Significance
Light is a powerful stimulant for human alertness and cognition that can be easily administered to improve performance or counteract the negative impact of sleepiness, even during the day. Here, we show that prior exposure to longer wavelength light (orange), relative to shorter wavelength (blue), enhances the subsequent impact of light on executive brain responses. These findings emphasize the importance of light for human cognitive brain function and constitute compelling evidence in favor of a cognitive role for melanopsin. This recently discovered photopigment may therefore provide a unique form of “photic memory” for human cognition and play a broader role than previously apprehended. Ultimately, these findings support the idea that the integration of light exposure over long periods of time can help optimize cognitive brain function.
Keywords: fMRI, non–image-forming
Abstract
Light is a powerful stimulant for human alertness and cognition, presumably acting through a photoreception system that heavily relies on the photopigment melanopsin. In humans, evidence for melanopsin involvement in light-driven cognitive stimulation remains indirect, due to the difficulty to selectively isolate its contribution. Therefore, a role for melanopsin in human cognitive regulation remains to be established. Here, sixteen participants underwent consecutive and identical functional MRI recordings, during which they performed a simple auditory detection task and a more difficult auditory working memory task, while continuously exposed to the same test light (515 nm). We show that the impact of test light on executive brain responses depends on the wavelength of the light to which individuals were exposed prior to each recording. Test-light impact on executive responses in widespread prefrontal areas and in the pulvinar increased when the participants had been exposed to longer (589 nm), but not shorter (461 nm), wavelength light, more than 1 h before. This wavelength-dependent impact of prior light exposure is consistent with recent theories of the light-driven melanopsin dual states. Our results emphasize the critical role of light for cognitive brain responses and are, to date, the strongest evidence in favor of a cognitive role for melanopsin, which may confer a form of “photic memory” to human cognitive brain function.
One of the major advances in neuroscience in the last decade was the discovery of a novel class of ocular photoreceptors: the intrinsically photosensitive retinal ganglion cells (ipRGCs) that express melanopsin (1), a photopigment maximally sensitive to blue light (2–4). The finding of a new inner retinal photopigment has led to a complete reexamination of the role of the eye, which is now viewed as the site of two distinct photoreceptive systems: one for vision, based mainly on rods and cones, and one for the non–image-forming functions of light, primarily dependent on melanopsin. Animal data have demonstrated that the melanopsin photoreception system directly mediates the impact of light on sleep/wake regulation (5). In humans, light also regulates sleep and wakefulness and constitutes a powerful stimulant for alertness and cognition (6, 7). However, evidence for the involvement of melanopsin in this human light-driven stimulating impact remains indirect (8, 9), due to the difficulty of selectively isolating contributions of ipRGCs, rods, and/or cones. Therefore, the contribution of melanopsin to the impact of light on human alertness and cognition remains to be established.
Photon capture by rod and cone photopigments converts the chromophore from a photosensitive to a photoinsensitive state, triggering phototransduction (10). To regain light sensitivity, the enzymatic retinoid cycle within the retinal pigment epithelium is required for regeneration of the chromophore back to the light-sensitive state. In contrast, melanopsin is a dual-state photopigment, in which photons drive both processes of phototransduction and part of chromophore regeneration (10–12). Recent rodent and human data suggest that exposure to longer wavelength light (590–620 nm; orange–red) triggers melanopsin chromophore regeneration and increases overall subsequent intrinsic photosensitivity of ipRGCs (13, 14). Conversely, exposure to shorter wavelength light (∼480 nm; blue) favors phototransduction and decreases overall subsequent ipRGCs intrinsic photosensitivity (13, 14). At the physiological level (12), the existence of two stable photon absorption states allows photoconversion of melanopsin between the 11-cis and all-trans isoforms of the photopigment-bound chromophore to drive both photic responses and restoration of light responsiveness (12, 14), similar to processes of invertebrate rhabdomeric photopigments (15). Melanopsin would thus act as a light-sensitive switch, with the 11-cis isoform maximally sensitive to 480-nm photons, whereas the all-trans isoform is most efficiently transformed by longer wavelengths. Prior short-wavelength light would therefore decrease the overall proportion of “phototransduction units” of ipRGCs, whereas longer-wavelength photons (∼590–620 nm) would increase the overall proportion of phototransduction units. At intermediate wavelengths near 515 nm, the two processes are in equilibrium, with the two effects counterbalancing each other (“isosbestic value”) (15).
The present study aimed at determining melanopsin influence on human cognitive brain function based on this photic history hypothesis of its dual states. Based on the spectral sensitivity of the two states of melanopsin, we hypothesized that the impact of a given test light on cognitive brain responses would be increased, decreased, or intermediate after prior exposure to longer, shorter, or intermediate wavelength light, respectively.
Results and Discussion
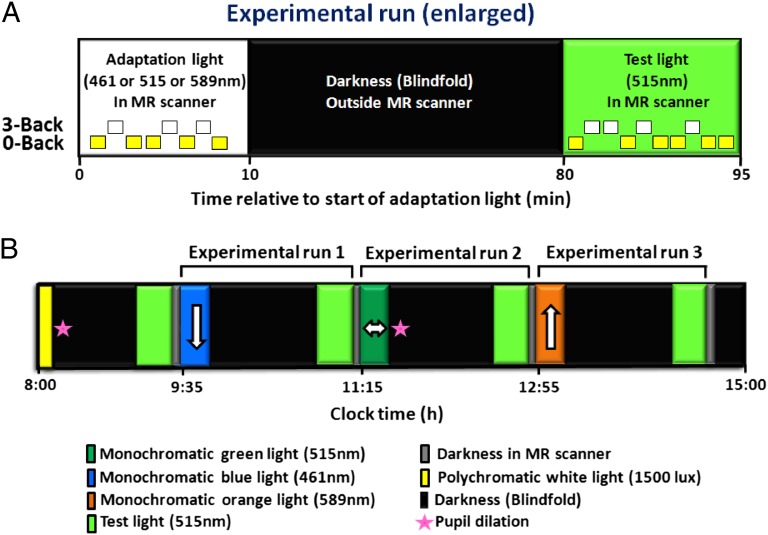
In a balanced cross-over design, 16 healthy young participants (Table S1) were exposed to 10 min of adaptation light, which could be of shorter (blue, 461 nm), intermediate (green, 515 nm), or longer (orange, 589 nm) wavelength (in a randomized manner), to modulate the subsequent impact of a test light on brain activity (Fig. 1). Adaptation light was administered in the magnetic resonance (MR) scanner, while participants performed auditory n-back tasks (0-back and 3-back). Afterwards, participants were kept in darkness (blindfolded outside the MR scanner) for 70 min to allow for complete readaptation of rods and cones. Subsequently, a test light (identical in each session) was administered for 15 min in the MR scanner, while participants performed auditory n-back tasks (0-back and 3-back). The test light consisted of a monochromatic green light (515 nm) of constantly changing irradiance level to separate possible linear time-on-task effects from the impact of test light on ongoing brain activity (see SI Materials and Methods for detailed methodology and see Figs. S1D and S2). Each test-light recording was followed by 5 min in complete darkness in the MR scanner. Responses to the simple letter-detection task (0-back) were subtracted from responses to the working memory task (3-back) in order to isolate executive brain responses and control for unspecific changes in baseline brain activity across recordings (changes in vigilance, boredom, circadian phase, etc.). Only data acquired during test-light exposure are considered here. Data from the first 15-min test-light recording were not preceded by exposure to an adaptation light (biased by an order effect) and therefore were not considered in the present analyses.
Fig. 1.
Experimental protocol. (A) Enlarged view of an experimental run, which started with 10-min exposure to an adaptation light, which could be of shorter (blue, 461 nm), intermediate (green, 515 nm), or longer (orange, 589 nm) wavelength (balanced order across subjects; constant irradiance, 6 × 1013 photons⋅cm−2⋅s−1). Adaptation light was administered in the MR scanner, while participants performed auditory n-back tasks (0-back and 3-back) to modulate subsequent impact of the test light on brain activity. Afterwards, participants were kept in darkness (blindfolded outside the MR scanner) for 70 min to allow for complete readaptation of rods and cones. Test light (515 nm; constantly changing irradiance) (see Materials and Methods and SI Materials and Methods) was then administered for 15 min in the MR scanner, while participants performed auditory n-back tasks (0-back and 3-back). (B) Schematic diagram of the entire study protocol (time relative to clock time, in hours). Order of adaptation-light wavelength was balanced across subjects. The white arrows indicate the predicted impact of the adaptation light on the subsequent impact of the test light.
Accuracy to both 3- and 0-back tasks was consistently high (>85%). Executive brain responses [i.e., 3-back to 0-back] were observed in the expected brain locations, such as the dorsolateral prefrontal cortex (DLPFC) and ventrolateral prefrontal cortex (VLPFC) (16) (Table S2 and Fig. S2). Behavioral measures and scores on multiple scales collected throughout the protocol revealed that task performance (see Fig. S5) and subjective feelings (e.g., sleepiness, light perception, etc.) (Figs. S3–S5) were similar across recordings. These results indicate that participants were well-engaged in the tasks, leaving prior light exposure administered 70 min earlier as the only difference between test-light recordings.
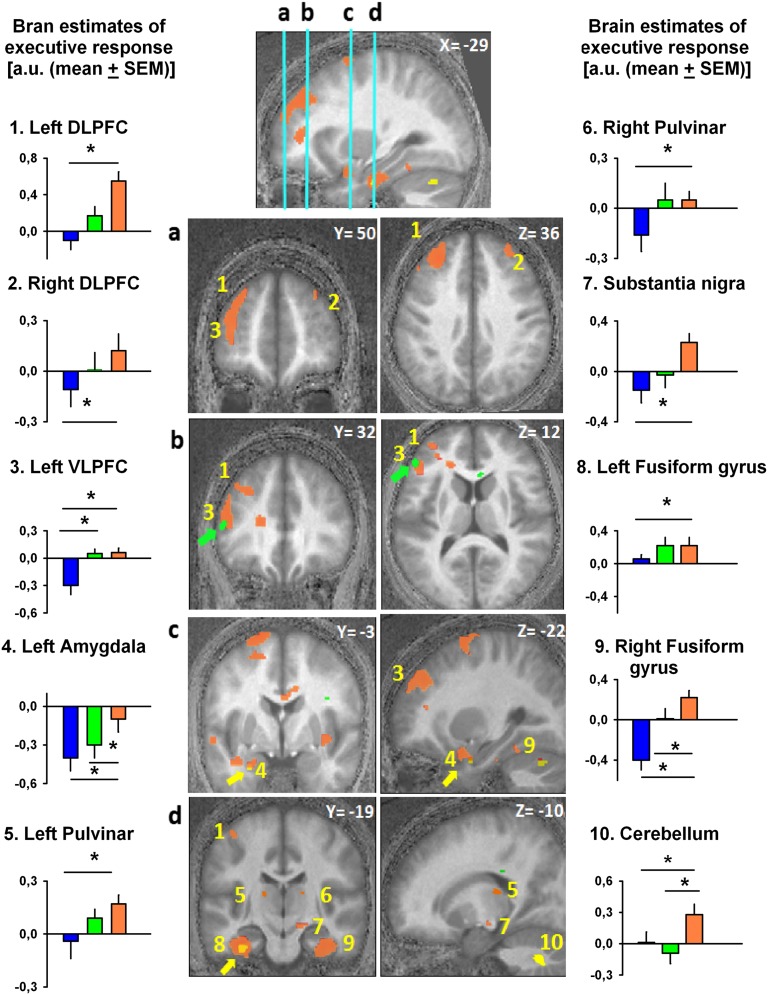
Intriguingly, despite identical scanning conditions across test-light recordings, analyses of the impact of test light on executive responses in each functional MRI (fMRI) recording revealed important dissimilarities depending on the wavelength of the adaptation light received before each recording (Fig. 2 and Table 1). Relative to prior blue-light exposure, prior orange-light exposure resulted in significantly higher impact of test light bilaterally in the superior and inferior DLPFC and in the left VLPFC. This widespread prefrontal impact of prior light encompasses regions involved in processes ranging from lower-order stimulus-driven cognitive control to higher-order executive control (17). Thus, prior light exposure had a modulatory effect on prefrontal cortex known to be implicated in various levels of executive control. Likewise, relative to prior blue-light exposure, prior orange-light exposure significantly increased test-light impact in the pulvinar. This region, which is essential to arousal and cognition regulation (18, 19), has been repeatedly suggested to play a key role in mediating the impact of light on alertness and cognition (7, 20, 21). Light impact on the pulvinar, which is a major relay between cortical areas, may facilitate information flow within the thalamocortical loops (7). Additionally, similar impacts of prior orange light were detected within the fusiform gyri, cerebellum, and amygdala, as well as in a subcortical area compatible with substantia nigra. The amygdala can be affected by the spectral quality of light (21) while engaged in emotional processes (22), presumably through direct or indirect ipRGC inputs (23, 24). Collectively, these results emphasize the importance of light history for human cognitive brain function and demonstrate that prior exposure to longer-wavelength light enhances the subsequent impact of light on brain structures important not only for executive functions, but also for emotion and alertness regulation. Further support for this concept builds up from the results of prior green-light exposure that, compared with prior blue exposure, resulted in an increased test-light impact in the same left VLPFC location as for prior orange light relative to prior blue light (Fig. 2, green arrow). In addition, compared with prior green light, prior orange-light exposure increased the impact of the test light on the left amygdala, fusiform gyrus, and bilateral cerebellum, in the vicinity of the locations reported for prior orange light relative to prior blue light (Fig. 2, yellow arrows). By contrast, all of the comparisons for a higher impact of prior shorter-wavelength vs. prior longer-wavelength light (prior green > orange; prior blue > orange; prior blue > green) revealed no significant differences.
Fig. 2.
Impact of the test light on executive brain responses depends on prior light. Orange blobs represent brain areas showing increased test-light impact after prior orange-light relative to prior blue-light exposure. Green blobs represent brain areas showing increased test-light impact after prior green-light relative to prior blue-light exposure (green arrows highlight these areas). Yellow blobs represent brain areas showing increased test-light impact after prior orange-light relative to prior green-light exposure (yellow arrows highlight these areas). Graphs show activity estimates of test-light impact on executive responses [3-back to 0-back; arbitrary units (a.u.); mean ± SEM] in the different brain areas after exposure to blue, green, and orange light. The numbers of the graphs correspond to brain locations on the central panels (as in Table 1). Graphs 1 and 2, left and right DLPFC; graph 3, left VLPFC; graph 4, left amygdala; graphs 5 and 6, left and right pulvinar; graph 7, substantia nigra; graphs 8 and 9, left and right fusiform gyrus; graph 10, cerebellum. *P < 0.05 corrected for multiple comparisons.
Table 1.
Significant effects of prior light exposure (blue, orange, and green) on the impact of test-light and executive-brain responses (3-back to 0-back)
| Contrast (graph no. in Fig. 2) | Side | X | Y | Z | Z-score | P value |
| Prior orange > prior blue | ||||||
| DLPFC (graphs 1 and 2) | L | −26 | 38 | 36 | 4.28 | 0.002 |
| L | −28 | 40 | 34 | 4.12 | 0.003 | |
| R | 32 | 6 | 66 | 4.03 | 0.003 | |
| L | −26 | 4 | 64 | 4.02 | 0.003 | |
| L | −18 | −2 | 72 | 4.01 | 0.003 | |
| L | −18 | 2 | 58 | 3.75 | 0.007 | |
| R | 30 | 44 | 34 | 3.68 | 0.005 | |
| L | −12 | −12 | 56 | 3.60 | 0.01 | |
| R | 16 | 4 | 56 | 3.13 | 0.03 | |
| VLPFC (graph 3) | L | −46 | 34 | 10 | 3.78 | 0.007 |
| L | −48 | 28 | 34 | 3.23 | 0.03 | |
| Fusiform gyrus (graphs 8 and 9) | L | −36 | 18 | 26 | 4.45 | 0.002 |
| R | 34 | −24 | −22 | 4.47 | 0.002 | |
| Amygdala (graph 4) | L | −26 | 4 | −20 | 3.62 | 0.01 |
| Pulvinar (graphs 5 and 6) | L | −16 | −20 | 18 | 3.76 | 0.01 |
| R | 18 | −24 | 16 | 3.22 | 0.04 | |
| Substantia nigra (graph 7) | R | 14 | −18 | −10 | 3.78 | 0.02 |
| Cerebellum (graph 10) | L | −6 | −82 | −42 | 4.04 | 0.003 |
| R | 28 | −70 | −36 | 3.39 | 0.02 | |
| Prior green > prior blue | ||||||
| VLPFC (graph 3) | L | −50 | 34 | 12 | 3.74 | 0.008 |
| Prior orange > prior green | ||||||
| Amygdala (graph 4) | L | −22 | −6 | −26 | 3.32 | 0.01 |
| Fusiform gyrus (graph 8) | L | −32 | 4 | −28 | 3.3 | 0.01 |
| Cerebellum | L | −14 | −68 | −28 | 3.64 | 0.007 |
| R | 20 | −60 | −36 | 3.51 | 0.01 | |
| Prior blue > prior orange | ||||||
| No significant voxel | ||||||
| Prior green > prior orange | ||||||
| No significant voxel | ||||||
| Prior blue > prior green | ||||||
| No significant voxel | ||||||
All P values are corrected for multiple comparisons over a priori small volume of interests (10-mm radius). DLPFC, dorsolateral prefrontal cortex; VLPFC, ventrolateral prefrontal cortex.
The effect of prior light exposure on the subsequent impact of light onto executive brain responses is in line with our hypothesis, such that, relative to shorter-wavelength light, prior exposure to longer-wavelength light would increase subsequent light impact. In our view, the only interpretation of our fMRI results is that prior exposure impact on the effect of light is achieved most plausibly through photoconversion of the melanopsin photopigment of ipRGCs. Light depolarizes ipRGCs directly through melanopsin activation and indirectly via synaptic pathways driven by rods and cones (2–4). Thus, all retinal photoreceptors should be accounted to the non–image-forming responses to light of different wavelength, duration, and irradiance levels (2–4). Involvement of both melanopsin, as well as cones and/or rods, certainly holds true for cognitive brain function. However, in our study, rods and cones were in the same state at the onset of each fMRI because the 70-min-darkness periods separating each run were more than twofold longer than required for complete dark adaptation (i.e., 30 min of darkness) (25). Their contribution to the difference between each test-light session is therefore highly unlikely. Conversely, melanopsin has longer kinetics for light–dark photic adaptation (26), and its sensitivity is deemed to be modulated by the wavelength of prior exposures. In vitro, heterologous expression of human melanopsin in a mouse paraneuronal cell line showed that exposure to longer-wavelength light (540 nm) triggered melanopsin chromophore regeneration and increased its subsequent light sensitivity (12). In vivo, mice preexposed to 630-nm light increased light-induced shifts in circadian locomotor activity and neuronal activity in the suprachiasmatic nucleus (14), site of the main circadian pacemaker, which receives most of the light input from ipRGCs (23). Likewise, human pupil light reflex increases after exposure to 590-nm orange light, compared with 460-nm blue-light exposure (13). In other words, our prior light effects on cognitive brain responses are strikingly similar to those of other human and rodent non–image-forming responses (13, 14). Collectively, these mice and human data suggest that the melanopsin dual-states system encodes prior light information that is retained and shapes subsequent responses to light.
We carefully designed a protocol in which behavioral changes during test-light recordings and across participants would be controlled for (e.g., wavelength-order randomization and task training to prevent learning and induce the ceiling of performance). Future research using different tasks and/or duration of light exposure are required to understand how these changes translate to behavior. Nevertheless, our results demonstrate that the brain dynamics required to perform cognitive tasks are influenced by prior light exposure, presumably by melanopsin-dependent ipRGCs intrinsic photosensitivity.
A recent study on rare blind individuals who retained non–image-forming responses to light (27) demonstrated the involvement of non–image-forming photoreception in the impact of light on human alertness and cognitive brain function, possibly through melanopsin-based ipRGC photosensitivity (28). The sample size of this study was relatively small (n = 3), due to the rarity of the recorded subjects. Furthermore, the impact of rod and cone degeneration and of the brain plasticity associated with blindness (29) remain unclear. In view of what is currently known, our data constitute the most compelling evidence to date in favor of a cognitive role for melanopsin, which mediates at least part of the impact of light on cognitive brain responses. Melanopsin may confer an important form of “photic memory” to human brain regions involved in various cognitive processes and may play a broader role than previously apprehended. Ultimately, these findings speak for an integration of light exposure over long periods of time when aiming at optimizing cognitive brain function.
Materials and Methods
Additional methodological descriptions are provided in SI Materials and Methods.
Participants.
Participants were right-handed, young, and healthy (n = 16; 7 women; 18–30 y old) (Table S1). They provided written informed consent, and the study was approved by the local ethics committee. Questionnaires were used to rule out medical, psychiatric, and sleep disorders and color blindness. Volunteers followed a regular sleep schedule during the 7 d before the laboratory setup, which was verified using actigraphy and sleep diaries.
Study Protocol.
During each experimental run, volunteers underwent 10 min of exposure to “adaptation” monochromatic light, which could be either one of three different wavelengths (blue, 461 nm; green, 515 nm; orange, 589 nm) (Fig. S1 A–C). Irradiance levels of each adaptation-light exposure were constant and set at 6 × 1013 photons⋅cm−2⋅s−1 (Fig. S1E). The order of adaptation-light wavelength was balanced across subjects. Afterwards, participants were kept in darkness (blindfolded outside the MR scanner) for 70 min. Subsequently, they were exposed to a monochromatic “test” light (515 nm) for 15 min in the MR scanner. The irradiance level of this light was pseudorandomly varied between 5 × 1012 photons⋅cm−2⋅s−1 and 6 × 1013 photons⋅cm−2⋅s−1, with periodic short periods of complete darkness (2–3 s), to ensure that any light-induced modulation of brain activity could be due only to light and not to other nonspecific effects (e.g., linear changes due to passage of time, changes in vigilance or boredom). The irradiance change pattern was identical for all subjects (Fig. S1D). After test-light exposure, subjects were kept under complete darkness for 5 min (0 lx), in the MR scanner.
Behavioral Task During the fMRI.
During both adaptation and test lights, volunteers continuously performed blocks of auditory n-back tasks (30), which varied between two different levels of difficulty. Volunteers performed the 3-back version of the task and were required to state whether or not a given consonant was identical to the consonant presented three items earlier, by using an MR-compatible keypad. Volunteers also performed a simple letter-detection 0-back version of the task and were required to state whether or not each consonant was a “k.” The 0-back task was implemented in the protocol to control for nonspecific changes across test-light recordings [e.g., changes in sleep pressure and circadian phase (20), or in vigilance or motivation levels] that could affect baseline brain activity and to isolate a large part of the executive component of the 3-back task in our fMRI analyses. Each type of task was preceded by a short vocal instruction. Subjective alertness and a visual analog scale for mood, concentration, motivation, and fatigue were collected every 35 min (before each test-light recording, after each adaptation light, and once during the dark adaptation period out of the scanner: 11 time points in total). Immediately after leaving the MR scanner (i.e., after each adaptation-light recording), mental effort and visual comfort scores were collected to assess the subjective feeling of light perception (4 time points in total).
MRI Data Acquisition.
MRI data were acquired on a 3T scanner (Allegra; Siemens). Multislice T2*-weighted functional images were acquired with a gradient-echo echo-planar imaging (EPI) sequence (34 axial slices; field of view, 192 × 192 mm2; voxel size, 3 × 3 × 3 mm3; 25% interslice gap; matrix size, 64 × 64 × 34; repetition time, 2,040 ms; echo time, 30 ms; flip angle, 90°) (31).
fMRI Data Analysis.
Functional volumes were analyzed using Statistical Parametric Mapping 8 (SPM8) (www.fil.ion.ucl.ac.uk/spm). Brain volumes were corrected for motion and distortion, coregistered to the structural image, normalized to the Montreal Neurological Institute (MNI) space, and smoothed. The fMRI data analysis was carried out in two steps, accounting, respectively, for fixed and random effects. For each test-light recording, separate boxcar functions, convolved with a canonical hemodynamic response function, modeled (i) blocks of 3-back and 0-back tasks (independent of light), (ii) linear change with time in brain responses to the 3-back and 0-back tasks (independent of light), (iii) irradiance profile for test light, and (iv) interaction light task (i.e., brain responses derived from interaction of light and task, and not explained by task and light effects alone). In each subject, the effects of interest included the following: (i) the difference between 3-back and 0-back tasks across the four test-light recordings; (ii) the difference between the interaction between test light and 3-back task (ITL_3b) and the interaction between test light and 0-back task, after blue-light exposure vs. after orange-light exposure [(ITL_3bxORANGE_test > ITL_0bxORANGE_test) vs. (ITL_3bxBLUE_test > ITL _0bxBLUE_test)]; (iii) the differences between ITL_3-back and ITL_0-back tasks after blue-light exposure vs. after monochromatic green-light exposure [(ITL_3bxGREEN_test > ITL_0bxGREEN_test) vs. (ITL_3bxBLUE_test > ITL_0bxBLUE_test)]; (iv) differences between ITL_3-back and ITL_0-back tasks after orange-light exposure vs. after green-light exposure [(ITL_3bxGREEN_test > ITL_0bxGREEN_test) vs. (ITL_3bxORANGE_test > ITL_0bxORANGE_ test)]. Contrasts of interest did not include the first test-light recording because this comparison would systematically be biased by an order effect. The second-level analysis corresponded to one sample t test threshold at P (uncorrected) < 0.001. Statistical inferences were performed after correction for multiple comparisons at a threshold of P < 0.05. Corrections for multiple comparisons (Family-Wise Error method) were computed on the entire brain volume or on small spherical volumes (10-mm radius) around a priori locations of activation.
Supplementary Material
Acknowledgments
We thank Pierre Maquet, Fabienne Collette, Brigitte Herbillon, Annick Claes, Benjamin Lauricella, Patrick Hawotte, Pascal Blain, Marc Déculot, and Fabian Languy for help. This study was funded by the Fond National de la Recherches Scientifique (FNRS) and Agence National de Recherche (Grant ANR-09-MNPS-040). J.Q.M.L., C.P., and G.V. are funded by FNRS. S.L.C. is funded by the University of Liège.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Commentary on page 5769.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1320005111/-/DCSupplemental.
References
- 1.Berson DM, Dunn FA, Takao M. Phototransduction by retinal ganglion cells that set the circadian clock. Science. 2002;295(5557):1070–1073. doi: 10.1126/science.1067262. [DOI] [PubMed] [Google Scholar]
- 2.Lucas RJ. Mammalian inner retinal photoreception. Curr Biol. 2013;23(3):029. doi: 10.1016/j.cub.2012.12.029. [DOI] [PubMed] [Google Scholar]
- 3.Hatori M, Panda S. The emerging roles of melanopsin in behavioral adaptation to light. Trends Mol Med. 2010;16(10):435–446. doi: 10.1016/j.molmed.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schmidt TM, Chen SK, Hattar S. Intrinsically photosensitive retinal ganglion cells: Many subtypes, diverse functions. Trends Neurosci. 2011;34(11):572–580. doi: 10.1016/j.tins.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hubbard J, Ruppert E, Gropp CM, Bourgin P. Non-circadian direct effects of light on sleep and alertness: Lessons from transgenic mouse models. Sleep Med Rev. 2013;17(6):445–452. doi: 10.1016/j.smrv.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 6.Chellappa SL, Gordijn MC, Cajochen C. Can light make us bright? Effects of light on cognition and sleep. Prog Brain Res. 2011;190:119–133. doi: 10.1016/B978-0-444-53817-8.00007-4. [DOI] [PubMed] [Google Scholar]
- 7.Vandewalle G, Dijk DJ. Neuroimaging the effects of light on non-visual brain functions. In: Nofzinger E, Maquet P, Thorpy M, editors. Neuroimaging of Sleep and Sleep Disorders. Cambridge, UK: Cambridge Univ Press; 2013. pp. 171–178. [Google Scholar]
- 8.Lockley SW, et al. Short-wavelength sensitivity for the direct effects of light on alertness, vigilance, and the waking electroencephalogram in humans. Sleep. 2006;29(2):161–168. [PubMed] [Google Scholar]
- 9.Chellappa SL, et al. Non-visual effects of light on melatonin, alertness and cognitive performance: Can blue-enriched light keep us alert? PLoS ONE. 2011;6(1):e16429. doi: 10.1371/journal.pone.0016429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sexton TJ, Golczak M, Palczewski K, Van Gelder RN. Melanopsin is highly resistant to light and chemical bleaching in vivo. J Biol Chem. 2012;287(25):20888–20897. doi: 10.1074/jbc.M111.325969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Terakita A, et al. Expression and comparative characterization of Gq-coupled invertebrate visual pigments and melanopsin. J Neurochem. 2008;105(3):883–890. doi: 10.1111/j.1471-4159.2007.05184.x. [DOI] [PubMed] [Google Scholar]
- 12.Melyan Z, Tarttelin EE, Bellingham J, Lucas RJ, Hankins MW. Addition of human melanopsin renders mammalian cells photoresponsive. Nature. 2005;433(7027):741–745. doi: 10.1038/nature03344. [DOI] [PubMed] [Google Scholar]
- 13.Mure LS, et al. Melanopsin bistability: A fly’s eye technology in the human retina. PLoS ONE. 2009;4(6):e5991. doi: 10.1371/journal.pone.0005991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mure LS, Rieux C, Hattar S, Cooper HM. Melanopsin-dependent nonvisual responses: Evidence for photopigment bistability in vivo. J Biol Rhythms. 2007;22(5):411–424. doi: 10.1177/0748730407306043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hillman P, Hochstein S, Minke B. Transduction in invertebrate photoreceptors: Role of pigment bistability. Physiol Rev. 1983;63(2):668–772. doi: 10.1152/physrev.1983.63.2.668. [DOI] [PubMed] [Google Scholar]
- 16.Collette F, Hogge M, Salmon E, Van der Linden M. Exploration of the neural substrates of executive functioning by functional neuroimaging. Neuroscience. 2006;139(1):209–221. doi: 10.1016/j.neuroscience.2005.05.035. [DOI] [PubMed] [Google Scholar]
- 17.Koechlin E, Ody C, Kouneiher F. The architecture of cognitive control in the human prefrontal cortex. Science. 2003;302(5648):1181–1185. doi: 10.1126/science.1088545. [DOI] [PubMed] [Google Scholar]
- 18.Coull JT, Jones ME, Egan TD, Frith CD, Maze M. Attentional effects of noradrenaline vary with arousal level: Selective activation of thalamic pulvinar in humans. Neuroimage. 2004;22(1):315–322. doi: 10.1016/j.neuroimage.2003.12.022. [DOI] [PubMed] [Google Scholar]
- 19.Saalmann YB, Pinsk MA, Wang L, Li X, Kastner S. The pulvinar regulates information transmission between cortical areas based on attention demands. Science. 2012;337(6095):753–756. doi: 10.1126/science.1223082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vandewalle G, et al. Effects of light on cognitive brain responses depend on circadian phase and sleep homeostasis. J Biol Rhythms. 2011;26(3):249–259. doi: 10.1177/0748730411401736. [DOI] [PubMed] [Google Scholar]
- 21.Vandewalle G, et al. Wavelength-dependent modulation of brain responses to a working memory task by daytime light exposure. Cereb Cortex. 2007;17(12):2788–2795. doi: 10.1093/cercor/bhm007. [DOI] [PubMed] [Google Scholar]
- 22.Vandewalle G, et al. Spectral quality of light modulates emotional brain responses in humans. Proc Natl Acad Sci USA. 2010;107(45):19549–19554. doi: 10.1073/pnas.1010180107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hattar S, et al. Central projections of melanopsin-expressing retinal ganglion cells in the mouse. J Comp Neurol. 2006;497(3):326–349. doi: 10.1002/cne.20970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morris JS, Ohman A, Dolan RJ. A subcortical pathway to the right amygdala mediating “unseen” fear. Proc Natl Acad Sci USA. 1999;96(4):1680–1685. doi: 10.1073/pnas.96.4.1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fain GL, Matthews HR, Cornwall MC, Koutalos Y. Adaptation in vertebrate photoreceptors. Physiol Rev. 2001;81(1):117–151. doi: 10.1152/physrev.2001.81.1.117. [DOI] [PubMed] [Google Scholar]
- 26.Drouyer E, Rieux C, Hut RA, Cooper HM. Responses of suprachiasmatic nucleus neurons to light and dark adaptation: Relative contributions of melanopsin and rod-cone inputs. J Neurosci. 2007;27(36):9623–9631. doi: 10.1523/JNEUROSCI.1391-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Czeisler CA, et al. Suppression of melatonin secretion in some blind patients by exposure to bright light. N Engl J Med. 1995;332(1):6–11. doi: 10.1056/NEJM199501053320102. [DOI] [PubMed] [Google Scholar]
- 28.Vandewalle G, et al. Blue light stimulates cognitive brain activity in visually blind individuals. J Cogn Neurosci. 2013;25(12):2072–2085. doi: 10.1162/jocn_a_00450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Collignon O, et al. Impact of blindness onset on the functional organization and the connectivity of the occipital cortex. Brain. 2013;136(Pt 9):2769–2783. doi: 10.1093/brain/awt176. [DOI] [PubMed] [Google Scholar]
- 30.Cohen JD, et al. Temporal dynamics of brain activation during a working memory task. Nature. 1997;386(6625):604–608. doi: 10.1038/386604a0. [DOI] [PubMed] [Google Scholar]
- 31.Helms G, Finsterbusch J, Weiskopf N, Dechent P. Rapid radiofrequency field mapping in vivo using single-shot STEAM MRI. Magn Reson Med. 2008;60(3):739–743. doi: 10.1002/mrm.21676. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.