Significance
Millions of people worldwide suffer from chronic nasal inflammation involving obstructed airflow and nasal discharge. Although nasal inflammation is often considered to be a reaction to allergens, approximately one-quarter of all cases are nonallergic rhinitis. The causes of this disease are unknown, but symptoms may be triggered or exacerbated by a variety of inhaled irritants or even seemingly innocuous odors. We report here that specialized chemosensory cells of the nasal epithelium of mice detect potential irritants and transmit this information to pain-sensing nerve terminals, which then release bioactive peptides to trigger an inflammatory response—all without the necessity for activity of the adaptive immune system. This previously unidentified pathway may offer therapeutic targets for intervention in nonallergic rhinitis.
Keywords: rhinitis, innate immunity, quorum sensing, chemesthesis, airway irritation
Abstract
Solitary chemosensory cells (SCCs) of the nasal cavity are specialized epithelial chemosensors that respond to irritants through the canonical taste transduction cascade involving Gα-gustducin and transient receptor potential melastatin 5. When stimulated, SCCs trigger peptidergic nociceptive (or pain) nerve fibers, causing an alteration of the respiratory rate indicative of trigeminal activation. Direct chemical excitation of trigeminal pain fibers by capsaicin evokes neurogenic inflammation in the surrounding epithelium. In the current study, we test whether activation of nasal SCCs can trigger similar local inflammatory responses, specifically mast cell degranulation and plasma leakage. The prototypical bitter compound, denatonium, a well-established activator of SCCs, caused significant inflammatory responses in WT mice but not mice with a genetic deletion of elements of the canonical taste transduction cascade, showing that activation of taste signaling components is sufficient to trigger local inflammation. Chemical ablation of peptidergic trigeminal fibers prevented the SCC-induced nasal inflammation, indicating that SCCs evoke inflammation only by neural activity and not by release of local inflammatory mediators. Additionally, blocking nicotinic, but not muscarinic, acetylcholine receptors prevents SCC-mediated neurogenic inflammation for both denatonium and the bacterial signaling molecule 3-oxo-C12-homoserine lactone, showing the necessity for cholinergic transmission. Finally, we show that the neurokinin 1 receptor for substance P is required for SCC-mediated inflammation, suggesting that release of substance P from nerve fibers triggers the inflammatory events. Taken together, these results show that SCCs use cholinergic neurotransmission to trigger peptidergic trigeminal nociceptors, which link SCCs to the neurogenic inflammatory pathway.
The respiratory tract is continually assaulted by a plethora of irritants and xenobiotics. The nasal cavity serves as the first line of defense against this chemically diverse array of noxious substances (1) and houses parallel systems for irritant detection―trigeminal free nerve endings and solitary chemosensory cells (SCCs)―both of which mediate protective airway reflexes (2). The dual chemodetector systems allow for responses to irritating substances with widely varied physical and chemical properties (1, 2). In the current study, we describe the mechanism and mediators by which the parallel warning systems of free nerve endings and SCCs evoke local inflammation and a proinflammatory response in the nasal epithelium.
Free nerve endings of the trigeminal nerve occur throughout the nasal respiratory epithelium and respond directly to many irritants through chemosensitive transient receptor potential (TRP) ion channels (3, 4). However, these intranasal trigeminal fibers terminate below the level of tight junctions at the surface of the epithelium (5), allowing lipophilic compounds to reach the receptors on the free nerve endings. Accordingly, peptidergic nociceptive trigeminal fibers are responsive to only a subset of potentially dangerous compounds entering the airway.
An alternative means by which irritants can activate the trigeminal system is through the agency of SCCs, which populate the respiratory epithelium of the nasal cavity (6–8). These SCCs extend microvillous sensory processes into the lumen of the nasal cavity and therefore, have access to potential irritants that cannot penetrate the epithelial barrier (6). SCCs use the same chemosensory transduction cascade as bitter-responsive taste cells, including Gα-gustducin, phospholipase Cβ2, and the monovalent-selective cation channel transient receptor potential melastatin 5 (TRPM5) (6, 9). SCCs respond to both traditional bitter compounds (e.g., denatonium) as well as bacterial metabolites [e.g., the quorum-sensing factor acyl-homoserine lactones (AHLs)] (7). Our previous studies have established that the activation of SCCs evokes protective respiratory reflexes through a well-characterized trigeminally mediated brainstem reflex (6, 7), suggesting that SCCs synapse onto trigeminal nerve sensory endings. SCCs show an accumulation of small vesicles typically associated with synaptic functions (6). Presumably, on stimulation, SCCs release a hitherto unidentified neurotransmitter that excites the trigeminal endings. Experiments in the present study suggest that nasal SCCs release acetylcholine to activate nicotinic cholinergic receptors (nAChRs) on the trigeminal nerve fibers, which in turn, evoke a neurogenic inflammation.
Inflammation is classically defined by pre-Galen physicians as the symptoms of sensitivity to pain (dolor), heat (calor), redness (rubor), and swelling (tumor) (10). The swelling or edema, which characterizes inflammation, results when chemical signals trigger changes in the endothelial cells that compose blood vessel walls, opening junctions between cells and resulting in leakage of plasma into the extracellular space (11).
Inflammation also can be characterized by the subsequent recruitment and activation of the immune system (12). Mast cells are components of the innate immune system that reside within epithelial tissues, such as the airways, and react to proinflammatory mediators by releasing cytoplasmic granules. These granules contain of a broad array of biologically active substances, including histamine, heparin, proteases, lipid-derived mediators, growth factors, cytokines, and chemokines (12–14). Previous studies have established that mast cells release their granules in response to a variety of agents, including neuropeptides such as substance P, that are released from peptidergic nerve fibers (12, 13, 15). The current study relies on mast cell degranulation as an index of activation of the immune system.
We postulated that stimulation of SCCs with irritants will excite peptiergic trigeminal fibers, ultimately resulting in local inflammation and activation of the innate immune system. Here, we describe the mechanisms and mediators by which different classes of irritants evoke these responses. Specifically, we confirm that peptidergic trigeminal fibers play an essential role in generating the SCC-mediated local inflammatory and early immune responses. Furthermore, we present evidence that SCCs release the neurotransmitter acetylcholine to activate nAChRs on trigeminal fibers. Finally, we show that the substance P released from peptidergic trigeminal fibers is the primary signal that induces both the edema characteristic of local inflammation and mast cell degranulation indicative of an early immune response.
Results
SCCs Are Cholinergic and Contact Peptidergic Nociceptors.
Solitary chemosensory cells in the nasal respiratory epithelium are taste cell-like chemosensors, which express key elements of the canonical taste transduction cascade, including TRPM5 and Gα-gustducin (Fig. 1A) (6–8). In the trachea, a related cell type, the chemosensory brush cell, releases acetylcholine (ACh) to activate vagal pain fibers (16–18), suggesting ACh as a candidate neurotransmitter for SCCs. Similarly, SCCs lining the vomeronasal duct of mice express choline acetyltransferase (ChAT), the synthetic enzyme of acetylcholine (19). To confirm whether SCCs in the nasal respiratory epithelium express ChAT, we examined the nasal epithelium of a transgenic mouse, which expresses τ-GFP driven by the ChAT promoter (20). In the nose of these mice, cells immunoreactive for gustducin also express ChAT-driven GFP (Fig. 1B). This result suggests that nasal SCCs, like tracheal brush cells, are capable of producing ACh for release onto nerve fibers (16, 17).
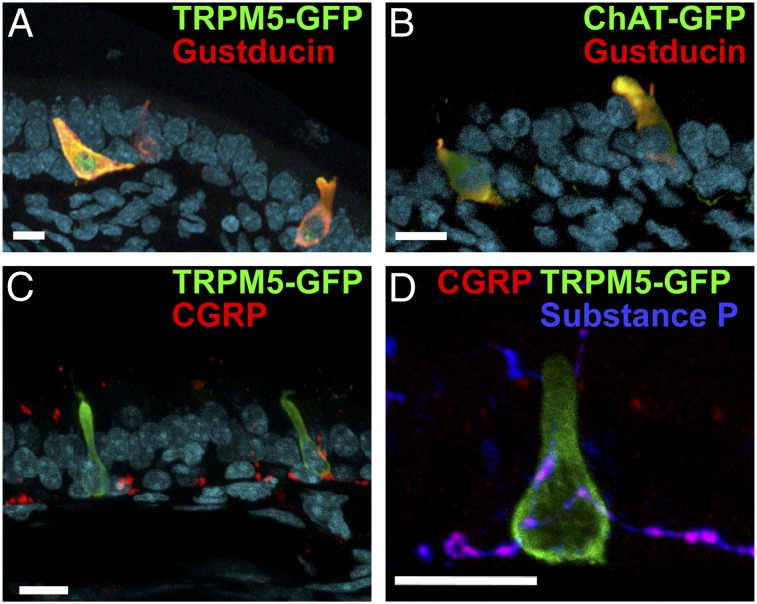
Fig. 1.
Cross-sections of nasal epithelium showing cellular properties and relationships of SCCs. (A) SCCs express both TRPM5 (detected by TRPM5-driven GFP; green) and gustducin (red), which are elements of the canonical taste transduction pathway. (B) SCCs express both gustducin (red) and ChAT (detected by ChAT-driven GFP; green), the synthesizing enzyme for ACh. (C) SCCs expressing TRPM5 (green) are intimately contacted by calcitonin gene-related peptide (CGRP) immunoreactive peptidergic nociceptive trigeminal fibers. (D) These peptidergic fibers (magenta) contact SCCs (TRPM5-GFP; green) and are immunoreactive for both CGRP (red) and substance P (blue). The nuclear counterstain DRAQ5 is shown in cyan in A–C. (Scale bars: 10 µm.)
The trigeminal nerve fibers that contact the SCCs are immunoreactive for substance P and calcitonin gene-related peptide (Fig. 1 C and D), both of which are inflammatory mediators typically coexpressed and released by nociceptive (e.g., pain) fibers (6, 21). The peptidergic trigeminal nociceptive fibers branch within the epithelium to both contact SCCs and terminate as free nerve endings in nearby epithelium (Fig. 1C). The anatomical arrangement of SCCs and trigeminal free nerve endings potentially offers dual pathways for trigeminal activation by irritants, because not only the SCCs but also, the free nerve endings themselves are chemosensitive.
Gustducin and TRPM5 Are Required for SCC-Mediated Inflammation.
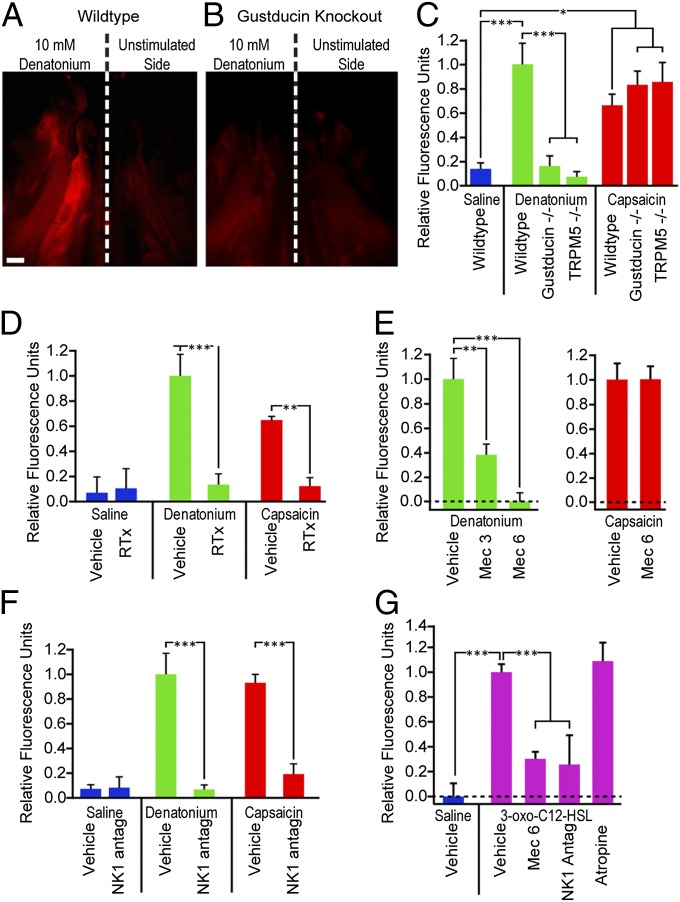
Direct chemical activation of trigeminal nasal nociceptive nerve fibers with capsaicin evokes neurogenic inflammation (22–24). To test the possible role of SCCs in irritant-induced nasal inflammation, we stimulated mice with capsaicin, denatonium (a model compound known to activate SCCs) (9), or 3-oxo-C12-homoserine lactone (3-oxo-C12-HSL), a bacterial quorum-sensing molecule of Pseudomonas aeruginosa and activator of SCCs (7). Capsaicin directly activates peptidergic trigeminal nociceptors through transient receptor potential vanilloid 1 (TRPV1) channels (25, 26), whereas denatonium and 3-oxo-C12-HSL activate SCCs in vitro and are capable of triggering SCC-mediated respiratory reflexes typical of trigeminal simulation in vivo (7, 9). Application of capsaicin or either of the two SCC activators to the nasal cavity resulted in a significant increase in two hallmarks of inflammation: plasma extravasation (Fig. 2 A–C and G and Fig. S1) and mast cell degranulation (Fig. 3 A–C). To test whether the inflammation provoked by denatonium but not capsaicin requires functional SCCs, we tested both compounds on mice with genetic deletion of either of the two elements of the canonical taste signaling cascade. Although both gustducin−/− and TRPM5−/− mice showed significant plasma extravasation when stimulated with capsaicin, an activator of TRPV1 on the nerve fibers (Fig. 2C), neither showed extravasation after exposure to denatonium (Fig. 2 B and C). These data are consistent with the hypothesis that functional taste-related transduction in SCCs is required for induction of plasma leakage by denatonium but not capsaicin.
Fig. 2.
Stimulation of SCCs or nociceptor nerve terminals activates a proinflammatory pathway, leading to plasma extravasation. (A and B) Fluorescence images of a whole mount of the hemisected nasal cavity from mice stimulated unilaterally with 10 mM denatonium benzoate and injected i.v. with Alexa 555-albumin showing fluorescence because of plasma leakage. Anterior is up. (Scale bar: 1 mm.) (A) WT mouse shows increased fluorescence on the stimulated side. (B) A Gustducin−/− mouse shows no significant fluorescence on either the stimulated or unstimulated sides. (C–G) Bar graphs illustrating the relative fluorescence of stimulated and unstimulated sides under various conditions and genotypes. Positive values indicate that the stimulated side was brighter than the unstimulated side. Bars represent mean + SEM. (C) WT mice stimulated with 10 mM denatonium (green) or 2 μM capsaicin (red) showed significant (P < 0.001 or P < 0.01, respectively) plasma extravasation on the stimulated side compared with saline-stimulated mice (blue). Two KO strains, Gustducin−/− and TRPM5−/−, showed significantly less (P < 0.001) extravasation than WT controls stimulated with denatonium but normal extravasation with capsaicin. (D) Mice treated with RTx to eliminate peptidergic nerve fibers were significantly different from vehicle-treated controls (P < 0.01 and P < 0.001) and showed no significant extravasation to either denatonium or capsaicin. (E) The nAChR antagonist mecamylamine (Mec) significantly reduced denatonium-induced extravasation at both 3 (P < 0.01) and 6 mg/kg (P < 0.001) but did not alter capsaicin-induced extravasation. (F) The NK1 antagonist L732138 (5 mg/kg i.p.), which blocks responses to substance P, significantly reduces plasma extravasation in response to both denatonium (P < 0.001) and capsaicin (P < 0.001). (G) Stimulation with the bacterial metabolite 3-oxo-C12-HSL (300 μM) provoked significant (P < 0.001) plasma extravastion compared with saline-stimulated controls. This HSL-induced plasma extravasation was significantly reduced (P < 0.001) by treatment with either the nicotinic antagonist mecamylamine or the NK1 antagonist L732138, but was not altered by the muscarinic AChR blocker atropine (1 mg/kg). *P < 0.05; **P < 0.01; ***P < 0.001 by one-way ANOVA with Tukey honest significant difference (HSD) test.
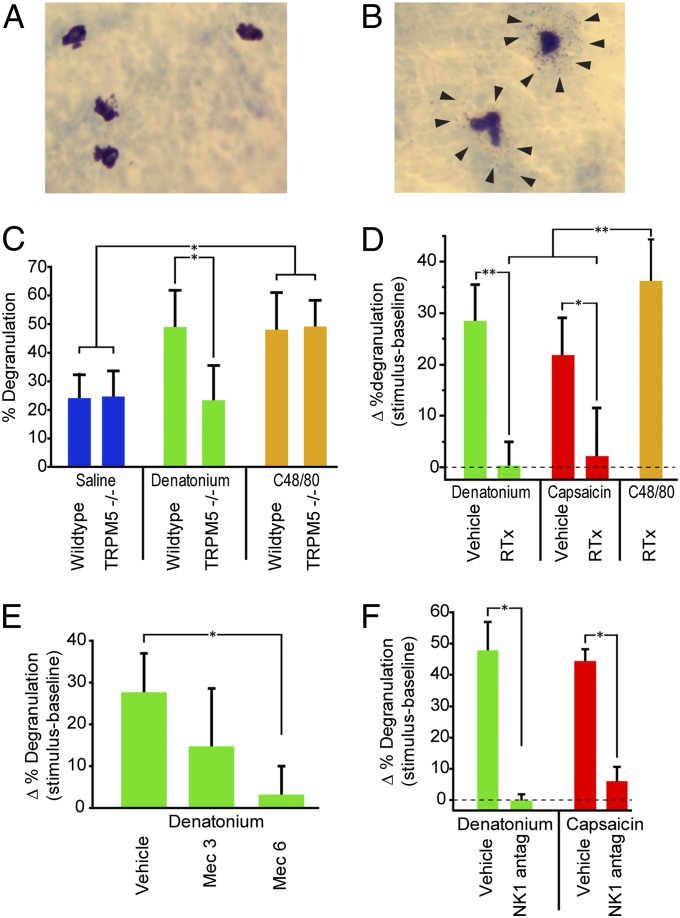
Fig. 3.
Stimulation of SCCs activates a proinflammatory pathway that triggers mast cell degranulation. Photos of (A) resting and (B) degranulated mast cells stained with acidified toluidine blue. Arrows point to granules forming a halo around the degranulated mast cells. (C) WT mice stimulated with 10 mM denatonium showed significantly more mast cell degranulation than TRPM5−/− animals (P < 0.05). Both WT and TRPM5−/− mice showed degranulation on exposure to the secretagague C48/80. (D) Mice treated with RTx to eliminate nerve fibers showed significantly less mast cell degranulation than vehicle-treated controls to both denatonium (P < 0.001) and capsaicin (P < 0.01) but were still able to respond to compound 48/80 (C48/80), which directly acts on mast cells to cause degranulation. (E) The nAChR antagonist mecamylamine (Mec) significantly reduced denatonium-induced mast cell degranulation at a dose of 6 mg/kg (P < 0.01). (F) The NK1 antagonist L732138 (5 mg/kg i.p.), which blocks responses to substance P, significantly reduces mast cell degranulation in response to both denatonium (P < 0.05) and capsaicin (P < 0.05). Bars represent mean + SEM. *P < 0.05; **P < 0.01 by one-way ANOVA with Tukey HSD test.
Similarly, in TRPM5−/− mice, SCC-mediated mast cell degranulation was deficient in response to denatonium but not capsaicin (Fig. 3C), suggesting that for some irritants, mast cell degranulation also depends on the taste transduction cascade. To test whether genetic deletion TRPM5 reduced the overall capacity for mast cell degranulation, we tested animals of each genotype with the mast cell secretagogue, compound 48/80. Both WT and TRPM5−/− mice showed similar levels of mast cell degranulation when injected with compound 48/80 (Fig. 3C). Taken together, these results indicate that SCCs and trigeminal free nerve endings offer dual proinflammatory pathways, detecting different chemicals but ultimately triggering the same local inflammatory response and early immune reactions.
Peptidergic Nociceptive Trigeminal Fibers Are Required for SCC-Mediated Inflammation.
The results above suggest that both SCCs and trigeminal free nerve endings can trigger similar inflammatory and immune reactions (Figs. 2C and 3C). Conceivably, SCCs might trigger this response independent of the nerve through paracrine intraepithelial signals. Alternatively, SCC-mediated inflammatory and immune reactions might be entirely dependent on the release of neuropeptides from nociceptive trigeminal fibers. To test if peptidergic trigeminal fibers are required for SCC-mediated inflammation and early immune response, we ablated peptidergic nociceptive fibers by treating mice repeatedly with resiniferatoxin (RTx) (27), which destroys TRPV1-expressing peptidergic trigeminal fibers (27) without causing any visible changes to SCCs (Fig. S2) (28).
In RTx-treated mice, capsaicin failed to evoke either plasma extravasation or mast cell degranulation, indicating that the ablated peptidergic trigeminal fibers were necessary for the responses to capsaicin (Figs. 2D and 3D). If SCCs were capable of triggering an inflammatory response through a paracrine pathway in the absence of innervation, then SCC activators, such as denatonium, should evoke inflammatory responses in the absence of peptidergic nerve fibers. However, denatonium fails to evoke any significant inflammatory response in RTx-treated mice in terms of either plasma extravasation (Fig. 2D) or mast cell degranulation (Fig. 3D), affirming the necessity for polymodal nociceptive nerve fibers in these responses. Collectively, these results indicate that SCC-mediated local inflammation and early immune response require the presence of peptidergic trigeminal fibers to convey signals from the SCCs to the postcapillary venules and mast cells (29).
SCC-Mediated Inflammation Requires nAChRs.
Our results above show that SCCs require the presence of nociceptive trigeminal nerve fibers to trigger inflammation (Figs. 2D and 3D). The presence of synapses between SCCs and trigeminal fibers (6) suggests that the SCCs release a neurotransmitter, which activates the sensory nerve endings. The expression of choline acetyltransferase suggests that nasal SCCs, like vomeronasal duct SCCs (19) and tracheal brush cells (17), may be cholinergic (Fig. 1B), and previous studies have shown the presence of nAChRs on nasal trigeminal fibers (30). To test whether nAChRs are necessary for SCC-mediated effects, we treated mice with mecamylamine, a blocker of nAChRs, and tested responses to denatonium and capsaicin. Mecamylamine significantly reduced responses to denatonium in a dose-dependent manner but had no significant effect on responsiveness to capsaicin (Fig. 2E), reflecting the necessity for cholinergic neurotransmission for SCC-mediated effects but not direct neural effects (Fig. 2E). Similarly, mecamylamine significantly reduced the proportion of dengranulated mast cells after denatonium stimulation (Fig. 3E).
To test whether a similar nicotinic receptor mechanism is used during activation of the SCC system by compounds from a natural source, we tested the necessity for cholinergic transmission in response to stimulation by an AHL quorum-sensing molecule. Mecamylamine significantly reduced plasma extravasation after stimulation by 3-oxo-C12-HSL, showing that nicotinic cholinergic transmission is necessary for responses to this AHL, just as it is for denatonium. Specificity for nicotinic receptors is established, because atropine (1 mg/kg), a muscarinic acetylcholine receptor blocker, had no effect (Fig. 2G). Taken together, these results indicate that nicotinic but not muscarinic AChRs are required for SCC-induced inflammatory responses.
Neurokinin 1 Receptors Underlie Both Plasma Extravasation and Mast Cell Degranulation.
In other systems, neural-induced plasma extravasation depends on the release of substance P or neurokinin A from nerve fibers, acting on neurokinin 1 (NK1) or neurokinin 2 receptors, respectively, on the endothelial cells (11). Because the trigeminal polymodal nociceptors of the nasal cavity express substance P, we used the NK1 antagonist L732138 to test whether NK1 receptors are required for irritant-induced inflammation. If so, than blocking NK1 receptors on the final common effector pathway should prevent plasma leakage and mast cell degranulation, regardless of the avenue of activation by the irritant (i.e., through SCCs or TRP channels on free nerve endings).
For both denatonium (P < 0.001) and capsaicin (P < 0.001), an injection of the antagonist L732138 (5 mg/kg i.p.) significantly reduced plasma extravasation compared with the vehicle-injected controls (Fig. 2F). The level of denatonium-evoked plasma extravasation in mice treated with the NK1 antagonist was not significantly different from control mice stimulated with saline (Fig. 2F). Additionally, L732138 treatment prevented plasma extravasation induced by the bacterial metabolite 3-oxo-C12-HSL (Fig. 2G). This antagonist also prevented mast cell degranulation in mice stimulated with either denatonium or capsaicin (Fig. 3F). Taken together, these results indicate that NK1 activation is necessary for both irritant-induced plasma extravasation and mast cell degranulation, regardless of whether the irritant acts through SCCs or directly on the nerve.
Discussion
Our results delineate the mechanism by which chemical activation of nasal epithelial chemosensors, SCCs, can evoke inflammation in the absence of an Ig-mediated response. SCCs use the canonical taste transduction cascade to generate responses to diverse noxious and innocuous airborne or bacterially derived substances. The SCCs then release the neurotransmitter ACh to activate nAChRs on peptidergic trigeminal nerve fibers within the epithelium (Fig. 4). Intramucosal collaterals of these nerve fibers, in turn, release substance P to activate NK1 receptors on the endothelial cells and mast cells, resulting in plasma leakage from the vessels and mast cell degranulation, respectively (Fig. 4). Because plasma extravasation and mast cell degranulation are hallmarks of early inflammatory states, this pathway provides an avenue by which nonallergenic irritants can evoke nasal inflammation.
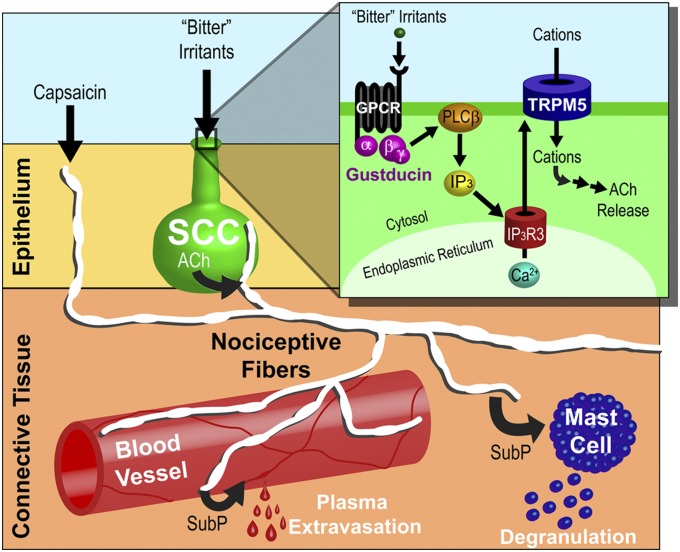
Fig. 4.
Parallel pathways for airway irritation. SCCs (green) respond to bitter substances, such as denatonium and bacterial metabolites, through the canonical taste transduction pathway. SCCs release ACh, which activates nAChRs on nociceptive trigeminal nerve fibers (white) that innervate the SCCs. These nociceptive trigeminal fibers also express TRPV1 and TRPA1, two chemosensitive ion channels that are responsive to irritants, such as capsaicin. Regardless of whether the nociceptive fiber is activated directly through TRP channels or indirectly through SCCs, the collateral terminals release inflammatory mediators. One of these mediators, substance P (SubP), activates NK1 receptors on blood vessels (red), resulting in plasma extravasation, and mast cells (blue), causing degranulation. (Inset) Irritants stimulate G protein-coupled receptors (GPCRs) to activate the βγ-subunit associated with α-gustducin, thereby producing inositiol trisphosphate (IP3) through a phospholipase Cβ2 (PLCβ2) -mediated cascade. In turn, IP3 binds to the type 3 IP3 receptor (IP3R3), releasing Ca2+ from the endoplasmic reticulum. Increases in cytosolic Ca2+ activate TRPM5, a nonspecific cation channel, and lead to depolarization and release of ACh.
Parallel Pathways for Airway Irritation.
In the airway, multiple pathways exist to trigger inflammatory responses to potentially dangerous substances. IgE- (31), nociceptor neural-, and SCC-mediated pathways may initiate a response to different types of irritating substances, but all three converge onto common downstream mechanisms. Additionally, although some elements of these pathways are convergent, portions of each may act independently. The existence of parallel proinflammatory pathways allows for a broadly tuned and redundant warning system for protection of the airway.
The best described airway proinflammatory pathway is IgE-mediated type I immune hypersensitivity, which underlies allergic rhinitis (31). Typically, the xenobiotics that activate the IgE pathway are large macromolecules, such as proteins in pollen (31).
A second proinflammatory pathway involves stimulation of peptidergic nociceptive nerve fibers, which respond directly to diverse irritants through chemosensitive TRP channels. However, trigeminal nociceptive endings, being situated beneath the surface of the epithelium, are limited in the types of noxious compounds to which they can respond. Specifically, lipophobic compounds in the lumen of the nasal cavity are largely incapable of crossing the epithelial barrier to reach the underlying nerve fibers. After they are activated, nociceptive fibers evoke neurogenic inflammation through an axon reflex mechanism—the release of neuropeptides from both the stimulated terminals and collateral branches of the same axon (3). The peptides calcitonin gene-related peptide and substance P released by axon reflex have multiple effects, including sensitization of nociceptors (1, 3), inflammatory edema (Fig. 2F), and mast cell degranulation (Fig. 3F). Substance P, released from peptidergic nociceptors, is capable of causing inflammation through NK1 receptors on blood vessel walls and mast cells (Figs. 2F and 3F) (32). Thus, substance P release links nociceptive nerve fibers to the immune system through mast cells (12, 33).
SCCs form the third proinflammatory pathway of the airway. In contrast to nerve fibers that are beneath the epithelial surface, SCCs extend their apex into the lumen of the nasal cavity. Metabolites and bacterially produced quorum factors activate SCCs through the canonical taste transduction pathway (7). When stimulated by these compounds, SCCs release ACh, which activates peptidergic trigeminal nociceptors through nAChRs (Fig. 2E). Trigeminal sensory neurons express 10 nAChR subunits (α2–α7, α9, and β2–β4) (30, 34). Of these subunits, α3, α4, and α6 are expressed in peptidergic sensory cells (35). In the brush cell-mediated irritant detection system in the trachea, ACh release from brush cells activates α3α5β4 nAChRs on vagal peptidergic nociceptors (17). Which nAChR subunits are involved in the nasal cholinergic responses remains to be clarified.
Having a specialized epithelial cell mechanism for detection of bacterial metabolites in the lumen of the airway allows the trigeminal system to respond to potentially pathogenic bacteria before the bacteria have achieved a sufficient density to transform their phenotype and coalesce into tenacious biofilms (7) likely to cause tissue damage. SCCs are entirely dependent on peptidergic nerve fibers to convey their proinflammatory signal (Figs. 2D and 3D). Activation of the trigeminal system by ACh results in substance P release (Figs. 2 E–G and 3 E and F), which can lead to inflammation and immune system response (12). However, SCCs may also release ACh in a paracellular manner to trigger local defenses in the airway, such as increasing ciliary beat frequency, mucus secretion, and nitric oxide release (36), which occurs independent of trigeminal innervation.
Although the IgE-, neuronal-, and SCC-mediated proinflammatory pathways are parallel, all three converge on blood vessels and mast cells to trigger inflammation. These convergences allow for interactions between the three pathways that could lead to sensitization.
Mast Cells—A Node in the Inflammatory Signaling Cascade.
Mast cell degranulation can underlie many of the symptoms of inflammation by release of signaling molecules (12). All three of the parallel inflammatory pathways of the airway can trigger mast cell degranulation, allowing for modulation or sensitization of inflammatory symptoms (12). Mast cells have been implicated in the recruitment of other immune cells (37–39), and therefore, mast cell degranulation could recruit other elements of the innate immune system to fight invasive bacteria and prevent additional damage to the epithelium. Many of the mediators released from mast cells have a synergistic effect on inflammation, creating positive feedback, which could lead to pathological inflammation (12, 31, 40) related to nonallergic rhinitis (NAR).
SCCs, One Component of the Airway Chemofensor Complex.
Since the initial reports of taste-like chemosensory transduction elements in SCCs, other investigators have reported potential protective roles for similar transduction elements in other cell types of the airway (17, 41–43). For example, stimulation of ciliated cells of the respiratory epithelium in the nose or trachea seems to promote protective mechanisms, such as the production of reactive oxygen species and antimicrobial peptides (41). Similarly, taste receptors in tracheal smooth muscle may also have a protective effect (43, 44). These discoveries have necessitated expansion of our conceptualization of the chemical senses. In recognition of this shifting paradigm, the term chemofensor complex was suggested to refer to chemoreceptors that alert an organism to harmful xenobiotics and toxic chemicals (45).
Multiple elements of the chemofensor complex (45) are capable of detecting a single compound. For example, the quorum-sensing molecule produced by P. aeruginosa, 3-oxo-c12-HSL (46), can activate both SCCs (7) and ciliated respiratory epithelial cells (41, 47). Calcium imaging experiments with dissociated murine nasal epithelium show that 3-oxo-c12-HSL activates SCCs (7). Similar experiments on both human and murine respiratory epithelium show that ciliated cells are also capable of responding to 3-oxo-c12-HSL (41, 47). In contrast to 3-oxo-c12-HSL, denatonium, the model compound used in the present study, seems to activate only SCCs (9) and not ciliated epithelial cells (41). Taken together, these reports indicate that the airway chemofensor complex consists of redundant mechanisms capable of detecting bacterial metabolites. However, although multiple cell types seem capable of responding to bacterial metabolites, only SCCs release ACh to trigger inflammation by activating nociceptive nerve fibers.
SCC Overstimulation—A Possible Pathology for Nonallergic (Idiopathic) Rhinitis?
In the present study, we show that stimulation of SCCs triggers a proinflammatory pathway reflecting common features with idiopathic NAR (11, 48–50). Patients with NAR, similar to patients afflicted with asthma or chronic obstructive pulmonary disease (COPD), report hyperresponsiveness to normally benign odorants (51), including many chemicals that activate SCCs (52). Essentially, our data elucidate a pathway by which commonly encountered chemicals and xenobiotics could lead to a chronic inflammatory state independent of an IgE-mediated mechanism—a prerequisite for any proposed cause of NAR. If SCCs are involved in creating or exacerbating the hyperinflammatory state of NAR, then nicotinic antagonists or NK1 antagonists may provide relief, whereas use of muscarinic antagonists, commonly used in the treatment of asthma, would be ineffective. Even when taken with the important caveat that SCCs have yet to be linked to inflammation in the human airway, our study raises the possibility that similar cells in the humans (8, 36) may be involved in NAR.
Summary.
The current study shows that activation of SCCs in the nasal epithelium of mice can lead to a rapid neurogenic local proinflammatory response. This fast proinflammatory response is potentially a defense mechanism to alert the downstream immune and inflammatory systems of a potential danger. On exposure to an irritant, SCCs release ACh, which activates nAChRs on peptidergic trigeminal nociceptors and in turn, releases substance P. This peptide then activates NK1 receptors to produce inflammatory edema and mast cell degranulation, consistent with the inflammation observed in individuals with chronic NAR.
Materials and Methods
RTx Ablation of TRPV1-Expressing Nerve Terminals.
To eliminate nerve endings containing the TRPV1 receptor, we used an established protocol (27) entailing multiple injections of the potent capsaicin analog, RTx. Mice were anesthetized with isoflurane and injected s.c. with increasing doses of RTx followed by a single final injection of 200 µg/kg RTx 11 d after the initial injections. Histological examination of nasal epithelium and cornea showed elimination of nearly all peptidergic nerve fibers after this treatment (Fig. S1).
Plasma Extravasation.
After anesthetization with 1.0 g/kg urethane (Sigma), mice were exposed to 20 μL saline (control), 1, 3, or 10 mM denatonium (Fig. S1), 2 μM capsaicin, or 300 μM 3-oxo-C12-HSL, all applied dropwise to the right naris. Five minutes after nasal stimulation, mice were injected in the tail vein with 25 mg/kg fluorescent albumin-A555 in saline. Then, 10 min later, they were perfused with saline followed by fixative. The heads were bisected in the midsagittal plane, and the nasal septum was removed. Photos were taken of the left and right nasal turbinates, and fluorescence intensity was quantified using ImageJ. Each experiment was analyzed by a one-way ANOVA and subsequent Tukey HSD test.
Mast Cell Degranulation.
To determine the ratio of degranulated to nondegranulated mast cells, we used toluidine blue staining of nasal epithelia from mice that had been stimulated retronasally by potential irritants. Mice were first anesthetized with 1 g/kg urethane, and a two-way tracheotomy was performed as described previously (7). Denatonium benzoate (10 mM), capsaicin (2 μM), or saline was passed retronasally to stimulate the nasal cavity. After 10 min, mice were perfused, and the respiratory epithelium was dissected free, stained with acidified toluidine blue, and mounted on slides. A mast cell was considered degranulated when five or more granules were observed in the immediate vicinity of the mast cell.
Pharmacology.
Mecamylamine (3 or 6 mg/kg), NK1 receptor antagonist L732138 (5 mg/kg), atropine methyl bromide (1 mg/kg), or compound 48/80 (5 mg/kg) was administered i.p. before anesthesia. SI Materials and Methods has detailed procedures.
Supplementary Material
Acknowledgments
We thank Sukumar Vijayaraghavan for the ChAT-τ-GFP mice and Robert Margolskee for the TRPM5-GFP, TRPM5−/−, and Gustducin−/− mice. Additionally, we thank Jennifer Strafford for statistical advice and Vijay Ramakrishnan and J. John Cohen for commenting on earlier drafts of this manuscript. This work was supported by National Institutes of Health Grants from the National Institute on Deafness and Other Communication Disorders R01DC009820 (to T.E.F.), R03DC012413 (to M.T.), and P30DC04657 (to D. Restrepo).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1402251111/-/DCSupplemental.
References
- 1.Silver W, Roe P, Saunders CJ. Functional neuroanatomy of the upper airway in experimental animals. In: Morris JB, Shusterman D, editors. Toxicology of the Nose and Upper Airways. New York: Informa Healthcare; 2010. pp. 45–64. [Google Scholar]
- 2.Silver WL, Finger TE. The anatomical and electrophysiological basis of peripheral nasal trigeminal chemoreception. Ann N Y Acad Sci. 2009;1170:202–205. doi: 10.1111/j.1749-6632.2009.03894.x. [DOI] [PubMed] [Google Scholar]
- 3.Bryant BP, Silver W. Chemesthesis: The common chemical sense. In: Finger TE, Silver W, Restrepo D, editors. Neurobiology of Taste and Smell. 2nd Ed. New York: Wiley-Liss; 2000. pp. 73–100. [Google Scholar]
- 4.Kobayashi K, et al. Distinct expression of TRPM8, TRPA1, and TRPV1 mRNAs in rat primary afferent neurons with adelta/c-fibers and colocalization with trk receptors. J Comp Neurol. 2005;493(4):596–606. doi: 10.1002/cne.20794. [DOI] [PubMed] [Google Scholar]
- 5.Finger TE, St Jeor VL, Kinnamon JC, Silver WL. Ultrastructure of substance P- and CGRP-immunoreactive nerve fibers in the nasal epithelium of rodents. J Comp Neurol. 1990;294(2):293–305. doi: 10.1002/cne.902940212. [DOI] [PubMed] [Google Scholar]
- 6.Finger TE, et al. Solitary chemoreceptor cells in the nasal cavity serve as sentinels of respiration. Proc Natl Acad Sci USA. 2003;100(15):8981–8986. doi: 10.1073/pnas.1531172100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tizzano M, et al. Nasal chemosensory cells use bitter taste signaling to detect irritants and bacterial signals. Proc Natl Acad Sci USA. 2010;107(7):3210–3215. doi: 10.1073/pnas.0911934107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barham HP, et al. Solitary chemosensory cells and bitter taste receptor signaling in human sinonasal mucosa. Int Forum Allergy Rhinol. 2013;3(6):450–457. doi: 10.1002/alr.21149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gulbransen BD, Clapp TR, Finger TE, Kinnamon SC. Nasal solitary chemoreceptor cell responses to bitter and trigeminal stimulants in vitro. J Neurophysiol. 2008;99(6):2929–2937. doi: 10.1152/jn.00066.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rather LJ. Disturbance of function (functio laesa): The legendary fifth cardinal sign of inflammation, added by Galen to the four cardinal signs of Celsus. Bull N Y Acad Med. 1971;47(3):303–322. [PMC free article] [PubMed] [Google Scholar]
- 11.Rodger IW, Tousignant C, Young D, Savoie C, Chan CC. Neurokinin receptors subserving plasma extravasation in guinea pig airways. Can J Physiol Pharmacol. 1995;73(7):927–931. doi: 10.1139/y95-128. [DOI] [PubMed] [Google Scholar]
- 12.Metcalfe DD, Baram D, Mekori YA. Mast cells. Physiol Rev. 1997;77(4):1033–1079. doi: 10.1152/physrev.1997.77.4.1033. [DOI] [PubMed] [Google Scholar]
- 13.Galli SJ, Nakae S, Tsai M. Mast cells in the development of adaptive immune responses. Nat Immunol. 2005;6(2):135–142. doi: 10.1038/ni1158. [DOI] [PubMed] [Google Scholar]
- 14.Gri G, et al. Mast cell: An emerging partner in immune interaction. Front Immunol. 2012;3:120. doi: 10.3389/fimmu.2012.00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lagunoff D, Martin TW, Read G. Agents that release histamine from mast cells. Annu Rev Pharmacol Toxicol. 1983;23:331–351. doi: 10.1146/annurev.pa.23.040183.001555. [DOI] [PubMed] [Google Scholar]
- 16.Saunders CJ, Reynolds SD, Finger TE. Chemosensory brush cells of the trachea. A stable population in a dynamic epithelium. Am J Respir Cell Mol Biol. 2013;49(2):190–196. doi: 10.1165/rcmb.2012-0485OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krasteva G, et al. Cholinergic chemosensory cells in the trachea regulate breathing. Proc Natl Acad Sci USA. 2011;108(23):9478–9483. doi: 10.1073/pnas.1019418108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krasteva G, et al. Cholinergic chemosensory cells in the auditory tube. Histochem Cell Biol. 2012;137(4):483–497. doi: 10.1007/s00418-012-0911-x. [DOI] [PubMed] [Google Scholar]
- 19.Ogura T, Krosnowski K, Zhang L, Bekkerman M, Lin W. Chemoreception regulates chemical access to mouse vomeronasal organ: Role of solitary chemosensory cells. PLoS ONE. 2010;5(7):e11924. doi: 10.1371/journal.pone.0011924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grybko MJ, et al. A transgenic mouse model reveals fast nicotinic transmission in hippocampal pyramidal neurons. Eur J Neurosci. 2011;33(10):1786–1798. doi: 10.1111/j.1460-9568.2011.07671.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Price TJ, Flores CM. Critical evaluation of the colocalization between calcitonin gene-related peptide, substance P, transient receptor potential vanilloid subfamily type 1 immunoreactivities, and isolectin B4 binding in primary afferent neurons of the rat and mouse. J Pain. 2007;8(3):263–272. doi: 10.1016/j.jpain.2006.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lundberg JM, Brodin E, Hua X, Saria A. Vascular permeability changes and smooth muscle contraction in relation to capsaicin-sensitive substance P afferents in the guinea-pig. Acta Physiol Scand. 1984;120(2):217–227. doi: 10.1111/j.1748-1716.1984.tb00127.x. [DOI] [PubMed] [Google Scholar]
- 23.Baluk P, Nadel JA, McDonald DM. Substance P-immunoreactive sensory axons in the rat respiratory tract: A quantitative study of their distribution and role in neurogenic inflammation. J Comp Neurol. 1992;319(4):586–598. doi: 10.1002/cne.903190408. [DOI] [PubMed] [Google Scholar]
- 24.Baluk P, Thurston G, Murphy TJ, Bunnett NW, McDonald DM. Neurogenic plasma leakage in mouse airways. Br J Pharmacol. 1999;126(2):522–528. doi: 10.1038/sj.bjp.0702323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Caterina MJ, et al. The capsaicin receptor: A heat-activated ion channel in the pain pathway. Nature. 1997;389(6653):816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 26.Lundblad L, Lundberg JM, Brodin E, Anggård A. Origin and distribution of capsaicin-sensitive substance P-immunoreactive nerves in the nasal mucosa. Acta Otolaryngol. 1983;96(5-6):485–493. doi: 10.3109/00016488309132735. [DOI] [PubMed] [Google Scholar]
- 27.Cavanaugh DJ, et al. Restriction of transient receptor potential vanilloid-1 to the peptidergic subset of primary afferent neurons follows its developmental downregulation in nonpeptidergic neurons. J Neurosci. 2011;31(28):10119–10127. doi: 10.1523/JNEUROSCI.1299-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gulbransen B, Silver W, Finger TE. Solitary chemoreceptor cell survival is independent of intact trigeminal innervation. J Comp Neurol. 2008;508(1):62–71. doi: 10.1002/cne.21657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McDonald DM, Thurston G, Baluk P. Endothelial gaps as sites for plasma leakage in inflammation. Microcirculation. 1999;6(1):7–22. [PubMed] [Google Scholar]
- 30.Alimohammadi H, Silver WL. Evidence for nicotinic acetylcholine receptors on nasal trigeminal nerve endings of the rat. Chem Senses. 2000;25(1):61–66. doi: 10.1093/chemse/25.1.61. [DOI] [PubMed] [Google Scholar]
- 31.MacGlashan DW., Jr IgE-dependent signaling as a therapeutic target for allergies. Trends Pharmacol Sci. 2012;33(9):502–509. doi: 10.1016/j.tips.2012.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O’Connor TM, et al. The role of substance P in inflammatory disease. J Cell Physiol. 2004;201(2):167–180. doi: 10.1002/jcp.20061. [DOI] [PubMed] [Google Scholar]
- 33.Gilfillan AM, Tkaczyk C. Integrated signalling pathways for mast-cell activation. Nat Rev Immunol. 2006;6(3):218–230. doi: 10.1038/nri1782. [DOI] [PubMed] [Google Scholar]
- 34.Liu L, Chang GQ, Jiao YQ, Simon SA. Neuronal nicotinic acetylcholine receptors in rat trigeminal ganglia. Brain Res. 1998;809(2):238–245. doi: 10.1016/s0006-8993(98)00862-2. [DOI] [PubMed] [Google Scholar]
- 35.Dussor GO, et al. Potentiation of evoked calcitonin gene-related peptide release from oral mucosa: A potential basis for the pro-inflammatory effects of nicotine. Eur J Neurosci. 2003;18(9):2515–2526. doi: 10.1046/j.1460-9568.2003.02935.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee RJ, et al. Bitter and sweet taste receptors regulate human upper respiratory innate immunity. J Clin Invest. 2014;124(3):1393–1405. doi: 10.1172/JCI72094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dawicki W, Marshall JS. New and emerging roles for mast cells in host defence. Curr Opin Immunol. 2007;19(1):31–38. doi: 10.1016/j.coi.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 38.Nakae S, et al. Mast cells enhance T cell activation: Importance of mast cell-derived TNF. Proc Natl Acad Sci USA. 2005;102(18):6467–6472. doi: 10.1073/pnas.0501912102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Suto H, et al. Mast cell-associated TNF promotes dendritic cell migration. J Immunol. 2006;176(7):4102–4112. doi: 10.4049/jimmunol.176.7.4102. [DOI] [PubMed] [Google Scholar]
- 40.Voedisch S, Rochlitzer S, Veres TZ, Spies E, Braun A. Neuropeptides control the dynamic behavior of airway mucosal dendritic cells. PLoS ONE. 2012;7(9):e45951. doi: 10.1371/journal.pone.0045951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee RJ, et al. T2R38 taste receptor polymorphisms underlie susceptibility to upper respiratory infection. J Clin Invest. 2012;122(11):4145–4159. doi: 10.1172/JCI64240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Krasteva G, Canning BJ, Papadakis T, Kummer W. Cholinergic brush cells in the trachea mediate respiratory responses to quorum sensing molecules. Life Sci. 2012;91(21-22):992–996. doi: 10.1016/j.lfs.2012.06.014. [DOI] [PubMed] [Google Scholar]
- 43.Deshpande DA, et al. Bitter taste receptors on airway smooth muscle bronchodilate by localized calcium signaling and reverse obstruction. Nat Med. 2010;16(11):1299–1304. doi: 10.1038/nm.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang CH, et al. The cellular and molecular basis of bitter tastant-induced bronchodilation. PLoS Biol. 2013;11(3):e1001501. doi: 10.1371/journal.pbio.1001501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Green BG. Chemesthesis and the chemical senses as components of a “chemofensor complex.”. Chem Senses. 2012;37(3):201–206. doi: 10.1093/chemse/bjr119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fuqua C, Greenberg EP. Listening in on bacteria: Acyl-homoserine lactone signalling. Nat Rev Mol Cell Biol. 2002;3(9):685–695. doi: 10.1038/nrm907. [DOI] [PubMed] [Google Scholar]
- 47.Lee RJ, Cohen NA. The emerging role of the bitter taste receptor T2R38 in upper respiratory infection and chronic rhinosinusitis. Am J Rhinol Allergy. 2013;27(4):283–286. doi: 10.2500/ajra.2013.27.3911. [DOI] [PubMed] [Google Scholar]
- 48.Shusterman D. Trigeminally-mediated health effects of air pollutants: Sources of inter-individual variability. Hum Exp Toxicol. 2007;26(3):149–157. doi: 10.1177/0960327107070550. [DOI] [PubMed] [Google Scholar]
- 49.Knipping S, Holzhausen HJ, Riederer A, Schrom T. Allergic and idiopathic rhinitis: An ultrastructural study. Eur Arch Otorhinolaryngol. 2009;266(8):1249–1256. doi: 10.1007/s00405-008-0898-z. [DOI] [PubMed] [Google Scholar]
- 50.Gelardi M, et al. Non-allergic rhinitis with eosinophils and mast cells constitutes a new severe nasal disorder. Int J Immunopathol Pharmacol. 2008;21(2):325–331. doi: 10.1177/039463200802100209. [DOI] [PubMed] [Google Scholar]
- 51.Hargreave FE, Dolovich J, O’Byrne PM, Ramsdale EH, Daniel EE. The origin of airway hyperresponsiveness. J Allergy Clin Immunol. 1986;78(5 Pt 1):825–832. doi: 10.1016/0091-6749(86)90226-5. [DOI] [PubMed] [Google Scholar]
- 52.Lin W, Ogura T, Margolskee RF, Finger TE, Restrepo D. TRPM5-expressing solitary chemosensory cells respond to odorous irritants. J Neurophysiol. 2008;99(3):1451–1460. doi: 10.1152/jn.01195.2007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.