Abstract
Background
We evaluated the safety and efficacy of percutaneous extracorporeal membrane oxygenation (ECMO) in patients with primary graft dysfunction after heart transplantation.
Methods
Of 65 patients (44 males and 21 females) who underwent heart transplantation from January 2006 to December 2012, 13 patients (group I) needed peripheral ECMO support due to difficulty in weaning from cardiopulmonary bypass (CPB) and 52 patients (group II) were weaned from CPB without mechanical support. The mean age of the patients at the time of operation was 54.4±13.6 years. There were no differences in the preoperative characteristics of the two groups. Multivariable analysis was performed to identify the risk factors for ECMO therapy.
Results
All group I patients were successfully weaned from ECMO after 53±9 hours of circulatory support. Early mortality occurred in four patients (1 [7.7%] in group I and 3 [5.8%] in group II, p>0.999). There were no differences in the postoperative complications between the two groups, with the exception of reoperation for bleeding. A greater number of group I patients underwent reoperation for bleeding (5 [38.5%] in group I vs. 6 [11.5%] in group II, p=0.035). In multivariable analysis, preoperative mechanical support (ECMO and intra-aortic balloon pump) and longer CPB time were the risk factors of ECMO therapy for graft dysfunction (odds ratio, 6.377; 95% confidence interval, 1.519 to 26.77; p=0.011 and odds ratio, 1.010; 95% confidence interval, 1.001 to 1.019; p=0.033).
Conclusion
Percutaneous ECMO support could be a viable option for rescuing patients when graft dysfunction refractory to medical management develops after heart transplantation.
Keywords: Heart transplantation, Extracorporeal circulation
INTRODUCTION
Heart transplantation remains the treatment of choice for patients with end-stage heart failure refractory to medical or surgical management [1]. Early graft dysfunction often occurs after implantation for various reasons including ischemia-reperfusion injury and failure of donor heart preservation [2,3]. Mechanical circulatory support for early graft dysfunction has been performed easily with a low complication rate since the introduction of peripheral cardiopulmonary support [3,4,5].
The purpose of this study was to evaluate the efficacy and safety of percutaneous veno-arterial extracorporeal membrane oxygenation (ECMO) in patients who developed early graft dysfunction after heart transplantation.
METHODS
1) Patient characteristics
From January 2006 to December 2012, 65 patients (44 males and 21 females) underwent heart transplantation at our institution. Thirteen patients (group I) needed peripheral ECMO support to be weaned from cardiopulmonary bypass (CPB). Fifty-two patients (group II) could be weaned from CPB without mechanical support. The mean patient age at the time of operation was 54.4±13.6 years. Diabetes (n=16, 24.6%) and hypertension (n=16, 24.6%) were the most common co-morbidities. There were no differences in the preoperative characteristics between the two patient groups (Table 1).
Table 1.
Preoperative characteristics of the study patients
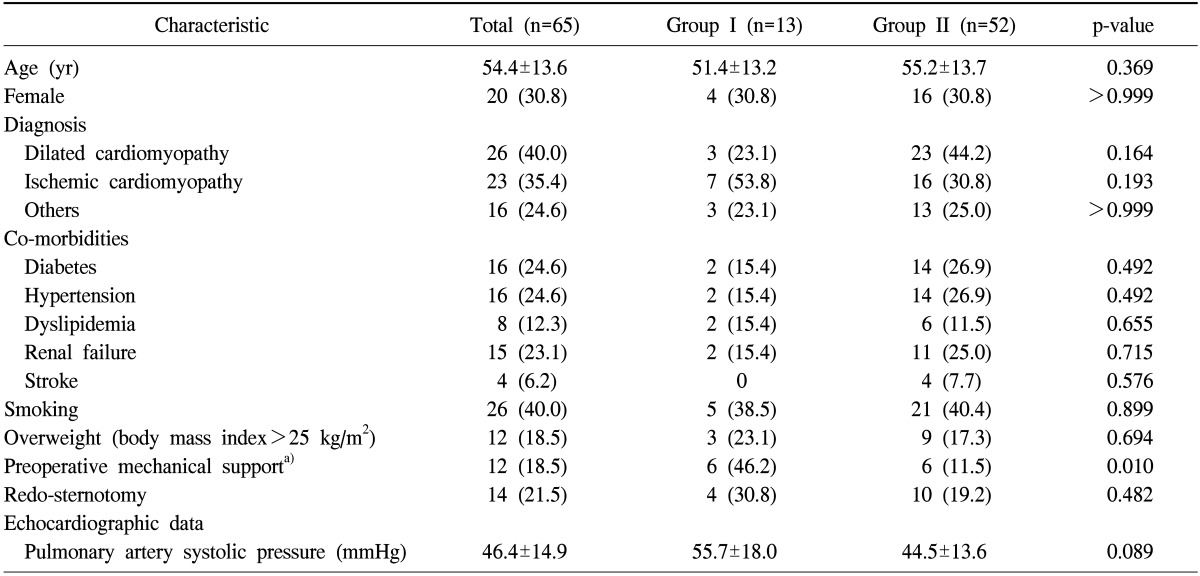
Values are presented as mean±standard deviation or number (%).
DCMP, dilated cardiomyopathy; ICMP, ischemic cardiomyopathy; PSAP, pulmonary artery systolic pressure.
a)Includes extracorporeal membrane oxygenation and intra-aortic balloon counterpulsation.
2) Operative strategies and perioperative management
The bicaval anastomotic technique for cardiac transplantation was used in all patients. During weaning from CPB after the completion of transplantation, graft function recovery was assessed by transthoracic echocardiography. When the recipient failed to be weaned from CPB, CPB support was maintained for an additional 10 minutes. Additionally, pharmacologic treatment, including dopamine, dobutamine, isoproterenol, and milrinone, was initiated. If the recipient was not weaned after three attempts of CPB support, ECMO insertion was considered.
Primary graft dysfunction was defined as the need for support with ECMO in the postoperative 48-hour period. The indications of mechanical support were as follows: (1) hemodynamic instabilities including systemic arterial pressure of <90 mmHg, pulmonary artery systolic pressure of >60 mmHg, and bradycardia (<80/min) under maximal pharmacologic support and (2) intraoperative transesophageal echocardiographic findings of decreased right ventricular or left ventricular function. ECMO was applied through the femoral artery and vein using a commercially available circuit (Capiox EBS; Terumo, Tokyo, Japan). Heparin was initially neutralized with protamine when stopping CPB and initiating ECMO support. If surgical bleeding was stopped at the intensive care unit, patients periodically received heparin for maintaining an activated clotting time of more than 180 seconds.
3) Evaluation of clinical results
Early mortality was defined as any death within 30 days after surgery. In-hospital mortality was defined as death during the same hospitalization. The occurrence of any short runs (>30 seconds) of atrial fibrillation during the hospital stay was considered to represent the development of atrial fibrillation. Respiratory complications included postoperative pneumonia or prolonged ventilator support of >48 hours. Acute renal failure was defined as an increase of >50% in the serum creatinine level from the preoperative value or a need for renal replacement therapy irrespective of the serum creatinine level. A risk factor analysis was performed for identifying the predictors of graft dysfunction requiring ECMO support.
4) Organ preservation and immune suppression protocol
Donor heart was preserved using a single flush of 10 to 20 mg/kg histidine-tryptophan-ketoglutarate solution. During transportation, the heart was immersed in a cold saline bag packed with ice. The immunosuppressive protocols included the following: (1) a calcineurin inhibitor (cyclosporine before 2009 and tacrolimus after 2009), (2) an antiproliferative agent (azathioprine before 1999 and mycophenolate mofetil after 1999), and (3) corticosteroids. In addition to these so-called triple regimens, interleukin-2 has been used since 2009. Intravenous methylprednisolone (500 mg) was administered just before releasing the aortic cross clamp, followed by three additional doses (150 mg q8h).
5) Statistical analyses
Statistical analyses were performed using the PASW SPSS ver. 18.0 (SPSS Inc., Chicago, IL, USA). A univariate analysis was performed using the χ2 test or Fisher's exact test for categorical variables and the Student t-test for continuous variables. A multivariate analysis was performed using a logistic regression analysis. Variables with a p-value of <0.2 in univariate analyses were entered into the multivariate analysis. A p-value of <0.05 was considered statistically significant.
RESULTS
1) Operative data
The mean cardiopulmonary bypass and donor ischemic times were 271±73 minutes and 154±53 minutes, respectively. There were no differences in the CPB time and the donor ischemic time between the two groups, with the exception of donor age; group I donors were older than group II donors (41.3±9.2 years vs. 34.0±11.3 years, p=0.036) (Table 2).
Table 2.
Operative data of the study patients
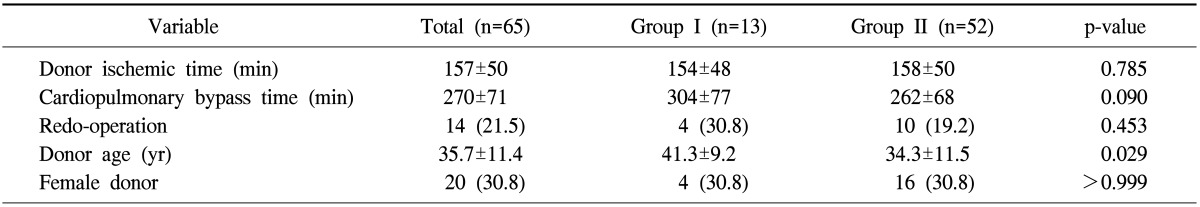
Values are presented as mean±standard deviation or number (%).
2) Extracorporeal membrane oxygenation results of group I patients
Heparin-induced anticoagulation was initiated 25±16 hours postoperatively. All patients were successfully weaned from ECMO after 53±9 hours of support. No patient suffered from ECMO-related complications such as vascular injury, lower leg ischemia, or thromboembolic complications.
3) Clinical outcomes
Early mortality occurred in four patients (1 [7.7%] in group I vs. 3 [5.8%] in group II, p>0.999). Three patients died of pneumonia on postoperative days 13, 22, and 28, and one patient died of sudden cardiac arrest on postoperative day 30. Postoperative morbidities included bleeding reoperation (n=11, 16.9%), respiratory complication (n=9, 13.8%), and stroke (n=4, 6.2%). A greater proportion of group I patients underwent reoperation for bleeding than group II patients (5 [38.5%] in group I vs. 6 [11.5%] in group II, p=0.035). All bleeding reoperations were performed before anticoagulation was re-initiated. There were no differences in other postoperative complications between the two groups (Table 3).
Table 3.
Early clinical results
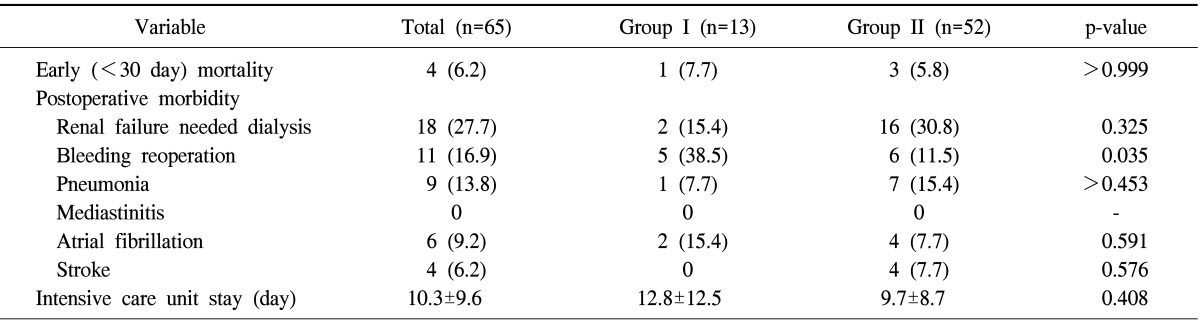
Values are presented as number (%) or mean±standard deviation.
4) Risk factor analysis for extracorporeal membrane oxygenation insertion
Univariate analyses revealed that high donor age and preoperative mechanical support (ECMO and intra-aortic balloon pump) were significant factors. In the multivariate logistic regression analysis, preoperative mechanical support and longer CPB time were the risk factors of ECMO requirement (Table 4).
Table 4.
Risk factor analysis for graft dysfunction requiring extracorporeal membrane oxygenation
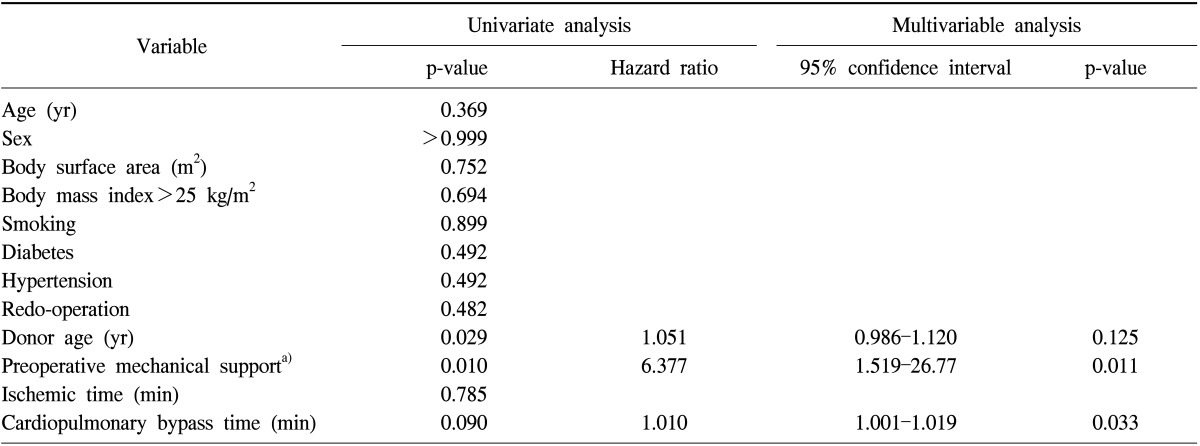
a)Includes extracorporeal membrane oxygenation and intra-aortic balloon counterpulsation.
DISCUSSION
The present study revealed two main findings. First, percutaneous ECMO could be a useful option for rescuing patients suffering from early postoperative graft dysfunction after heart transplantation. Second, preoperative mechanical support and longer CPB time are risk factors of ECMO requirement.
Primary graft failure after heart transplantation is a rare but catastrophic complication; it is responsible for one-third of the deaths in the early post-transplantation period [6]. The prevalence of graft failure was reported to be 2.4% to 20% [10,11,12,13,14], and the survival rate after primary graft dysfunction varied from 3.7% to 40% [6,7,15]. The wide variability in outcomes is explained by the differences in the definitions of graft dysfunction and risk profiles of the study patients [6]. The etiologies of early graft dysfunction are believed to be multifactorial and include ischemia-reperfusion injury, failure of donor heart preservation, acute rejection, and pre-existing high pulmonary vascular resistance [2,3]. When a failed graft is refractory to medical management, including maximal inotropic support and the use of inhaled nitric oxide, mechanical circulatory support could be an option for rescuing patients with early graft dysfunction [2,3,6]. In the current study, the proportion of patients requiring ECMO application was higher than that in previous studies. We applied peripheral ECMO early upon discovering any sign of graft dysfunction to ensure that percutaneous mechanical circulatory support could be offered easily with minimal complications [3,4]. Furthermore, all patients were weaned from ECMO 53±9 hour after application without vascular injury and thromboembolic complications. Early mortality in the ECMO group occurred in one patient and was not related to ECMO application. The high ECMO weaning and survival rates probably resulted from the prevention of irreversible deterioration of the transplanted heart owing to early ECMO application. One of the major complications in the ECMO group was the higher rate of bleeding reoperation than that in the non-ECMO group. In the present study, protamine was administered for normalizing the prolonged activated clotting time after CPB weaning, and heparin was restarted after the cessation of surgical bleeding was confirmed in the intensive care unit. However, bleeding reoperation before restarting anticoagulation occurred in 38.5% of the patients. Longer CPB times and ECMO circuit-induced coagulopathy might have elevated the risk of bleeding.
Previous studies suggested preoperative mechanical circulatory support, high donor age, and long ischemic time as the risk factors of early graft dysfunction [7,8]. The risk of graft failure increased by 43% with the passage of every hour of ischemic time after hour 4 [9]. In the present study, preoperative mechanical circulatory support and longer CPB time were the risk factors of ECMO support. A greater inflammatory response, which might have resulted from preoperative mechanical circulatory support and longer CPB times, could have influenced the occurrence of primary graft failure [7]. The ischemic time was not a significant risk factor probably because it did not exceed 4 h in a majority of the patients. Given that the causes of primary graft dysfunction were known to be multifactorial, efforts to decrease the incidence of organ dysfunction should be made, including the following: 1) appropriate donor selection, 2) optimal donor heart preservation, 3) minimization of the CPB and donor ischemic times via co-ordination between the donor procurement and recipient operation teams, and 4) effective immunosuppressive therapy.
There are certain limitations of the present study that must be recognized. First, the study was a retrospective observational study conducted in a single institution. Second, the number of enrolled patients was relatively small to draw a definite conclusion. Third, ECMO indications were not precisely defined because of the retrospective nature of this study.
In conclusion, when graft dysfunction after heart transplantation is refractory to medical management, percutaneous ECMO insertion might be a valuable option for rescuing patients. Strict coagulopathy control seems to be necessary for avoiding bleeding complications.
Footnotes
Presented at the 44th Annual Meeting of The Korean Society for Thoracic and Cardiovascular Surgery, Pusan, Korea, November 1st-3rd, 2012.
No potential conflict of interest relevant to this article was reported.
References
- 1.Taghavi S, Zuckermann A, Ankersmit J, et al. Extracorporeal membrane oxygenation is superior to right ventricular assist device for acute right ventricular failure after heart transplantation. Ann Thorac Surg. 2004;78:1644–1649. doi: 10.1016/j.athoracsur.2004.04.059. [DOI] [PubMed] [Google Scholar]
- 2.Taghavi S, Ankersmit HJ, Wieselthaler G, et al. Extracorporeal membrane oxygenation for graft failure after heart transplantation: recent Vienna experience. J Thorac Cardiovasc Surg. 2001;122:819–820. doi: 10.1067/mtc.2001.115692. [DOI] [PubMed] [Google Scholar]
- 3.Marasco SF, Vale M, Pellegrino V, et al. Extracorporeal membrane oxygenation in primary graft failure after heart transplantation. Ann Thorac Surg. 2010;90:1541–1546. doi: 10.1016/j.athoracsur.2010.05.066. [DOI] [PubMed] [Google Scholar]
- 4.Sung K, Lee YT, Park PW, et al. Improved survival after cardiac arrest using emergent autopriming percutaneous cardiopulmonary support. Ann Thorac Surg. 2006;82:651–656. doi: 10.1016/j.athoracsur.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 5.Lee WS, Chee HK, Song MG, et al. Short-term mechanical circulatory support with a centrifugal pump: results of peripheral extracorporeal membrane oxygenator according to clinical situation. Korean J Thorac Cardiovasc Surg. 2011;44:9–17. doi: 10.5090/kjtcs.2011.44.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mihaljevic T, Jarrett CM, Gonzalez-Stawinski G, et al. Mechanical circulatory support after heart transplantation. Eur J Cardiothorac Surg. 2012;41:200–206. doi: 10.1016/j.ejcts.2011.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.D'Alessandro C, Golmard JL, Barreda E, et al. Predictive risk factors for primary graft failure requiring temporary extra-corporeal membrane oxygenation support after cardiac transplantation in adults. Eur J Cardiothorac Surg. 2011;40:962–969. doi: 10.1016/j.ejcts.2011.01.064. [DOI] [PubMed] [Google Scholar]
- 8.D'Alessandro C, Aubert S, Golmard JL, et al. Extra-corporeal membrane oxygenation temporary support for early graft failure after cardiac transplantation. Eur J Cardiothorac Surg. 2010;37:343–349. doi: 10.1016/j.ejcts.2009.05.034. [DOI] [PubMed] [Google Scholar]
- 9.Marasco SF, Esmore DS, Negri J, et al. Early institution of mechanical support improves outcomes in primary cardiac allograft failure. J Heart Lung Transplant. 2005;24:2037–2042. doi: 10.1016/j.healun.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 10.Taylor DO, Edwards LB, Aurora P, et al. Registry of the International Society for Heart and Lung Transplantation: twenty-fifth official adult heart transplant report--2008. J Heart Lung Transplant. 2008;27:943–956. doi: 10.1016/j.healun.2008.06.017. [DOI] [PubMed] [Google Scholar]
- 11.Russo MJ, Iribarne A, Hong KN, et al. Factors associated with primary graft failure after heart transplantation. Transplantation. 2010;90:444–450. doi: 10.1097/TP.0b013e3181e6f1eb. [DOI] [PubMed] [Google Scholar]
- 12.Young JB, Hauptman PJ, Naftel DC, et al. Determinants of early graft failure following cardiac transplantation, a 10-year, multi-institutional, multivariable analysis. J Heart Lung Transplant. 2001;20:212. doi: 10.1016/s1053-2498(00)00460-5. [DOI] [PubMed] [Google Scholar]
- 13.Tissot C, Buckvold S, Phelps CM, et al. Outcome of extracorporeal membrane oxygenation for early primary graft failure after pediatric heart transplantation. J Am Coll Cardiol. 2009;54:730–737. doi: 10.1016/j.jacc.2009.04.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Segovia J, Pulpon LA, Sanmartin M, et al. Primary graft failure in heart transplantation: a multivariate analysis. Transplant Proc. 1998;30:1932. doi: 10.1016/s0041-1345(98)00485-0. [DOI] [PubMed] [Google Scholar]
- 15.Chou NK, Chi NH, Ko WJ, et al. Extracorporeal membrane oxygenation for perioperative cardiac allograft failure. ASAIO J. 2006;52:100–103. doi: 10.1097/01.mat.0000196514.69525.d9. [DOI] [PubMed] [Google Scholar]


