Significance
Mammalian extinction during the past several hundred thousand years has been a major focus for evolutionary biologists, geologists, and archaeologists, often being linked to climate change and human overhunting. Until relatively recently, study has been largely restricted to the Americas, Europe, and Australasia. We present the oldest well-dated sequence of mammalian faunas for the Indian subcontinent, demonstrating continuity of 20 of 21 identified mammals from at least 100,000 y ago to the present. We suggest that, although local extirpations occurred, the majority of taxa survived or adapted to substantial ecological pressures in fragmented habitats. These results complement data from Africa and elsewhere that demonstrate the necessity of a nuanced ecological understanding of such extinctions in different areas of the world.
Keywords: Kurnool, fossil mammals, OSL dating, Theropithecus
Abstract
Mammalian extinction worldwide during the Late Pleistocene has been a major focus for Quaternary biochronology and paleoecology. These extinctions have been variably attributed to the impacts of climate change and human interference. However, until relatively recently, research has been largely restricted to the Americas, Europe, and Australasia. We present the oldest Middle–Late Pleistocene stratified and numerically dated faunal succession for the Indian subcontinent from the Billasurgam cave complex. Our data demonstrate continuity of 20 of 21 identified mammalian taxa from at least 100,000 y ago to the present, and in some cases up to 200,000 y ago. Comparison of this fossil record to contemporary faunal ranges indicates some geographical redistribution of mammalian taxa within India. We suggest that, although local extirpations occurred, the majority of taxa survived or adapted to substantial ecological pressures in fragmented habitats. Comparison of the Indian record with faunal records from Southeast and Southwest Asia demonstrates the importance of interconnected mosaic habitats to long-term faunal persistence across the Asian tropics. The data presented here have implications for mammalian conservation in India today, where increasing ecological circumscription may leave certain taxa increasingly endangered in the most densely populated region of the world.
The extinction of many Late Pleistocene mammals has been described as a “major event in recent Earth history” (1). For a long time, attention has focused on terrestrial “megafauna” (>44 kg), with 97 of 150 genera becoming extinct worldwide by 10 thousand years ago (10 ka) (2). Climate change and overhunting or habitat disruption by humans are typically cited as causes for the widespread disappearance of these mammals, but research in Africa and a growing understanding of fauna–ecosystem interactions suggests that single-factor explanations for faunal extinctions are overly simplistic (2–5). Furthermore, the simultaneous disappearance of smaller mammalian taxa in some regions (6), but not in others (3), suggests that Late Pleistocene mammalian population analyses should also consider multiple causes acting on different taxa at a range of spatial and temporal scales and that wider ecological perspectives should be embraced (1). Securely dated Pleistocene fossil assemblages from less-well-documented regions of the world therefore offer considerable potential to further our knowledge about the factors affecting past faunal distributions and diversity.
The Indian subcontinent preserves a mosaic of ecosystems, ranging from tropical rainforests to grassland savannahs and deserts (7). This ecological diversity would have supported a large range of organisms (8), yet little research has been undertaken on Pleistocene mammalian population changes in the region, owing to a lack of stratified finds. Indian ecosystems are subject to the influence and strength of the Indian summer monsoon (9), and the subcontinent is likely to have been one of the first regions reached by early modern humans leaving Africa in the Late Pleistocene (10, 11), with potential repercussions for mammalian communities. Human impact on mammals in regions beyond India has been much discussed (1, 3), but the influence of modern human arrival on faunal populations in South Asia remains unknown, as does the possible effect of the ∼74 ka Toba volcanic supereruption (12). The Indian subcontinent therefore presents a critical, but poorly documented, region for investigating faunal responses to climatic, volcanic, and anthropogenic-driven change during the Pleistocene. In this work, we report the earliest well-dated, stratified Middle–Late Pleistocene faunal sequence from the Indian subcontinent.
Results and Discussion
The Stratified Faunal Sequence.
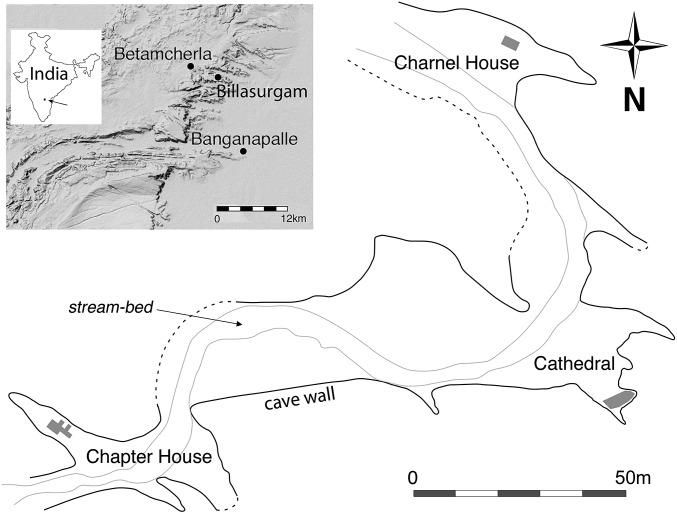
The Billasurgam cave complex, Kurnool District, Andhra Pradesh (N15°26.153′ E7°11.122′) (Fig. 1) lies in a semiarid tropical landscape, with an average annual rainfall of 700 mm. The cave complex consists of an interconnected series of large chambers within Paleozoic carbonate host rock. Excavations have focused on the thick (>10 m) sequences of Quaternary sediments preserved within the major chambers of Charnel House Cave and Cathedral Cave. Although the abundance of fossils has led to intermittent research and excavation of the Billasurgam caves since the 19th century (13, 14), the faunal sequence has not previously been dated or systematically analyzed to understand past biodiversity and faunal dynamics. Previous excavations at Billasurgam and in nearby caves reportedly produced Upper Paleolithic artifacts alongside cut-marked bones (15, 16); however, no such evidence has been identified in recent excavations, and bone striations and damage have been attributed to animal gnawing (17, 18). The sedimentary sequence within the cave complex is dominated by silt-grade siliciclastic deposits [referred to as “cave earths” by Foote (13)]. These deposits are punctuated by occasional layers of coarse breccia related to minor periodic roof collapse. The majority of the sedimentary input to the cave complex has been via surface runoff into a well-developed network of sinkholes. There is also a minor component of wind-blown material in the sequences near the main entrance. There is no evidence of significant fluvial input or reworking of the sediments.
Fig. 1.
Map of the Billasurgam cave complex, showing the positions of the excavations at Charnel House Cave and Cathedral Cave at its northern and southern ends, respectively.
Optically Stimulated Luminescence Dating.
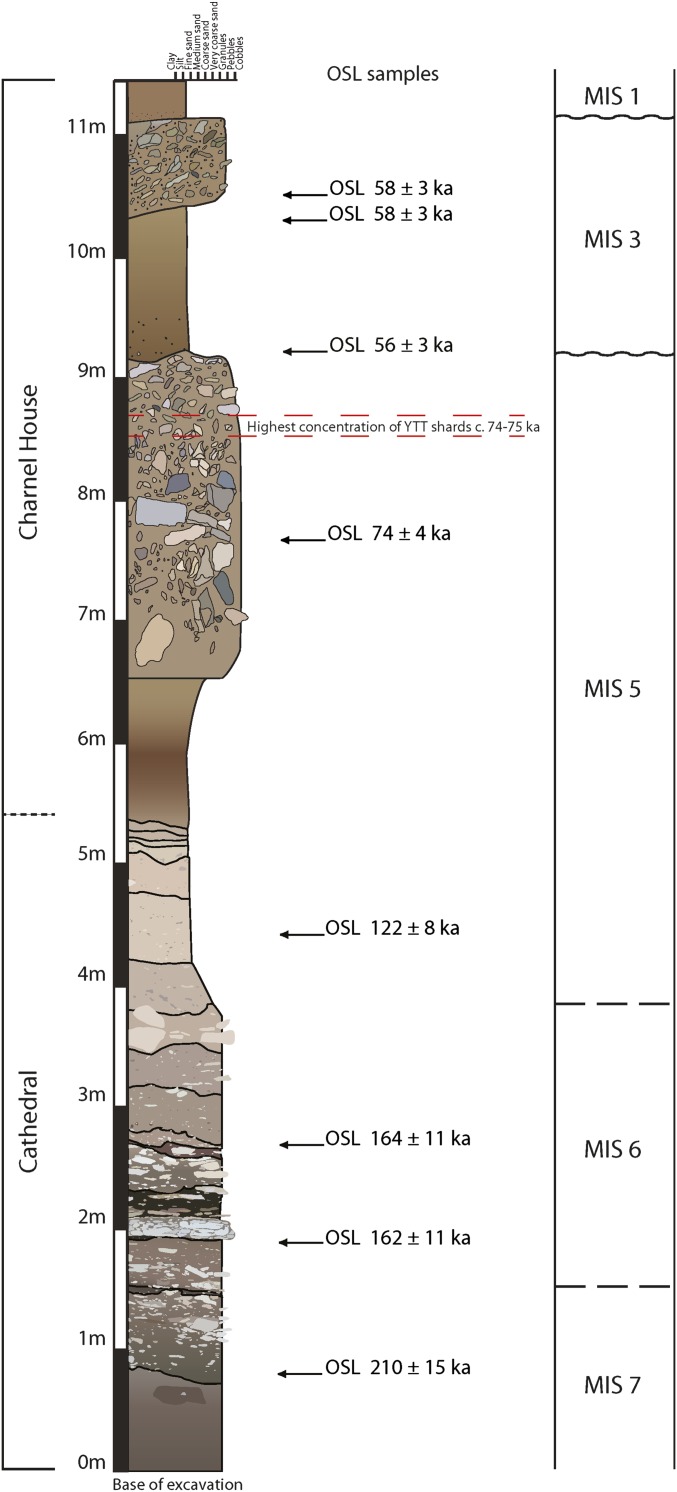
Eight sediment samples were dated by optically stimulated luminescence (OSL) from the Charnel House Cave and Cathedral Cave sequences. The four samples from Charnel House Cave indicate that the sampled deposits are largely dated to between 74 and 57 ka (Fig. 2; SI Appendix, Table S10). This result complements previous publications of tephra analysis undertaken on the Charnel House Cave sequence that demonstrated the presence of ash shards from the Toba eruption (19) (Fig. 2). Finds of pottery date the uppermost levels of Charnel House Cave to the Holocene.
Fig. 2.
Composite sedimentary log of the sequences in the Charnel House and Cathedral chambers of the Billasurgam cave complex. The deposits are composed of silt-dominated cave earths, punctuated by bands of angular blocky limestone conglomerate (from roof/wall collapse) and more rounded, mixed pebble conglomerate bands (input by land surface runoff via sinkhole depositional processes). Inferred correlation of the layers by MIS is based on stratigraphic inferences, OSL dating of the sediments, and the identification of Youngest Toba Tuff (YTT) ash shards. Wavy lines indicate disconformities with missing sediment; dashed lines are estimated boundaries in semicontinuous sections. Several of the OSL ages fall on or near the ages assigned to MIS boundaries, so the plotted boundary positions and MIS designations are approximate.
OSL dating of the Cathedral Cave sediments indicates deposition between ∼210 and 120 ka (Fig. 2; SI Appendix, Table S10), with at least 4.5 m of overlying (younger) deposits removed by 19th-century excavations (13).
Fig. 2 shows the scientific dating results at the two sites and their correlation with Marine Isotope Stages (MIS). The dating confirms that the Billasurgam cave complex represents the oldest Middle–Late Pleistocene stratified and scientifically dated faunal succession for the Indian subcontinent, stretching from MIS 7 (starting 240 ka) to the Holocene (MIS 1).
Paleontological Identification.
At Cathedral Cave, 144 of 5,281 macrovertebrate bone fragments have been identified to genus or species level (SI Appendix, Table S3), and in Charnel House Cave, 37 of 288 macrovertebrate bone fragments have been similarly identified to genus or species level (SI Appendix, Table S4). The relatively low number of identified specimens is a result of taphonomic processes associated with external weathering and sedimentary transport of the remains into the caves.
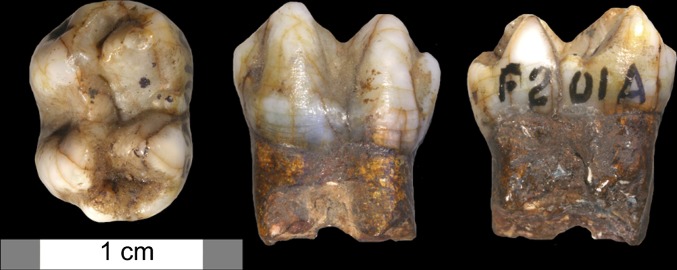
Table 1 displays the presence or absence of genera and species from each MIS represented by the Billasurgam sequence. Overall, the two Billasurgam caves present evidence for a diverse range of Middle–Late Pleistocene mammals, including small to large carnivores, primates, small to large bovids and cervids, rhinoceros, and wild Equus. Crucially, every Pleistocene taxon identified survives in the Indian subcontinent today, with the exception of one primate based on a single specimen recovered in the 19th century excavations—here identified as Theropithecus cf gelada (Fig. 3; SI Appendix, SI Methods and Table S6A).
Table 1.
Faunal succession at the Billasurgam Caves, arranged by MIS
| Taxa | Modern India* | Modern Andhra Pradesh† | MIS 1 | MIS 3 | MIS 5 | MIS 6 | MIS 7 |
| Carnivora | |||||||
| Ursus sp. | + | + | — | — | — | + | — |
| Felis chaus | + | + | — | — | — | + | — |
| Panthera pardus | + | + | — | — | + | — | — |
| Herpestes griseus | + | + | — | — | + | + | — |
| Canis sp. | + | + | — | — | + | + | — |
| Vulpes sp. | + | + | — | — | + | + | — |
| Primates | |||||||
| Theropithecus cf gelada | — | — | — | — | + | — | — |
| Semnopithecus entellus subsp. | + | + | — | — | +‡ | — | — |
| Artiodactyla | |||||||
| Bos sp. | + | + | — | — | + | + | — |
| Boselaphus sp. | + | + | — | — | + | + | + |
| Antilope cervicapra | + | + | — | — | + | + | — |
| Gazella sp. | + | + | — | — | + | + | + |
| Tetracerus quadricornis | + | + | — | + | + | — | — |
| Axis axis | + | + | + | — | + | + | — |
| Muntjacus sp. | + | + | — | — | + | — | |
| Sus sp. | + | + | — | — | + | + | — |
| Other | |||||||
| Equus sp. | + | — | — | + | + | + | + |
| Rhinoceros sp. | + | — | — | + | + | + | — |
| Lepus sp. | + | + | + | + | + | + | — |
| Hystrix crassidens | + | + | — | + | + | + | — |
| Erinaceus sp. | + | + | — | — | + | — | — |
+, Specimens identified in this study, including our reexamination of Theropithecus cf gelada specimen from 19th-century excavations; —, not identified in this study.
As known in the records of the International Union for Conservation of Nature (www.iucnredlist.org).
As known from modern faunal studies of Andhra Pradesh (20).
Provides a terminus ante quem. Theropithecus specimen comes from Foote’s excavations, which removed layers that are now minimally dated to MIS 5. The actual date remains unknown due to the removal of these layers (SI Appendix, SI Methods).
Fig. 3.
Theropithecus cf gelada lower second (or possibly first) molar from Bed M of Charnel House Cave, correlated here to at least 74 ka, MIS 5 or older. From left to right are occlusal, buccal, and lingual views (photographs by R.L.C.).
Spatial Comparison of Faunal Presence.
Genera and species in the Billasurgam sequence have been compared with the modern distributions of these taxa in the southern Indian state of Andhra Pradesh (20) (www.iucnredlist.org) and India as a whole (www.iucnredlist.org). This comparison indicates that the majority of taxa in the Billasurgam record are still present in modern Andhra Pradesh (20) (www.iucnredlist.org). However, Felis chaus and Ursus labiatus are absent from the Billasurgam sequence following MIS 6, and Herpestes griseus and Panthera pardus are absent after MIS 5. Although Herpestes griseus, F. chaus, U. labiatus, and P. pardus are all still present in the wider Andhra Pradesh region today, they have a patchy distribution here and in India as a whole. P. pardus is considered “near threatened” (www.iucnredlist.org), and the jungle habitat of F. chaus has been greatly restricted in southern India. The broader continuity of these species in Andhra Pradesh is therefore tempered by restrictions to their previous distribution.
The fossil faunal record also includes mammalian taxa that are no longer present in the modern Billasurgam region. This finding has different implications for identifications depending on the taxonomic level being analyzed. Changes in the spatial distribution of a particular genus could be the result of either a spatial redistribution of species within that genus following local extinctions or a new speciation event that has facilitated the persistence of a genus in new regions or environments. Although a genus as a whole may demonstrate continuity, this level of identification cannot account for the extinction of past unidentified species within that genus. For example, although present in the Pleistocene layers of Billasurgam, no Rhincoceros sp. can be found anywhere in southern India today. Indeed, the Indian rhinoceros is currently restricted to heavily forested and wetland habitats in northeast India and Nepal, with two-thirds of the population confined to the Kaziranga National Park, Assam (www.iucnredlist.org). This status could either imply a restriction of range of the Pleistocene Rhinoceros sp. or a speciation event within that genus at a later stage that led to the modern species. Similarly, Equus hemionus khur (Indian wild ass) is historically known from arid habitats of the subcontinent, including the Thar Desert and Baluchistan, but this endangered subspecies is currently restricted to the mesic Little Rann of Kutch (www.iucnredlist.org). Again, its absence from modern Billasurgam could represent the restriction of range of a Pleistocene species or a more recent speciation event.
By contrast, changes in the spatial distribution of a particular species within India must be the result of range restriction in that species. Primate taxa are absent from the MIS 3 and 1/2 layers of the Billasurgam sequence (Table 1). However, comparisons of Lydekker’s faunal identifications (14) with ours (SI Appendix, SI Methods) suggest that Semnopithecus entellus subspecies (subsp.) is present in layers corresponding to MIS 1 and 2. Fossil teeth of S. entellus from Billasurgam are closer in size to those of S. entellus schistaceus, today found in the Himalayan zone, than to those of the smaller subspecies S. entellus entellus found abundantly at Billasurgam today (SI Appendix, SI Methods). This finding suggests that the range of the larger northern form may have extended significantly farther south during the later Pleistocene under cooler climatic conditions or perhaps that local populations increased in size under those conditions following Bergmann’s rule (21).
Of special interest is the presence of Theropithecus cf gelada in the cave sequence during MIS 5 or earlier (Table 1; SI Appendix, SI Methods). Extinct species of this genus are known from numerous sites in Africa between ∼4 million years ago (Ma) and 200 ka, whereas the extant T. gelada is today restricted to the highlands of Ethiopia (22). How a gelada-sized species of Theropithecus might have reached southern India near the Middle–Late Pleistocene boundary is uncertain, although it suggests significant mobility of mammals during the later Pleistocene (23). Theropithecus specimens with larger inferred body size are known outside Africa in several areas: at ∼1.6 Ma in Israel, by 1.0 Ma in Europe (24), and at or after 1 Ma in northern India (25). Given the general trend in size increase over time in molar teeth of Theropithecus oswaldi, it is unlikely that any of these larger and older assemblages pertain to the ancestry of the Billasurgam species. However, it is not possible to determine whether the Billasurgam Theropithecus cf gelada was part of an isolated group or belonged to a more widespread occurrence or when these relatives (or potentially conspecifics) of modern geladas first arrived in India.
Discussion
The fossil and contemporary data generally illustrate a continuation of mammalian genera and species in the Indian subcontinent from the late Middle Pleistocene to the present, a period encompassing two complete glacial–interglacial cycles, the deposition of ash from the Toba volcanic supereruption, and the arrival of modern humans. The Billasurgam caves are close to abundant freshwater springs, recharged by rainfall from the humid Western Ghats, and as such they would have provided diverse wetland habitats capable of supporting a variety of mammals during periods of increased aridity. Continuity in faunal diversity at Billasurgam from MIS 7–5 suggests that mammalian groups survived the penultimate glacial maximum in MIS 6. Although our data indicate that a number of genera and species were no longer present in the Billasurgam record after MIS 5, a nearby rock-shelter excavation indicates the survival of some of the smaller of these taxa well into MIS 3 (26), and a number of these taxa are present in the wider Andhra Pradesh region today (20).
The continuity demonstrated by the Indian record is worth comparing to the well-studied faunal records from Southeast and Southwest Asia. The persistence of mammalian taxa in both the Indian and Sri Lankan Late Pleistocene records (27) indicates that the fauna of the Indian subcontinent were relatively resistant to climatic and human impacts associated with this period. Climatic instability during the last glacial cycle (28, 29) may have resulted in local faunal turnover, such as the possible disappearance of large (>44 kg) carnivores in the Billasurgam record. Similarly, the disappearance of certain mammalian groups (especially those >44 kg) from the Billasurgam record after MIS 5, and particularly in MIS 3, could have been driven by increasing competition for fragmenting ecological resources from growing human populations, armed with microlithic technologies (30, 31). However, the relative stability of rainfall and topography across the Indian subcontinent as a whole meant that habitat survival in patches facilitated faunal recombination, migration, and general long-term persistence.
Resistance of mammalian fauna to environmental and human change has also been highlighted in records from mainland and island Southeast Asia (32). As in India, mixed, interconnected habitats facilitated long-term faunal persistence (33), including regional survival through the impact of the 74 ka Toba supereruption (34). Although Southeast Asia witnessed the extinction or reduction in range of several genera (including Stegodon, Hexaprotodon, Pongo, Crocuta, Hyaena, Tapirus, and Rhinoceros) during the late Middle Pleistocene, and increased faunal turnover and redistribution during the Late Pleistocene, this shift appears to have been generally limited to coastal areas vulnerable to eustatic changes in sea level and climate change linked to glacial cycles (33, 35). Here, rising sea levels fragmented the linked, mosaic habitats permanently, resulting in isolated islands—such as Java, Sumatra, and Flores—that were potentially more vulnerable to human, climatic, and ecological change.
The importance of stable mosaic habitats for faunal continuity is further demonstrated in the faunal records of Southwest Asia. The Levant (including Israel, Jordan, Lebanon, and Syria) is often discussed in the context of environmental connectivity facilitating the migration of Pleistocene faunas (36). Whereas cycles of aridity stimulated faunal turnover in the south and east of the Levant (37), the mosaic environment afforded by the foothills and forests of Israel’s Mediterranean coastline facilitated stability of small and large mammalian taxa across the Middle and Late Pleistocene (38). As in the Indian record, the Israeli sequences indicate limited species turnover throughout the climatic cycles encompassed by MIS 7–2 (39, 40), with a resilience of Mediterranean fauna including Gazella gazella and Dama mesopotamica. By contrast, the lack of mosaic environments in the deserts of the Arabian Peninsula meant that Arabian faunal communities were particularly vulnerable to extreme environmental fluctuations (41). Remains recovered from Middle Pleistocene lacustrine deposits demonstrate that species such as Crocuta crocuta, Elaphas sp., and Hexaprotodon could survive in the deserts of the Arabian Peninsula under wetter conditions (42, 43). However, the return of aridity and the disappearance of freshwater sources led to the disappearance of these species from this region.
The Indian faunal record indicates long-term persistence of some mammals. On a genus level, this finding is indicative of either new speciation events or species redistribution, whereas at a species level this finding demonstrates persistence of mammals within the subcontinent. The continued presence of 20 of 21 mammal taxa in the face of large-scale climatic changes, the Toba volcanic supereruption, and human impacts throughout the Late Pleistocene mirrors the faunal record of general survivorship in Africa (2, 44). The continuation of faunal assemblages in Africa and India contrasts with the situation in northern Eurasia, Australia, Madagascar, and the Americas, where many mammalian taxa became extinct before the Holocene (1–5), perhaps due in part to the influx of modern humans, among other factors. Comparisons of the Indian faunal record with those from Southeast and Southwest Asia suggest that long-term faunal continuity is to be expected in the Asian tropics, unless disrupted by significant changes in rainfall or sea-level. In particular, interconnected mosaic habitats, facilitated by stability in topography and precipitation, have been essential to the long-term persistence of mammalian fauna across the Indian subcontinent. Recognition of the importance of ecological mosaics to mammalian survival is increasing in modern conservation studies in India and elsewhere in Asia (45). Moreover, increasing ecological circumscription in the face of demographic growth and environmental change in the most densely populated region of the world will present a significant challenge to mammals and those invested in their protection.
Methods
OSL Dating and Stratigraphic Correlations.
OSL dating provides an estimate of the time elapsed since luminescent mineral grains were last exposed to sufficient heat or sunlight to empty the relevant electron traps. The method is based on the absorption of radiation energy by grains (quartz in this study) after the last heating or bleaching event, resulting in the trapping of electronic charge at defects in the crystal lattice. The population of trapped electrons increases with time after burial, in response to the supply of ionizing radiation from natural environmental sources (dose rate). The dose rate is mostly derived from the radioactive decay of 238U, 235U, 232Th (and their daughter products), and 40K in the deposits surrounding the dated mineral grains, together with smaller contributions from cosmic rays and from radioactive inclusions internal to the grains. Given the primary mode of sediment input to the Billasurgam cave complex (surface runoff and wind transport) and the lack of significant postdepositional reworking, the quartz grains are likely to have been well bleached by sunlight at the time of burial and remained undisturbed thereafter.
For the OSL samples in this study, we measured the beta dose rates by low-level beta counting in the laboratory and the gamma dose rates in the field using in situ gamma-ray spectrometry. The cosmic-ray dose rates were determined by using published equations, and a small contribution was assumed for the internal dose rate. Supporting data are provided in SI Appendix, SI Methods. The radiation energy absorbed by the grains during the period of burial (equivalent dose) was estimated in the laboratory by measuring the UV OSL emissions from individual grains of quartz (180–212 µm in diameter), to benefit from the advantages of single-grain OSL dating. A green laser was used to stimulate the most light-sensitive electron traps in each grain, and a dose–response curve was generated by using the single-aliquot regenerative-dose procedure, from which the equivalent dose was estimated by interpolation. The OSL data were analyzed by using well-established and objective quality-assurance criteria and tests based on sound statistical principles. The burial age of each OSL sample was obtained by dividing the weighted mean equivalent dose (after rejecting outliers) by the dose rate. Details and data are given in SI Appendix, SI Methods, Figs. S1–S3, and Tables S9–S11.
Following numerical dating, the fossil record of faunal specimens was correlated with MIS. This correlation was inferred on the basis of stratigraphic information (Fig. 2), the results of the OSL dating, and the previous identification of Toba ash in the sequence of Charnel House Cave (19) (Fig. 2).
Paleontological Sampling and Identification.
Faunal samples were excavated and collected by hand whenever possible. Samples were grouped on the basis of sediments excavated by a combination of natural layers and 10-cm spits within these layers. All sediments were dry sieved using 1- or 0.5-mm mesh to retrieve smaller elements.
Taxonomic identifications were made with the aid of comparative modern osteological and late Quaternary fossil material housed in the Archaeozoology Laboratory at Deccan College (Pune, India). Faunal analysts P. K. Thomas (Retired Reader, Department of Archaeology, Deccan College, Pune, India) and P. Joglekar (Department of Archaeology, Deccan College, Pune, India) helped with the analysis of several difficult specimens. SI Appendix, Tables S1 and S2 demonstrate the skeletal elements used to identify different genera and species. In all cases, elements were only identified to genus or species level when an excavated element was considered indistinguishable from the modern and fossil reference taxa. Carnivora from the two sites were identified on the basis of dental, phalangeal, humeral, and femoral measurements. Primates were primarily identified by using dental measurements, although some cranial comparisons were also possible. Bovids, cervids, and other Artiodactyla were similarly taxonomically categorized on the basis of dental fragments, astragali, distal phalanges, and long bone measurements. The identification of Equus sp. from both sites relied upon a comparison of dental remains with the modern collections, whereas Rhinoceros sp. identification was based on dental remains at Cathedral Cave and rib fragments at Charnal House Cave. Further details of each taxonomic identification are provided in SI Appendix, Tables S1 and S2.
Given the highly fragmentary nature of the skeletal material, taxonomic abundances were quantified according to specimen counts (number of identifiable faunal specimens). Following specimen identification, the presence or absence of each identified genus/species was recorded relative to the correlated MIS. This method provides the best representation of faunal diversity at Billasurgam through time. For further detail, see SI Appendix, SI Methods.
Spatial Comparison of Faunal Presence.
Following correlation of the presence of fossil taxa with the MIS chronology, the fossil faunal record was compared with modern faunal diversity studies from the State of Andhra Pradesh, of which Billasurgam is a part (20). The fossil faunal record was also compared to records held by the International Union for Conservation of Nature (www.iucnredlist.org) of modern faunal distributions across modern India. This process was done in order to track changes in the spatial distributions of fauna in India and surrounding regions through time.
Supplementary Material
Acknowledgments
We thank Miriam Belmaker, John de Vos, and Chris Stimpson for suggestions regarding faunal continuity in the Levant, Southeast Asia, and the Arabian Peninsula, respectively. We thank the Archaeological Survey of India for permission to conduct the fieldwork and the international team for their contributions to the excavations. This work was supported by grants from the British Academy, the Leverhulme Trust, and European Research Council Grant 295719 (to M.D.P.), and the Australian Research Council (to R.G.R.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1323465111/-/DCSupplemental.
References
- 1.Rule S, et al. The aftermath of megafaunal extinction: Ecosystem transformation in Pleistocene Australia. Science. 2012;335(6075):1483–1486. doi: 10.1126/science.1214261. [DOI] [PubMed] [Google Scholar]
- 2.Koch PL, Barnosky AD. Late Quaternary extinctions: State of the debate. Annu Rev Ecol Evol Syst. 2006;37:215–250. [Google Scholar]
- 3.Prideaux GJ, et al. Timing and dynamics of Late Pleistocene mammal extinctions in southwestern Australia. Proc Natl Acad Sci USA. 2010;107(51):22157–22162. doi: 10.1073/pnas.1011073107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lorenzen ED, et al. Species-specific responses of Late Quaternary megafauna to climate and humans. Nature. 2011;479(7373):359–364. doi: 10.1038/nature10574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prescott GW, Williams DR, Balmford A, Green RE, Manica A. Quantitative global analysis of the role of climate and people in explaining late Quaternary megafaunal extinctions. Proc Natl Acad Sci USA. 2012;109(12):4527–4531. doi: 10.1073/pnas.1113875109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brace S, et al. Serial population extinctions in a small mammal indicate Late Pleistocene ecosystem instability. Proc Natl Acad Sci USA. 2012;109(50):20532–20536. doi: 10.1073/pnas.1213322109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boivin N, Fuller DQ, Dennell R, Allaby R, Petraglia MD. Human dispersal across diverse environments of Asia during the Upper Pleistocene. Quat Int. 2013;300:32–47. [Google Scholar]
- 8.Badam GL. Pleistocene Fauna of India. Pune, India: Deccan College Postgraduate and Research Institute; 1979. [Google Scholar]
- 9.Clift PD, Plumb RA. The Asian Monsoon: Causes, History and Effects. Cambridge, UK: Cambridge Univ Press; 2008. [Google Scholar]
- 10.Petraglia MD, Haslam M, Fuller DQ, Boivin N, Clarkson C. Out of Africa: New hypotheses and evidence for the dispersal of Homo sapiens along the Indian Ocean rim. Ann Hum Biol. 2010;37(3):288–311. doi: 10.3109/03014461003639249. [DOI] [PubMed] [Google Scholar]
- 11.Mellars P, Gori KC, Carr M, Soares PA, Richards MB. Genetic and archaeological perspectives on the initial modern human colonization of southern Asia. Proc Natl Acad Sci USA. 2013;110(26):10699–10704. doi: 10.1073/pnas.1306043110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blinkhorn J, Parker AG, Ditchfield P, Haslam M, Petraglia M. Uncovering a landscape buried by the super-eruption of Toba, 74,000 years ago: A multi-proxy environmental reconstruction of landscape heterogeneity in the Jurreru Valley, south India. Quat Int. 2012;258:135–147. [Google Scholar]
- 13.Foote RB. Notes on the results of Mr H.B. Foote’s further excavations in the Billa Surgam caves. Records of the Geological Survey of India. 1885;18:227–235. [Google Scholar]
- 14.Lydekker R. The fauna of the Kurnool Caves. Palaeontologica Indica Series X. 1886;4:23–58. [Google Scholar]
- 15.Murty MLK. A Late Pleistocene cave site in southern India. Proc Am Philos Soc. 1974;118:196–230. [Google Scholar]
- 16.Reddy KT. Billasurgam: An Upper Palaeolithic cave Site in South India. Asian Perspect. 1977;20:206–277. [Google Scholar]
- 17.Petraglia MD, et al. Human occupation, adaptation and behavioral change in the Pleistocene and Holocene of South India: Recent investigations in the Kurnool District, Andhra Pradesh. Eurasian Prehistory. 2009;6:119–166. [Google Scholar]
- 18.Haslam M, et al. In Foote’s steps: The history, significance and recent archaeological investigation of the Billa Surgam caves in southern India. S Asian Stud. 2010;26(1):1–19. [Google Scholar]
- 19.Lane C, et al. Cryptotephra from the 74 ka BP Toba super-eruption in the Billa Surgam caves, southern India. Quat Sci Rev. 2011;30:1819–1824. [Google Scholar]
- 20.Srinivasulu C, Nagulu V. Mammalian and avian diversity of the Nallamala Hills, Andhra Pradesh. Zoos’ Print J. 2002;17(1):675–684. [Google Scholar]
- 21.Meiri S, Dayan T. On the validity of Bergmann’s rule. J Biogeogr. 2003;30:331–351. [Google Scholar]
- 22.Jablonski NG, Frost SR. Cercopithecoidea. In: Werdelin L, Sanders WJ, editors. Cenozoic Mammals of Africa. Berkeley, CA: Univ of California Press; 2010. pp. 393–428. [Google Scholar]
- 23.Hughes JK, Elton S, O’Regan HJ. Theropithecus and ‘Out of Africa’ dispersal in the Plio-Pleistocene. J Hum Evol. 2008;54(1):43–77. doi: 10.1016/j.jhevol.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 24.Rook L, Martínez-Navarro B. The large sized cercopithecoid from Pirro Nord and the importance of Theropithecus in the Early Pleistocene of Europe: Faunal marker for hominins dispersal outside Africa. Palaeontographica Abteilung A. 2013;298:107–112. [Google Scholar]
- 25.Delson E. Theropithecus fossils from Africa and India and the taxonomy of the genus. In: Jablonski NG, editor. Theropithecus: Rise and Fall of a Primate Genus. Cambridge, UK: Cambridge Univ Press; 1993. pp. 157–189. [Google Scholar]
- 26.Clarkson C, et al. The oldest and longest enduring microlithic sequence in India: 35 000 years of modern human occupation and change at the Jwalapuram Locality 9 rockshelter. Antiquity. 2009;83(320):326–348. [Google Scholar]
- 27.Perera N, et al. People of the ancient rainforest: Late Pleistocene foragers at the Batadomba-lena rockshelter, Sri Lanka. J Hum Evol. 2011;61(3):254–269. doi: 10.1016/j.jhevol.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 28.Prabhu CN, et al. A 200-ka pollen and oxygen-isotopic record from two sediment cores from the eastern Arabian Sea. Palaeogeogr Palaeocl. 2004;214(4):309–321. [Google Scholar]
- 29.Caley T, et al. New Arabian Sea records help decipher orbital timing of Indo-Asian monsoon. Earth Planet Sci Lett. 2011;308(3–4):433–444. [Google Scholar]
- 30.Atkinson QD, Gray RD, Drummond AJ. mtDNA variation predicts population size in humans and reveals a major Southern Asian chapter in human prehistory. Mol Biol Evol. 2008;25(2):468–474. doi: 10.1093/molbev/msm277. [DOI] [PubMed] [Google Scholar]
- 31.Petraglia M, et al. Population increase and environmental deterioration correspond with microlithic innovations in South Asia ca. 35,000 years ago. Proc Natl Acad Sci USA. 2009;106(30):12261–12266. doi: 10.1073/pnas.0810842106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Louys J. Mammal community structure of Sundanese fossil assemblages from the Late Pleistocene, and a discussion on the ecological effects of the Toba eruption. Quat Int. 2012;258:80–87. [Google Scholar]
- 33.Louys J, Meijaard E. Palaeoecology of Southeast Asian megafauna-bearing sites from the Pleistocene and a review of environmental changes in the region. J Biogeogr. 2010;37(8):1432–1449. [Google Scholar]
- 34.Louys J. Limited effect of the Quaternary’s largest super-eruption (Toba) on land mammals from Southeast Asia. Quat Sci Rev. 2007;26(25–28):3108–3117. [Google Scholar]
- 35.Long VT, de Vos J, Ciochon RL. The fossil mammal fauna of the Lang Trang Caves, Vietnam compared with Southeast Asian fossil and recent mammal faunas: The geographica; implications. Bull Indo Pac Pre Hi. 1996;14(1):101–109. [Google Scholar]
- 36.Tchernov E. The biogeographical history of the southern Levant. In: Yom-Tov Y, Tchernov E, editors. The Zoogeography of Israel. Dordrecht, The Netherlands: Dr. Junk; 1988. pp. 159–250. [Google Scholar]
- 37.Belmaker M. Hominin adaptability and patterns of faunal turnover in the Lower-Middle Pleistocene transition in the Levant. In: Camps M, Chauhan PR, editors. A Sourcebook of Paleolithic Transitions: Methods, Theories and Interpretations. New York: Springer; 2009. pp. 211–227. [Google Scholar]
- 38.Belmaker M, Hovers E. Ecological change and the extinction of the Levantine Neanderthals: Implications from a diachronic study of micromammals from Amud Cave, Israel. Quat Sci Rev. 2011;30(21–22):3196–3209. [Google Scholar]
- 39.Mercier N, et al. Hayonim Cave: A TL-based chronology for this Levantine Mousterian sequence. J Archaeol Sci. 2007;34(7):1064–1077. [Google Scholar]
- 40.Marder O, et al. Mammal remains at Rantis Cave, Israel, and Middle-Late Pleistocene human subsistence and ecology in the Southern Levant. J Quaternary Sci. 2011;26(8):769–780. [Google Scholar]
- 41.Groucutt HS, Petraglia MD. The prehistory of the Arabian peninsula: Deserts, dispersals, and demography. Evol Anthropol. 2012;21(3):113–125. doi: 10.1002/evan.21308. [DOI] [PubMed] [Google Scholar]
- 42.Thomas H, et al. First Pleistocene faunas from the Arabian Peninsula: An Nafud desert, Saudi Arabia. CR Acad Sci II A. 1998;326:145–152. [Google Scholar]
- 43.Rosenberg TM, et al. Middle and Late Pleistocene humid periods recorded in palaeolake deposits of the Nafud desert, Saudi Arabia. Quat Sci Rev. 2013;70:109–123. [Google Scholar]
- 44.Faith JT. Late Pleistocene and Holocene mammal extinctions on continental Africa. Earth Sci Rev. 2014;128:105–121. [Google Scholar]
- 45.Cranbrook E, Piper PJ. Paleontology to policy: The Quaternary history of Southeast Asian tapirs (Tapiridae) in relation to large mammal species turnover, with a proposal for conservation of Malayan tapir by reintroduction to Borneo. Integr Zool. 2013;8(1):95–120. doi: 10.1111/j.1749-4877.2012.00319.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.