulated with vehicle control, 100 nM fMLP (positive control chemoattractant) or 100 nM CNO. All cells responded to fMLP whereas only Di3- or Di-expressing cells responded to CNO. Mean ± SEM for n = 3 replicates is shown. (D) Cell migration of HL-60 neutrophils transiently transfected with engineered GPCRs was quantitated in a porous transwell Boyden-chamber assay. All cells migrated in response to fMLP whereas only Di3- or Di-expressing cells migrated in response to CNO. Drug concentrations used: 100 nM CNO, 100 nM fMLP. Mean ± SEM for n = 3 replicates is shown. (E) Polarization and cell migration in neutrophils involves Rac and PI3K activation. Di-expressing HL-60 neutrophils were treated with 100 nM fMLP or 100 nM CNO before immunoblotting for phosphorylated Akt and phosphorylated PAK as readouts for PI3K and Rac activity, respectively. Peak levels of phospho-Akt and phospho-PAK are shown for each condition. Both were increased by CNO stimulation in Di cells but not in control cells (P < 0.01 by Student t test). Stimulation with fMLP increased phospho-Akt and phospho-PAK levels in both Di and control cells (P < 0.01 by Student t test), but Di cells showed higher peak levels of phospho-Akt than did control cells (P < 0.01 by Student t test). Three (for CNO) or four (for fMLP) independent experiments were performed and mean ± SEM are shown.
Results
Identification of Orthogonal GPCR That Controls HL-60 Neutrophil Motility.
To rapidly test whether this family of engineered orthogonal receptors could be used to control cell morphology and motility, we first transiently expressed several variants of these receptors (Dq, Di3, and Di) along with green fluorescent protein (GFP) in HL-60 neutrophils. Transfection efficiencies were routinely 40–45%, as measured by coelectroporation with GFP and determination of % GFP-positive cells via flow cytometry. We tested these engineered cells in a high-throughput impedance-based adhesion/spreading assay in which cells are plated on a fibronectin-coated electrode array and exposed to putative chemoattractants (Fig. 1C). Cells that morphologically respond to the chemoattractant adhere tightly to the surface and spread out, and this cytoskeletal change is measured as an increase in electrical impedance in real-time. We found that cells expressing the Gαi-coupled receptors Di3 and Di responded to the drug CNO whereas cells expressing the Gαq-coupled receptor Dq did not. This result was consistent with the known fact that many natural Gαi-coupled receptors are associated with chemotaxis (25). None of the cells responded to vehicle treatment, and all of the cells maintained a strong response to the positive control chemoattractant formyl-Met-Leu-Phe (fMLP), which strongly attracts neutrophils (Fig. 1C and Fig. S2). fMLP also induced a strong cell-spreading response in Di receptor and vector control-transfected HL-60 neutrophils (Fig. S3).
We tested whether HL-60 neutrophils expressing the same three engineered receptors would migrate directionally through a porous membrane in response to a gradient of the drug CNO in a Boyden-chamber transwell migration assay (Fig. 1D). The number of migrating cells was quantitated by flow cytometry using a fluorescent bead-counting standard. Consistent with the results of the cell-spreading assay, cells expressing the Gαi-coupled receptors Di3 and Di migrated in response to a gradient of CNO whereas cells expressing the Gαq-coupled receptor Dq did not. All of the cells maintained a strong migratory response to the positive control chemoattractant, fMLP (Fig. 1D).
It is well known that polarization and cell migration in neutrophils involves highly conserved cellular signaling and positive feedback loops that include the activation of the Rho-family GTPase Rac and the generation of phosphatidylinositol-(3,4,5)-Tris-phosphate by phosphotidylinositol 3-kinase (PI3K) at the leading edge of the migrating cell. To confirm that these pathways are activated in Di-expressing HL-60 neutrophils in response to CNO stimulation, we stimulated cells in suspension and performed immunoblotting for phosphorylated Akt and phosphorylated PAK as readouts for PI3K activity and Rac activity, respectively (Fig. 1E and Fig. S4). We observed that, upon CNO stimulation, levels of phosphorylated Akt and PAK significantly increased in Di-expressing, but not control, cells. In contrast, upon stimulation with the natural chemoattractant fMLP, levels of phosphorylated Akt and PAK increased in both Di and control cells. Interestingly, the amplitude and duration of phospho-Akt and phospho-PAK were slightly higher in Di-expressing cells, both in response to CNO and fMLP (Fig. S4).
Finally, we tested whether uniform stimulation with CNO is sufficient to induce polarization, symmetry breaking, and random motility in unpolarized Di-expressing HL-60 neutrophils, as is known to be the case with natural chemoattractants, including fMLP. HL-60 neutrophils were serum-starved for 45 min, plated on fibronectin-coated glass, and treated with CNO while being observed via time-lapse microscopy. We observed that Di-expressing HL-60 neutrophils did indeed undergo the expected morphological changes and motile behaviors characteristic of neutrophils undergoing chemokinesis upon treatment with CNO (Movie S1).
Directed Migration in a CNO Gradient.
Next, we used a micropipet migration assay with time-lapse microscopy to visualize the dynamic process of migration. This assay allows for visualization of individual cell behavior and provides (i) a very steep local concentration gradient and (ii) the ability to rapidly move the source of the gradient (Fig. S5A). Transiently transfected HL-60 neutrophils expressing Di and GFP (as a coelectroporation control) migrated robustly and directionally to the micropipet point source of CNO whereas cells transfected with an irrelevant plasmid control exhibited random migration (Fig. 2A and Movies S2–S4). Further, cells migrating to CNO were able to reorient to a changing gradient of the drug as can be appreciated when the micropipet is moved in Movie S3.
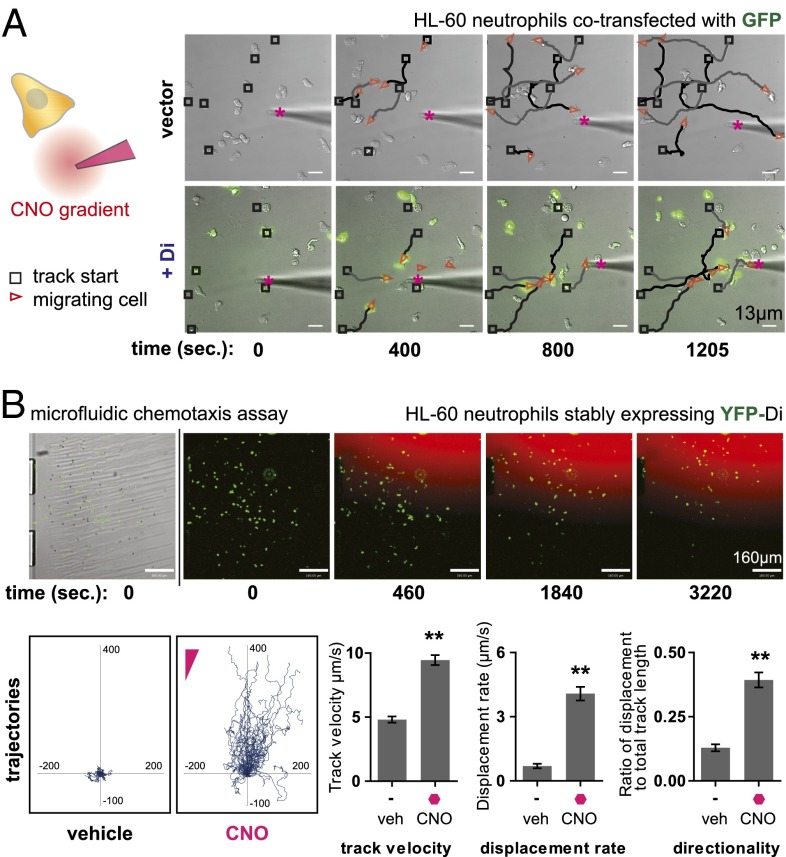
Fig. 2.
Microscopic analysis of HL-60 neutrophil polarization and cell migration in response to CNO. (A) HL-60 neutrophils coelectroporated with Di and GFP were plated on a fibronectin-coated glass surface and observed by time-lapse microscopy in the presence of a steep, micropipette-generated gradient of CNO. Di- and GFP-expressing cells migrated directionally toward the micropipet. Fluorescent dye Alexa 594 tracer is mixed with CNO solution in micropipet to visualize the diffusive gradient. The micropipet gradient source is marked by a magenta asterisk. Track start locations are marked by black squares, and red triangles mark cell location and direction in each frame. Traces (black and gray) connect track start locations (black squares) and cell location (red triangles). Drug concentration used (at source): 1μM CNO. See Movies S2–S4 for full movies. (B) HL-60 neutrophils stably expressing Di were placed in the fibronectin-coated viewing area of a microfluidic chemotaxis assay device capable of generating a smooth, stable gradient of CNO. Time-lapse microscopy was used to track cell migration, and cell-tracking software was used to quantitate various migration metrics. Cells migrated toward the CNO gradient (trajectories plotted with cell start locations at origin) and show increased track velocity, displacement rate, and directionality compared with basal motility in the presence of vehicle control. Drug concentration used (at source): 200 nM CNO. Mean ± SEM is shown for n = 61 cells tracked (**P < 0.0001 by Student t test). See Movie S5 for full movie.
To facilitate further quantitation of migration metrics of engineered HL-60 neutrophil chemotaxis in vitro, we used a microfluidic gradient generator developed and optimized in collaboration with the CellASIC Corporation. The microfluidic device is capable of generating a smooth, steady gradient over a relatively large area, allowing the user to track and analyze many cells within a field of view that are all experiencing a fairly consistent chemical gradient environment (Fig. S5B). To improve the homogeneity of receptor expression, we also generated HL-60 cell lines stably expressing the Di receptor with a YFP fluorescent protein fusion. Cells were loaded into the microfluidic device and allowed to adhere to the fibronectin-coated glass surface, and unbound cells were washed away, as can be seen at the beginning of Movie S5. A diffusive CNO gradient was applied (visualized by a fluorescent red tracer dye), and cells were tracked by time-lapse microscopy. Image analysis was performed, and cell tracks were generated, with initial cell positions plotted at the origin (Fig. 2B). Di receptor-expressing cells migrated directionally in response to the CNO gradient compared with vehicle control, as determined by comparing the track velocity, displacement rate, and directionality metrics between the two treatment conditions (Fig. 2B). In a separate experiment, we observed that Di receptor-expressing cells also migrated directionally toward the positive control chemoattractant fMLP, with grossly comparable fold increases in track velocity, displacement rate, and directionality in the presence versus the absence of chemoattractant as in CNO experiments (Fig. S6 and Movie S6).
Orthogonal Control of Chemotaxis in Diverse Cell Types.
Having established that the Di receptor is a potent mediator of CNO chemotaxis in HL-60 neutrophils, we asked whether this engineered chemotaxis receptor is “portable” to other cell types. We therefore generated a lentiviral vector to efficiently express an mCherry fluorescent protein-tagged Di receptor construct in a variety of cell types. Stable Di receptor-expressing cell lines were then established from HL-60 cells, primary human T lymphocytes, primary neonatal human epidermal keratinocytes, and primary human umbilical vein endothelial cells (HUVECs) (Fig. 3A). Expression of the Di receptor did not cause alterations in gross cellular morphology, and cells expressing, and not expressing, Di appeared indistinct on microscopic examination (Fig. S7).
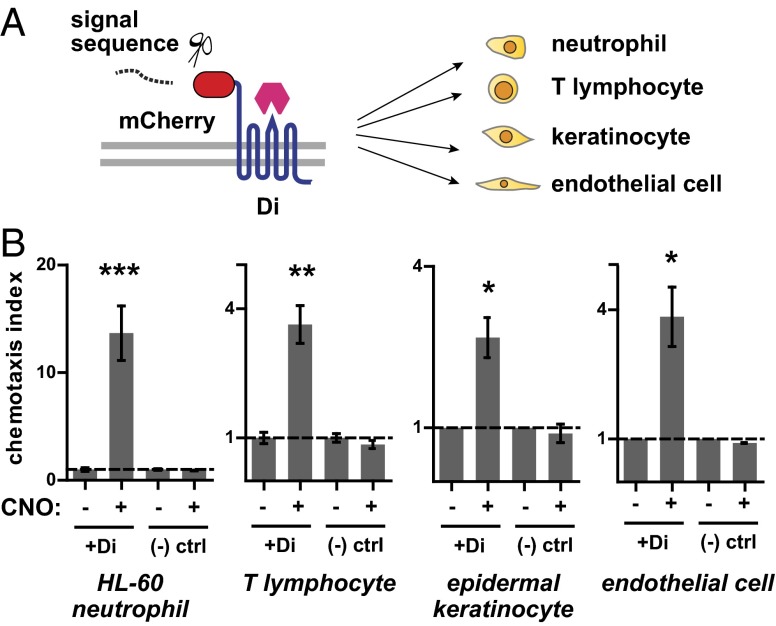
Fig. 3.
The engineered chemotaxis receptor Di is portable to a range of cell types. (A) Gene construct with N-terminal signal sequence followed by mCherry fluorescent protein fused to Di was inserted into a lentiviral plasmid backbone for viral expression in various cell types. (B) HL-60 neutrophils, primary human T lymphocytes, primary human epidermal keratinocytes, and primary human umbilical vein endothelial cells were transduced to stably express the Di receptor and tested for migration in the presence of a CNO gradient in Transwell experiments. All of the above cell types exhibited increased migration through the Transwell membrane in the presence of CNO compared with vehicle control. Drug concentrations used: 25 nM CNO for T lymphocytes, 100 nM CNO for all other cell types. Mean ± SEM is shown for three repeats (***P < 1e−4, **P = 0.001, *P = 0.02 by Student t test).
We tested each of the above cell types in Boyden-chamber transwell migration assays. In each case, Di receptor-expressing cells migrated in response to a gradient of CNO. Control cells not expressing the Di receptor did not migrate in response to CNO (Fig. 3B). We also performed transwell checkerboard control experiments, in which the putative chemoattractant is placed in the top and/or bottom chamber of the transwell in all combinations to distinguish between cellular chemotaxis (directed migration up a gradient of chemoattractant) and chemokinesis (increased motility in the presence of chemoattractant). In these experiments, HL-60 neutrophils and T lymphocytes exhibited directed migration toward CNO (chemotaxis) whereas keratinocytes and endothelial cells showed only increased motility in the presence of CNO (chemokinesis) (Fig. S8).
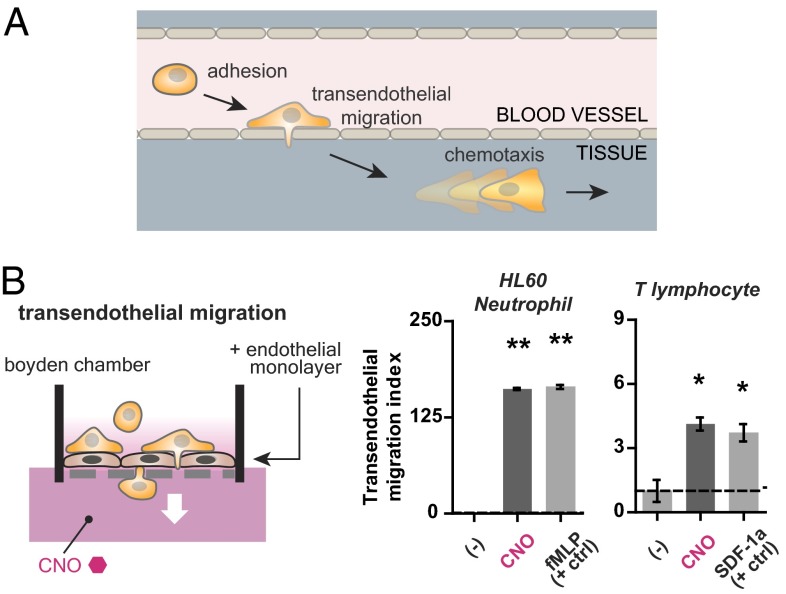
Cellular migration in the body is complicated by mammalian anatomy. A critical step in homing for cells that travel via the bloodstream to reach target sites is exiting blood vessels to enter surrounding tissues—a process known as diapedesis or transendothelial migration (Fig. 4A). Therefore, in our next experiment, we tested whether motile cells expressing the Di receptor could migrate through an endothelial monolayer in vitro in response to a gradient of CNO. We grew a tight monolayer of HUVECs on a fibronectin-coated porous transwell membrane for 4 d. Monolayer integrity was assessed by an observed increase in transendothelial electrical resistance from a baseline of <7 Ω to >60 Ω and barrier function in an FITC-dextran permeability assay (Fig. S9). We then proceeded with a transwell migration assay using HL-60 neutrophils and primary human T lymphocytes as the motile cell types. Both engineered HL-60 neutrophils and primary human T lymphocytes exhibited a directed transendothelial migration response to CNO as well as to a positive control chemoattractant (fMLP for HL-60 neutrophils and SDF-1a for T lymphocytes) (Fig. 4B).
Fig. 4.
The engineered receptor Di is sufficient to mediate both chemotaxis and transendothelial migration in immune cells. (A) Transendothelial migration is a critical step in the overall process of cellular homing that also includes adhesion to endothelium and chemotaxis. (B) HL-60 neutrophils and primary human T lymphocytes stably expressing Di were tested for their ability to transmigrate through a tight endothelial monolayer grown on a porous fibronectin-coated transwell membrane in response to both a CNO gradient as well as a positive control chemoattractant (100 nM fMLP and 50 ng/mL SDF-1a, respectively). Both cell types exhibited migration in the presence of CNO. Mean ± SEM for n = 3 (HL-60) or n = 4 (T lymphocytes) replicates is shown (**P < 1e−4, *P = 0.02 by Student t test).
Cells with Engineered Receptor Home to CNO Signal in Vivo.
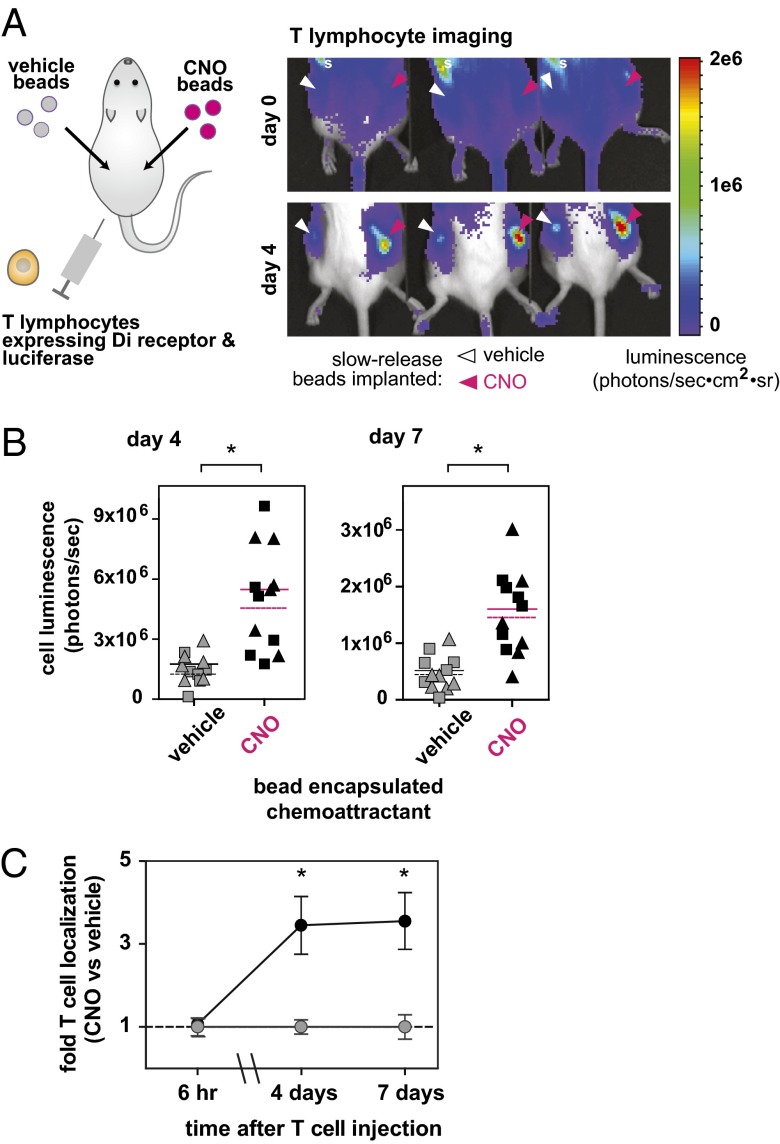
Finally, we tested whether our approach of redirecting cellular homing using a small-molecule drug could be feasible for use in vivo. T lymphocytes are highly motile cells of the adaptive immune system that play critical roles in cell-mediated immunity. Their use is currently being heavily explored in cell-based therapeutic applications in human clinical trials and in preclinical models, especially in cancer and autoimmunity (1, 2, 26, 27). We therefore tested whether the homing of engineered T lymphocytes could be redirected to the orthogonal CNO signal in a mouse. Mouse T lymphocytes were retrovirally transduced with a bicistronic construct encoding both an mCherry-tagged Di receptor and an enhanced firefly luciferase to allow tracking of modified cells (28). Biodegradable CNO-loaded poly-lactide-coglycolide (PLGA) microspheres were formulated using standard techniques to generate a slow-release source of CNO in the body (Fig. S10). The encapsulated drug concentration was determined to be 4.1 μg/mg (encapsulation efficiency of 19.6%). Vehicle control microspheres were generated in parallel by omission of CNO in the protocol. CNO-loaded and vehicle control microspheres were injected s.c. (suspension in PBS) into opposing flanks of albino B6 mice. Di receptor- and luciferase-transduced T lymphocytes were injected i.v. via the lateral tail vein.
In this experiment, we observed that the Di receptor-expressing T lymphocytes preferentially localized to sites of injection of CNO-loaded beads versus vehicle control beads injected on the contralateral flanks (Fig. 5A). This preferential localization was also observed in mice where the injected flanks were switched (CNO-left and vehicle-right versus CNO-right and vehicle-left flank) (data points combined and analyzed together in Fig. 5B). The luminescence of the T cells localized at each site was quantitated at 6 h, 4 d, and 7 d after T-cell injection (Fig. 5 B and C). This study was also performed with T cells expressing luciferase but not the Di receptor—in this case, these negative control cells did not show preferential localization to CNO slow-release microspheres (Fig. S11).
Fig. 5.
Intravenously administered primary T lymphocytes expressing Di specifically localize to an s.c. implanted depot of CNO slow-release biodegradable microspheres. (A) Mouse T lymphocytes expressing Di and firefly luciferase (to enable in vivo bioluminescent imaging) were systemically administered (intravenously) to mice in which CNO-releasing biodegradable PLGA microspheres were implanted s.c. Bioluminescent imaging was used to track cell localization. T lymphocytes expressing Di specifically localized to CNO-releasing microspheres compared with vehicle control microspheres implanted on the contralateral flank. Location of spleen is denoted by the letter “s.” (B) Quantitative analysis of bioluminescent imaging was performed. Specific localization of T lymphocytes persists for at least 7 d. Quantitation shown for 4 and 7 d postinjection of T lymphocytes and for two different doses of implanted microspheres (analyzed for statistical significance separately). Microsphere injection doses were 2 mg (triangles) and 6 mg (squares). Mean shown for n = 6 mice for each microsphere dose (dashed line for 2-mg dose, solid line for 6-mg dose) (*P = 0.013 for day 4, 2 mg; P = 0.022 for day 4, 6 mg; P = 0.017 for day 7, 2 mg; P < 0.001 for day 7, 6 mg) (by Student t test). (C) Fold-differences in T lymphocyte luminescent signal in CNO microsphere-injected flanks (black circles) versus vehicle microsphere-injected flanks (gray circles) at 6 h, 4 d, and 7 d after T lymphocyte injection. Mean ± SEM shown for n = 6 mice (*P < 0.01 by Student t test).
Discussion
The technology we describe here represents a step forward in the development of generalizable genetic tools with user-defined orthogonal control for the study of cell migration in vitro and in vivo. Of course, further work remains to optimize this technology. For example, the small-molecule drug could be modified through synthetic chemistry to optimize its properties as a gradient-generating homing molecule. Alternative delivery formulations of the drug (such as smart liposomes with antibody-based targeting and triggered release characteristics) (29) could be used for delivery to sites of disease in a targeted manner. In the longer term, it may be possible to develop genetically encodable orthogonal receptor/ligand pairs to allow for biological expression of the homing signal by cells. Protein engineering of the receptor could also be used to develop variants with altered drug affinity, recycling properties, or signaling capabilities. Such tools will allow researchers to uncouple the control of motility from other signals and give them the ability to systematically perturb motility and understand its role in diverse processes such as development, immune response, wound healing, and regeneration.
An orthogonal tool to control cell migration like the one described here could be of value not only as a research tool, but also in the future as applied in the emerging field of cell-based therapeutics. For example, antitumor T-cell trafficking into tumors is often quite inefficient, despite being critical for antitumor activity: It has been observed that increased tumor infiltration by T cells correlates with better prognoses in mouse studies and in human clinical trials (10–15, 19, 30–34). There are currently limited ways for physicians to steer cells to desired sites, however. Most cells that are currently used in clinical trials, including immune cells and stem cells, largely rely on the natural “tropism” of particular cell types for certain tissues (e.g., hematopoietic stem cell homing to the bone marrow niche) (35) or for disease-associated signals [e.g., mesenchymal and neural stem cell homing to inflammation (9, 18, 36, 37) or monocytes into tumors (38, 39)]. The use of a simple system to guide cellular localization in the body to arbitrary locations could in principle allow physicians to more effectively harness powerful cellular therapeutic activities, including cell killing, repair/regeneration, sensing disease (40, 41), and delivering therapeutic molecules (42–44) to treat disease, and potentially broaden the range of uses for cells in medicine.
The use of a bioinert drug to orthogonally direct engineered cell migration is conceptually distinct from (and complementary to) past strategies reported in the literature for directing cell migration. Other groups have described interesting approaches, including the chemical or enzymatic modification of the cell surface with specific adhesion molecules (45), materials engineering of artificial scaffolds and tunable matrices to direct cell adhesion and migration (46), expression or direct injection of natural homing ligands such as chemokines into sites where increased cell migration is desired (47, 48), and the expression in therapeutic cells of natural receptors such as chemokine receptors whose ligands are up-regulated in inflammation or cancer (8, 7, 16, 17). These strategies rely on naturally existing homing receptors and ligands, and they are powerful because they tap into cells’ native migration axes. However, many homing ligands are present in multiple locations throughout the body, the expression of these ligands may vary in time throughout the natural course of disease or in response to therapy, many ligands (such as chemokines) interact with multiple receptors and vice versa, and native receptors for natural ligands can sometimes be found not only on therapeutic cell types but also on cell types that are detrimental for therapy (47, 49–52). In contrast, the work we have demonstrated here benefits from the use of an orthogonal receptor–drug pair. The drug has a low toxicity profile, which decreases concerns of side effects in therapeutic settings. The homing receptor for the drug is expressed uniquely on the cell type of one's choosing (and not on native cells). The user can better control when, where, and how much drug is present at a given site, and the drug cannot naturally be produced at off-target sites. Cellular homing can be directed not only to sites of disease where there are known chemotactic ligands or migration signals, but also to any site where a drug can be delivered.
Another intriguing strategy to gain control over cell migration is the use of light-sensitive proteins such as photoactivatable Rac or opsin photoreceptors to tap into cell motility signaling pathways (53–55). These types of tools have already yielded valuable insights into the basic biology and mechanism of cell migration in vitro as well as in vivo in the optically transparent zebrafish model. So far, however, the requirement for consistent in vivo delivery of light remains an obstacle to the broader use of so-called optogenetic tools in vivo and in therapeutic contexts.
We have demonstrated a simple approach to directing cell migration in vitro and in vivo in a variety of cell types. A paradigm of gaining synthetic control over complex cellular behaviors using engineered proteins that respond to orthogonal chemical signals is likely to be generally useful for basic research and in future biotechnological and therapeutic applications.
Materials and Methods
A complete detailed description of materials and methods is provided in SI Materials and Methods. Gene constructs were cloned using standard molecular biology methods. The DREADD constructs were a generous gift from Dr. Bryan Roth (University of North Carolina Medical School, Chapel Hill, NC). The enhanced firefly luciferase gene (effLuc) was a generous gift from Dr. Brian Rabinovich (M. D. Anderson Cancer Center, Houston). Standard sterile culture methods were used for cell culture and viral supernatant production. The xCELLigence RTCA MP impedance array assay platform (ACEA Biosciences/Roche) was used to monitor cytoskeletal changes (adhesion/spreading) of HL-60 neutrophils on fibronectin-coated wells in response to agonist. Boyden-chamber assays were used to assess the migration of cells through porous membranes. Standard cell lysis and immunoblotting procedures were used to assay protein phosphorylation in stimulated cells by Western blot. Micropipet gradients for chemotaxis assays were generated using the Narishige MM-89 micromanipulator and glass capillaries pulled on a Sutter Model P-97. The ONIX microfluidic platform with M04G gradient generator plate (CellASIC/EMD Biosciences) was used to study HL-60 neutrophil migration. Biodegradable microspheres loaded with CNO were generated in a sterile environment using a standard oil-in-water emulsion method. Animal studies were conducted with the University of California, San Francisco (UCSF) Preclinical Therapeutics Core under a protocol approved by the UCSF Institutional Animal Care and Use Committee.
Supplementary Material
Acknowledgments
We thank the University of California, San Francisco (UCSF) Preclinical Therapeutics Core Facility (especially Byron Hann, Don Hom, Donghui Wang, and Paul Phojanakong) for mouse experimental support and helpful discussions. We also acknowledge the 2009 UCSF International Genetically Engineered Machine (iGEM) competition team (especially Katja Kolar, Ryan Liang, Cathy Liu, Hansi Liu, Jackie Tam, and Eric Wong) for their work on HL-60 neutrophil chemotaxis experiments and molecular cloning. This work was supported by National Institutes of Health (NIH) Grant R01 HL60664-07 (to B.R.C.), pilot study funds from the Gladstone Institutes, NIH Nanomedicine Development Center Grant PN2EY016546 (The Cell Propulsion Laboratory: Center for Synthetic Signaling and Motility Systems Engineering) (to W.A.L.), NIH Grant P50 GM08187 (to W.A.L.), the National Science Foundation Synthetic Biology Engineering Research Center, NIH Grant R01 GM084040 (to O.D.W.), a California Institute for Regenerative Medicine fellowship (Grant TG2-01153) (to J.S.P.), and the Howard Hughes Medical Institute (W.A.L.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1402087111/-/DCSupplemental.
References
- 1.Tang Q, Bluestone JA, Kang S-M. CD4(+)Foxp3(+) regulatory T cell therapy in transplantation. J Mol Cell Biol. 2012;4(1):11–21. doi: 10.1093/jmcb/mjr047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burrell BE, Nakayama Y, Xu J, Brinkman CC, Bromberg JS. Regulatory T cell induction, migration, and function in transplantation. J Immunol. 2012;189(10):4705–4711. doi: 10.4049/jimmunol.1202027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Restifo NP, Dudley ME, Rosenberg SA. Adoptive immunotherapy for cancer: Harnessing the T cell response. Nat Rev Immunol. 2012;12(4):269–281. doi: 10.1038/nri3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Riddell SR, et al. Restoration of viral immunity in immunodeficient humans by the adoptive transfer of T cell clones. Science. 1992;257(5067):238–241. doi: 10.1126/science.1352912. [DOI] [PubMed] [Google Scholar]
- 5.Lu P, et al. Long-distance growth and connectivity of neural stem cells after severe spinal cord injury. Cell. 2012;150(6):1264–1273. doi: 10.1016/j.cell.2012.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bliss T, Guzman R, Daadi M, Steinberg GK. Cell transplantation therapy for stroke. Stroke. 2007;38(2) Suppl:817–826. doi: 10.1161/01.STR.0000247888.25985.62. [DOI] [PubMed] [Google Scholar]
- 7.Kershaw MH, et al. Redirecting migration of T cells to chemokine secreted from tumors by genetic modification with CXCR2. Hum Gene Ther. 2002;13(16):1971–1980. doi: 10.1089/10430340260355374. [DOI] [PubMed] [Google Scholar]
- 8.Moon EK, et al. Expression of a functional CCR2 receptor enhances tumor localization and tumor eradication by retargeted human T cells expressing a mesothelin-specific chimeric antibody receptor. Clin Cancer Res. 2011;17(14):4719–4730. doi: 10.1158/1078-0432.CCR-11-0351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ankrum J, Karp JM. Mesenchymal stem cell therapy: Two steps forward, one step back. Trends Mol Med. 2010;16(5):203–209. doi: 10.1016/j.molmed.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cohen PJ, Lotze MT, Roberts JR, Rosenberg SA, Jaffe ES. The immunopathology of sequential tumor biopsies in patients treated with interleukin-2: Correlation of response with T-cell infiltration and HLA-DR expression. Am J Pathol. 1987;129(2):208–216. [PMC free article] [PubMed] [Google Scholar]
- 11.Cole DJ, et al. Histopathological analysis of metastatic melanoma deposits in patients receiving adoptive immunotherapy with tumor-infiltrating lymphocytes. Cancer Immunol Immunother. 1994;38(5):299–303. doi: 10.1007/BF01525507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pockaj BA, et al. Localization of 111indium-labeled tumor infiltrating lymphocytes to tumor in patients receiving adoptive immunotherapy: Augmentation with cyclophosphamide and correlation with response. Cancer. 1994;73(6):1731–1737. doi: 10.1002/1097-0142(19940315)73:6<1731::aid-cncr2820730630>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 13.Galon J, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313(5795):1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 14.Tosolini M, et al. Clinical impact of different classes of infiltrating T cytotoxic and helper cells (Th1, th2, treg, th17) in patients with colorectal cancer. Cancer Res. 2011;71(4):1263–1271. doi: 10.1158/0008-5472.CAN-10-2907. [DOI] [PubMed] [Google Scholar]
- 15.Mitchell MS, et al. Phase I trial of adoptive immunotherapy with cytolytic T lymphocytes immunized against a tyrosinase epitope. J Clin Oncol. 2002;20(4):1075–1086. doi: 10.1200/JCO.2002.20.4.1075. [DOI] [PubMed] [Google Scholar]
- 16.Craddock JA, et al. Enhanced tumor trafficking of GD2 chimeric antigen receptor T cells by expression of the chemokine receptor CCR2b. J Immunother. 2010;33(8):780–788. doi: 10.1097/CJI.0b013e3181ee6675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Di Stasi A, et al. T lymphocytes coexpressing CCR4 and a chimeric antigen receptor targeting CD30 have improved homing and antitumor activity in a Hodgkin tumor model. Blood. 2009;113(25):6392–6402. doi: 10.1182/blood-2009-03-209650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karp JM, Leng Teo GS. Mesenchymal stem cell homing: The devil is in the details. Cell Stem Cell. 2009;4(3):206–216. doi: 10.1016/j.stem.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 19.Rosenberg SA, et al. Treatment of patients with metastatic melanoma with autologous tumor-infiltrating lymphocytes and interleukin 2. J Natl Cancer Inst. 1994;86(15):1159–1166. doi: 10.1093/jnci/86.15.1159. [DOI] [PubMed] [Google Scholar]
- 20.Armbruster BN, Li X, Pausch MH, Herlitze S, Roth BL. Evolving the lock to fit the key to create a family of G protein-coupled receptors potently activated by an inert ligand. Proc Natl Acad Sci USA. 2007;104(12):5163–5168. doi: 10.1073/pnas.0700293104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Conklin BR, et al. Engineering GPCR signaling pathways with RASSLs. Nat Methods. 2008;5(8):673–678. doi: 10.1038/nmeth.1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakajima K, Wess J. Design and functional characterization of a novel, arrestin-biased designer G protein-coupled receptor. Mol Pharmacol. 2012;82(4):575–582. doi: 10.1124/mol.112.080358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bender D, Holschbach M, Stöcklin G. Synthesis of n.c.a. carbon-11 labelled clozapine and its major metabolite clozapine-N-oxide and comparison of their biodistribution in mice. Nucl Med Biol. 1994;21(7):921–925. doi: 10.1016/0969-8051(94)90080-9. [DOI] [PubMed] [Google Scholar]
- 24.Ray RS, et al. Impaired respiratory and body temperature control upon acute serotonergic neuron inhibition. Science. 2011;333(6042):637–642. doi: 10.1126/science.1205295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Neptune ER, Bourne HR. Receptors induce chemotaxis by releasing the betagamma subunit of Gi, not by activating Gq or Gs. Proc Natl Acad Sci USA. 1997;94(26):14489–14494. doi: 10.1073/pnas.94.26.14489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fischbach MA, Bluestone JA, Lim WA. Cell-based therapeutics: The next pillar of medicine. Sci Transl Med. 2013;5(179):ps7. doi: 10.1126/scitranslmed.3005568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rosenberg SA. Raising the bar: The curative potential of human cancer immunotherapy. Sci Transl Med. 2012;4(127):ps8. doi: 10.1126/scitranslmed.3003634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rabinovich BA, et al. Visualizing fewer than 10 mouse T cells with an enhanced firefly luciferase in immunocompetent mouse models of cancer. Proc Natl Acad Sci USA. 2008;105(38):14342–14346. doi: 10.1073/pnas.0804105105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Allen TM, Cullis PR. Liposomal drug delivery systems: From concept to clinical applications. Adv Drug Deliv Rev. 2013;65(1):36–48. doi: 10.1016/j.addr.2012.09.037. [DOI] [PubMed] [Google Scholar]
- 30.Rubin JT, Elwood LJ, Rosenberg SA, Lotze MT. Immunohistochemical correlates of response to recombinant interleukin-2-based immunotherapy in humans. Cancer Res. 1989;49(24 Pt 1):7086–7092. [PubMed] [Google Scholar]
- 31.Ogawa M, et al. Enhanced induction of very late antigen 4/lymphocyte function-associated antigen 1-dependent T-cell migration to tumor sites following administration of interleukin 12. Cancer Res. 1997;57(11):2216–2222. [PubMed] [Google Scholar]
- 32.Pagès F, et al. Effector memory T cells, early metastasis, and survival in colorectal cancer. N Engl J Med. 2005;353(25):2654–2666. doi: 10.1056/NEJMoa051424. [DOI] [PubMed] [Google Scholar]
- 33.Musha H, et al. Selective infiltration of CCR5(+)CXCR3(+) T lymphocytes in human colorectal carcinoma. Int J Cancer. 2005;116(6):949–956. doi: 10.1002/ijc.21135. [DOI] [PubMed] [Google Scholar]
- 34.Ohta M, et al. The high expression of Fractalkine results in a better prognosis for colorectal cancer patients. Int J Oncol. 2005;26(1):41–47. [PubMed] [Google Scholar]
- 35.Peled A, et al. Dependence of human stem cell engraftment and repopulation of NOD/SCID mice on CXCR4. Science. 1999;283(5403):845–848. doi: 10.1126/science.283.5403.845. [DOI] [PubMed] [Google Scholar]
- 36.Imitola J, et al. Directed migration of neural stem cells to sites of CNS injury by the stromal cell-derived factor 1alpha/CXC chemokine receptor 4 pathway. Proc Natl Acad Sci USA. 2004;101(52):18117–18122. doi: 10.1073/pnas.0408258102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wagner J, Kean T, Young R, Dennis JE, Caplan AI. Optimizing mesenchymal stem cell-based therapeutics. Curr Opin Biotechnol. 2009;20(5):531–536. doi: 10.1016/j.copbio.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 38.De Palma M, Murdoch C, Venneri MA, Naldini L, Lewis CE. Tie2-expressing monocytes: Regulation of tumor angiogenesis and therapeutic implications. Trends Immunol. 2007;28(12):519–524. doi: 10.1016/j.it.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 39.Lewis CE, Pollard JW. Distinct role of macrophages in different tumor microenvironments. Cancer Res. 2006;66(2):605–612. doi: 10.1158/0008-5472.CAN-05-4005. [DOI] [PubMed] [Google Scholar]
- 40.Folcher M, Fussenegger M. Synthetic biology advancing clinical applications. Curr Opin Chem Biol. 2012;16(3-4):345–354. doi: 10.1016/j.cbpa.2012.06.008. [DOI] [PubMed] [Google Scholar]
- 41.Khalil AS, Collins JJ. Synthetic biology: Applications come of age. Nat Rev Genet. 2010;11(5):367–379. doi: 10.1038/nrg2775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ruoslahti E, Bhatia SN, Sailor MJ. Targeting of drugs and nanoparticles to tumors. J Cell Biol. 2010;188(6):759–768. doi: 10.1083/jcb.200910104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stephan MT, Moon JJ, Um SH, Bershteyn A, Irvine DJ. Therapeutic cell engineering with surface-conjugated synthetic nanoparticles. Nat Med. 2010;16(9):1035–1041. doi: 10.1038/nm.2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yoo J-W, Irvine DJ, Discher DE, Mitragotri S. Bio-inspired, bioengineered and biomimetic drug delivery carriers. Nat Rev Drug Discov. 2011;10(7):521–535. doi: 10.1038/nrd3499. [DOI] [PubMed] [Google Scholar]
- 45.Sarkar D, et al. Engineered cell homing. Blood. 2011;118(25):e184–e191. doi: 10.1182/blood-2010-10-311464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lutolf MP, Hubbell JA. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat Biotechnol. 2005;23(1):47–55. doi: 10.1038/nbt1055. [DOI] [PubMed] [Google Scholar]
- 47.Homey B, Müller A, Zlotnik A. Chemokines: Agents for the immunotherapy of cancer? Nat Rev Immunol. 2002;2(3):175–184. doi: 10.1038/nri748. [DOI] [PubMed] [Google Scholar]
- 48.Chada S, Ramesh R, Mhashilkar AM. Cytokine- and chemokine-based gene therapy for cancer. Curr Opin Mol Ther. 2003;5(5):463–474. [PubMed] [Google Scholar]
- 49.Tan MCB, et al. Disruption of CCR5-dependent homing of regulatory T cells inhibits tumor growth in a murine model of pancreatic cancer. J Immunol. 2009;182(3):1746–1755. doi: 10.4049/jimmunol.182.3.1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Penn MS, et al. An open-label dose escalation study to evaluate the safety of administration of nonviral stromal cell-derived factor-1 plasmid to treat symptomatic ischemic heart failure. Circ Res. 2013;112(5):816–825. doi: 10.1161/CIRCRESAHA.111.300440. [DOI] [PubMed] [Google Scholar]
- 51.Abastado J-P. The next challenge in cancer immunotherapy: Controlling T-cell traffic to the tumor. Cancer Res. 2012;72(9):2159–2161. doi: 10.1158/0008-5472.CAN-11-3538. [DOI] [PubMed] [Google Scholar]
- 52.Hong M, et al. Chemotherapy induces intratumoral expression of chemokines in cutaneous melanoma, favoring T-cell infiltration and tumor control. Cancer Res. 2011;71(22):6997–7009. doi: 10.1158/0008-5472.CAN-11-1466. [DOI] [PubMed] [Google Scholar]
- 53.Wu YI, et al. A genetically encoded photoactivatable Rac controls the motility of living cells. Nature. 2009;461(7260):104–108. doi: 10.1038/nature08241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yoo SK, et al. Differential regulation of protrusion and polarity by PI3K during neutrophil motility in live zebrafish. Dev Cell. 2010;18(2):226–236. doi: 10.1016/j.devcel.2009.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Levskaya A, Weiner OD, Lim WA, Voigt CA. Spatiotemporal control of cell signalling using a light-switchable protein interaction. Nature. 2009;461(7266):997–1001. doi: 10.1038/nature08446. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.