Significance
Searching for drugs to prevent conversion of host-encoded prion protein (PrPC) to its infectious conformation (PrPSc) is a key strategy in the pursuit of therapies for prion disorders: fatal, transmissible epidemic diseases of unpredictable occurrence and uncertain zoonotic potential. Despite quinacrine’s ability to reduce mouse PrPSc in cell models, its use to treat patients has been unsuccessful. Here, we show that quinacrine augments PrPSc and intensifies replication of prions, causing chronic wasting disease of deer, elk, and moose, and that the resulting prions have altered conformational and transmission properties. Our finding, that a drug capable of restraining PrPSc in one species acts to improve replicative ability and induce mutation in another, forces reexamination of current strategies to combat these diseases.
Keywords: prion therapeutics, prion enhancing drugs
Abstract
Quinacrine’s ability to reduce levels of pathogenic prion protein (PrPSc) in mouse cells infected with experimentally adapted prions led to several unsuccessful clinical studies in patients with prion diseases, a 10-y investment to understand its mechanism of action, and the production of related compounds with expectations of greater efficacy. We show here, in stark contrast to this reported inhibitory effect, that quinacrine enhances deer and elk PrPSc accumulation and promotes propagation of prions causing chronic wasting disease (CWD), a fatal, transmissible, neurodegenerative disorder of cervids of uncertain zoonotic potential. Surprisingly, despite increased prion titers in quinacrine-treated cells, transmission of the resulting prions produced prolonged incubation times and altered PrPSc deposition patterns in the brains of diseased transgenic mice. This unexpected outcome is consistent with quinacrine affecting the intrinsic properties of the CWD prion. Accordingly, quinacrine-treated CWD prions were comprised of an altered PrPSc conformation. Our findings provide convincing evidence for drug-induced conformational mutation of prions without the prerequisite of generating drug-resistant variants of the original strain. More specifically, they show that a drug capable of restraining prions in one species/strain setting, and consequently used to treat human prion diseases, improves replicative ability in another and therefore force reconsideration of current strategies to screen antiprion compounds.
Prions are protein-based infectious agents that cause an increasing variety of fatal, infectious neurodegenerative disorders, frequently as epidemics. Variant Creutzfeldt–Jakob disease (CJD) resulting from bovine spongiform encephalopathy exemplifies prion zoonosis, and the continued, unpredictable emergence of additional epidemics in other species, particularly chronic wasting disease (CWD) in deer, elk, and moose, raises additional concerns. Its unprecedented contagious spread, widening host range, and conflicting evidence surrounding its zoonotic potential place CWD at the forefront of public health concerns. Although the notion, that prion replication occurs by corruption of host-encoded cellular prion protein (PrPC) by abnormally conformed PrPSc, is now widely accepted, the details of this mechanism remain enigmatic.
Except under certain experimental conditions, treatments capable of arresting or of effectively modifying the course of disease do not exist for prion disorders. Compounds targeting prevention of PrP misfolding have been aggressively pursued as therapeutics. Cells chronically infected with rodent prions are used as a means either to screen compounds inhibiting PrPSc accumulation (1–5) or to confirm the proposed antiprion effects of compounds discovered by other means (6, 7). Such strategies rest on the assumption that compounds with efficacy against experimentally adapted rodent prions will be effective against naturally occurring prions, in particular those causing human diseases. Exemplifying this approach, quinacrine, a compound that has been repeatedly shown to reduced PrPSc levels in rodent cells chronically infected with experimentally adapted mouse prions (8, 9), was used to treat patients with human prion diseases in several settings (10–15), albeit unsuccessfully.
Our development of cultured cells in which CWD infection is stably and indefinitely maintained (16) provided a unique and convenient means of exploring CWD therapeutics. Our first approach was to assess the efficacy of compounds with known effects on rodent prions. Consistent with its effect on mouse prion infectivity, sustained treatment of Elk21+ cells with dextran sulfate 500 (DS-500) resulted in PrPSc clearance, which did not reemerge in the resulting Elk21− cells after more than 40 passages, and inoculation of susceptible transgenic (Tg) mice showed that Elk21− cells were cured of CWD prion infection (16). The present study was initially designed to assess the effects of quinacrine on CWD. We found that, far from inhibiting PrPSc accumulation and curing infection, quinacrine augmented PrPSc levels and stimulated CWD prion replication in cells expressing elk or deer PrP. We performed additional studies to address the properties of quinacrine-enhanced CWD prions.
Results
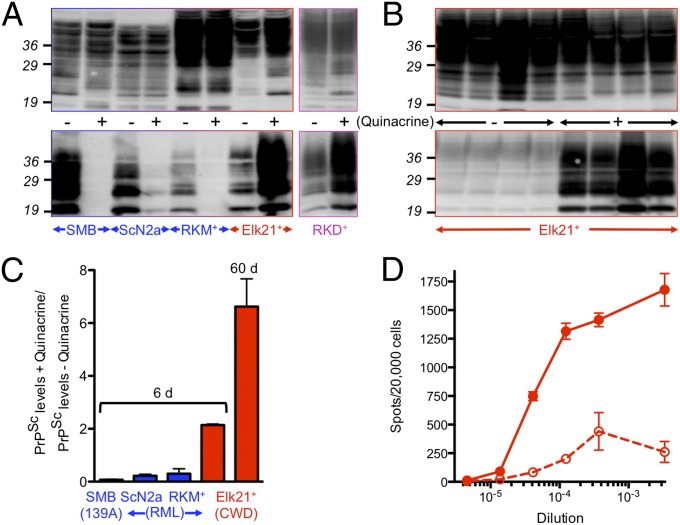
We confirmed previous observations (8, 9, 17–21) that treatment of ScN2A or SMB cells chronically infected with rodent prions with 1 μM quinacrine for 6 d resulted in 5- to 14-fold reductions of PrPSc depending on cell type (n = 3 independent comparisons, P ≤ 0.05 in both cases) (Fig. 1 A and C). Surprisingly, when Elk21+ cells chronically infected with CWD prions (16) were treated with the same amount of quinacrine for the same time, PrPSc levels, assessed by Western blotting, increased by more than twofold compared with untreated Elk21+ (n = 11 comparisons, P ≤ 0.05) (Fig. 1 A and C). To address whether these effects were cell-specific, we produced RK13 cells expressing mouse PrPC persistently infected with mouse RML prions, referred to as RKM+. Treatment of RKM+ with 1 μM quinacrine for 6 d reduced mouse PrPSc levels more than threefold (n = 3 comparisons, P ≤ 0.05) (Fig. 1 A and C). We also produced RK13 cells stably expressing deer PrPC, referred to as RKD, that were readily and stably infected with elk CWD isolate 012–09442 from diseased Tg(DeerPrP)1536+/− mice (22), to produce RKD+ cells. Similar to Elk21+, treatment of RKD+ with 1 μM quinacrine increased PrPSc levels, assessed by Western blotting, by more than twofold compared with untreated RKD+, albeit after longer treatment times (Fig. 1A). Enhancement of PrPSc was more pronounced when Elk21+ were treated for 10 passages (∼60 d) with 1 μM quinacrine, after which time PrPSc levels increased approximately sevenfold (n = 5 comparisons, P ≤ 0.001) (Fig. 1 B and C).
Fig. 1.
Discrepant effects of quinacrine on mouse and cervid PrPSc accumulation and prion replication. (A and B) Equal amounts of proteins from quinacrine treated (+) and untreated (−) cells were either not treated (Upper) or digested with PK to detect PrPSc (Lower). Blots were probed with mAb 6H4. (A) Cells were treated with 1 μM quinacrine for 6 d, except RKD+ were treated for eight passages. (B) Replicate plates of Elk21+ were treated with 1 μM quinacrine for 10 passages compared with untreated cells maintained for the same time. (C) Densitometric analysis of PrPSc levels in quinacrine-treated and untreated SMB (n = 3 pairs), ScN2a (n = 3 pairs), RKM+ (n = 3 pairs), and Elk21+ (n = 11 pairs) for 6 d, or Elk21+ for 60 d (n = 5 pairs). (D) Numbers of prion-infected Elk21+ cells following quinacrine treatment (solid line, filled circles) or no treatment (dashed line, open circles) determined by CPCA. Mouse, elk, or deer PrP indicated in blue, red, and magenta respectively.
We previously developed a cell-based assay for quantifying CWD prions with sensitivity comparable with the CWD bioassay in susceptible Tg mice (16). We used this cervid prion-cell assay (CPCA) to compare levels of CWD prions in Elk21+ with quinacrine-treated cells. Untreated Elk21+ accumulated 1.38 × 106 CPCA units/g whereas treatment with 1 μM quinacrine for 60 d resulted in a greater than 13-fold increase of 1.86 × 107 CPCA units/g (Fig. 1D).
Treatment of Elk21+ with various quinacrine concentrations for 6 d produced a dose-dependent increase in the number of CWD-infected cells compared with untreated cells, when equivalent cell numbers were applied to enzyme-linked immunosorbent spot (ELISpot) plates. A maximal fourfold increase occurred at 1.2 μM (P ≤ 0.0001) (Fig. 2A). We also observed an ∼60% increase in the number of CWD-infected RKD+ after eight passages in the presence of 1 μM quinacrine (n = 4 comparisons, P ≤ 0.0001) (Fig. 2A). In accordance with previous reports describing cytotoxicity or inhibition of cell growth of N2a-derived and SMB cells at drug concentrations ranging from 1.0 μM to ≥2.0 μM (8, 17), we observed lower numbers of adherent cells and significant reductions in cell viability when Elk21+ were treated with quinacrine concentrations of 2 μM and higher (Fig. 2B). During protein-misfolding cyclic amplification (PMCA), quinacrine failed to enhance or inhibit elk PrPSc amplification at these, or higher, drug concentrations (Fig. 2C).
Fig. 2.
Effects of various quinacrine concentrations on CWD propagation and cell viability in Elk21+ and RKD+ cells, and during protein misfolding cyclic amplification. (A) Dose–response effects in Elk21+ (red) treated with quinacrine for 6 d, and RKD+ cells treated with 1 μM quinacrine for 60 d (magenta). (B) Effect of quinacrine on numbers of adherent Elk21+ (left y axis; red line, circles), and cell viability assessed by MTT assay (right y axis; blue line, squares). For each treatment, 16 replicates were assessed. The statistical difference of means was assessed by one-way ANOVA. (C) Densitometric analysis of western blotted PrPSc following PMCA performed in the absence or presence of quinacrine (n = 3 samples for each concentration). Error bars refer to standard errors of the mean (SEM). ns, P > 0.05; *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001; ****P ≤ 0.0001.
To address whether the enhancing effects of quinacrine on PrPSc were permanent, we treated Elk21+ continuously for up to 10 passages and compared PrPSc levels in untreated, continuously treated, and cells maintained for a further five passages after withdrawal of drug. Although PrPSc levels in quinacrine-treated Elk21+ gradually increased compared with untreated cells, after withdrawal of drug, PrPSc returned to original levels within five passages, regardless of the duration of quinacrine treatment (Fig. 3).
Fig. 3.
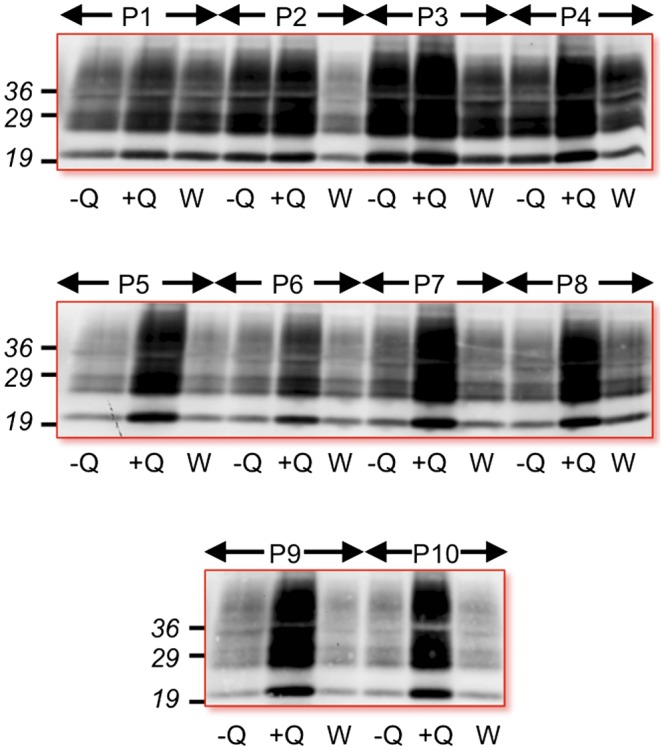
Continuous drug treatment is required to sustain enhanced CWD PrPSc levels in Elk21+. Western blots of PrPSc in Elk21+ treated with 1 μg/mL quinacrine (+Q) for up to 10 passages (P1–P10) compared with untreated cultures (−Q) and treated cells withdrawn from drug and subsequently maintained for a further five passages (W).
We compared the conformational stability of PrPSc produced in Elk21+ exposed to 1 μM quinacrine for 6 d, with that of PrPSc produced in untreated cells. To do so, we established a sensitive, quantitative cell-based conformational stability assay (CSA), referred to as C-CSA, where numbers of PrPSc-producing cells were assessed by ELISpot analysis following exposure to various concentrations of guanidinium hydrochloride (GdnHCl). The C-CSA denaturation curves of PrPSc from untreated and quinacrine-treated Elk21+ were reproducibly different. The 2.58-M concentration of GdnHCl at the midpoint of the sigmoidal transition (GdnHCl1/2) for quinacrine-treated Elk21+ was 13% higher than the 2.29-M value of untreated Elk21+ (n = 16 replicates, P ≤ 0.0001) (Fig. 4A). We confirmed the differences in conformational stabilities of PrPSc produced in the presence and absence of 1 μM quinacrine, by adapting standard procedures used to assess the conformational stabilities of PrPSc in infected brains (23) to our cell-culture system. When we treated cell extracts with increasing GdnHCl concentrations and compared conformational stabilities following proteinase K (PK) treatment of materials applied to PVDF membranes, according to previously published approaches (24, 25), the 1.57-M GdnHCl1/2 of PrPSc isolated from quinacrine-treated Elk21+ reflected a significantly greater conformational stability than the 0.94-M GdnHCl1/2 of PrPSc from untreated Elk21+ (n = 8 replicates, P ≤ 0.0001) (Fig. 4B). We also performed C-CSA on quinacrine-treated and untreated RKD+. In accordance with our previously published results showing differences in the unfolding characteristics of PrPSc from CWD-infected Tg mice expressing elk or deer PrP (26), C-CSA showed that the 3.61-M GdnHCl1/2 of deer PrPSc in RKD+ was considerably higher than the 2.29-M GdnHCl1/2 of elk PrPSc in Elk21+ (P ≤ 0.0001) (Fig. 4 A and B). Consistent with its effect on increasing the stability of elk PrPSc, quinacrine treatment of RKD+ resulted in an additional 10% increase in conformational stability of deer PrPSc (GdnHCl1/2 = 3.97 M; P ≤ 0.0001) (Fig. 4A). We refer to prions formed from the more stable quinacrine-treated conformers as Q-CWD.
Fig. 4.
Quinacrine alters the conformation of elk and deer PrPSc and the transmission properties of elk CWD prions. (A) Conformational stability of elk PrPSc in Elk21+ treated with 1 μg/mL quinacrine (solid line, filled red symbols) or untreated (dashed line, open red symbols) for 6 d (n = 16), assessed by C-CSA. RKD+ were treated with 1 μg/mL quinacrine (sold line, filled magenta symbols) or untreated (dashed line, open magenta symbols) for 60 d (n = 4). (B) Conformational stability of elk PrPSc extracted from Elk21+ treated with 1 μg/mL quinacrine (solid line, filled red symbols) or untreated (dashed line, open red symbols) for 6 d (n = 8), assessed by densitometric analysis of PrPSc applied to dot blots. ****P ≤ 0.0001 refers to differences between GdnHCl1/2 from best-fitted curves. (C) Incubation times of Q-CWD prions are prolonged compared with CWD prions. Times to disease onset in Tg(DeerPrP)1536+/− and Tg(ElkPrP)5037+/− mice (red and magenta lines/symbols, respectively) inoculated with Elk21+ either with 1 μg/mL quinacrine (filled circles) or untreated Elk21+ (open circles). (D) Quinacrine treatment of Tg(ElkPrP)5037+/− mice fails to affect time to onset of CWD. CWD-inoculated Tg(ElkPrP)5037+/− mice orally dosed with quinacrine, filled squares; animals similarly inoculated, but not receiving quinacrine, open squares. ***P ≤ 0.001. Incubation times (mean ± SEM) of the numbers of diseased mice are shown for each transmission.
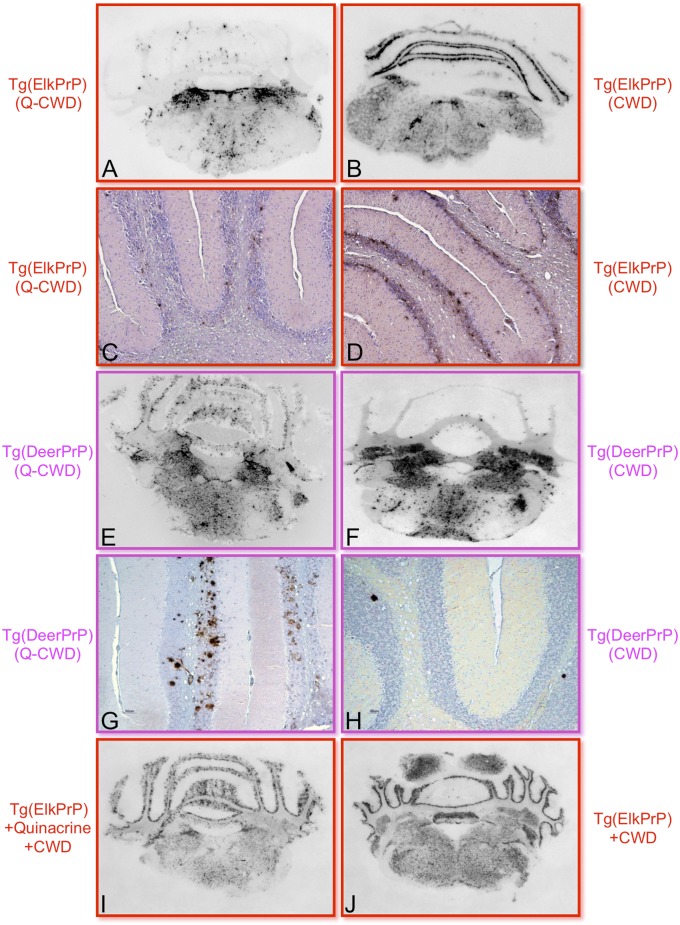
The mean 172 ± 2 d (± SEM) time to disease onset in Tg(ElkPrP)5037+/− mice inoculated with Q-CWD prions from quinacrine-treated Elk21+ was 54% longer than the 112 ± 1 d mean time to disease onset in mice receiving prions from untreated Elk21+ (P ≤ 0.001) (Fig. 4C). Similarly, mean incubation times were 27% longer in Tg(DeerPrP)1536+/− mice inoculated with elk Q-CWD prions (403 ± 12 d compared with 318 ± 10 d, P ≤ 0.001) (Fig. 4C). Immunohistochemical and histoblot analyses revealed reproducibly distinct differences in PrPSc distribution, particularly in the cerebellum of mice receiving elk Q-CWD or elk CWD prions (Fig. 5). Although PrPSc-containing plaques in the granular layer of the cerebellum were observed in Tg(ElkPrP)5037+/− mice receiving Elk21+ extracts that had not been exposed to quinacrine, this feature was not reproduced in Q-CWD–infected Tg(ElkPrP)5037+/− mice. In contrast, although elk Q-CWD prions produced relatively large PrPSc-containing plaques in the molecular layer of the cerebellum in diseased Tg(DeerPrP)1536+/− mice, the same sections of diseased Tg(DeerPrP)1536+/− mice inoculated with untreated Elk21+ extracts contained only occasional deposits in these areas, but relatively intense PrPSc staining in the medulla.
Fig. 5.
Q-CWD prions produce altered patterns of PrPSc deposition compared with untreated CWD prions. PrPSc deposition patterns in the region of the cerebellum assessed by histoblotting (A, B, E, F, I, and J) or immunohistochemistry (C, D, G, and H) in the brains of Tg(ElkPrP)5037+/− mice (A–D, I, and J) or Tg(DeerPrP)1536+/− mice (E–H) inoculated with Elk21+ treated with 1 μg/mL quinacrine (Left) or untreated Elk21+ (Right). (I and J) Histoblots of diseased, CWD-infected Tg(ElkPrP)5037+/− mice either treated (I) or not treated (J) with quinacrine.
To determine whether quinacrine affected disease outcome when directly administered to CWD-infected mice, Tg(ElkPrP)5037+/− mice were orally dosed with 30 mg/kg quinacrine per day, 2 wk before inoculation with elk CWD prions. Animals continued to receive quinacrine daily until manifesting terminal signs of prion disease. Quinacrine-treated mice developed disease with a mean onset of 153 ± 3 d, which was similar to the 150 ± 2 d incubation time in Tg(ElkPrP)5037+/− mice that did not receive drug (Fig. 4D). Levels and distribution of PrPSc in the brains of diseased mice from both groups were indistinguishable. Patterns of deposition in the cerebellum in both groups of mice were similar to Tg(ElkPrP)5037+/− mice infected with prions from untreated Elk21+, but distinct from Tg(ElkPrP)5037+/− mice infected with Q-CWD prions (Fig. 5).
Discussion
In stark contrast to its well-documented inhibitory effects on PrPSc accumulation in cells chronically infected with experimentally adapted rodent prions (8, 9, 17–21), the same range of quinacrine concentrations increased elk and deer PrPSc accumulation in cells persistently infected with CWD prions. This paradoxical dose- and time-dependent enhancement was associated with a greater than order of magnitude increase in CWD prion titers in drug-treated Elk21+. Even though the enhancing effects of 2 μM quinacrine on the number of CWD-infected Elk21+ cells are extremely (P ≤ 0.0001) significant (Fig. 2A), we speculate that the decreases at this and higher concentrations may correspond to detrimental effects of the drug on cell viability.
Although quinacrine is reported to inhibit PMCA of mouse PrPSc (17), it failed to increase levels of CWD PrPSc during PMCA. Augmentation of PrPSc in cells, but not during cell-free amplification, suggests that quinacrine-enhanced CWD propagation depends on processes linked to cellular integrity. The contradictory effects of quinacrine on mouse and CWD prions seem unrelated to effects specific to RK13 cells because we show that quinacrine reduces mouse PrPSc in RK13 cells expressing mouse PrPC infected with RML prions, while improving CWD replication in RK13 cells expressing either elk or deer PrPC. The failure of long-term quinacrine treatment to affect disease outcome in CWD-infected Tg(ElkPrP)5037+/− mice is consistent with previous results showing a similar lack of effect of quinacrine on mouse prions in vivo (17, 27, 28).
Previous studies showed that quinacrine destabilized detergent-resistant membrane domains by redistributing cholesterol, leading to down-regulation of PrP in this compartment (20). Whether quinacrine differentially affects the cellular localization of distinct PrP primary structures remains to be determined. Quinacrine has been shown to bind nonspecifically to stabilize the PrPC conformation (29). Consistent with PrPC stabilization, additional studies indicated that quinacrine confers a structural modification that prevents recombinant PrP oligomerization (30). However, other studies indicate that quinacrine binds to specific residues in the C terminus of recombinant human PrP (31), most prominently tyrosines at residues 225 and 226 and glutamine at 227. Whether the variable effects of quinacrine on mouse and cervid prion propagation result from its differential binding to species-specific PrP primary structures or particular PrP conformations is unclear, but it is worth noting that, whereas human and cervid PrP encode glutamine at residue 227, aspartate occurs at this location in mouse PrP.
The second important aspect of our studies is that quinacrine altered the transmission properties of CWD prions, as well as the biochemical characteristics of the constitutive PrPSc. Despite accumulating significantly higher prion titers, Q-CWD prions from Elk21+ cells produced prolonged incubation times in Tg(DeerPrP)1536+/− and Tg(ElkPrP)5037+/− mice compared with CWD from untreated Elk21+ cells. Because kinetics of disease onset in prion-infected animals is inversely related to the titer of a given prion strain, this unusual outcome is consistent with quinacrine affecting the intrinsic properties of the CWD prion. In accordance with this notion, although the deposition patterns of PrPSc in the brains of diseased Tg(DeerPrP)1536+/− and Tg(ElkPrP)5037+/− mice receiving prions from Elk21+ are concordant with our previously published descriptions following transmissions of naturally occurring or PMCA-generated CWD prions (32), these distinctive patterns were not recapitulated in either line of Tg mice receiving Q-CWD prions. Prion incubation times and neuronal targeting are the biological criteria by which prion strains are defined. Previous studies showed that strain properties are enciphered within the conformation of PrPSc (32, 33) and that cervid prion incubation times positively correlate with PrPSc conformational stability (32). It is therefore significant that the longer incubation times of Q-CWD prions and altered patterns of PrPSc deposition in both Tg models were associated with an increase in the relative stability of PrPSc conformers constituting Q-CWD prions.
The properties of Q-CWD prions described here provide convincing evidence for conformational mutation that was suggested, but could not be observed, in previous studies of mouse-adapted scrapie prions in cell cultures treated with swainsonine, an inhibitor of Golgi α-mannosidase II (34). Because the swainsonine-induced properties of cell-derived mouse prions reverted to their original characteristics when propagated in vivo, and there were no detectable differences in the conformations of PrPSc generated under conditions of swainsonine treatment compared with untreated cells, claims of strain mutation were indirectly based on the differing properties of prions from swainsonine treated and untreated cells using a cell-panel assay. In contrast, we show that CWD and Q-CWD prions are comprised of distinct PrPSc conformers that produce discernable phenotypes in two lines of Tg mice.
Apart from limited investigations in infected cell cultures (35), conformational stability analyses are generally conducted on PrPSc produced in infected brains. This constraint relates, in large part, to relatively low PrPSc levels in infected cell cultures and a paucity of sensitive cell-based analytical approaches. We devised the C-CSA to circumvent these drawbacks. C-CSA provides the advantage of direct in situ detection of PrPSc without isolation or purification and is a quantitative, sensitive, and accurate means of discerning the unfolding characteristics of PrPSc. In accordance with our previous Western blotting-based analyses showing higher conformational stabilities of PrPSc produced in the brains of CWD-infected Tg(DeerPrP)1536+/− mice compared with Tg(ElkPrP)5037+/− mice (26), C-CSA confirmed that the conformation of deer PrPSc in RKD+ was more stable than elk PrPSc in Elk21+. Because Elk21+ and RKD+ propagate CWD prions from the same source (elk CWD isolate 012–09442), we hypothesize that these differences in conformational stability result from the effects of residue 226 (glutamate in elk PrP and glutamine in deer PrP), the sole primary structural difference between elk and deer PrP. Although each method used to ascertain conformational stability, including Western blots of brain material, dot blots of PrPSc extracted from cultured cells, and C-CSA, produces different GdnHCl1/2 values, the effects of both quinacrine treatment and species-specific primary structural differences on deer and elk PrPSc are internally consistent from assay to assay.
Because it inhibited mouse prion propagation in ScN2a cells and traversed the blood–brain barrier, quinacrine was considered to be of promise for treating human prion diseases (36), and several subsequent clinical studies invested considerable efforts to assess its effects in patients. Tragically, in no case did treatment provide discernible benefits (10–15). Possibly related to this failure, studies in mice lacking the multiple drug resistant gene inoculated with RML prions and orally dosed with quinacrine resulted in selection and propagation of quinacrine-resistant prions (28). Similarly, IND24, although effective at prolonging the lifespan of RML-infected mice, also produced drug-resistant prions but had no effect in CJD-infected Tg mice (37). Our findings are distinct from such cases because they show that a compound with demonstrated, albeit transient, efficacy against a strain in one species acts to directly enhance prion replication in a different species and produce prions with altered biological properties without the prerequisite of generating drug-resistant variants of the original strain.
Several practical repercussions arise from quinacrine’s prion-enhancing and mutagenic effects. First, the effects of “antiprion” compounds are clearly species/strain-dependent, in that drugs found to restrain prions in one situation may improve replicative ability in another. Based on initial reports of quinacrine’s effects on mouse prion propagation in cell culture, a plethora of studies were designed to discern the mechanism of its presumed antiprion effects (19, 20, 29–31, 38–45), to assess its pharmacokinetics (21, 36, 42, 46) and to derive similar compounds with improved antiprion efficacy (18, 20, 47–52, 53–59). These studies continue to this day, despite multiple failed clinical studies in patients with human prion diseases (10–15). The findings reported here force a reconsideration of current strategies to screen antiprion drugs. In particular, it seems imperative that cells with susceptibilities to cervid, ovine, bovine, and human prions be used to identify drugs capable of treating prions affecting those species. To date, no such resources have been reported for human prions, and the reasons for this drawback remain enigmatic, given the relatively facile production of cell lines with susceptibilities to ovine, murine, bank vole, and cervid prions.
Second, to the best of our knowledge, the effect of quinacrine on CWD represents the first reported example of a drug capable of facilitating cellular prion replication. On a positive note, our results raise the possibility of using prion-enhancing compounds, in addition to previously described effects of retroviral GAG expression on cellular prion susceptibility (16), as a strategy to heighten the vulnerability of otherwise resistant cell cultures to novel prion infections: for example, to improve cellular susceptibility to human prions.
Materials and Methods
Cell Culture.
SMB and ScN2a cells were cultured in DMEM containing 10% (vol/vol) FBS and 1 μg/mL penicillin/100 U/mL streptomycin. Rabbit kidney epithelial (RK13) cells were obtained from the American Type Culture Collection (Cat. no. CCL-37; ATCC). Mouse, deer, and elk PrP coding sequences were cloned into pIRESpuro3 vector. HIV-1 GAG precursor protein coding sequence was cloned into pcDNA3.1-neomycin or pcDNA3.1-hygromycin. RKD+ expressing deer PrP and HIV-1 GAG were cultured in the presence of 1 μg/mL puromycin and 250 μg/mL hygromycin; Elk21+ were cultured in the presence of puromycin and 1 mg/mL G418; RKM+ were cultured in the presence of puromycin. RK13 cells stably transfected with pIRESpuro3 vector, referred to as RKV, were used as negative controls for prion infections. Cells were passaged at a ratio of 1:10 every 5 d. Quinacrine (Sigma-Aldrich) was dissolved in PBS and filtered with a 0.22-μm filter. Cell culture medium was replaced every other day.
Cell Viability.
A 12-mM solution of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) (Life Technologies) was prepared in PBS. Cell viability was assessed as described previously (60). Cell numbers were assessed with a TC20 Automated Cell Counter (Bio-Rad).
Western Blotting.
Western blotting was performed and analyzed as previously described (24).
Protein Misfolding Cyclic Amplification.
PMCA reactions were performed as described previously (32) at a seed to substrate ratio of 1:4,000, digested with PK at a final concentration of 0.33 μg/μL, and analyzed by Western blotting using anti-PrP mAb 6H4 (Prionics AG) or PRC5 (61).
Cervid Prion Cell Assay.
The CPCA using Elk21− cells was performed as previously described (16), with the following modifications. Images were scanned with CTL Immunospot S6UNIV equipment, and spot numbers were determined using Immunospot 5.0 Analysis and 5.1 Counting software (Cellular Technology).
Cell-Based Conformational Stability Assay.
Twenty thousand cells were filtered onto ELISpot plates (EMD Millipore) and air-dried at 50 °C for 2 h. Cells were denatured with 100 μL of GdnHCl at concentrations ranging from 0 to 3.5 M in 0.25-M increments. Following removal of denaturant, plates were washed twice with PBS, followed by a 5 μg/mL PK digestion for 90 min at 37 °C, and then denaturation in guanidinium thiocyanate. PrPSc-producing cells were detected as described for the CPCA (16), except that a semiautomated approach was used, in which the operator manually adjusted software sensitivity to optimize detection of infected cells at each GdnHCl concentration. Spot numbers were plotted against the concentration of applied guanidine hydrochloride using Prism 6.0d for Mac OS X (GraphPad Software). Datasets were normalized to a common scale, and the sigmoidal dose–response was best fitted by a variable-slope four-parameter algorithm and nonlinear least-squares fit using Prism 6.0d for Mac OS X, with maxima and minima constrained. Results are presented as fractions of the apparent spot counts (Fapp). Statistical differences between curves deriving from the various GdnHCl treatment series were assessed and reported as differences between the concentrations of GdnHCl at the midpoint of the sigmoidal transitions.
Conformational Stability Assay of PrPSc from Cell Extracts.
Cell extracts from quinacrine-treated and untreated Elk21+ cells containing 50 μg and 150 μg of total protein, respectively, were incubated with various concentrations of GdnHCl for 1 h at room temperature as previously described for brain samples (24) except membranes were treated with 1 μg/mL PK, and membranes were blocked with Odyssey Blocking Buffer (LI-COR Biosciences) for 1 h, probed with mAb 6H4 overnight at 4 °C, followed by IRDye 800 CW goat anti-Mouse IgG secondary antibody (LI-COR Biosciences) for 1 h. After three washes with Tris-buffered (pH 7.6) saline and 0.05% Tween 20, the membrane was rinsed with PBS and scanned with the Odyssey CLx infrared imaging system (LI-COR Biosciences), and signals were analyzed with Odyssey CLx Image Studio software (LI-COR Biosciences).
Preparation of Brain and Cell Homogenates.
Preparation of brain and cell homogenates was performed as previously described (24).
In Vivo Studies.
Tg(DeerPrP)1536+/− and Tg(ElkPrP)5037+/− mice have been described previously (22, 62). Tg(ElkPrP)5037+/− mice were orally dosed with quinacrine (30 mg⋅kg−1⋅d−1) by gavage, 2 wk before intracerebral inoculation with elk CWD prions. Animals continued to receive quinacrine daily until manifesting terminal signs of prion disease. Ten percent brain or cell homogenates in PBS lacking calcium and magnesium ions were diluted a further 10-fold before inoculation. Incubation time assays were performed as described (24). Following diagnosis, animals were euthanized by CO2 asphyxiation. All work with animals was performed in compliance with University of Kentucky and Colorado State University Institutional Animal Care and Use Committees.
Histoblotting.
Histoblots were produced and analyzed according to previously described protocols (32). Images were captured with a NikonDMX 1200F digital camera and analyzed using MetaMorph imaging software (Molecular Devices).
Immunohistochemistry.
Immunohistochemistry was performed as previously described (32) using mAb 6H4 as primary antibody and IgG1 biotinylated goat anti-mouse as secondary antibody (SouthernBiotech). Digitized images were obtained by light microscopy using a Nikon Eclipse E600 microscope equipped with a Nikon DMX 1200F digital camera.
Acknowledgments
The authors acknowledge technical assistance from Tanya Seward and Dana Napier. We thank Dr. Charles Merrill for critically reading the manuscript and providing advice for improvements. We thank Prionics for mAb 6H4. This work was supported by National Institutes of Health Grants R01 NS040334 and P01 AI077774.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Lu D, et al. Biaryl amides and hydrazones as therapeutics for prion disease in transgenic mice. J Pharmacol Exp Ther. 2013;347(2):325–338. doi: 10.1124/jpet.113.205799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li Z, et al. Discovery and preliminary SAR of arylpiperazines as novel, brainpenetrant antiprion compounds. ACS Med Chem Letters. 2013;4(4):397–401. doi: 10.1021/ml300472n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kimata A, et al. New series of antiprion compounds: Pyrazolone derivatives have the potent activity of inhibiting protease-resistant prion protein accumulation. J Med Chem. 2007;50(21):5053–5056. doi: 10.1021/jm070688r. [DOI] [PubMed] [Google Scholar]
- 4.Geissen M, et al. From high-throughput cell culture screening to mouse model: Identification of new inhibitor classes against prion disease. ChemMedChem. 2011;6(10):1928–1937. doi: 10.1002/cmdc.201100119. [DOI] [PubMed] [Google Scholar]
- 5.Ghaemmaghami S, May BC, Renslo AR, Prusiner SB. Discovery of 2-aminothiazoles as potent antiprion compounds. J Virol. 2010;84(7):3408–3412. doi: 10.1128/JVI.02145-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nicoll AJ, et al. Pharmacological chaperone for the structured domain of human prion protein. Proc Natl Acad Sci USA. 2010;107(41):17610–17615. doi: 10.1073/pnas.1009062107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kuwata K, et al. Hot spots in prion protein for pathogenic conversion. Proc Natl Acad Sci USA. 2007;104(29):11921–11926. doi: 10.1073/pnas.0702671104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Doh-Ura K, Iwaki T, Caughey B. Lysosomotropic agents and cysteine protease inhibitors inhibit scrapie-associated prion protein accumulation. J Virol. 2000;74(10):4894–4897. doi: 10.1128/jvi.74.10.4894-4897.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Korth C, May BC, Cohen FE, Prusiner SB. Acridine and phenothiazine derivatives as pharmacotherapeutics for prion disease. Proc Natl Acad Sci USA. 2001;98(17):9836–9841. doi: 10.1073/pnas.161274798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mead S, et al. PRION-1 scales analysis supports use of functional outcome measures in prion disease. Neurology. 2011;77(18):1674–1683. doi: 10.1212/WNL.0b013e3182364890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Collinge J, et al. Safety and efficacy of quinacrine in human prion disease (PRION-1 study): A patient-preference trial. Lancet Neurol. 2009;8(4):334–344. doi: 10.1016/S1474-4422(09)70049-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Benito-León J. Combined quinacrine and chlorpromazine therapy in fatal familial insomnia. Clin Neuropharmacol. 2004;27(4):201–203. doi: 10.1097/01.wnf.0000134853.36429.0e. [DOI] [PubMed] [Google Scholar]
- 13.Nakajima M, et al. Results of quinacrine administration to patients with Creutzfeldt-Jakob disease. Dement Geriatr Cogn Disord. 2004;17(3):158–163. doi: 10.1159/000076350. [DOI] [PubMed] [Google Scholar]
- 14.Kobayashi Y, Hirata K, Tanaka H, Yamada T. [Quinacrine administration to a patient with Creutzfeldt-Jakob disease who received a cadaveric dura mater graft: An EEG evaluation] Rinsho Shinkeigaku. 2003;43(7):403–408. [PubMed] [Google Scholar]
- 15.Geschwind MD, et al. Quinacrine treatment trial for sporadic Creutzfeldt-Jakob disease. Neurology. 2013;81(23):2015–2023. doi: 10.1212/WNL.0b013e3182a9f3b4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bian J, et al. Cell-based quantification of chronic wasting disease prions. J Virol. 2010;84(16):8322–8326. doi: 10.1128/JVI.00633-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barret A, et al. Evaluation of quinacrine treatment for prion diseases. J Virol. 2003;77(15):8462–8469. doi: 10.1128/JVI.77.15.8462-8469.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mays CE, et al. Prion inhibition with multivalent PrPSc binding compounds. Biomaterials. 2012;33(28):6808–6822. doi: 10.1016/j.biomaterials.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 19.Fasano C, et al. Gene expression profile of quinacrine-cured prion-infected mouse neuronal cells. J Neurochem. 2008;105(1):239–250. doi: 10.1111/j.1471-4159.2007.05140.x. [DOI] [PubMed] [Google Scholar]
- 20.Klingenstein R, et al. Tricyclic antidepressants, quinacrine and a novel, synthetic chimera thereof clear prions by destabilizing detergent-resistant membrane compartments. J Neurochem. 2006;98(3):748–759. doi: 10.1111/j.1471-4159.2006.03889.x. [DOI] [PubMed] [Google Scholar]
- 21.Ryou C, et al. Differential inhibition of prion propagation by enantiomers of quinacrine. Lab Invest. 2003;83(6):837–843. doi: 10.1097/01.lab.0000074919.08232.a2. [DOI] [PubMed] [Google Scholar]
- 22.Browning SR, et al. Transmission of prions from mule deer and elk with chronic wasting disease to transgenic mice expressing cervid PrP. J Virol. 2004;78(23):13345–13350. doi: 10.1128/JVI.78.23.13345-13350.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peretz D, et al. Strain-specified relative conformational stability of the scrapie prion protein. Protein Sci. 2001;10(4):854–863. doi: 10.1110/ps.39201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saijo E, et al. Epigenetic dominance of prion conformers. PLoS Pathog. 2013;9(10):e1003692. doi: 10.1371/journal.ppat.1003692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Makarava N, et al. Genesis of mammalian prions: From non-infectious amyloid fibrils to a transmissible prion disease. PLoS Pathog. 2011;7(12):e1002419. doi: 10.1371/journal.ppat.1002419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Angers RC, et al. Prion strain mutation determined by prion protein conformational compatibility and primary structure. Science. 2010;328(5982):1154–1158. doi: 10.1126/science.1187107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Collins SJ, et al. Quinacrine does not prolong survival in a murine Creutzfeldt-Jakob disease model. Ann Neurol. 2002;52(4):503–506. doi: 10.1002/ana.10336. [DOI] [PubMed] [Google Scholar]
- 28.Ghaemmaghami S, et al. Continuous quinacrine treatment results in the formation of drug-resistant prions. PLoS Pathog. 2009;5(11):e1000673. doi: 10.1371/journal.ppat.1000673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kamatari YO, Hayano Y, Yamaguchi K, Hosokawa-Muto J, Kuwata K. Characterizing antiprion compounds based on their binding properties to prion proteins: Implications as medical chaperones. Protein Sci. 2013;22(1):22–34. doi: 10.1002/pro.2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Georgieva D, et al. Interactions of recombinant prions with compounds of therapeutical significance. Biochem Biophys Res Commun. 2006;344(2):463–470. doi: 10.1016/j.bbrc.2006.03.135. [DOI] [PubMed] [Google Scholar]
- 31.Vogtherr M, et al. Antimalarial drug quinacrine binds to C-terminal helix of cellular prion protein. J Med Chem. 2003;46(17):3563–3564. doi: 10.1021/jm034093h. [DOI] [PubMed] [Google Scholar]
- 32.Green KM, et al. Accelerated high fidelity prion amplification within and across prion species barriers. PLoS Pathog. 2008;4(8):e1000139. doi: 10.1371/journal.ppat.1000139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Telling GC, et al. Evidence for the conformation of the pathologic isoform of the prion protein enciphering and propagating prion diversity. Science. 1996;274(5295):2079–2082. doi: 10.1126/science.274.5295.2079. [DOI] [PubMed] [Google Scholar]
- 34.Li J, Browning S, Mahal SP, Oelschlegel AM, Weissmann C. Darwinian evolution of prions in cell culture. Science. 2010;327(5967):869–872. doi: 10.1126/science.1183218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ghaemmaghami S, et al. Conformational transformation and selection of synthetic prion strains. J Mol Biol. 2011;413(3):527–542. doi: 10.1016/j.jmb.2011.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yung L, et al. Pharmacokinetics of quinacrine in the treatment of prion disease. BMC Infect Dis. 2004;4:53. doi: 10.1186/1471-2334-4-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Berry DB, et al. Drug resistance confounding prion therapeutics. Proc Natl Acad Sci USA. 2013;110(44):E4160–E4169. doi: 10.1073/pnas.1317164110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zawada Z, et al. Quinacrine reactivity with prion proteins and prion-derived peptides. Amino Acids. 2013;44(5):1279–1292. doi: 10.1007/s00726-013-1460-x. [DOI] [PubMed] [Google Scholar]
- 39.Dearmond SJ, Bajsarowicz K. PrPSc accumulation in neuronal plasma membranes links Notch-1 activation to dendritic degeneration in prion diseases. Mol Neurodegener. 2010;5:6. doi: 10.1186/1750-1326-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Spilman P, et al. A gamma-secretase inhibitor and quinacrine reduce prions and prevent dendritic degeneration in murine brains. Proc Natl Acad Sci USA. 2008;105(30):10595–10600. doi: 10.1073/pnas.0803671105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huang Y, et al. Quinacrine is mainly metabolized to mono-desethyl quinacrine by CYP3A4/5 and its brain accumulation is limited by P-glycoprotein. Drug Metab Dispos. 2006;34(7):1136–1144. doi: 10.1124/dmd.105.008664. [DOI] [PubMed] [Google Scholar]
- 42.Gayrard V, et al. A possible pharmacological explanation for quinacrine failure to treat prion diseases: Pharmacokinetic investigations in a ovine model of scrapie. Br J Pharmacol. 2005;144(3):386–393. doi: 10.1038/sj.bjp.0706072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sandberg MK, Wallén P, Wikström MA, Kristensson K. Scrapie-infected GT1-1 cells show impaired function of voltage-gated N-type calcium channels (Ca(v) 2.2) which is ameliorated by quinacrine treatment. Neurobiol Dis. 2004;15(1):143–151. doi: 10.1016/j.nbd.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 44.Farrelly PV, et al. Quinacrine blocks PrP (106-126)-formed channels. J Neurosci Res. 2003;74(6):934–941. doi: 10.1002/jnr.10849. [DOI] [PubMed] [Google Scholar]
- 45.Turnbull S, Tabner BJ, Brown DR, Allsop D. Quinacrine acts as an antioxidant and reduces the toxicity of the prion peptide PrP106-126. Neuroreport. 2003;14(13):1743–1745. doi: 10.1097/00001756-200309150-00017. [DOI] [PubMed] [Google Scholar]
- 46.Dohgu S, et al. Uptake and efflux of quinacrine, a candidate for the treatment of prion diseases, at the blood-brain barrier. Cell Mol Neurobiol. 2004;24(2):205–217. doi: 10.1023/B:CEMN.0000018617.21378.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chung E, et al. Styryl-based and tricyclic compounds as potential anti-prion agents. PLoS ONE. 2011;6(9):e24844. doi: 10.1371/journal.pone.0024844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nguyen TH, et al. Antiprion activity of functionalized 9-aminoacridines related to quinacrine. Bioorg Med Chem. 2008;16(14):6737–6746. doi: 10.1016/j.bmc.2008.05.060. [DOI] [PubMed] [Google Scholar]
- 49.Nguyen T, Sakasegawa Y, Doh-Ura K, Go ML. Anti-prion activities and drug-like potential of functionalized quinacrine analogs with basic phenyl residues at the 9-amino position. Eur J Med Chem. 2011;46(7):2917–2929. doi: 10.1016/j.ejmech.2011.04.016. [DOI] [PubMed] [Google Scholar]
- 50.Villa V, et al. Efficacy of novel acridine derivatives in the inhibition of hPrP90-231 prion protein fragment toxicity. Neurotox Res. 2011;19(4):556–574. doi: 10.1007/s12640-010-9189-8. [DOI] [PubMed] [Google Scholar]
- 51.Tran HN, Bongarzone S, Carloni P, Legname G, Bolognesi ML. Synthesis and evaluation of a library of 2,5-bisdiamino-benzoquinone derivatives as probes to modulate protein-protein interactions in prions. Bioorg Med Chem Lett. 2010;20(6):1866–1868. doi: 10.1016/j.bmcl.2010.01.149. [DOI] [PubMed] [Google Scholar]
- 52.Csuk R, et al. Synthesis of monomeric and dimeric acridine compounds as potential therapeutics in Alzheimer and prion diseases. Arch Pharm (Weinheim) 2009;342(12):699–709. doi: 10.1002/ardp.200900065. [DOI] [PubMed] [Google Scholar]
- 53.Doh-ura K, et al. Chelating compound, chrysoidine, is more effective in both antiprion activity and brain endothelial permeability than quinacrine. Cell Mol Neurobiol. 2007;27(3):303–316. doi: 10.1007/s10571-006-9122-0. [DOI] [PubMed] [Google Scholar]
- 54.Dollinger S, Löber S, Klingenstein R, Korth C, Gmeiner P. A chimeric ligand approach leading to potent antiprion active acridine derivatives: Design, synthesis, and biological investigations. J Med Chem. 2006;49(22):6591–6595. doi: 10.1021/jm060773j. [DOI] [PubMed] [Google Scholar]
- 55.Sebestík J, Safarík M, Stibor I, Hlavácek J. Acridin-9-yl exchange: A proposal for the action of some 9-aminoacridine drugs. Biopolymers. 2006;84(6):605–614. doi: 10.1002/bip.20590. [DOI] [PubMed] [Google Scholar]
- 56.Kocisko DA, Caughey B. Mefloquine, an antimalaria drug with antiprion activity in vitro, lacks activity in vivo. J Virol. 2006;80(2):1044–1046. doi: 10.1128/JVI.80.2.1044-1046.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Murakami-Kubo I, et al. Quinoline derivatives are therapeutic candidates for transmissible spongiform encephalopathies. J Virol. 2004;78(3):1281–1288. doi: 10.1128/JVI.78.3.1281-1288.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.May BC, et al. Potent inhibition of scrapie prion replication in cultured cells by bis-acridines. Proc Natl Acad Sci USA. 2003;100(6):3416–3421. doi: 10.1073/pnas.2627988100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.May BC, et al. Structure-activity relationship study of 9-aminoacridine compounds in scrapie-infected neuroblastoma cells. Bioorg Med Chem Lett. 2006;16(18):4913–4916. doi: 10.1016/j.bmcl.2006.06.050. [DOI] [PubMed] [Google Scholar]
- 60.Carmichael J, DeGraff WG, Gazdar AF, Minna JD, Mitchell JB. Evaluation of a tetrazolium-based semiautomated colorimetric assay: Assessment of radiosensitivity. Cancer Res. 1987;47(4):943–946. [PubMed] [Google Scholar]
- 61.Kang HE, et al. Characterization of conformation-dependent prion protein epitopes. J Biol Chem. 2012;287(44):37219–37232. doi: 10.1074/jbc.M112.395921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Angers RC, et al. Chronic wasting disease prions in elk antler velvet. Emerg Infect Dis. 2009;15(5):696–703. doi: 10.3201/eid1505.081458. [DOI] [PMC free article] [PubMed] [Google Scholar]