Abstract
We present a case of a 55-year-old woman who complained of chest pain at rest. A mass was detected adjacent to her left atrium. The mass was completely excised, and a pathologic examination revealed it to be a schwannoma. Schwannomas are tumors that originate in the nerve sheath and are rarely detected in the heart. Here, we describe a rare case of primary schwannoma of the left atrium.
Keywords: Schwannoma, Cardiac tumor, Heart neoplasms
CASE REPORT
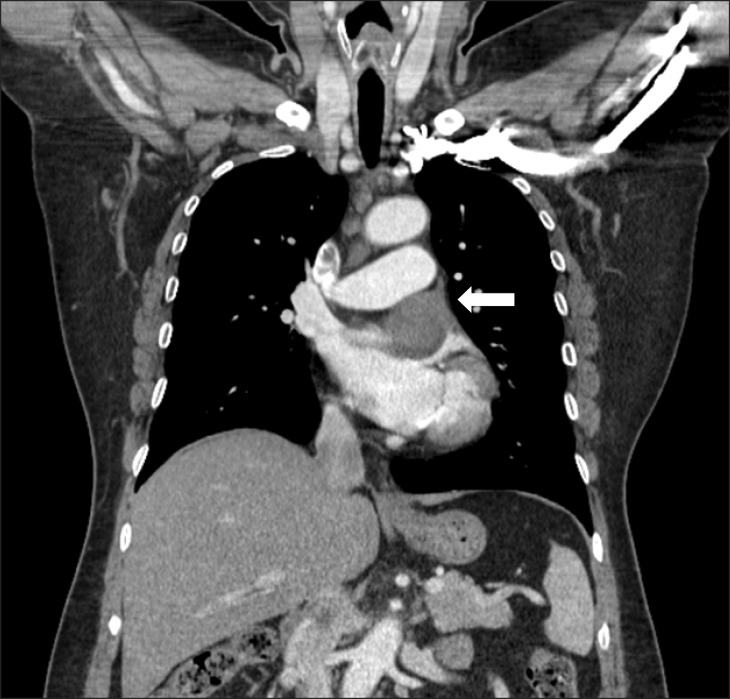
The patient was a 55-year-old woman with a four-year history of chest pain at rest. The symptom was aggravated at night when she was lying down. She visited a cardiologist and was examined by treadmill testing, but no abnormal findings were noted. The patient subsequently developed orthopnea, for which she was medically treated at a clinic; however, this treatment was not successful. After experiencing sudden left-sided chest pain, she visited an emergency department, at which time she was examined by chest radiography and computed tomography (CT) (Fig. 1). The CT scan indicated the presence of a mass in the left pericardial area. The mass was located between the left superior pulmonary vein and the left atrial appendage with a pericardial tail. Therefore, the patient visited our medical center, where she was examined by magnetic resonance imaging. The size of the mass was approximately 4.4×3.5×4.3 cm with a hemorrhagic formation. The cine image showed a sliding motion between the pulmonary artery and the left atrium. We further examined the patient with two-dimensional echocardiography, which showed a mixture of high and low echogenicity, indicating the presence of a mixed echogenic mass that was 4.5×2.5 cm in size. The left ventricle was not compressed, but the mass caused a mild flow acceleration in the pulmonary artery. We believed that this was the cause of the patient's orthopnea and dyspnea.
Fig. 1.
Computed tomography image indicating the presence of a left atrial mass (arrow). The mass was located between the left superior pulmonary vein and left atrial appendage with a pericardial tail.
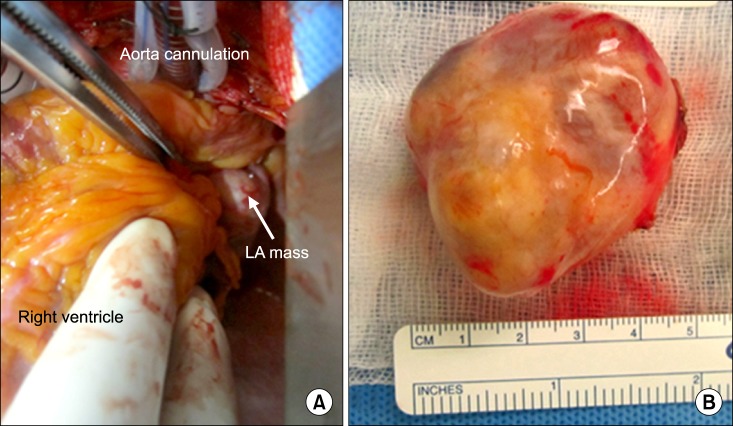
Intraoperatively, we noted that the mass was located adjacent to the left atrium (Fig. 2A). The mass was attached to the left atrial appendage, and the stalk did not have a peduncle. We attempted to perform direct excision under cardiopulmonary bypass, but the heart was very compressed when it was moved laterally in order to achieve a secure operative field. Therefore, we clamped the ascending aorta and administered cardioplegics, and then, resected the mass.
Fig. 2.
(A) The mass was located adjacent to the left atrium (LA). (B) The mass is ovoid in shape and well capsulated.
Upon macroscopic examination, we noted that the tumor was a pinkish-yellow ovoid soft tissue mass (dimensions: 4.3×4×3 cm) (Fig. 2B). Focal necrosis and cystic changes were noted on the cut surface. Following the excision of the mass, a 3-cm defect was noted in the left atrial appendage, which was closed using bovine pericardium. Upon pathological examination, the patient was diagnosed with a schwannoma. Histologically, the tumor had the typical biphasic pattern of a schwannoma with a compact spindle cell area (Antoni A) and a loosely formed hypocellular area (Antoni B) (Fig. 3). Verocay bodies, formed by palisading cells, are occasionally identified in compact Antoni A areas. The loosely formed Antoni B areas generally contain thick-walled hyalinized vessels.
Fig. 3.
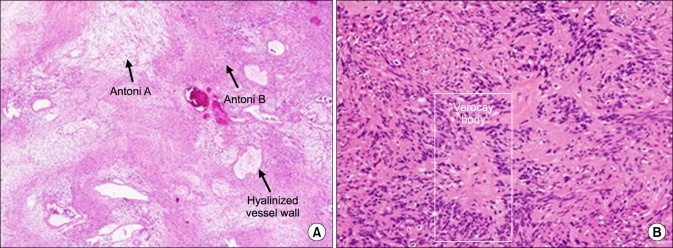
(A) Biphasic tumor with compact Antoni A and loose Antoni B areas (H&E stain, ×40). (B) Verocay bodies in Antoni A area (H&E stain, ×200).
Following surgery, the patient was transferred to the intensive care unit (ICU). Her cardiac output was 3.4 L/min, and the cardiac index was 1.8 L/min/m2. We initiated the administration of dopamine followed by dobutamine, which resulted in improved cardiac function, with a cardiac output of 5.3 L/min and a cardiac index of 2.7/min/m2. The patient was extubated on the day after the surgery. Thereafter, the inotropes were tapered, but her cardiac index decreased. Subsequently, we started epinephrine (0.02 mcg/kg/min) since heart traction in the operating room resulted in the failure of cardiac function to a certain extent. We monitored the patient in the ICU for 4 days. Echocardiography indicated that no remnant mass was present on postoperative day 4. The patient was discharged 9 days after surgery. She regularly visited an outpatient clinic for 1 year. Her follow-up cardiac echocardiography showed normal cardiac function and no remnant mass.
DISCUSSION
Primary schwannoma is believed to originate from the cardiac plexus or the cardiac branch of the vagus nerve [1,2]. It is located primarily on the right side of the heart, particularly in the right atrium [1]. Primary cardiac schwannoma is an extremely rare disease; thus far, only 16 cases of this disease have been reported in the literature.
Echocardiography is a useful tool for the diagnosis of cardiac masses, particularly transthoracic echocardiography, which is non-invasive and widely available. This modality evaluates the tumor size, shape, attachment, and mobility [3]. However, magnetic resonance imaging has emerged as a useful tool for a detailed evaluation of cardiac masses. It is non-invasive, has a large field of view, and allows for direct multiplanar imaging [4].
When surgical resection is considered, the following should be used as a guideline in the operating room, during tumor resection: 1) aim to provide an adequate operation field, while not pulling the heart excessively, 2) aim to perform a complete resection without injury to adjacent structures, and 3) consider reconstruction with bovine pericardium or autologous pericardium for post-resection defects. Pathologic findings are also important, and therefore, obtaining a frozen biopsy specimen may prove useful in the operating room for ensuring a complete resection margin and for avoiding misdiagnosis.
Histological features of the schwannoma include the biphasic architecture of Antoni A and B patterns, as well as nuclear palisading (Verocay bodies) and fibrous capsules containing the cells derived from the nerves. The Antoni A pattern contains elongated fascicles in the areas of moderate-to-high cellularity with a small stromal matrix. In the Antoni B pattern, the tumor is less densely cellular with a loose meshwork of cells along with microcysts and myxoid changes [5].
Immunohistochemical examination is also a useful diagnostic method. The S-100 protein is a specific marker for schwannoma. It is obtained from the neural crest origin tumors from which the melanocytes and Schwann cells are derived [6]. Thus, we report a rare case of primary cardiac schwannoma.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.D'Amato N, Correale M, Ireva R, Di Biase M. A rare cause of acute heart failure: malignant schwannoma of the pericardium. Congest Heart Fail. 2010;16:82–84. doi: 10.1111/j.1751-7133.2009.00124.x. [DOI] [PubMed] [Google Scholar]
- 2.Morishita T, Yamazaki J, Ohsawa H, et al. Malignant schwannoma of the heart. Clin Cardiol. 1988;11:126–130. doi: 10.1002/clc.4960110213. [DOI] [PubMed] [Google Scholar]
- 3.Butany J, Nair V, Naseemuddin A, Nair GM, Catton C, Yau T. Cardiac tumours: diagnosis and management. Lancet Oncol. 2005;6:219–228. doi: 10.1016/S1470-2045(05)70093-0. [DOI] [PubMed] [Google Scholar]
- 4.Gulati G, Sharma S, Kothari SS, Juneja R, Saxena A, Talwar KK. Comparison of echo and MRI in the imaging evaluation of intracardiac masses. Cardiovasc Intervent Radiol. 2004;27:459–469. doi: 10.1007/s00270-004-0123-4. [DOI] [PubMed] [Google Scholar]
- 5.Cotran RS, Kumar V, Collins T, Robbins SL. Robbinsons pathologic basis of disease. 6th ed. Philadelphia: Elsevier Saunders; 1999. [Google Scholar]
- 6.Stefansson K, Wollmann R, Jerkovic M. S-100 protein in soft-tissue tumors derived from Schwann cells and melanocytes. Am J Pathol. 1982;106:261–268. [PMC free article] [PubMed] [Google Scholar]