Abstract
Background
We aimed to investigate the epidemiological, clinical, paraclinical, and treatment aspects of elastofibroma dorsi through a retrospective study of 76 patients who underwent surgery between January 2008 and December 2012 in our department.
Methods
Our study is retrospective between January 2008 and December 2012. We admitted 79 patients with a subscapular mass, and only 76 patients had ED. The others (n=2) had high associated risk of anesthesia and were managed by a medical treatment and one patient had a subscapular sclerotic hemangioma.
Results
The average age of the patients was 49 years (range, 38 to 70 years), with a female predominance (54 females and 22 males). Subscapular location was constant. The right, left, and bilateral form was noted in 41, 15 and 20 cases, respectively. The diagnosis was clinical in 60 cases. Ultrasound and computerized tomography scans confirmed the diagnosis of an ill-defined mass in a subscapular location in all cases. Surgical treatment consisted of complete resection of the mass. The clinical diameter of the mass remained significantly lower than that of the surgical specimen (7 cm versus 12 cm) because the major hidden part of the mass in the subscapular area was inaccessible to palpation. Complications were noted in 9 cases (11.8%), seroma in 8 cases (10.5%), infection of wound site in 4 cases (5%), and parietal textilome in one case (1%). No case of recurrence was noted.
Conclusion
Surgery of elastofibroma is unique because of the subscapular location of the parietal tumor, whose histological fibrous nature makes it very adherent to the chest wall.
Keywords: Elastofibroma, Subscapular mass, Surgery
INTRODUCTION
In 1961, Jarvi and Saxen [1] first described an uncommon, non-encapsulated, benign tumor in the subscapular region characterized by the proliferation of elastin fibers in a stroma of collagen and fatty connective tissue. Elastofibroma dorsi (ED) is a relatively rare soft-tissue pseudo-tumor localized in the infra- or periscapular area (under the rhomboid and serratus anterior muscles). It usually occurs between the fourth and the seventh decade of life and is more common in females [2,3]. Further, it is a degenerative pseudo-tumor with the clinical appearance of a malignant tumor because clinically, the lesion is firmly attached to the rib cage. Recognition of the lesion is important because the differential diagnosis includes malignant tumors. Less common elastofibroma sites include the olecranon, orbits, greater trochanter, deltoid muscle, foot, inguinal region, tricuspid valve, stomach, greater omentum, axilla, and the intraspinal space [3].
In this study, we retrospectively describe a series of 76 patients with ED and discuss its clinical and radiological findings as well as its pathologic and therapeutic features. In addition, we present an algorithm to diagnose and treat this lesion while avoiding the use of unnecessary medical, radiological, or surgical interventions to establish the diagnosis.
METHODS
Our retrospective study was from January 2008 to December 2012. We admitted 79 patients with a subscapular mass, and only 76 patients had ED (54 females and 22 males). The mean age was 49 years (range, 38 to 70 years). Among the others, 2 patients had a high associated risk of anesthesia and were managed with a medical treatment, and 1 patient had a subscapular sclerotic hemangioma.
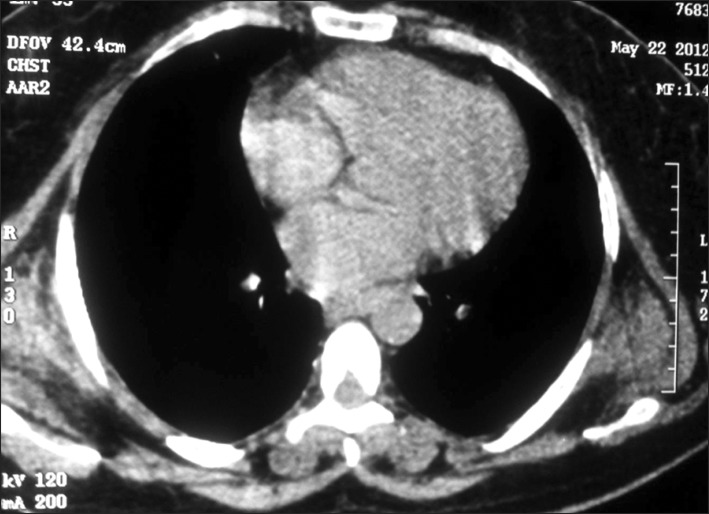
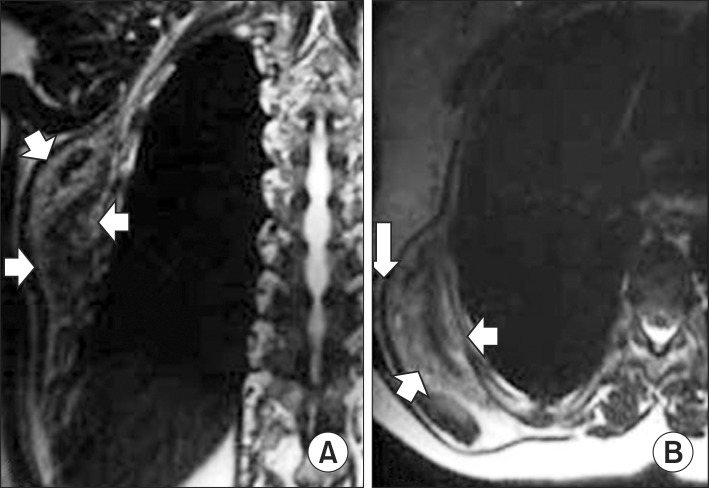
We proceeded with the diagnostic and treatment protocols that we usually follow for patients with thoracic tumors: clinical history, physical examination with special attention to clinical signs of the tumor, chest X-ray, ultrasound (US) of soft tissue, and computed tomography (CT) (Fig. 1) of the thorax or magnetic resonance imaging (MRI) (Fig. 2). We did not use preoperative histological evaluation, and we suggest biopsy only when the mass has enlarged rapidly within a few months or presents atypical features.
Fig. 1.
Computed chest scan showing a bilateral solid ill-defined mass of the subscapular area.
Fig. 2.
Magnetic resonance imaging features of typical elastofibroma dorsi. (A) An axial and (B) sagittal T1-weighted image showing a heterogeneous, lenticular, and poorly circumscribed mass (arrows) deep into the muscles and adjacent to the chest wall. The intermediate signal intensity of the lesion is similar to that of the adjacent skeletal muscle, with interspersed linear areas of increased signal intensity similar to that of fat.
All patients were operated on under general anesthesia in a lateral decubitus position, thus enabling free movement of the affected arm. The lesion was totally excised via a posterior thoracotomy. All patients were followed-up once in a month for at least six months, and at intervals of 3 months subsequently.
RESULTS
Repetitive professional microtrauma was found in 19 cases (25%), and only 1 patient had a history of recent chest trauma. The dominant clinical symptoms noted in the physical exam were pain in movement and the mass. ED was bilateral in 20 cases (26%). The results are summarized in Table 1.
Table 1.
Distribution of clinical radiologic and pathologic findings of a patient with a subscapular mass
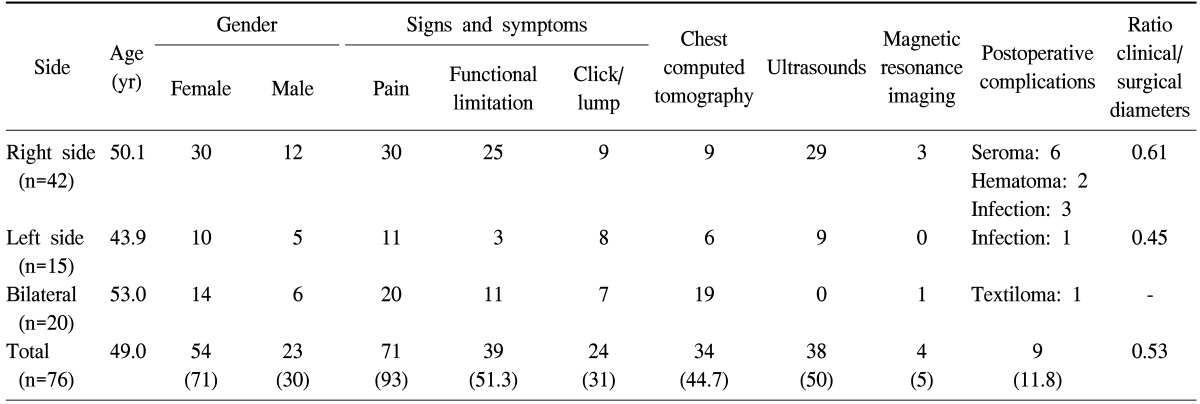
Values are presented as number or number (%).
Surgical treatment was performed if the lesion was symptomatic. In the case of bilateral lesions, we begin from the most painful, function-limiting side or from the biggest lesion's side.
In surgery, the lesion was evident underneath the latissimus dorsi muscle, adhering tenaciously to the deeper planes, but cleavable from the overlying muscle plane. Macroscopically, it was un-capsulated and relatively ill defined compared with its surroundings, rubbery, and contained grayish white fibrous tissue with small interposing areas of adipose tissue.
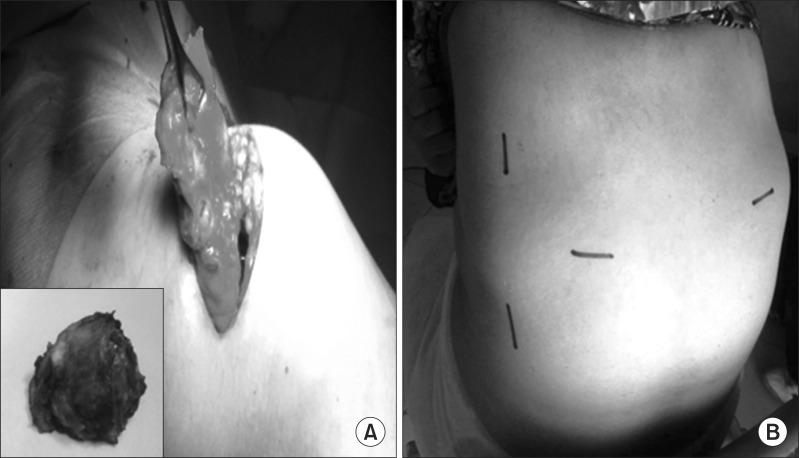
In 5 cases (6.5%), partial detachment of the rhomboids was obligatory for enabling resection. The clinical diameter (Fig. 3) of the mass ranged from 6 to 18 cm in the greatest dimension. The real diameters of the excised lesions were 10 to 22 cm.
Fig. 3.
(A) Clinical findings and (B) preoperative view of elastofibroma dorsi.
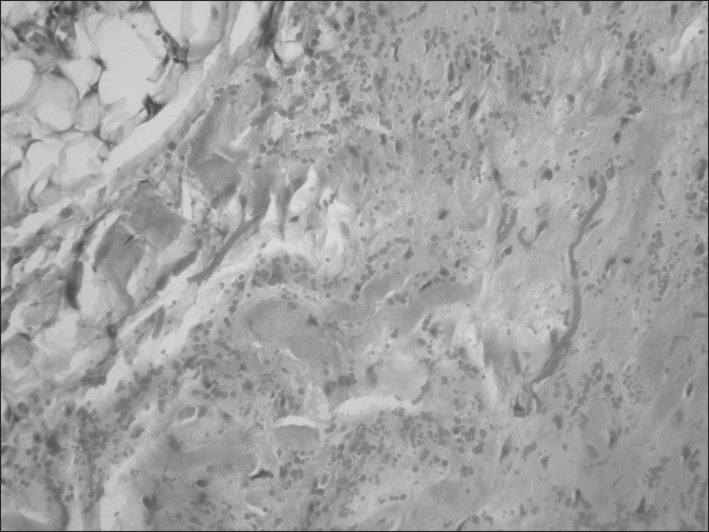
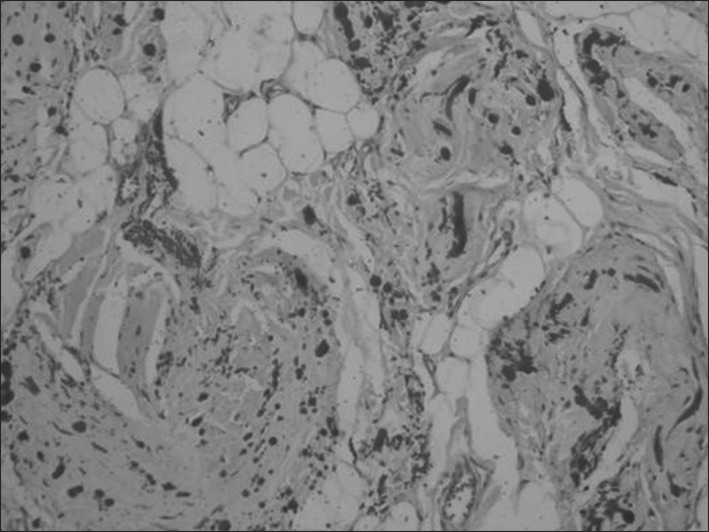
Synchronic bilateral resection was not performed in any case. We performed sequential surgery to allow free activity of the non-operated shoulder. A majority of patients (85.5%, n=65) were discharged with the removal of a drain on the third day following surgery. Eleven patients underwent anticoagulant therapy; therefore, we maintained the drain for 5 days after surgery to prevent postoperative seroma of the wound site. Postoperative seroma requiring needle aspiration developed in 8 patients, infections of the wound site developed in 4 patients, and 1 patient had textiloma. In fact, the textiloma was not postoperative but occurred during the management of an infected wound site. No patient had residual symptoms or local recurrence. The histological study conducted with standard staining (hematoxylin-eosin) revealed the presence of islets of fatty tissue and a scantly cellular component (fibroblasts and myofibroblasts) associated with intensely eosinophilic bands composed of collagen and elastic fibers that define this type of lesion (Fig. 4). Elastic stains (Weigert) showed that the large branched or unbranched fibers had a dense core and irregular serrated margins. Although the elastin-like material was removed by prior treatment of the sections with pancreatic elastase, it was more resistant to digestion than the control skin (Fig. 5).
Fig. 4.
Original pathologic findings showing fibrotic tissue with few cells and eosinophilic, partly swollen, plump collagen fibers and fatty tissue. Elastic stain highlights the abnormal elastic fibers (×100).
Fig. 5.
Weigert's elastic stain highlights the beadlike arrangement of the abnormal elastic fibers (×100).
In one case where the patient had recently suffered chest trauma, the pathologic diagnosis showed a tumor with various amounts of hemangiomatous components and focal hemorrhagic areas suggestive of a sclerotic hemangioma.
DISCUSSION
The literature on ED consists mostly of isolated case reports and small series, furthering the misconception that ED is a rare condition. The etiology of ED remains to be fully understood, but most researchers believe that ED results from reactive hyperproliferation of fibroblastic tissue rather than a degenerative process, while others have suggested that it may be a consequence of collagen degeneration due to repeated mechanical friction between the chest wall and the scapular tip [4]. However, a lack of association between trauma and tumor growth has been reported by several authors [5,6].
Based on the discovery of 'pre-elastofibroma changes' in their autopsy series, Giebel et al. [4] speculated that ED might be the result of a physiologic aging process secondary to vascular insufficiency as opposed to abnormal elastogenesis or degeneration. The few published cases of multiple familial occurrences supported the possibility of a genetic enzymatic defect, as proposed by Fukuda et al. [7], Nagamine et al. [8], and Enjoji et al. [9]. Recently, cytogenetic analyses of elastofibroma have revealed significant chromosomal instability manifested as both clonal and nonclonal structural alterations [10]. In asymptomatic patients, the estimated prevalence of ED is 2% [5]. In an autopsy series, individuals over the age of 50 presented a prevalence of subclinical ED, reaching 24% in women and 11% in men [11]. Although the prevalence of bilaterality reached (7%) in the autopsy series [11,12], ED is unilateral in most cases, and bilateral occurrence is very rare; we found very few cases reports on the latter in the English and French literature. ED is usually asymptomatic and is incidentally discovered by patients with the development of a mass in the subscapular region. The symptoms are often mild and include pain, snapping, clicking, or scapular clunking with movement. Occasionally, clinical signs can vary, and ED can be misdiagnosed as a rotator cuff tear or subacromial bursitis.
Radiographs are usually normal and may show elevation of the scapula and a soft-tissue mass without calcification in the subscapular region. Therefore, imaging-based diagnosis is usually commenced with US, and a sub- or periscapular, inhomogeneous, fasciculated pattern is most frequently seen in addition to the highly characteristic location of the lesion [13]. On CT (Fig. 1), ED is a poorly circumscribed mass, isodense with the surrounding musculature, and with characteristic hypodense striations suggestive of dense fat. Bone erosion and invasion are rare, having been reported in only one case of an intra-articular elastofibroma [14]. MRI (Fig. 2), which is considered the main imaging technique for its diagnosis, clearly shows a heterogeneous ill-defined lesion with an alternating pattern of fibrous tissue and fatty tissue [15]. On T1-weighted MRI, EDs are isointense with the muscle tissue, which explains why these tumors are often overlooked. Both T1- and T2-weighted images show alternating linear and curvilinear, hyperintense areas representing fat [16]. Lastly, in positron emission tomography-CT images, ED is seen as a non-encapsulated mass with low-grade, diffuse 18F fluorodeoxyglucose uptake [17].
In our opinion, incomplete radiological investigations (US, CT, and MRI) can succeed in clarifying the nature of the lesion because the differential diagnosis is with other soft tissue neoplasms (sarcoma, metastases, fibromatosis, and so on). Our diagnostic strategy is always based on US and less frequently on CT. According to some authors, only CT and MRI can rule out malignancy. In contrast, several authors have reported that US is a useful diagnostic adjunct for this lesion [18]. Preoperative biopsy is not our standard practice. We believe that biopsy is necessary only when the mass has enlarged rapidly within a few months or the clinical or radiological signs are atypical.
The treatment of ED remains controversial. Excision may be offered to symptomatic patients, with curative marginal resection proving to be sufficient and preferred over radical resection; conservative treatment is recommended in elderly, asymptomatic patients because malignant transformation has not been reported [19].
In general, surgery is suggested depending on the severity of symptoms and patient preference. Our own approach is simple: In the case of a single asymptomatic mass at the typical location, clinical follow-up is sufficient. However, if the mass is symptomatic, marginal excision is prescribed. Therefore, we insist that resection be R0 (negative macroscopic and microscopic margins) to enable free follow-up of the disease. Furthermore, the few reported recurrences in the literature resulted from incomplete excision [8]. Postoperative complications such as seroma or hematoma were reported [18]. The use of postoperative tube drainage and compressing bandage reduces the incidence of these complications. Considering that the patients are usually of an advanced age, to avoid immobilization with the risk of residual stiffness in the shoulder girdle, we proceeded with the punctuation of the seroma. The size of the lesion at presentation was usually inferior to the real diameter obtained by surgery (clinical/surgical diameters=0.53) (Table 1), and we ascribe this difference to the involvement of the lesion between the scapula and the chest wall. A few anecdotal reports mentioned good results with radiotherapy as well [20].
Histologically, fibrous collagenous strands with eosinophilic, plump, elongated elastic fibers are seen. The lesion is hypocellular, and it contains entrapped islands of adipose tissue with benign fibrocytic and fibroblastic cells without atypia or mitotic activity [8,21]. The differential diagnosis of subscapular ED includes tumors with dominant fibrous composition signal in low MRI (fibroma, desmoid tumor, neurofibroma, and malignant tumors) because the mass is uncapsulated and poorly circumscribed, tumors with fatty tissue (lipoma, liposarcoma, and differentiated hemangioma), or collections containing hemoglobin degradation products (subacute hematoma and tumors with necrosis) [22].
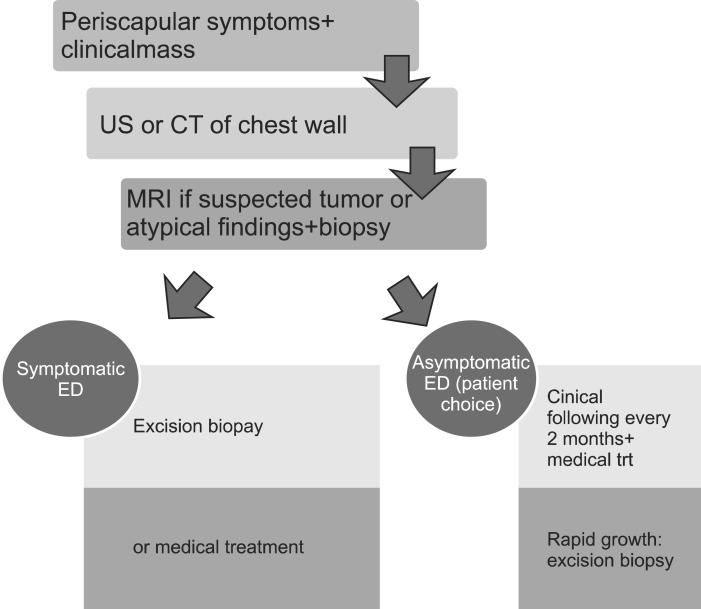
In conclusion, ED is not a rare condition, but a benign lesion of the sub- and periscapular area. Its clinical and radiological diagnosis based on the typical location and very suggestive preoperative biopsy. Surgery, if indicacharacteristics allowed a majority of the authors to abandon ted, yielded good results. We propose our diagnostic strategy in the form of the algorithm in Fig. 6.
Fig. 6.
Algorithm of our diagnostic and therapeutic strategy in the management of elastofibroma dorsi. US, ultrasound; CT, computed tomography; MRI, magnetic resonance imaging; ED, elastofibroma dorsi.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Jarvi O, Saxen E. Elastofibroma dorse. Acta Pathol Microbiol Scand Suppl. 1961;51(Suppl 144):83–84. [PubMed] [Google Scholar]
- 2.Kara M, Dikmen E, Kara SA, Atasoy P. Bilateral elastofibroma dorsi: proper positioning for an accurate diagnosis. Eur J Cardiothorac Surg. 2002;22:839–841. doi: 10.1016/s1010-7940(02)00475-x. [DOI] [PubMed] [Google Scholar]
- 3.Schick S, Zembsch A, Gahleitner A, et al. Atypical appearance of elastofibroma dorsi on MRI: case reports and review of the literature. J Comput Assist Tomogr. 2000;24:288–292. doi: 10.1097/00004728-200003000-00020. [DOI] [PubMed] [Google Scholar]
- 4.Giebel GD, Bierhoff E, Vogel J. Elastofibroma and pre-elastofibroma: a biopsy and autopsy study. Eur J Surg Oncol. 1996;22:93–96. doi: 10.1016/s0748-7983(96)91781-3. [DOI] [PubMed] [Google Scholar]
- 5.Marin ML, Perzin KH, Markowitz AM. Elastofibroma dorsi: benign chest wall tumor. J Thorac Cardiovasc Surg. 1989;98:234–238. [PubMed] [Google Scholar]
- 6.Stemmermann GN, Stout AP. Elastofibroma dorsi. Am J Clin Pathol. 1962;37:499–506. doi: 10.1093/ajcp/37.5.499. [DOI] [PubMed] [Google Scholar]
- 7.Fukuda Y, Miyake H, Masuda Y, Masugi Y. Histogenesis of unique elastinophilic fibers of elastofibroma: ultrastructural and immunohistochemical studies. Hum Pathol. 1987;18:424–429. doi: 10.1016/s0046-8177(87)80026-6. [DOI] [PubMed] [Google Scholar]
- 8.Nagamine N, Nohara Y, Ito E. Elastofibroma in Okinawa: a clinicopathologic study of 170 cases. Cancer. 1982;50:1794–1805. doi: 10.1002/1097-0142(19821101)50:9<1794::aid-cncr2820500925>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 9.Enjoji M, Sumiyoshi K, Sueyoshi K. Elastofibromatous lesion of the stomach in a patient with elastofibroma dorsi. Am J Surg Pathol. 1985;9:233–237. doi: 10.1097/00000478-198503000-00007. [DOI] [PubMed] [Google Scholar]
- 10.Batstone P, Forsyth L, Goodlad J. Clonal chromosome aberrations secondary to chromosome instability in an elastofibroma. Cancer Genet Cytogenet. 2001;128:46–47. doi: 10.1016/s0165-4608(01)00394-6. [DOI] [PubMed] [Google Scholar]
- 11.Kourda J, Ayadi-Kaddour A, Merai S, Hantous S, Miled KB, Mezni FE. Bilateral elastofibroma dorsi. A case report and review of the literature. Orthop Traumatol Surg Res. 2009;95:383–387. doi: 10.1016/j.otsr.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 12.Turna A, Yilmaz MA, Urer N, Bedirhan MA, Gurses A. Bilateral elastofibroma dorsi. Ann Thorac Surg. 2002;73:630–632. doi: 10.1016/s0003-4975(01)02862-4. [DOI] [PubMed] [Google Scholar]
- 13.Battaglia M, Vanel D, Pollastri P, et al. Imaging patterns in elastofibroma dorsi. Eur J Radiol. 2009;72:16–21. doi: 10.1016/j.ejrad.2009.05.024. [DOI] [PubMed] [Google Scholar]
- 14.Kastner M, Salai M, Fichman S, Heller S, Dudkiewicz I. Elastofibroma at the scapular region. Isr Med Assoc J. 2009;11:170–172. [PubMed] [Google Scholar]
- 15.Malghem J, Baudrez V, Lecouvet F, Lebon C, Maldague B, Vande Berg B. Imaging study findings in elastofibroma dorsi. Joint Bone Spine. 2004;71:536–541. doi: 10.1016/j.jbspin.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 16.Kransdorf MJ, Meis JM, Montgomery E. Elastofibroma: MR and CT appearance with radiologic-pathologic correlation. AJR Am J Roentgenol. 1992;159:575–579. doi: 10.2214/ajr.159.3.1503030. [DOI] [PubMed] [Google Scholar]
- 17.Tetikkurt C, Tetikkurt S, Bayar N. Diagnosis of elastofibroma. Can Respir J. 2008;15:217–218. doi: 10.1155/2008/638624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parratt MT, Donaldson JR, Flanagan AM, et al. Elastofibroma dorsi: management, outcome and review of the literature. J Bone Joint Surg Br. 2010;92:262–266. doi: 10.1302/0301-620X.92B2.22927. [DOI] [PubMed] [Google Scholar]
- 19.Daigeler A, Vogt PM, Busch K, et al. Elastofibroma dorsi: differential diagnosis in chest wall tumours. World J Surg Oncol. 2007;5:15. doi: 10.1186/1477-7819-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deutsch GP. Elastofibroma dorsalis treated by radiotherapy. Br J Radiol. 1974;47:621–623. doi: 10.1259/0007-1285-47-561-621. [DOI] [PubMed] [Google Scholar]
- 21.Chandrasekar CR, Grimer RJ, Carter SR, et al. Elastofibroma dorsi: an uncommon benign pseudotumour. Sarcoma. 2008;2008:756565. doi: 10.1155/2008/756565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sundaram M, McGuire MH, Schajowicz F. Soft-tissue masses: histologic basis for decreased signal (short T2) on T2-weighted MR images. AJR Am J Roentgenol. 1987;148:1247–1250. doi: 10.2214/ajr.148.6.1247. [DOI] [PubMed] [Google Scholar]