Significance
Blood platelets, the small circulating cells that coordinate hemostasis, are produced by specialized bone marrow cells called megakaryocytes. The cytokine thrombopoietin (TPO) is a key regulator of platelet production acting via its specific cell receptor, Mpl. Via genetic modification of the Mpl allele in mice, we precisely define the bone marrow cells that express Mpl and, by genetically removing Mpl from megakaryocytes and platelets, we show TPO signaling via Mpl is not required in megakaryocytes for their expansion, maturation, or platelet production. Rather, Mpl expression on megakaryocytes is essential for regulating TPO availability in the bone marrow microenvironment to prevent myeloproliferation, a model we suggest is important for human disease.
Keywords: bone marrow, essential thrombocythemia
Abstract
Thrombopoietin (TPO) acting via its receptor, the cellular homologue of the myeloproliferative leukemia virus oncogene (Mpl), is the major cytokine regulator of platelet number. To precisely define the role of specific hematopoietic cells in TPO-dependent hematopoiesis, we generated mice that express the Mpl receptor normally on stem/progenitor cells but lack expression on megakaryocytes and platelets (MplPF4cre/PF4cre). MplPF4cre/PF4cre mice displayed profound megakaryocytosis and thrombocytosis with a remarkable expansion of megakaryocyte-committed and multipotential progenitor cells, the latter displaying biological responses and a gene expression signature indicative of chronic TPO overstimulation as the underlying causative mechanism, despite a normal circulating TPO level. Thus, TPO signaling in megakaryocytes is dispensable for platelet production; its key role in control of platelet number is via generation and stimulation of the bipotential megakaryocyte precursors. Nevertheless, Mpl expression on megakaryocytes and platelets is essential to prevent megakaryocytosis and myeloproliferation by restricting the amount of TPO available to stimulate the production of megakaryocytes from the progenitor cell pool.
Thrombopoietin (TPO) is the principal hematopoietic cytokine that regulates platelet production at steady state and is required for rapid responses to platelet loss. TPO acts by binding to a specific cell surface receptor, the cellular homologue of the myeloproliferative leukemia virus oncogene (Mpl), leading to receptor dimerization, activation of intracellular signal transduction pathways, and responses of target cells. Mice lacking TPO or Mpl are severely thrombocytopenic and deficient in megakaryocytes and their progenitor cells, a phenotype consistent with a role for TPO in maintaining appropriate megakaryocyte numbers in vivo. In addition to its role in megakaryopoiesis, TPO is also an indispensible regulator of hematopoietic stem cells (HSC), essential for maintenance of quiescence and self-renewal (1).
TPO is produced primarily in the liver (2) and upon binding to the Mpl receptor on target cells, is internalized and degraded. The prevailing model posits that circulating TPO concentration is inversely proportional to the “Mpl mass” contributed by the total number of megakaryocytes and platelets. In normal individuals, this model describes an effective feedback system to regulate TPO-driven megakaryocyte and platelet production according to need. The reciprocal relationship between platelet number and circulating TPO level is clearly evident in bone marrow transplant patients (1), and the key role of the TPO receptor is illustrated by the elevated circulating TPO in Mpl−/− mice (3) and the modest elevation of platelet counts in transgenic mice expressing low levels of Mpl (4, 5). However, the relationship between circulating TPO concentration and peripheral platelet counts is not always conserved in pathological states of thrombocytosis and thrombocytopenia (6–9), suggesting that a simple relationship among megakaryocyte and platelet Mpl mass, circulating TPO concentration, and the degree of stimulation of megakaryopoiesis may not always hold.
Whereas expression of Mpl on megakaryocytes and platelets contributes to regulation of available TPO, the role of direct TPO stimulation of megakaryocytes for effective platelet production is unclear. Administration of TPO in vivo or stimulation of bone marrow in vitro elevates megakaryocyte numbers and increases mean DNA ploidy (10, 11), and the thrombocytopenia in TPO−/− mice is accompanied by reduced megakaryocyte ploidy (12). However, although exposure of megakaryocytes to TPO stimulates intracellular signaling (13), in vitro studies suggesting direct action of TPO on megakaryocytes to increase DNA ploidy, promote cytoplasmic maturation, or to stimulate proplatelet production (14–16) are balanced by reports that TPO is dispensable for these megakaryocyte functions (15, 17–19).
To comprehensively define Mpl-expressing stem and progenitor cell populations in vivo and resolve the specific requirements for Mpl expression in megakaryocytes and platelets for platelet production and feedback control of TPO levels, we generated a mouse strain in which green fluorescent protein (GFP) is expressed from the Mpl locus and, when crossed to Platelet Factor 4(PF4)cre transgenic mice (20), specifically lacks Mpl expression in megakaryocytes and platelets.
Results
A targeting vector was constructed for generation, via homologous recombination in embryonic stem cells, of a modified Mpl allele (Mplfl) designed to retain Mpl expression and provide a green fluorescent protein (GFP) reporter for transcriptional activity of the Mpl locus. The inclusion of loxP sites allowed for cre recombinase-mediated deletion of the GFP reporter as well as exons 11 and 12, resulting in a null allele (Fig. 1A and SI Appendix, SI Materials and Methods). PF4-cre transgenic mice (20) have been reported to allow specific and efficient deletion of conditional alleles in megakaryocytes and platelets. To verify the activity and cell-type specificity of PF4-cre mice, particularly in view of a recent report of activity in HSCs (21), we crossed PF4-cre mice to mice in which enhanced yellow fluorescent protein (EYFP) is expressed in a cre-dependent manner (Rosa26EYFP; ref. 22). Whereas, as expected, EYFP was not expressed in the absence of cre recombinase, in Rosa26EYFPPF4Cre mice, EYFP was expressed in megakaryocytes and platelets, consistent with previous reports (20) and not in erythroid, lymphoid, or myeloid cells (SI Appendix, Fig. S1A). Megakaryocyte formation progresses from a series of TPO-responsive bipotential erythroid-megakaryocyte progenitors (23). EYFP was not expressed in lineage-negative Sca-1+ Kit+ (LSK) bone marrow cells, the population containing stem and primitive multipotential progenitor cells, nor in any progenitor cell fraction tested, including the BEMP and CD150+CD9hi megakaryocyte/erythroid-restricted bipotential megakaryocyte-erythroid progenitor population and CFU-E erythroid progenitor population (SI Appendix, Table S1 and Fig. S1 B and C). EYFP expression was observed in a proportion of cells in a previously uncharacterized CD150+FcγR+ population (Lin−Sca-1−Kit+CD150+IL7Rα−FcγRII/III+EndoglinloCD9hi; SI Appendix, Fig. S1 B and C) that comprised ∼0.01% of the bone marrow (SI Appendix, Fig. S2A) and which demonstrated potent erythro-megakaryocytic potential in vitro and in vivo (SI Appendix, Fig. S2 B and C). We conclude that the CD150+FcγR+ fraction includes megakaryocyte precursors downstream from the previously characterized CD150+CD9hi fraction, revealed by the onset of PF4 promoter activity that, given the lack of YFP expression in CFU-E in Rosa26EYFPPF4Cre mice, may define a megakaryocyte-restricted precursor within the bipotential CD150+FcγR+ fraction. Thus, cre-dependent recombination using the PF4-cre transgenic mouse was restricted to late megakaryocyte progenitors, megakaryocytes, and platelets and absent in other progenitor cells and hematopoietic lineages.
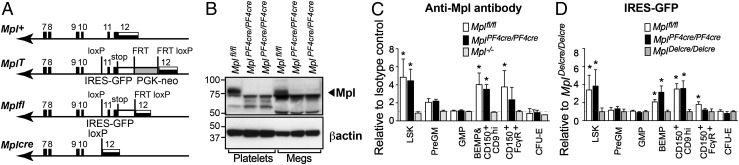
Fig. 1.
Targeted modification of the Mpl locus in mice and Mpl expression in MplPF4cre/PF4cre mice. (A) Generation of a GFP reporter allele of Mpl expression that also allows conditional, cre-mediated Mpl inactivation. Mpl+, wild type Mpl allele. Exons 7–12 (filled boxes), 3′ untranslated region of exon 12 (striped). MplT, targeting vector incorporated into the Mpl locus. IRES-GFP cassette (white), PGK-neo selection cassette (shaded). Mplfl, GFP reporter for expression from the Mpl locus. PGK-neo excised by intercrossing with Flp recombinase transgenic mice. Mplcre, Mpl null allele generated via cre-mediated excision. (B) Western blot of protein extracted from platelets (100 μg) and megakaryocytes (Megs, 188 μg) pooled from two to three independent genotype-matched Mplfl/fl and MplPF4cre/PF4cre mice per lane. (C) The mean fluorescence intensity of Mpl expression on stem and progenitor cell populations from Mplfl/fl, MplPF4cre/PF4cre and Mpl−/− mice is shown relative to the isotype control (SI Appendix, Fig. S3C). Mean and SD shown, n = 4 mice per genotype. *P < 0.005 by two-tailed Student t test. (D) Transcriptional activity of the Mpl locus via expression of the IRES-GFP cassette in stem and progenitor populations. GFP mean fluorescence intensity from Mplfl/fl and MplPF4cre/PF4cre cells is shown relative to MplDelcre/Delcre mice (SI Appendix, Fig. S3D). Mean and SD shown, n = 3–8 mice per genotype. *P < 0.002.
Mpl Expression Is Absent in Megakaryocytes and Platelets, but Retained in Megakaryocyte Progenitor and Stem Cells in MplPF4cre/PF4cre Mice.
To investigate the physiological effects of Mpl deletion on megakaryocytes and platelets, we crossed Mplfl/fl mice to PF4cre mice to produce progeny in which the intracellular domain of Mpl and the IRES-GFP cassette had been specifically deleted in megakaryocytes and platelets (MplPF4cre/PF4cre). Western blot analysis of extracts from megakaryocytes and platelets demonstrated efficient ablation of Mpl expression in MplPF4cre/PF4cre mice (Fig. 1B). This finding was confirmed by analysis of Mpl-GFP reporter expression: although megakaryocytes generated in culture from Mplfl/fl mice expressed GFP, GFP expression was lost in MplPF4cre/PF4cre mice, consistent with recombination and deletion of the targeted Mplfl intracellular domain and IRES-GFP cassette in these cells (SI Appendix, Fig. S3A and Fig. 1A) and, consequently, absence of GFP in platelets (SI Appendix, Fig. S3B). As anticipated, no activity of the Mpl locus was evident in lymphocytes, granulocytes, or erythroid cells (SI Appendix, Fig. S3B). In LSK cells, MplPF4cre/PF4cre mice expressed Mpl at equivalent levels to Mplfl/fl controls, both via flow cytometry with anti-Mpl antibody and via the Mpl-GFP reporter (Fig. 1 C and D and SI Appendix, Fig. S3 C and D). As expected, Mpl expression was not detected in pregranulocyte-macrophage (preGM) or granulocyte-macrophage (GMP) progenitor cells, nor in CFU-E. In Mplfl/fl control megakaryocyte progenitor cells, Mpl expression first became apparent in BEMP and was maintained in maturing CD150+CD9hi and CD150+FcγR+ progenitor cells. As expected, Mpl was not detected in any cell populations examined in previously generated Mpl−/− mice (24), nor via the Mpl-GFP reporter in MplDelcre/Delcre control mice, in which Mplfl/fl mice were crossed to Deleter-cre transgenic mice (25) to generate mice in which the Mplfl locus including the IRES-GFP reporter cassette were deleted throughout the animal. Importantly, Mpl expression was unperturbed in MplPF4cre/PF4cre BEMP and CD150+CD9hi megakaryocyte-biased bipotential progenitors, but reduction in Mpl IRES-GFP transcription and cell surface Mpl expression was apparent in MplPF4cre/PF4cre CD150+FcγR+ megakaryocyte precursors.
Thus, in MplPF4cre/PF4cre mice, Mpl expression was intact in LSK and early bipotential erythro-megakaryocytic progenitor cells, reduced in later progenitors with megakaryocyte potential, and absent in megakaryocytes and platelets. The pattern of ablation of Mpl expression in MplPF4cre/PF4cre mice was entirely consistent with the activity of PF4-cre defined in Rosa26EYFPPF4Cre mice.
MplPF4cre/PF4cre Mice Develop a Marked Thrombocytosis and Megakaryocytosis.
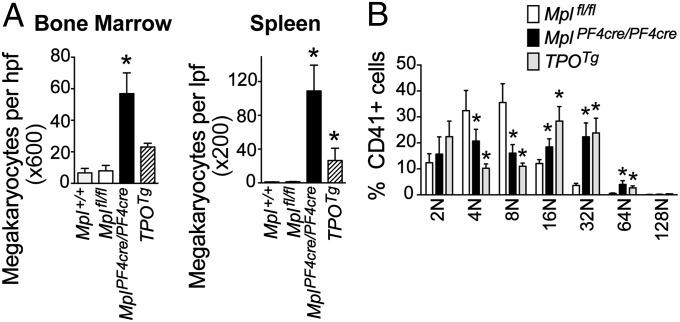
Whereas MplPF4cre/PF4cre mice were healthy and displayed no outward abnormalities, analysis of peripheral blood (Table 1) revealed a remarkable thrombocytosis with a 10-fold increase in the number of circulating platelets in comparison with Mplfl/fl or Mpl+/+ controls. In contrast, and as expected, MplDelcre/Delcre exhibited a profound thrombocytopenia identical in phenotype to previously reported Mpl−/− mice (24). In contrast, the numbers of other blood cells in MplPF4cre/PF4cre mice were not significantly different from Mplfl/fl or Mpl+/+ controls. Significant splenomegaly was evident in MplPF4cre/PF4cre mice (MplPF4cre/PF4cre, 210 ± 46 mg; Mplfl/fl, 76 ± 15 mg; n = 4 per genotype, P = 0.0012) with histology revealing gross megakaryocytosis in the bone marrow and spleen, with the numbers of megakaryocytes in these organs even greater than observed in transgenic mice (TPOTg; ref. 3) engineered to express high amounts of TPO (Fig. 2A and SI Appendix, Fig. S4A). Both MplPF4cre/PF4cre and TPOTg mice had a significant increase in the proportion of megakaryocytes with ploidy of 16N or greater relative to Mplfl/fl controls (Fig. 2B). Clonogenic assays of bone marrow and spleen cells revealed a profound increase in the numbers of megakaryocyte colony-forming cells in MplPF4cre/PF4cre mice (Table 2). The profound thrombocytosis in MplPF4cre/PF4cre was not accompanied by significant extension in platelet lifespan (SI Appendix, Fig. S4B). Together, these data suggest that the thrombocytosis in MplPF4cre/PF4cre mice is caused primarily by excess production of megakaryocytes and their progenitors.
Table 1.
Peripheral blood cells in MplPF4cre/PF4cre and control mice
| Mpl+/+ | Mpl−/− | TPOTg | Mplfl/fl | MplDelcre/Delcre | MplPF4cre/PF4cre | |
| n | 17 | 8 | 6 | 21 | 12 | 15 |
| Platelet count, x109/L | 1,070 ± 209 | 154 ± 44* | 3,381 ± 303* | 1,235 ± 163* | 95 ± 43* | 11,070 ± 2102* |
| Hematocrit, % | 56.9 ± 2.0 | 54.7 ± 1.4 | 50.9 ± 5.4 | 57.7 ± 3.2 | 56.7 ± 2.7 | 50.5 ± 3.2 |
| White cell count, x109/L | 10.0 ± 1.7 | 5.7 ± 1.2 | 13.3 ± 5.2 | 9.6 ± 2.2 | 9.2 ± 1.5 | 17.4 ± 6.8 |
| Neutophil, x109/L | 0.8 ± 0.3 | 0.7 ± 0.7 | 3.2 ± 2.7 | 0.7 ± 0.2 | 0.7 ± 0.2 | 1.8 ± 0.5 |
| Lymphocyte, x109/L | 8.6 ± 1.5 | 4.7 ± 1.3 | 9.4 ± 2.5 | 8.2 ± 2.0 | 8.0 ± 1.3 | 12.8 ± 2.6 |
| Monocyte, x109/L | 0.2 ± 0.1 | 0.1 ± 0.0 | 0.2 ± 0.1 | 0.2 ± 0.1 | 0.2 ± 0.1 | 0.5 ± 0.2 |
| Eosinophil, x109/L | 0.2 ± 0.1 | 0.1 ± 0.0 | 0.2 ± 0.1 | 0.2 ± 0.1 | 0.2 ± 0.1 | 0.3 ± 0.1 |
Blood was analyzed at 7–12 wk of age. *P < 0.05 Tukey's multiple comparison test.
Fig. 2.
Expanded megakaryopoiesis in MplPF4cre/PF4cre mice. (A) Number of megakaryocytes per high powered field from bone marrow (Left) and spleen (Right) of Mpl+/+, Mplfl/fl, MplPF4cre/PF4cre, and TPOTg mice. *P < 0.03 by Student's unpaired two-tailed t test, n = 3–9 mice per genotype. (B) Ploidy of bone marrow megakaryocytes from Mplfl/fl, MplPF4cre/PF4cre, and TPOTg mice. Mean and SD shown, n = 4–6 mice per genotype. *Padj < 0.03 by Student's unpaired two-tailed t test using Bonferroni testing for multiple comparisons.
Table 2.
Numbers of clonogenic hemopoietic progenitor cells in MplPF4cre/PF4cre and control mice
| Genotype (n) | Blast | G | GM | M | Eo | Meg | Total |
| Bone marrow | |||||||
| Mplfl/fl (6) | 4.5 ± 2.2 | 30 ± 5 | 22 ± 11 | 30 ± 11 | 3.5 ± 1.9 | 21 ± 9 | 111 ± 30 |
| MplPF4cre/PF4cre (6) | 40 ± 15* | 70 ± 55 | 85 ± 30* | 73 ± 54 | 4.8 ± 3.6 | 88 ± 39* | 360 ± 80* |
| TPOTg (4) | 12 ± 4* | 48 ± 20 | 63 ± 17* | 42 ± 10* | 2.8 ± 1.0 | 39 ± 12* | 204 ± 55* |
| Spleen | |||||||
| Mplfl/fl (3) | 1.0 ± 1.0 | 1.0 ± 1.7 | 1.3 ± 2.3 | 3.0 ± 4.4 | 0.0 ± 0.0 | 8.0 ± 8.2 | 14 ± 13 |
| MplPF4cre/PF4cre (3) | 35 ± 18* | 41 ± 10* | 89 ± 49* | 45 ± 19* | 1.3 ± 1.2 | 204 ± 48* | 415 ± 131* |
| TPOTg (2) | 2.0 ± 0.0 | 1.5 ± 0.7 | 1.0 ± 1.4 | 1.0 ± 1.4 | 0.0 ± 0.0 | 24 ± 14 | 29 ± 12 |
Numbers of colonies from 25,000 unfractionated bone marrow cells or 50,000 spleen cells cultured in stem-cell factor (SCF, 100 ng/mL), interleukin-3 (IL-3, 10 ng/mL), and erythropoietin (EPO, 2 U/mL) with the type and number of colonies scored after 7 d. Eo, eosinophil; G, granulocyte; GM, granulocyte-macrophage; M, macrophage; Meg, megakaryocyte. *P < 0.03 compared with Mpl+/+ by Student's unpaired two-tailed t test.
MplPF4cre/PF4cre Mice Have Perturbed Stem and Progenitor Cell Compartments and Develop Features of Myeloproliferation.
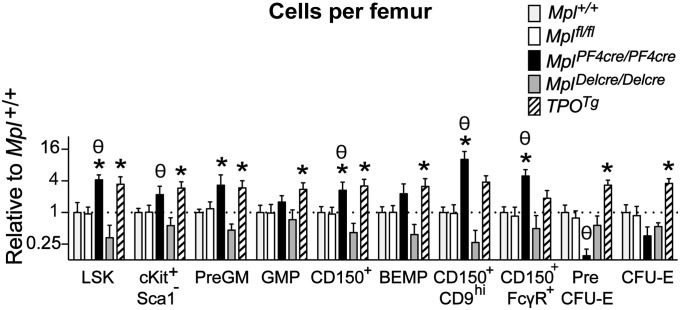
In addition to excess numbers of megakaryocyte progenitor cells, the total number of myeloid progenitor cells in the bone marrow and spleen of MplPF4cre/PF4cre mice was also significantly elevated, with a particular increase in the number of blast colony-forming cells, a phenotype also observed in the bone marrow of TPOTg mice (Table 2). HSC and myeloid progenitor cell populations were analyzed by flow cytometry, with particular emphasis on cells with megakaryocyte potential. MplPF4cre/PF4cre mice displayed a two- to fourfold increase in bone marrow LSK cells, Lin−cKit+Sca1− myeloid progenitors, pregranulocyte-macrophage (PreGM) progenitors, GM progenitors (GMP), and CD150+CD9hi and CD150+FcγR+ bipotential erythroid-megakaryocyte progenitor populations, with a reduction in erythroid progenitors (preCFU-E and CFU-E) compared with Mpl+/+ and/or Mplfl/fl controls (Fig. 3 and SI Appendix, Fig. S5). This observation was similar to the effects caused by chronic excessive in vivo TPO stimulation (TPOTg; Fig. 3 and SI Appendix, Fig. S5; ref. 23), but was significantly more marked in MplPF4cre/PF4cre mice.
Fig. 3.
Expanded progenitors in MplPF4cre/PF4cre and TPOTg mice. Numbers of cells in flow cytometrically defined bone marrow stem and progenitor cell fractions from Mplfl/fl (n = 8), MplPF4cre/PF4cre (n = 6), MplDelcre/Delcre (n = 3), and TPOTg (n = 5) mice shown as cells per femur relative to Mpl+/+ (n = 4) controls. For definitions of cell populations, see SI Appendix, Fig. S5 and Table S1. Mean and SD shown. *Padj < 0.05 by two-tailed Student's unpaired t test compared with Mpl+/+; θPadj < 0.05 by two-tailed Student's unpaired t test specifically for MplPF4cre/PF4cre compared with Mplfl/fl with Bonferroni testing for multiple comparisons.
Blast cell colonies, which represent primitive multipotential preprogenitor cells (26) predominantly derived from the LSK population (27, 28), were picked from primary cultures and replated. Blast cell colonies from MplPF4cre/PF4cre mice had a significantly increased propensity to formation of secondary colonies, particularly secondary blast cell and megakaryocyte colonies. This effect was also evident for blast colonies derived from TPOTg mice (SI Appendix, Fig. S6). This finding demonstrates a greater in vitro capacity for MplPF4cre/PF4cre preprogenitor cell self-renewal and secondary colony formation compared with Mplfl/fl controls. This data therefore provides evidence at the single-cell level that MplPF4cre/PF4cre multipotential preprogenitor cells, like those in TPOTg mice, have characteristics of chronic excessive TPO exposure.
Stem and Progenitor Cells Expressing Mpl Are Responsible for TPO Clearance in MplPF4cre/PF4cre Mice.
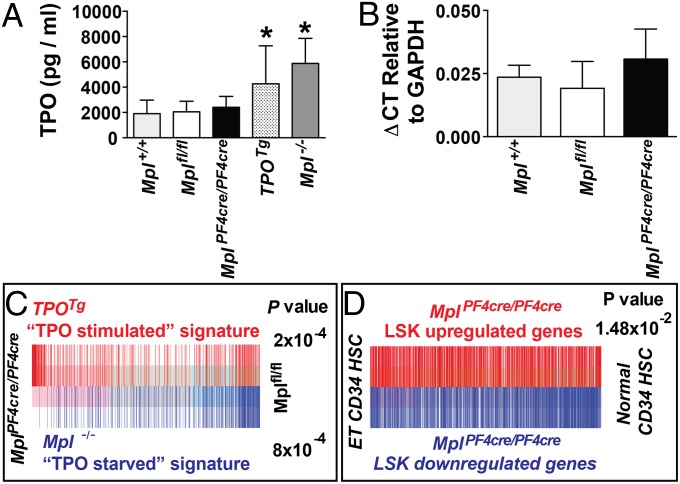
Circulating TPO concentrations were assayed to determine how serum TPO levels were affected by loss of Mpl expression on megakarycytes and platelets in MplPF4cre/PF4cre mice. As expected, TPOTg mice, engineered to express excess TPO, and Mpl−/− mice, which have no capacity to clear serum TPO by Mpl receptor-mediated endocytosis, had elevated levels of serum TPO (Fig. 4A). Surprisingly, the circulating TPO level in MplPF4cre/PF4cre mice was not significantly different from Mplfl/fl controls. Transcription of TPO in the liver, the major site of TPO production, was unaltered in MplPF4cre/PF4cre mice (Fig. 4B). This finding implies that Mpl-expressing LSK and megakaryocyte/erythroid progenitor cells in MplPF4cre/PF4cre mice, which are expanded significantly in number, clear circulating TPO to levels observed in Mpl-replete mice, even in the absence of clearance by megakaryocytes and platelets.
Fig. 4.
TPO transcription, circulating TPO concentration, and gene expression changes in LSK cells of MplPF4cre/PF4cre mice. (A) TPO concentration by immunoassay in serum from Mpl+/+, Mplfl/fl, MplPF4cre/PF4cre, Mpl−/−, and TPOTg mice. *P < 0.04, n = 10–17 mice per genotype. (B) TPO expression in livers determined by quantitative RT-PCR with ΔCT shown as mean and SD relative to GAPDH expression. There were no significant differences among Mpl+/+, Mplfl/fl, and MplPF4cre/PF4cre genotypes, n = 3–5 mice per group. (C) Barcode plot showing ability of TPOTg “TPO stimulated” LSK signature genes and Mpl−/− “TPO starved” signature genes (SI Appendix, Tables S3 and S4) to distinguish between MplPF4cre/PF4cre LSKs and Mplfl/fl LSKs, with corresponding P values derived from rotational gene set testing using ROAST. Red bars designate up-regulated genes in the TPOTg TPO stimulated LSK signature set (P = 2 × 10−4); blue bars designate Mplfl/fl −/− TPO starved LSK signature set (P = 8 × 10−4); demonstrating gene expression in MplPF4cre/PF4cre LSKs is strongly correlated with a TPO stimulated gene signature. (D) Barcode plot showing the TPO stimulated gene expression signature of MplPF4cre/PF4cre LSKs (red bars, up-regulated genes; blue bars, down-regulated genes; SI Appendix, Table S2) can distinguish between CD34+ bone marrow cells of patients with essential thromboocythemia from controls (ref. 33; GEO database accession no. GSE9827, P = 1.48 × 10−2). P value derived from rotational gene set testing by using ROAST with the MplPF4cre/PF4cre LSK gene signature weighted by log fold-change (SI Appendix, SI Materials and Methods, Table S2, and Fig. S7).
Gene Expression Profiling of MplPF4cre/PF4cre Stem and Primitive Progenitors Demonstrate a TPO Stimulation Signature.
Gene expression profiling was undertaken on sorted LSK populations from Mpl+/+, TPOTg, Mpl−/−, Mplfl/fl, and MplPF4cre/PF4cre mice by using Illumina WG version 2 bead-chip microarrays. Pairwise comparison between MplPF4cre/PF4cre and Mplfl/fl LSKs was performed to obtain differentially expressed genes by using a linear modeling and an empirical Bayes approach (ref. 29; SI Appendix, Table S2 and SI Materials and Methods). Signature genes of TPOTg LSKs and Mpl−/− LSKs were defined as those that were significantly differentially expressed in a positive direction by pairwise comparison with wild-type LSKs (SI Appendix, Tables S3 and S4). Rotational gene set tests were then performed by using ROAST (30) with the “TPO overstimulation” gene signature from TPOTg LSKs and the “TPO starvation” gene signature from Mpl−/− LSKs. This analysis revealed that genes associated with TPO overstimulation in TPOTg LSKs were strongly correlated with up-regulated genes in MplPF4cre/PF4cre LSKs, whereas the gene signature associated with TPO starvation in Mpl−/− LSKs was associated with down-regulated genes in MplPF4cre/PF4cre LSKs (Fig. 4C). This data confirms at a genetic level that Mpl expressing stem and progenitor cells in MplPF4cre/PF4cre mice were exposed to excessive TPO stimulation, explaining the behavior of their preprogenitor blast colonies at the single-cell level and the mechanistic basis for the myeloproliferation, megakaryocytosis, and thrombocytosis obverved in MplPF4cre/PF4cre mice.
Human CD34+ Stem and Progenitor Cells Demonstrate a MplPF4cre/PF4cre LSK Gene Expression Signature.
Megakaryocytes and platelets in human myeloproliferative neoplasms have been shown to express reduced levels of MPL (31, 32). To explore the possibility that reduced MPL expression on megakaryocytes and platelets in human disease might contribute to the myeloproliferative phenotype via a mechanism analagous to the excessive TPO stimulation of stem and progenitor cells evident in the MplPF4cre/PF4cre model, we compared the gene expression signature of MplPF4cre/PF4cre LSK cells (SI Appendix, Table S2) to gene expression in CD34+ bone marrow cells from patients with mutated JAK2V617F and JAK2 wild-type essential thrombocythemia [Gene Expression Omnibus (GEO) database accession no. GSE9827; ref. 33]. Rotational gene set testing demonstrated significant enrichment of the MplPF4cre/PF4cre LSK gene signature (P = 1.48 × 10−2; Fig. 4D) and TPOTg and Mpl−/− LSK gene signatures (P = 8 × 10−4; SI Appendix, Fig. S7) in CD34+ cells from patients with disease. Thus, reduced expression of Mpl in megakaryocytes and platelets resulting in the excessive TPO stimulation of stem and progenitor cells that characterizes MplPF4cre/PF4cre mice may, in part, underpin the pathogenesis of megakaryocytosis and thrombocytosis in human myeloproliferative disease.
Discussion
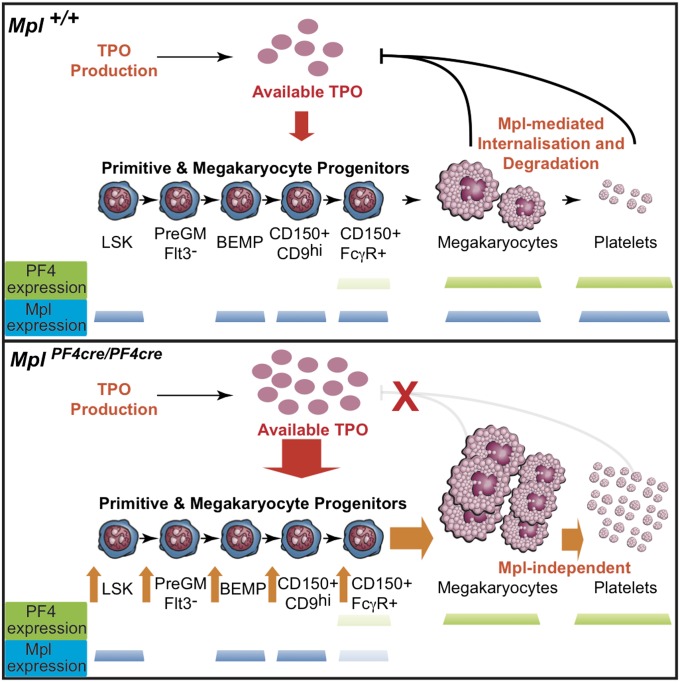
Although it has been established that TPO signaling is required for maintaining steady state platelet numbers and response to crises requiring rapid platelet production, the precise cellular mechanisms by which TPO actions are achieved remained largely undefined. Our results definitively establish that (i) Mpl expression on megakaryocytes is dispensable for high-level platelet production and megakaryocyte maturation, (ii) the primary mechanism by which TPO signaling stimulates thrombopoiesis is via Mpl-expressing hematopoietic progenitors, and (iii) Mpl receptor on megakaryocytes and platelets acts predominantly, if not solely to regulate, via internalization and degradation, the amount of TPO available to hematopoietic stem and progenitor cell populations (Fig. 5).
Fig. 5.
Model for regulation of TPO and control of megakaryopoiesis. (Upper) Clearance of TPO by Mpl-expressing megakaryocytes in bone marrow and the peripheral blood platelet pool maintains TPO homeostasis at steady state and in situations of acute thrombocytopenia. For definitions of cell populations, see SI Appendix, Table S1. Mpl expression is shown as blue bars for each population in Mplfl/fl mice. (Lower) Loss of TPO clearance by megakaryocytes and platelets leads to excessive TPO stimulation of Mpl-expressing HSCs and progenitor cells, multilineage progenitor expansion, and differentiation toward the megakaryocyte lineage from bipotential megakaryocyte-erythroid progenitors, with consequent myeloproliferation, megakaryocytosis, and thrombocytosis. Whereas the availability of TPO for stimulation of stem/progenitor cells is increased, consumption by the expanded numbers of these cells normalizes circulating TPO concentration. PF4-cre expression is shown as green bars and Mpl expression is shown as blue bars for each population in MplPF4cre/PF4cre mice.
Regarding the role of Mpl expression on megakaryocytes, whereas increased megakaryocyte ploidy has been associated with high TPO activity and the converse has been observed in TPO/Mpl-deficient mouse models (3, 10–12), a shift to higher ploidy megakaryocytes accompanied marked thrombocytosis in MplPF4cre/PF4cre mice was observed despite the absence of Mpl expression in megakaryocytes. Thus, thrombopoiesis and the development of high DNA ploidy in megakaryocytes is independent of TPO signaling in these cells, the latter likely reflecting alternative mechanisms associated with states of increased megakaryocyte production. A role for TPO in sensitizing platelets to specific activators (34) may explain the retention of Mpl signaling pathways in megakaryocytes and platelets because our data do not exclude TPO, when functional Mpl is present, having a positive effect on megakaryocyte or platelet function.
Notably, MplPF4cre/PF4cre mice exhibited all of the phenotypic hallmarks and gene expression changes in stem/primitive multipotential progenitor cells, indicative of chronic excessive TPO stimulation. This phenotype occurred despite MplPF4cre/PF4cre mice having normal concentrations of serum TPO. These data support a model in which the absence of megakaryocyte and platelet Mpl mass allows greater available TPO for stimulation of Mpl-expressing stem/progenitor cells, the expansion of which normalizes the circulating TPO concentration via Mpl-mediated internalization. Thus, the circulating TPO concentration in this model is determined by the Mpl mass of both the megakaryocyte/platelet pool and Mpl-expressing stem and progenitor cells. Moreover, MplPF4cre/PF4cre mice were observed to have a more profound myeloproliferative phenotype with a greater degree of thrombocytosis relative to TPOTg counterparts, despite the latter having a higher circulating TPO level. This observation suggests local TPO availability within the stem and progenitor cell microenvironment of the bone marrow may be regulated in significant part by megakaryocytes: TPOTg mice would retain megakaryocyte Mpl-mediated regulation of local TPO contentration, whereas absence of functional Mpl on megakaryocytes in MplPF4cre/PF4cre mice would significantly impair local TPO regulation. Increasingly, evidence has suggested a close physical relationship between megakaryocytes and bone marrow stem/progenitor cell niches. Imaging studies have identified megakaryocytes colocalizing with CD150+CD48−Lin− HSCs adjacent to sinusoidal endothelium (35) and in close proximity to Nestin+ mesenchymal stem cells, which form a significant functional component of the HSC niche (36).
The importance of TPO as a multifunctional regulator of hematopoiesis was highlighted. Chronic TPO overstimulation in MplPF4cre/PF4cre and TPOTg mice resulted in excess numbers of primitive multipotential preprogenitor blast colonies that are principally derived from LSK cells, a population that expresses Mpl. The increased propensity of preprogenitor cells to self-renew and to produce increased numbers of progenitors of multiple myeloid lineages with chronic TPO overstimulation, provides a mechanistic rationale for the effectiveness of the TPO mimetic agent eltrombopag in the restoration of bone marrow cellularity and multilineage haemopoiesis in patients with severe refractory aplastic anemia (37). Additionally, megakaryocyte lineage skewing of preprogenitor cells derived from MplPF4cre/PF4cre mice suggests excessive TPO stimulation may also act during these earlier stages in the hematopoietic hierarchy for megakaryocyte lineage specification. In more differentiated progenitors, identification of Mpl receptor expression on bipotential progenitor populations that were expanded in MplPF4cre/PF4cre mice and were associated with megakaryocytosis, and a relative paucity of erythroid precursors, confirms our previous findings (23). Thus, bipotenital erythro-megakaryocytic progenitors express Mpl and are critical effector cells of TPO-dependent thrombopoiesis that are driven to differentiate into megakaryocytes in response to TPO, and which under conditions of excessive TPO stimulation, are skewed toward megakaryocytopoiesis at the expense of erythropoiesis. Despite an increased number of diverse myeloid progenitor cells in MplPF4cre/PF4cre mice, the number of circulating white blood cells was not consistently elevated, suggesting the contribution of other regulatory mechanisms in control of mature granulocyte and macrophage number.
Finally, the demonstration that the chronic TPO stimulation gene expression signature in LSK cells of MplPF4cre/PF4cre mice was also evident in the gene expression changes in human bone marrow CD34+ cells from patients with essential thrombocythemia provides an important potential explanation for the observation that abnormally low expression of MPL in platelets and megakaryocytes of human patients with myeloproliferative disorders is associated with thrombocytosis (31, 32). Our data support a model that such disorders may be, in part, underpinned by insufficient MPL mass within the platelet/megakaryocyte pool resulting in increased TPO stimulation of the MPL expressing stem and progenitor cells similar to that observed in MplPF4cre/PF4cre mice.
Materials and Methods
Mice were analyzed at age 7–12 wk. TPOTg (3) and Mpl−/− (24) mice have been described. See SI Appendix, SI Materials and Methods for generation of Mplfl/fl, MplDelcre/Delcre, and MplPF4cre/PF4cre mice. Experiments were performed by using procedures approved by The Walter and Eliza Hall Institute of Medical Research Animal Ethics Committee. Haematology, histology, clonal analysis of bone marrow cells semisolid agar cultures, liquid cultures of progenitor cells, flow cytometry, megakaryocyte analysis, Western blot analysis, platelet life span, serum TPO measurement, reverse transcription PCR, statistical analysis, and bioinformatic analysis are described in SI Appendix, SI Materials and Methods. Microarray data are available at Array Express (www.ebi.ac.uk/arrayexpress) under accession no. E-MTAB-2389.
Supplementary Material
Acknowledgments
We thank Janelle Lochland, Jason Corbin, Emilia Simankowicz, Melanie Howell, Lauren Wilkins, and Keti Stoev for skilled assistance. This work was supported by Australian National Health and Medical Research Council Program Grants 1016647 and 490037; Fellowship 575501 (to W.S.A.); Independent Research Institutes Infrastructure Support Scheme Grant 361646; the Carden Fellowship of the Cancer Council, Victoria (to D.M.); the Cure Cancer Australia/Leukaemia Foundation Australia Post Doctoral Fellowship (to A.P.N.); Lions Fellowship, Cancer Council of Victoria (to A.P.N.); the Australian Cancer Research Fund; and Victorian State Government Operational Infrastructure Support.
Footnotes
The authors declare no conflict of interest.
Data deposition: The sequences reported in this paper have been deposited in Array Express database, www.ebi.ac.uk/arrayexpress (accession no. E-MTAB-2389).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1404354111/-/DCSupplemental.
References
- 1.Kuter DJ. Thrombopoietin Mimetics. In: Michelson AD, editor. Platelets. London: Academic; 2013. pp. 1217–1242. [Google Scholar]
- 2.Qian S, Fu F, Li W, Chen Q, de Sauvage FJ. Primary role of the liver in thrombopoietin production shown by tissue-specific knockout. Blood. 1998;92(6):2189–2191. [PubMed] [Google Scholar]
- 3.de Graaf CA, et al. Regulation of hematopoietic stem cells by their mature progeny. Proc Natl Acad Sci USA. 2010;107(50):21689–21694. doi: 10.1073/pnas.1016166108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lannutti BJ, Epp A, Roy J, Chen J, Josephson NC. Incomplete restoration of Mpl expression in the mpl-/- mouse produces partial correction of the stem cell-repopulating defect and paradoxical thrombocytosis. Blood. 2009;113(8):1778–1785. doi: 10.1182/blood-2007-11-124859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tiedt R, et al. Pronounced thrombocytosis in transgenic mice expressing reduced levels of Mpl in platelets and terminally differentiated megakaryocytes. Blood. 2009;113(8):1768–1777. doi: 10.1182/blood-2008-03-146084. [DOI] [PubMed] [Google Scholar]
- 6.Emmons RV, et al. Human thrombopoietin levels are high when thrombocytopenia is due to megakaryocyte deficiency and low when due to increased platelet destruction. Blood. 1996;87(10):4068–4071. [PubMed] [Google Scholar]
- 7.Griesshammer M, et al. High levels of thrombopoietin in sera of patients with essential thrombocythemia: Cause or consequence of abnormal platelet production? Ann Hematol. 1998;77(5):211–215. doi: 10.1007/s002770050445. [DOI] [PubMed] [Google Scholar]
- 8.Nurden AT, Viallard JF, Nurden P. New-generation drugs that stimulate platelet production in chronic immune thrombocytopenic purpura. Lancet. 2009;373(9674):1562–1569. doi: 10.1016/S0140-6736(09)60255-5. [DOI] [PubMed] [Google Scholar]
- 9.Uppenkamp M, Makarova E, Petrasch S, Brittinger G. Thrombopoietin serum concentration in patients with reactive and myeloproliferative thrombocytosis. Ann Hematol. 1998;77(5):217–223. doi: 10.1007/s002770050446. [DOI] [PubMed] [Google Scholar]
- 10.Arnold JT, et al. A single injection of pegylated murine megakaryocyte growth and development factor (MGDF) into mice is sufficient to produce a profound stimulation of megakaryocyte frequency, size, and ploidization. Blood. 1997;89(3):823–833. [PubMed] [Google Scholar]
- 11.Broudy VC, Lin NL, Kaushansky K. Thrombopoietin (c-mpl ligand) acts synergistically with erythropoietin, stem cell factor, and interleukin-11 to enhance murine megakaryocyte colony growth and increases megakaryocyte ploidy in vitro. Blood. 1995;85(7):1719–1726. [PubMed] [Google Scholar]
- 12.de Sauvage FJ, et al. Physiological regulation of early and late stages of megakaryocytopoiesis by thrombopoietin. J Exp Med. 1996;183(2):651–656. doi: 10.1084/jem.183.2.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Drachman JG, Sabath DF, Fox NE, Kaushansky K. Thrombopoietin signal transduction in purified murine megakaryocytes. Blood. 1997;89(2):483–492. [PubMed] [Google Scholar]
- 14.Leven RM, Clark B, Tablin F. Effect of recombinant interleukin-6 and thrombopoietin on isolated guinea pig bone marrow megakaryocyte protein phosphorylation and proplatelet formation. Blood Cells Mol Dis. 1997;23(2):252–268. doi: 10.1006/bcmd.1997.0142. [DOI] [PubMed] [Google Scholar]
- 15.Tajika K, Nakamura H, Nakayama K, Dan K. Thrombopoietin can influence mature megakaryocytes to undergo further nuclear and cytoplasmic maturation. Exp Hematol. 2000;28(2):203–209. doi: 10.1016/s0301-472x(99)00138-1. [DOI] [PubMed] [Google Scholar]
- 16.Zucker-Franklin D, Kaushansky K. Effect of thrombopoietin on the development of megakaryocytes and platelets: An ultrastructural analysis. Blood. 1996;88(5):1632–1638. [PubMed] [Google Scholar]
- 17.Bunting S, et al. Normal platelets and megakaryocytes are produced in vivo in the absence of thrombopoietin. Blood. 1997;90(9):3423–3429. [PubMed] [Google Scholar]
- 18.Choi ES, et al. The role of megakaryocyte growth and development factor in terminal stages of thrombopoiesis. Br J Haematol. 1996;95(2):227–233. doi: 10.1046/j.1365-2141.1996.d01-1920.x. [DOI] [PubMed] [Google Scholar]
- 19.Vitrat N, et al. Compared effects of Mpl ligand and other cytokines on human MK differentiation. Stem Cells. 1998;16(Suppl 2):37–51. doi: 10.1002/stem.5530160707. [DOI] [PubMed] [Google Scholar]
- 20.Tiedt R, Schomber T, Hao-Shen H, Skoda RC. Pf4-Cre transgenic mice allow the generation of lineage-restricted gene knockouts for studying megakaryocyte and platelet function in vivo. Blood. 2007;109(4):1503–1506. doi: 10.1182/blood-2006-04-020362. [DOI] [PubMed] [Google Scholar]
- 21.Calaminus SD, et al. Lineage tracing of Pf4-Cre marks hematopoietic stem cells and their progeny. PLoS ONE. 2012;7(12):e51361. doi: 10.1371/journal.pone.0051361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Srinivas S, et al. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev Biol. 2001;1:4. doi: 10.1186/1471-213X-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ng AP, et al. Characterization of thrombopoietin (TPO)-responsive progenitor cells in adult mouse bone marrow with in vivo megakaryocyte and erythroid potential. Proc Natl Acad Sci USA. 2012;109(7):2364–2369. doi: 10.1073/pnas.1121385109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alexander WS, Roberts AW, Nicola NA, Li R, Metcalf D. Deficiencies in progenitor cells of multiple hematopoietic lineages and defective megakaryocytopoiesis in mice lacking the thrombopoietic receptor c-Mpl. Blood. 1996;87(6):2162–2170. [PubMed] [Google Scholar]
- 25.Rodríguez CI, et al. High-efficiency deleter mice show that FLPe is an alternative to Cre-loxP. Nat Genet. 2000;25(2):139–140. doi: 10.1038/75973. [DOI] [PubMed] [Google Scholar]
- 26.Metcalf D, et al. Two distinct types of murine blast colony-forming cells are multipotential hematopoietic precursors. Proc Natl Acad Sci USA. 2008;105(47):18501–18506. doi: 10.1073/pnas.0810072105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Metcalf D, Ng A, Mifsud S, Di Rago L. Multipotential hematopoietic blast colony-forming cells exhibit delays in self-generation and lineage commitment. Proc Natl Acad Sci USA. 2010;107(37):16257–16261. doi: 10.1073/pnas.1011881107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Metcalf D, et al. Murine hematopoietic blast colony-forming cells and their progeny have distinctive membrane marker profiles. Proc Natl Acad Sci USA. 2009;106(45):19102–19107. doi: 10.1073/pnas.0910354106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smyth GK. Linear models and empirical bayes methods for assessing differential expression in microarray experiments. Stat Appl Genet Mol Biol. 2004;3 doi: 10.2202/1544-6115.1027. Article3. [DOI] [PubMed] [Google Scholar]
- 30.Wu D, et al. ROAST: Rotation gene set tests for complex microarray experiments. Bioinformatics. 2010;26(17):2176–2182. doi: 10.1093/bioinformatics/btq401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Horikawa Y, et al. Markedly reduced expression of platelet c-mpl receptor in essential thrombocythemia. Blood. 1997;90(10):4031–4038. [PubMed] [Google Scholar]
- 32.Harrison CN, et al. Platelet c-mpl expression is dysregulated in patients with essential thrombocythaemia but this is not of diagnostic value. Br J Haematol. 1999;107(1):139–147. doi: 10.1046/j.1365-2141.1999.01667.x. [DOI] [PubMed] [Google Scholar]
- 33.Catani L, et al. Molecular profile of CD34+ stem/progenitor cells according to JAK2V617F mutation status in essential thrombocythemia. Leukemia. 2009;23(5):997–1000. doi: 10.1038/leu.2008.357. [DOI] [PubMed] [Google Scholar]
- 34.Kuter DJ. New thrombopoietic growth factors. Blood. 2007;109(11):4607–4616. doi: 10.1182/blood-2006-10-019315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kiel MJ, et al. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005;121(7):1109–1121. doi: 10.1016/j.cell.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 36.Méndez-Ferrer S, et al. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 2010;466(7308):829–834. doi: 10.1038/nature09262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Olnes MJ, et al. Eltrombopag and improved hematopoiesis in refractory aplastic anemia. N Engl J Med. 2012;367(1):11–19. doi: 10.1056/NEJMoa1200931. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.