Abstract
Lung cancer is one of the most common malignant tumors, and it has the highest death rate. Oncothermia is a feasible and successful treatment for lung cancer. Results show a remarkable survival benefit for patients, with a good quality of life. The treatment has no, or in some cases mild, side-effects and could decrease the adverse effects of the complementary treatment. Applying oncothermia together with other treatment methods could increase the effects and result in better performance. A comparison of studies demonstrates a good correspondence in the data, which strengthens the reliability of the studies, and clearly shows the feasibility of the application of oncothermia to treating all kinds of pulmonary malignancies including non-small-cell and small-cell primary tumors, and all of the metastatic diseases of the pulmonary system.
Keywords: Lung neoplasms, Fever, Meta-analysis, Hyperthermia
INTRODUCTION
Cancer morbidity worldwide is growing. However in the United States, the overall morbidity slightly decreased in 2013, (by >0.6%/yr in men, but it was stable in women), while the mortality rates decreased by 1.8%/yr in men and by 1.5%/yr in women [1]. The lung was the site of the highest cancer mortality in both the sexes (28% and 26% in men and women, respectively, while the second highest mortality rates, prostate cancer in men and breast cancer in women (10% and 14%, respectively), were nearly half those of the lung cancer rates [1]. While the disease, when recognized in its localized state, has good five-year survival (52%), the regional or far distant stages have survival rates of only 25% and 4%, respectively. However, at the first diagnosis, 56% of cases already show distant metastatic lesions, while only 22% are regional and 15% are local only [2]. This is why the overall survival data shows a sad picture: relative survival rates (male/female) are 29.4%/33%, 7.8%/9.3%, and 4.9%/5.9% for one-, five-, and ten-year-survival studied in 2005 to 2009 [3]. Due to the sad fact that lung cancer is one of the leading causes of mortality for humans, there have been numerous attempts to stop this trend [4]. Despite the well developing treatment modalities, the ratio of lung cancer mortality rate to the incidence (0.8) is more than double the average mortality/incidence ratio (0.3) among the population <65 years [5]. The incidence of lung cancer between the ≥65 years and <65 years old patients differs in 14%.
The Korean statistics are similar to the worldwide trends [6,7]. Furthermore, special circumstances were observed in the case of Korean patients: The profiles of EML4-ALK fusion gene variants of non-small-cell lung carcinoma (NSCLC) may differ from those in other populations of other ethnicities [8]. The need to improve the rate of cure has remained of central importance in the field.
The majority of cases (range, 75% to 85%) are of the non-small-cell histology, and half of these are adenocarcinomas [9]. The intense challenge is that most of the NSCLCs are first diagnosed when they have already in advanced stage or have already become rapidly metastatic [10]. The majority of patients present with either locally advanced disease (stage III) or metastatic disease (stage IV). The standard first-line strategy for the treatment of advanced stage NSCLCs is a limited number of chemotherapy cycles to achieve tumor regression or at least stabilization [11]. Importantly, patients who undergo curative surgical resection for apparent localized disease have survival rates ranging between 50% and 80%, implying the need for better systemic treatment to cure occult micrometastatic disease. Therefore, the majority of patients either presents with advanced disease requiring chemotherapy or require chemotherapy at the time of relapse after surgical resection. While efforts to cut down on smoking are crucial to the eventual control of the disease, newer treatments for patients who currently have the disease are critical.
As a class, NSCLC is relatively insensitive to chemotherapy, compared to small cell carcinoma (SCLC). When possible, they are primarily treated by surgical resection with curative intent; however, chemotherapy is increasingly being used both preoperatively (neoadjuvant chemotherapy) and postoperatively (adjuvant chemotherapy).
Modern lung-cancer treatment is based on platinum-containing doublets (carboplatin and cisplatin) and more recently on gemcitabine, paclitaxel (Taxol and Doxitaxel), vinorelbine (Navelbine). The analysis of 52 clinical studies shows the advantages of cisplatin-based therapies (10% 1-year survival increase), which reduce the risk of exitus by 27% [12], compared to the applied supportive therapies. In general, the median survival with such chemotherapy is 7 months.
The gemcitabine-based triplets and doublets (paclitaxel/carboplatin/gemcitabine; paclitaxel/carboplatin/vinorelbine; paclitaxel/gemcitabine; gemcitabine/vinorelbine); had 37%, 29%, 40%, and 49% one-year survival and 9.6, 9.9, 8.7, and 10.7 months median survival, respectively [13]. The gemcitabine-based doublets had a lower response rate (RR), but longer survival and less adverse effects. In general, the median survival ranges between 6 and 12 months, with 7 months on average. The one year survival is 24% to 51%, and 25% to 30% on average.
An extended large study including in total 1,207 patients [14] with advanced NSCLC was performed to compare the randomly assigned treatment to a reference regimen of cisplatin and paclitaxel or to one of three protocols: cisplatin and docetaxel, cisplatin and gemcitabine, or carboplatin and paclitaxel. The result was somewhat disappointing: none of the four chemotherapy protocols showed significant advantage over the others in the treatment of advanced NSCLC. The RR was 19%, with a median survival of 7.9 months, the first-year survival rate was 33%, while the second-year survival rate was 11%. Two other chemo-protocols were also compared, pemetrexed and docetaxel. The clinical efficacy was equivalent, but significantly fewer side effects were observed with the docetaxel regime in the second-line treatment of patients with advanced NSCLC [15].
Considering the side effects of the present treatment options, including surgery, radiation, and platinum-based doublet chemotherapy, another promising drug family was developed connected to growth factor receptors (GFR) to treat NSCLC. To increase the efficacy of such growth factor receptor tyrosine kinase inhibitors, the coinhibition of GFR signaling pathways and combination of inhibitors along with radiation or chemotherapy have been studied [16]. This is a clinically validated therapy targeting the signaling pathways of either the vascular endothelial GFR or epidermal GFR (e.g., gefitinib, cetuximab, erlotinib, bevacizumab), but the complex signaling system of solid tumors could adapt to the situation [17], so the targeting of multiple regulatory pathways makes sense [16]. The development of multitargeted tyrosine kinase inhibitors (TKIs) is thus a concern. Although there is increasing research interest in these kinds of drugs, their therapy-related adverse effects and their safety remain controversial. As we discussed above, some clinical trials have been stopped early because of toxicity issues with multitargeted TKIs, and some other trials did not achieve primary improvement in overall survival; there is still a need for exploring more convenient, newer pathways as well as to develop insight into the coinhibition of existing pathways. It is important that greater attention be drawn to the signaling pathways that are modified by the use of kinase inhibitors. Genomic landscapes for patient-specific evaluation should be provided to appropriately select patients who are most likely to benefit from RTK (receptor tyrosine kinase)-inhibition therapy.
SCLC comprises about 15% of all primary lung cancer cases, and it is usually the consequence of smoking. SCLC is a major clinical problem, with an aggressive clinical appearance and short survival time. Its treatment protocol is different from that of NSCLC [18]. Currently, the optimal treatment is platinum-etoposide chemotherapy (four cycles) applied with complementary thoracic irradiation in patients in early stages, but radiotherapy is not applied in the late stage.
Many promising clinical approaches are available for lung cancer, but unfortunately, the breakthrough in this kind of malignancy has not been reached yet, and lung cancer treatments remain a high priority in clinical practice.
Our present paper reviews the feasibility of oncothermia treatment for both NSCLC and SCLC. This study concentrates on the significance of the survival time as one of the most important factors for measuring the success of a treatment in oncology.
HYPERTHERMIA IN PULMONARY CANCERS
Hyperthermia (HT), combined with radiotherapy and chemotherapy, seems to be a promising method for cancer treatment [19], although many of the underlying molecular mechanisms of this combination treatment are still not clearly understood. Preliminary results with NSCLC recruiting a limited number of patients (range, 5 to 80 patients) also seem encouraging where HT was combined with chemotherapy, radiotherapy, or both. The co-administration of HT was proven to be safe [20,21,22]. A great number of studies show that HT inhibits angiogenesis, enhances chemo- and radio-sensitivity, and induces a high concentration of drugs within a tumor [23,24,25].
However, there are some restrictions on HT, in general, that hamper its use in lung cancer treatment. Namely, it could aggravate preexisting pleural liquids, the intensive cooling by actual breathing is contra-effective for the overall temperature increase. Furthermore, the increasingly constrained blood flow supports the tumor by glucose, which is an important contra-action, together with the increased risk of the dissemination. Focusing of the energy is limited by the intensive movement of the organ by breathing, and maintains the raised temperature on the place where the energy is focused. In fact, it has even more complications in a good heat-conducting environment than in thermal spreading in other organs. However, some successful clinical trials have shown the feasibility of the HT method for lung cancer. Most of these are combined with radiotherapy, having a 14÷70 Gy dose in the given session. The measured RR was surprisingly high: RR=75% (n=12) [26] and RR=100% (n=13) [26]. In this last study, the mean survival time (MST) was 15 months, (mean-value, 17 months) on the tumors, which had a size on average of 22 cm3. The average total dose was 60 Gy, average heating time was 52.3 minutes, and average total session in the cycle was 27. Others had a comparison to a control-arm (not randomized), studying the RR (Table 1).
Table 1.
Response and survival rates in controlled double-arm studies for non-small cell lung cancer treated by capacitive hyperthermia (8 MHz)
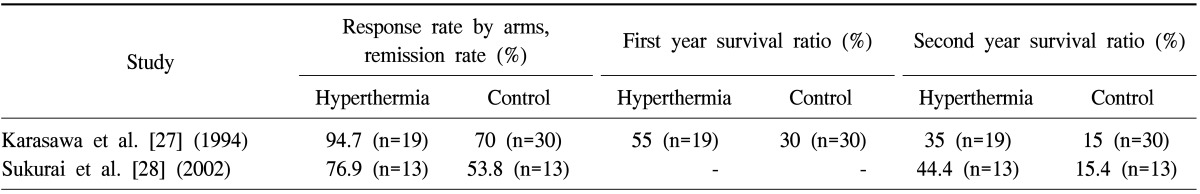
Other trials had a comparison to a control-arm (not randomized), growing the RR from RR=70% (n=30) and RR=53.8% (n=13), to RR=94.7% (n=19) [27] and RR=76.9% (n=13) [28], respectively. The second year survival also increased remarkably: from 15% and 15.4%, to 35% and 44.4%, respectively. The first year survival was measured as well, increasing from 30% to 55% [27].
A study on advanced NSCLC patients (n=13, capacitive coupling, f=8 MHz) to control local chest invasion [29] showed similar good results, and the pain relief was also surprisingly good (Table 2).
Table 2.
The results of radio-thermotherapy for advanced non-small cell lung cancer patients
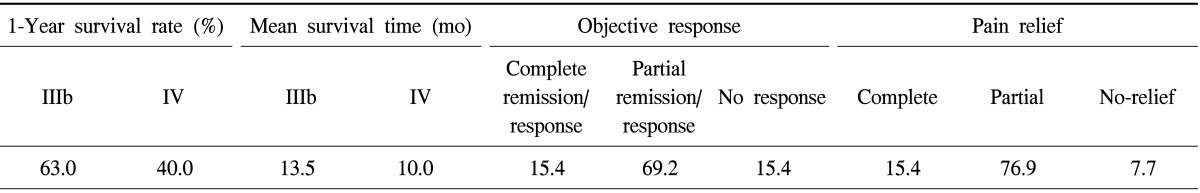
IIIb and IV are the staging categories according to the World Health Organization.
The chemo-thermotherapy combination was also investigated for NSCLC with success. In laboratory studies, cisplatin was shown to be effective [30], so the clinical studies concentrated on this drug combination. A special case report has shown its feasibility [31], and has demonstrated a median survival gain (from 15 [n=20] to 25 [n=32] months) [32]. The median survival was measured in another study [33] as 19.2 months, with RR=73% and a 1 year-survival of 75%. The 13.5 months median survival of the historical control was increased to 17.5 months using postoperative (lobectomy or pneumonectomy) application of intrathoracic chemo-thermotherapy by capacitive coupled HT [32,34].
Local control by capacitive HT in combination with radiotherapy was also successful for locally advanced non-small lung cancer [27]. The complete remission rates were 26% and 0% with and without HT, respectively, while the full RRs were 95% and 70%, respectively. Others have also achieved good results from complementary radiotherapy with capacitive HT [26].
The survivals for the treatment group (resection+postoperative intrathoracic chemo-thermotherapy [PICT], n=32) and the historical control groups: resection only (n=20) or exploratory thoracotomy (n=11) had median survival rates of 25, 15, and 10 months, respectively [32,34]. The survival curve of the treatment group was significantly better than those of the control groups. Furthermore, the local relapse-free survival for the treatment group (resection+PICT, n=32) and for the historical control group (resection only n=20) was drastically and significantly increased, having more than double the relapsed-free survival in the HT treated group than in its historical counterpart after 48 months. The postoperative application was successful in other studies too [31,34]. The 5-year median survival was measured in another study [34], showing rather high numbers (24.5%, for patients with N0+N1 status [n=14]). The median survival with PICT was measured, and definite gain was found (from 15 [n=20] to 25 [n=32] months) (capacitive coupling, 8 MHz and 13.56 MHz). The MST was 10, 15, and 25 months for treatment group (resection+PICT) and the historical control groups (resection only and exploratory thoracotomy only), respectively. The survival curve of the treatment group was significantly better than those of the control groups. Measuring the local relapse-free survival for the treatment group (resection+PICT) and the historical control group (resection only) also revealed a significant benefit with HT treatment. Another chemo-thermotherapy study [35] showed fairly good results: an MST of 19.2 months, RR=73%, and a 1 year-survival of 75%.
The chemo-thermotherapy combination was also investigated for NSCLC with success. In laboratory studies, the synergy between the gemcitabine and HT in NSCLC was shown in vitro, and in vivo in a nude mouse xenograft model [36]. The decrease in the tumor size and a significant inhibitory effect of growth were shown, and HT supported gemcitabine-induced apoptosis.
Locally advanced NSCLC was studied (n=32) with fractional radiation (180.300 cGy/fraction, 5 fractions/wk, median dose 5.58 Gy) [37] (Table 3). The results indicate differences, but they were not significant.
Table 3.
The measured data in locally advanced non-small cell lung cancer patients
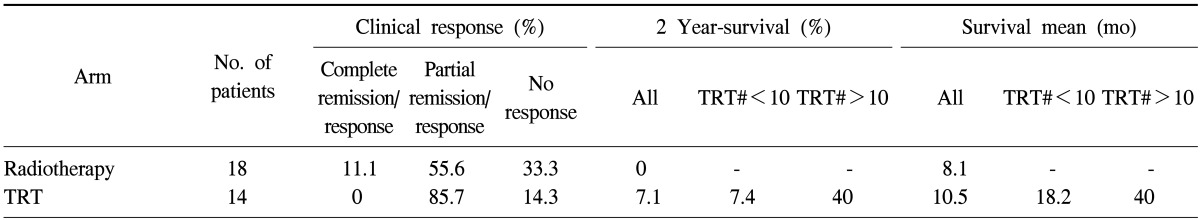
TRT#, number of thermoradiotherapy treatments.
To search for significant improvements in outcomes of thermotherapy, a large study involving two randomized, controlled, multicenter phase III studies was sponsored by a nonmedical international organization, the International Atomic Energy Organization to avoid any bias in medical sponsoring: for uterine cervix (n=110) [38] and for NSCLC (n=80) [39]. Both studies were performed in combination with radiotherapy and with or without HT. The results of both studies were disappointing.
The differences in the complete clinical response (complete remission) as well as overall survival rates in combined and single radiotherapy arms were statistically insignificant (p=0.49 and p=0.868, respectively), while the local progression-free survival was significantly better in the combined arm (p=0.036). The authors concluded that there was no substantial benefit in treating locally advanced NSCLC by adding HT to radiotherapy, although there was some improvement in local progression-free survival.
Other kinds of HT (whole-body heating and very local ablative heating) have also been attempted. Whole-body HT was also applied to treating advanced lung cancer [40]. A benefit of HT was found (n=49), which was more substantial in elderly (>60 years) patients. The remission rate was 50%, and the MST was 7 months (mean is 12.7 months) in primary and 5.5 months for metastatic diseases.
Percutaneous ablation by radiofrequency (RF) [41,42] and by laser-induced interstitial thermotherapy [43] are also used for pulmonary tumors. In addition, intrapleural HT by perfusion is being used in clinical practice [44]. The breathable perfluorochemical liquid used in convective HT also appears to be a feasible alternative for lung cancer treatment [45].
ONCOTHERMIA METHOD
There is a serious need for a stable HT method that is free from controversy and adverse effects, not only because of the high incidence of pulmonary malignancies, but also because of the variety of primary tumors that frequently metastasize to the lungs. The most common types include breast cancer, colon cancer, prostate cancer, and bladder cancer.
A primary lung lesion can also quickly develop metastatic lung malignancies in other lobes. This causes the patient to progress quickly to advanced disease and requires a high stage of care (frequently stage IV).
Furthermore, due to the lack of multiple effective therapies for high-line treatment (third-line or over), no real treatment options are available in such cases, which is another cause of the high mortality rate. Good supportive and sensitizing complementary therapies are missing from the application spectra.
Another important factor demands new therapies: the quality of life. Patients with lung malignancies rapidly lose their Karnowski Performance Score (KPS), so any new therapy should have minimal adverse effects and improve the quality of life of the patient.
One of the most advanced HT modalities devoted to oncology is oncothermia [46,47]. This method aims to preserve all positive effects of conventional HT while improving on the imperfections and answering the challenges posed by conventional approaches. The key is nano-range energy liberation instead of overall heating of the mass of the target [48]. This review will not explain the method of oncothermia in detail, which has already been done elsewhere [46,49], or describe its technical realization [50]. However, this paper will note some important factors of the method that are closely related to pulmonology applications.
Oncothermia is a highly sophisticated method that addresses some of the limitations of HT [51] and makes it possible to reintroduce HT by new standards [52]. It is based on a strong synergy between the temperature and the electric field [53]. The RF current is chosen with a proper amplitude-modulated RF (13.56 MHz) [54], which is absorbed in the nanoscopic range by the malignant cells [55]. The physiological differences of the malignant cells from their healthy counterparts [51] distinguish the malignancy, which is self-selected by additional time-fractal modulation [56], which means that this is a highly customized treatment method [57]. The malignant cell is destroyed by apoptosis [58]. The apoptotic process uses an outer pathway [59] (not the mitochondrial internal pathway that is used in normal HT [60]), forming a damage-associated molecular pattern [61] and inducing immunogenic cell death [62], which could be the basis of the directed abscopal effect too [63]. These effects start to emerge in lung cancer management [64,65], and could be the basis of a long-awaited feature of oncology: personalized care [66].
The treatment technique is simple and very user-friendly. It is safe and rarely has any side effects; patients are relaxed during the treatment thanks to the comfortable waterbed below containing the lower electrode. The upper electrode is a thermally and electrically balanced water bolus, which conforms to any body shape. The RF current flows through the patient self-selectively, irrespective of the movements of the tumor. The current flows along the best path for conduction, which is also the best pathway for treatment.
CLINICAL RESULTS
Given that oncothermia is usually applied in very advanced cases, mostly third-line therapy and beyond, it is rather complicated to assemble an appropriate cohort for a prospective study. The present clinical results are dominated by case reports and few more extensive studies.
SOME CASE REPORTS
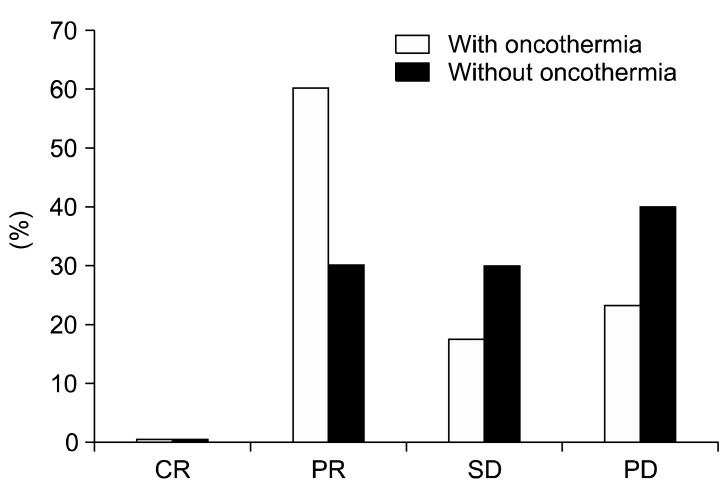
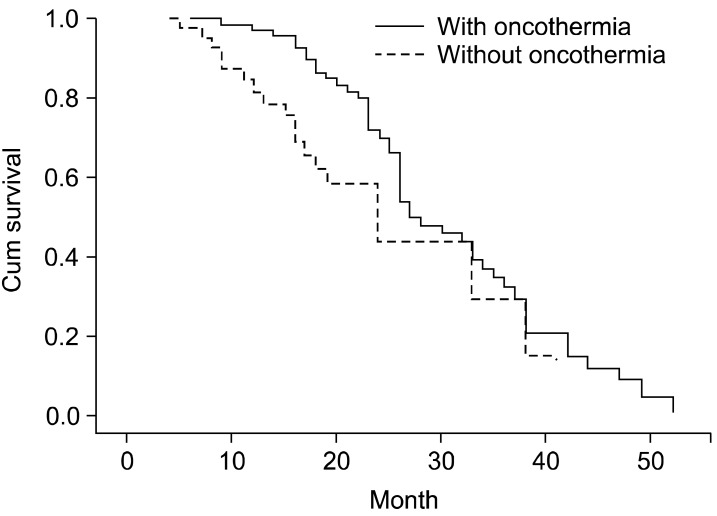
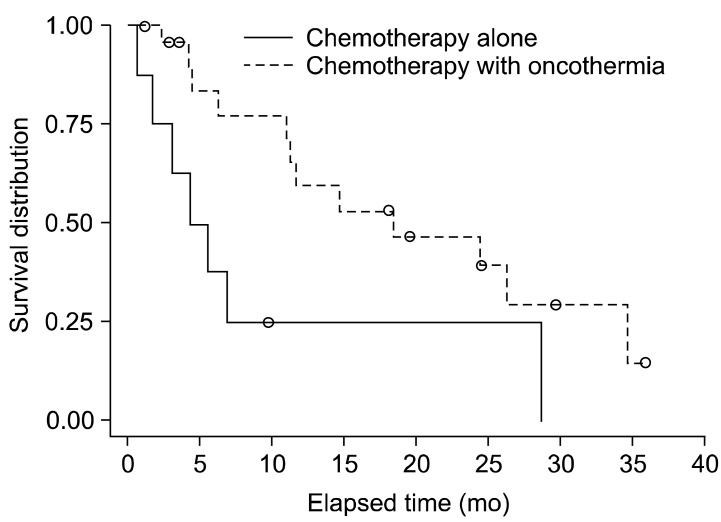
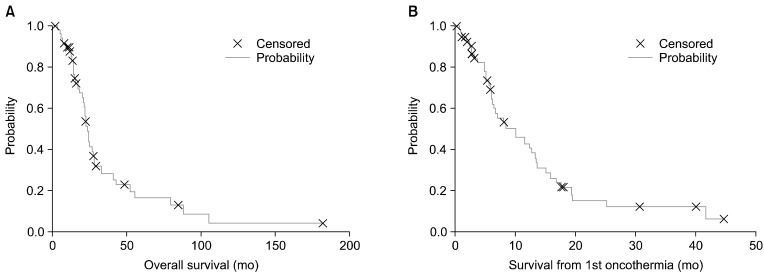
Several reports on cases of oncothermia application worldwide have been informative (Figs. 1, 2, 3, 4).
Fig. 1.
Comparison of the local control in the two arms. CR, complete remission/response, PR, partial remission/response; SD, stable disease; PD, progress disease.
Fig. 2.
The Kaplan-Meyer plot of survival showing a clear significant benefit of the oncothermia-treated arm.
Fig. 3.
The Kaplan-Meyer plot shows a statistically significant benefit for the oncothermia group.
Fig. 4.
(A) Overall and (B) oncothermia treatment time survivals by Kaplan-Meier plot of the patients in PFY (Peterfy Hospital, Budapest, Hungary) study.
Some important messages from these cases are:
1) Do not evaluate too early by imaging, due to the slow apoptotic process [46].
2) It is better to measure the actual change of the tumor marker from serum [67]. Their clinical effectiveness is relatively good [68], and corresponds well with the genetic abnormalities of the cancer [69]. Tumor markers may be used to monitor any response to the treatment and determine whether the cancer has recurred after treatment, but in general, tumor markers alone cannot be used to diagnose cancer; they must be combined with other tests. The guidelines of the National Academy of Clinical Biochemistry USA are best followed [70].
3) The optimization of the actual treatment has to be personalized. The trimodal therapies (oncothermia and two jointly applied 'gold standards' like radio- and chemotherapies) have good outcomes, but bimodal application is also suitable in most cases. In the cases when the gold standards are inapplicable, monotherapy with oncothermia also has good results, but in these cases, the number of sessions in a cycle is approximately doubled [71].
4) In chemotherapy applications, old 'simple' drugs like mitomycin-C could be reapplied [46].
5) The new chemo-categories (like the addition of bevacizumab to conventional platinum derivatives) are also applicable to oncothermia [72]. Independently of oncothermia, we expect various chemo-agents from the literature to be synergistic; for example, the addition of bevacizumab to cisplatin-based chemotherapy significantly prolonged progression free survival and overall survival in phase III [73,74] and phase IV trials [75], proved to be safe in treating over 5,000 patients [76,77,78], and in the maintenance setting, also demonstrated a beneficial effect on progression free survival [79,80], but the addition of oncothermia to this combination was recently approved for the first time [72].
6) Even in the cases where the local control is not so effective (poor partial remission/response or no response), the quality of life improved, which is a great advantage of oncothermia.
7) The method is safe and apparently free of adverse effects in any combined or monotherapy applications.
8) In the mild trimodal application (low-dose radiotherapy, an immune activator, and oncothermia), the abscopal effect is clearly observable [81], which was also seen in laboratory model experiments without the addition of ionizing radiation [63].
9) Oncothermia can be applied as concurrent therapy even in a fifth-line chemo-companion [82] in highly advanced disseminated cases.
10) The strategies of advanced cases have multiple vices [83], but oncothermia could be successfully applied in far advanced cases too [84,85].
11) Oncothermia can be applied not only in primary lung malignancies, but also for metastatic cases as well [86,87].
STUDIES
Advanced lung cancer was studied in a double-arm prospective study [88] with real endpoints of survival time and quality of life. In the active arm (n=75), stage IIIb and IV lung cancer patients were included, while a same patient characteristics cohort (n=40) was on control arm (Table 4). These patients were inoperable. Oncothermia was applied in 20 sessions, every other day, 60 min/each. The treatment efficacy, survival rate, and life of quality were assessed before the treatment, after the treatment, and every sixth month.
Table 4.
Distribution of the patients in the study
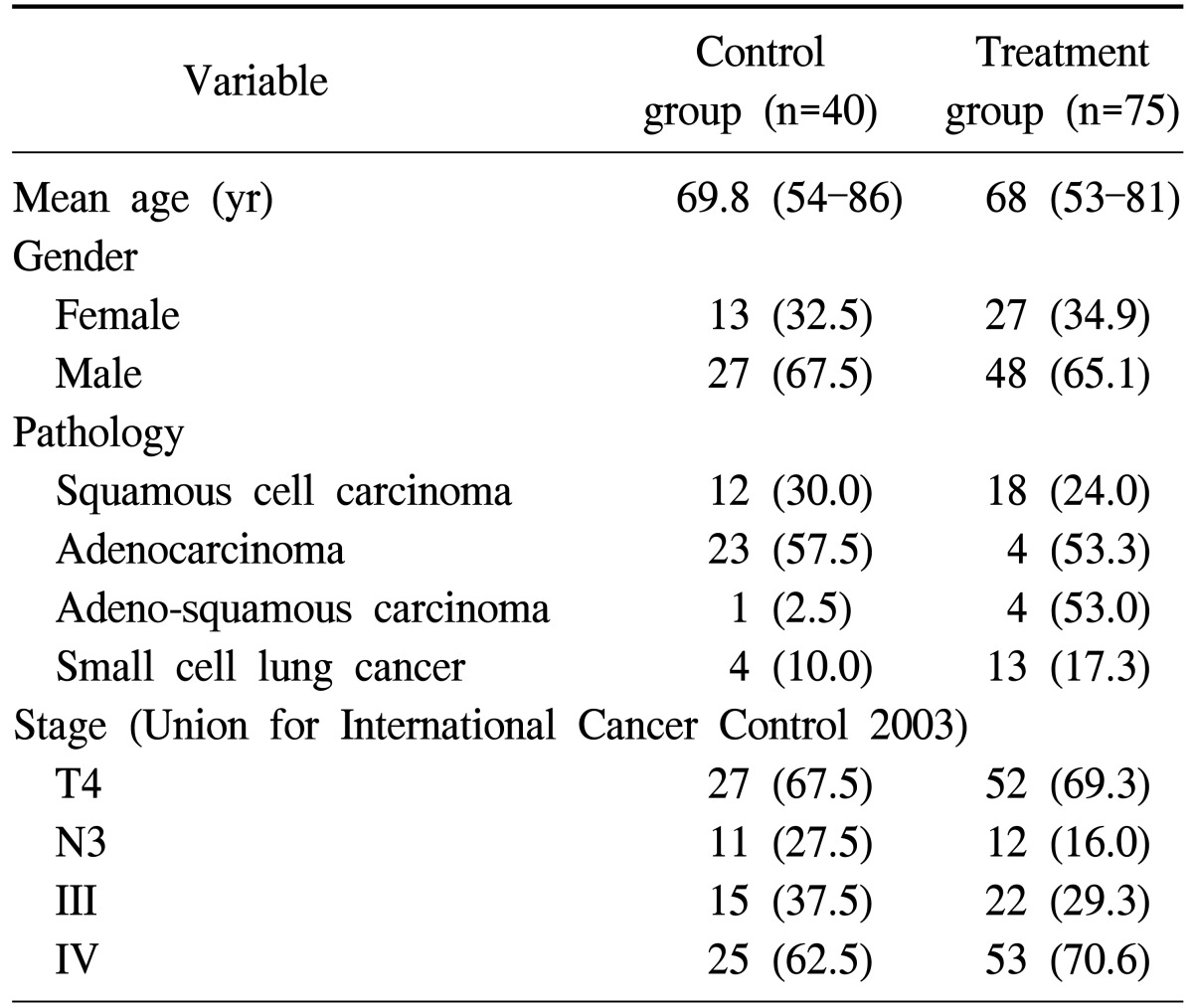
Values are presented as mean (range) or number (%).
The local control (remission rate) (Fig. 1), as well as the overall survival (Fig. 2), was significantly superior in the oncothermia combined (active) arm of the study in comparison with the control. In addition, the treatment group showed statistically significant differences in quality of life functions, emotions, general conditions, pain, shortness of breath, loss of appetite, cough, hemoptysis, and chest pain.
Another study examined SCLC [89]. The double-armed study included treatment with (n=23) and without (n=8) oncothermia complementary to chemotherapy for SCLC. Chemotherapy was the first-line treatment: irinotecan (60 mg/m2), and cisplatin (60 mg/m2) three times. The status was evaluated by chest computed tomography. When the progression of the tumor or metastases was detected, the chemotherapy was changed to etoposide (110 mg/m2) and cisplatin (70 mg/m2) in the second line. Oncothermia was performed from the first chemotherapy treatment period up to 150 W, 1,490.5 kJ energy for 60 minutes, every second day, with a rise in temperature to 38.5℃ to 42.5℃. In this study, we used an electrode with a 30-cm diameter applied to the thorax, with at least 12 sessions in 1 cycle. The results showed promising improvement of the survival compared to chemotherapy alone. The overall survival showed a significant benefit for the oncothermia treated group (Fig. 3).
An extended clinical investigation is in progress [87]. Presently, 10 NSCLC patients and 3 metastatic lung malignancies are included to study the safety (phase I) and the efficacy (phase II) in a well-controlled study. The work is in progress.
A retrospective clinical study of NSCLC patients was performed by two hospitals in Budapest [90,91,92], clearly demonstrating the feasibility and efficacy of oncothermia for advanced cases [93]. The first study was obtained from an open-label, single-arm, monocentric study (n=61) carried out in Peterfy Hospital, Budapest, Hungary (referred to as 'PFY') [91,92]. The patients included were analyzed according to an intention-to-treat schedule. The primary endpoints of the study were the overall survival time and the survival time from the first oncothermia treatment. The dates of exitus were checked by the National Death Register, so that accurate data were collected. Inclusion criteria were: 1) Inoperable or sub-totally resected, or recurrent primary pancreas tumor, 2) progression after radio- and/or chemo-therapy, and 3) KPS ≥30%, and the inclusion was irrespective of the localization of the lesion in the lung. Patients started the oncothermia process in their late/advanced stages, where most of them had failed to respond to any of the applied conventional therapies. The exclusion criteria were only the well-known contraindications of the oncothermia method (metallic implants or replacements in the treated area, missing heat sensitivity in the treated area, or a pacemaker or other field-sensitive implant in the patient).
The age distribution of n=61 patients was near normal (p=0.82); no outlier was present. The median age was 58 years (range, 38 to 77 years), the mean-age was 58.97 years (standard error [SE]=1.17). The gender distribution was 21/40 female/male (34.4%/65.6%). The proportion of the elderly (>68 years) patients was 21.3%. Oncothermia was performed in 2 to 3 sessions per week. The treatment time per session was 60 minutes. The power was gradually and linearly raised depending on the patient's tolerance from 40-80 W to 100-150 W. The applied average energy was 300 kJ/treatment (range, 250 to 450). The applicators used were 3.1 dm2 and 7.1 dm2, depending on the tumor volume.
Most of the patients (n=49, 80.3%) had distant metastases. They were heavily pre-treated; all of the patients had received at least one line of chemotherapy and had undergone other treatments (surgery, radio- or second-line chemo-therapy).
The actual staging was made at the first diagnosis (44% was in advanced [World Health Organization IIIb or IV] stages) and at the first oncothermia treatment (75% was in an advanced stage). The median of the elapsed time from the first diagnosis to the first oncothermia treatment was 8 months (range, 0.4 to 172 months), while its mean was 16.3 months (SE=3.1). The elapsed time ratio to the overall survival was more than 50% (median, 59.9% [6.5-99.1]; mean, 59.4 [SE=3.5]); the patients received their first oncothermia in the second half of their survival time. The oncothermia treatment was provided twice a week; the treatment number averaged 8.1 (SE=0.55) sessions with a median of 8 (range, 2 to 23).
The Kaplan-Meier plots of the overall survival (median, 16.4 months [1.7-181.9]; mean, 25.6 months [SE=3.8]) and the survival from the first oncothermia treatment (median, 5.7 months [0.1-44.9]; mean, 9.2 months, [SE=1.3]) are shown in Fig. 4. For the elderly patients, neither the overall survival nor the oncothermia survival differed between the groups (p=0.68).
The elapsed time to the first oncothermia treatment represents an important parameter. Namely, this is of course smaller (p=0.0019) for the patients with advanced disease at their first diagnosis (n=34, median, 13.0 months [1.5-142]; mean, 24.0 months [SE=5.2]; and n=27, median, 6.5 months [0.4-19.9]; mean, 6.67 months, [SE=0.83] for non-advanced and advanced, respectively). However, the opposite was found (p=0.14) when the staging at the first oncothermia was studied (n=15, median, 4.10 months [1.5-29.3]; mean, 8.9 months [SE=2.3]; and n=46, median, 8.3 months [0.4-142]; mean, 18.78 months [SE=4.0]; for non-advanced and advanced, respectively).
This tendency is more obvious when the overall survival and oncothermia survival are evaluated depending on the ratio of the elapsed time until the first oncothermia treatment to the overall survival, dividing the patients into 'early oncothermia' and 'late oncothermia' categories. The overall survival shows the expected result: the low survival cases start earlier (p=0.0065) with oncothermia (n=31, median, 16.4 months [4.7-79.7]; mean, 19.62 months [SE=2.61]) than those with long survival, (n=30, median, 17.4 months [1.7-182]; mean, 31.7 months [SE=7.07]). On the other hand, the oncothermia survival was opposite (p=0.073): those with an early start (n=31, median, 8.4 months [2.4-44.9]; mean 12.7 months [SE=1.9]) had longer survival, than those with a late start (n=30, median, 2.7 months [0.1-40.0]; mean, 5.6 months [SE=1.6]).
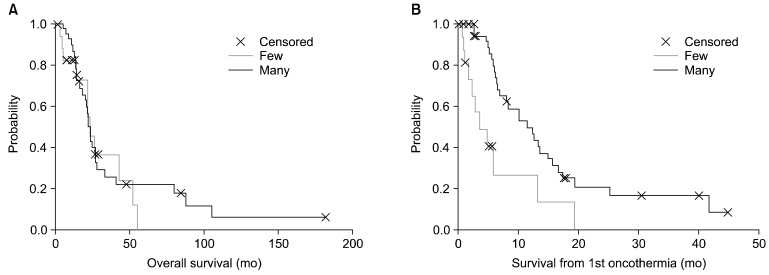
The number of treatments does not influence the overall survival significantly (p=0.61), but the oncothermia survival (p=0.0023) and the follow-up time after the last oncothermia (p=0.01) greatly depend on the number of oncothermia treatments (Fig. 5). Operability was especially beneficial for survival (p=0.0005), but the other pre-treatments did not relate significantly to either the overall survival or the oncothermia survival rates.
Fig. 5.
Survival times for patients according to the treatment session time ('few' lower than the median number, 'many' higher than the median number of treatments).
The effect of the length of treatment grouped by before and after the median time of the data was measured also. In the early experience group (nee=33), the median overall survival was 22.3 months (1.7-181) and mean was 33.7 (SE=6.4); the oncothermia survival median was 8.0 months (0.1-45) and mean was 11.6 (SE=2.07); and the median elapsed time to the first oncothermia treatment was 10.3 months (1.5-142) and mean was 22.1 (SE=5.3). Later, according to our experience (nle=28), the data were: median overall survival, 12.3 months (3.6-51.9) and mean, 15.9 (SE=2.2); median oncothermia survival, 5.0 months (0.1-25.1) and mean, 6.37 (SE=1.24); median elapsed time to first oncothermia treatment, 5.9 months (43.77) and mean, 61.1 (SE=1.8). The differences between the early and late experiences were significant in the case of overall survival (p=0.028) and the elapsed time to the first oncothermia treatment (p=0.012), but not significant for oncothermia survival (p=0.19). The significantly better survival values in the first half of the study time compared to the second one probably originated from the fact that the patient spectrum had shifted to more advanced cases. In the early experience group, the proportion of the advanced cases was 33%, while in the late experience group, 57% of the cases were advanced, but both of them increased (76% and 75%, respectively) when measured at the first oncothermia treatment. The nearly equal percentage of the advanced cases in both categories (rising from very different starting points) indicates that the patients can be assumed to start the oncothermia treatment at nearly the same stage irrespective of the elapsed time from the original diagnosis to the first oncothermia treatment.
Another Hungarian study [90,91,92,93] was made by HTT-Med (HTT), a specialized HT clinic. The inclusion and exclusion criteria as well as the complete protocol were identical with the PFY study. The age distribution of patients (n=197) was acceptably normal (p=0.59); no outlier was present. The median age was 57 years (range, 16 to 84), the mean-age was 56.71 years (SE=0.77). The gender distribution was 62/135 female/male (31.5%/68.5%). The proportion of the elderly (>68 years) patients was 20.3%. Most of the patients (157, 79.7%) had distant metastases (one, two, and three metastases were observed for 101, 43, and 13 patients, respectively). They were heavily pre-treated; most of them (93.4%) had undergone surgery and subsequent radiation therapy. The actual staging was performed at the first diagnosis (46.2% were in advanced [World Health Organization IIIb or IV] stages) and at the first oncothermia treatment they were at a more advanced status.
The median of the elapsed time from the first diagnosis to the first oncothermia treatment was 5.5 months (0.2-111.3), while its mean was 10.6 months (SE=1.0). The elapsed time ratio to the overall survival was near 50% (median, 45.4% [1.6-96.7]; mean, 45.7 [SE=3.9]).
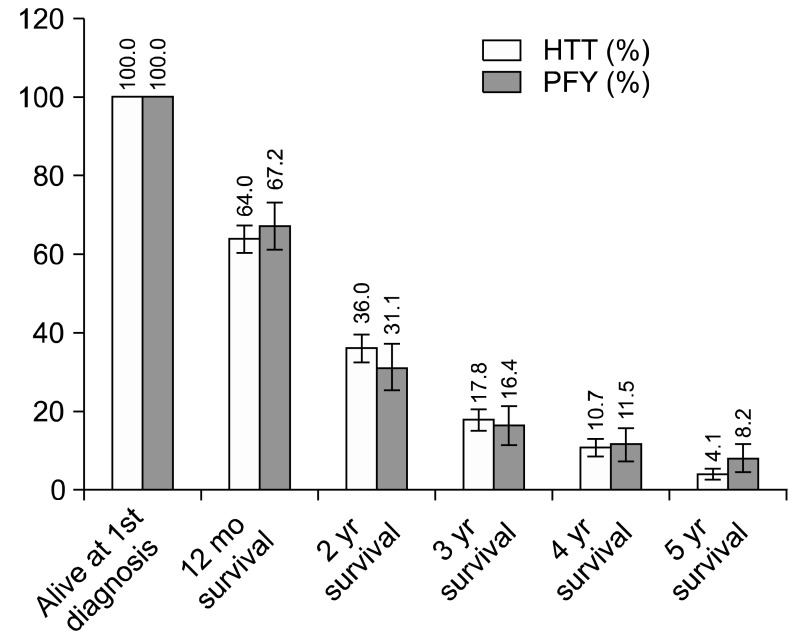
The oncothermia treatment was provided twice a week; the number of treatments was on average 7.9 (SE=0.4) and its median 6 (3.40). The median treatment time was 60 minutes (45-135) and the mean was 69.6 minutes (SE=1.3), while the median equivalent temperature was 52℃ (43℃-59℃) and its mean was 51.4℃ (SE=0.3). Note that the equivalent temperature is not the real temperature. It is the calculated value from the actual energy absorption and the impedance, meaning the actual destruction rate, which is as high as it would have been in the purely temperature-oriented case.
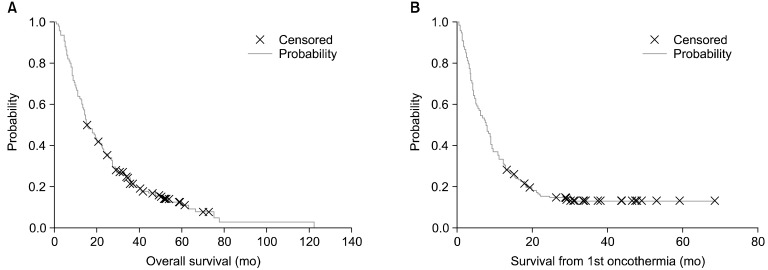
The Kaplan-Meier plots of the overall survival (median, 15.6 months [1.1-122.1]; mean, 22.4 months [SE=1.31]) and the survival from the first oncothermia treatment (median, 7.57 months [0.1-68.6]; mean, 11.8 months [SE=0.91]) are shown in Fig. 6. For elderly patients, neither the overall survival nor the oncothermia survival differed (p=0.37 and p=0.49, respectively).
Fig. 6.
(A) Overall survival, and (B) survival from the first oncothermia treatment for the patients that entered the HTT (HTT-Med) study.
The differences between the patients without or with metastases in their overall survival and oncothermia survival were not significant (p=0.33 and p=0.07, respectively). The number of treatments significantly influences the overall survival (p=0.048) and the oncothermia survival (p=0.00046) and the follow-up time after the last oncothermia treatment (p=0.0017) greatly depends on the number of oncothermia treatments. The operability here also gave a significant benefit for survival.
We studied the effect of the experience of treatment by the data before and after the median time of the study. In the early experience group (nee=94), the median overall survival was 15.3 months (2.4-122.1) and mean, 24.0 (SE=2.17); the median oncothermia survival, 7.2 months (0.3-68.6) and mean, 11.8 (SE=1.5); and the median of the elapsed time to the first oncothermia treatment was 5.37 months (0.4-111.3) and mean, 12.2 (SE=1.8). In the late experience (nle=103) the data were: median overall survival, 15.83 months (1.1-77.7) and mean, 21.0 (SE=1.5); median oncothermia survival, 8.13 months (0.1-43.9) and mean, 11.8 (SE=1.1); and the median elapsed time to the first oncothermia treatment, 5.6 months (0.2-64.8) and mean, 9.1 (SE=1.1). The differences between the early and late experiences were not significant in case of the overall survival (p=0.85), oncothermia survival (p=0.17), and the elapsed time to the first oncothermia treatment (p=0.21).
Due to the identical protocols, a simple meta-analysis was performed on these data [93]. The age distribution of the 258 patients together was near to normal (p=0.71), and no outlier was present. The median age was 57 years (range, 16 to 84), and the mean-age was 57.2 years (SE=0.7). In the spectrum of the PTF, a slight shift to the elderly patients was observed. The overall gender distribution was 83/175 female/male (32%/68%), and no significant difference could be measured between the places. The ratio of the elderly (>68 years) patients was 20.5%, (20.3% and 21.3% in PFY and HTT, respectively). The PFY/HTT patient ratio was 61/197 (24%/76%).
Eighty percent of the patients had distant metastases in both study locations and half of them were in an advanced stage at the first diagnosis of the disease. The patients were heavily pre-treated; in PFY, chemo-therapy, and in HTT, the surgery was the most frequent modality. The median elapsed time to the first oncothermia treatment from the first diagnosis (ETO) was significantly shorter in HTT than in PFY (p=0.028). The oncothermia treatment was provided twice a week, the number of treatments in average was more in the PFY than in the HTT procedures.
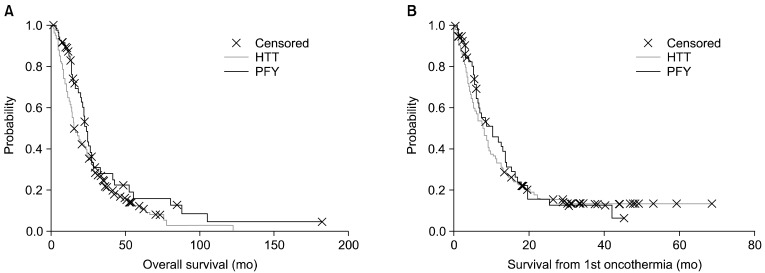
The overall survival and the survival from the first oncothermia treatment (OSO) are shown in Figs. 6, 7. The overall survivals are significantly lower in HTT (p=0.044), but in the oncothermia survival there are no significant differences (p=0.53). Survival after the treatment was not different in the two locations (p=0.55). However, for elderly patients, neither the overall survival nor the oncothermia survival was different (p=0.38 and p=0.86, respectively).
Fig. 7.
The difference between (A) the overall and (B) oncothermia survival, for patients involved in the studies. HTT, HTT-Med; PFY, Peterfy Hospital, Budapest, Hungary.
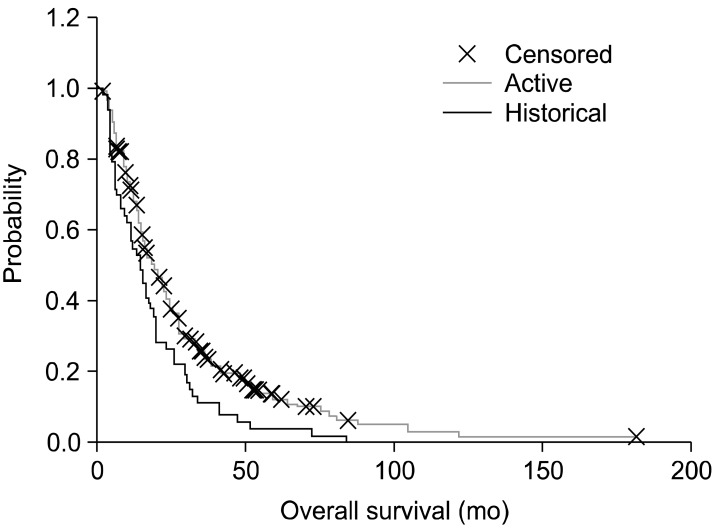
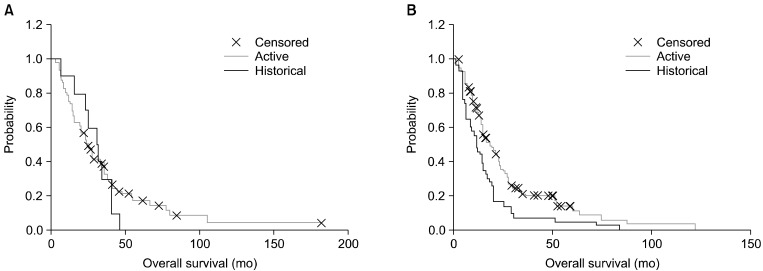
We added a Hungarian historical (retrospective) control (n=53), which was collected from the St. Borbala Hospital (Tatabanya, Hungary), for comparison. The data-set was collected from the patients of the leading author of a meta-analysis [93]. The overall survival Kaplan-Meier plot shows a significant benefit of the oncothermia (p=0.033; median, 15.8 months (1-182); mean, 23.1 months (SE=1.3) for oncothermia; median, 14.0 months (1-84); mean, 18.5 months (SE=2.3) for the historical control) (Fig. 8).
Fig. 8.
Kaplan-Meier plot for the historical and active arms in the study (n=311, active=258, control=53). The difference is significant (p=0.033).
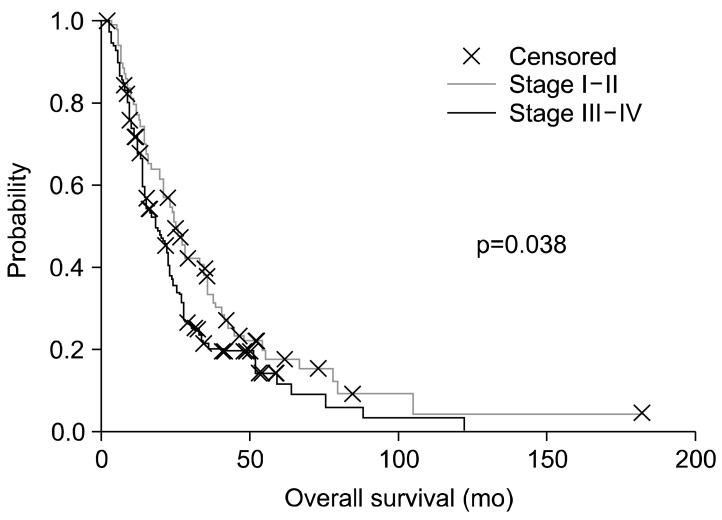
The patients were divided to subgroups of advanced (III, IIIa, IIIb, IV) (n=140) and not-advanced (I, Ia, Ib, II, IIa, IIb) (n=77) stages (data were not available for a smaller group (n=41); their data were not used in this evaluation). It is not surprising that the two subgroups are significantly different (p=0.038) by their survival (Fig. 9).
Fig. 9.
Survival difference between (p=0.038) advanced and non-advanced non-small-cell lung cancer subgroups.
The non-advanced subgroup (stages I, Ia, Ib, II, IIa, IIb) had 87 patients altogether (77 active, 10 control) and the advanced subgroup (stages III, IIIa, IIIb, IV) had 183 patients (140 active, 43 control). When we compared both subgroups with the relevant subgroup of the historical control, the effect of oncothermia became more pronounced in advanced cases (III+IV) (active arm: median, 14.7 months [n=132]; control arm: median, 11.0 months [n=43]) The result is more significant, p=0.023 than in the non-advanced case subgroup, where the differences were not large, and were not significant Fig. 10.
Fig. 10.
Comparison of subgroups with the relevant part of the historical data. Non-advanced subgroup: n=87, (77, 10), p=0.63; advanced subgroup: n=183, (140, 43), p=0.0017.
The above two studies were performed by the same guidelines but in entirely independent hospitals, with no overlap in medical personnel. The two retrospective data sets can be regarded as independent. The study of the expertise of the personnel in the two places was the same; their training was sufficient to make the optimal performance from the very start of the treatment.
The patients' pretreatments were mostly dominated by surgery and chemotherapy in HTT and PFY, respectively. Like the ETO; surprisingly the overall survival was also significantly lower as significantly different having the earlier start of oncothermia in HTT. It seems the patients treated by HTT were more advanced at their first diagnosis (more metastases were detected) than the PFY counterparts, which explains the difference. Despite the difference in overall survival, the OSO does not differ significantly between the two places. The yearly survival rates could be regarded as identical (p>0.99) within the measurement error (Fig. 11). This could be indication of the oncothermia leveling action as well.
Fig. 11.
The yearly survivals of the patients in the two institutions (no significant difference exists). In both locations, most of the patients reported subjective improvement of their quality of life. No extra toxicity or safety problem was observed during the treatments. HTT, HTT-Med; PFY, Peterfy Hospital, Budapest, Hungary.
CONCLUSION
Our present paper showed a strong indication on the oncothermia benefit to treat pulmonary malignances. The results clearly indicate the feasibility and the benefit of the oncothermia treatment for both SCLC and NSCLC for a number of reasons: 1) oncothermia showed valid treatment potential and safe application; 2) the survival time was increased by oncothermia for the patients; 3) oncothermia increased the quality of life of the patients; 4) due to the limited effectiveness of the established therapies, oncothermia could be one of the important future methods to improve our treatment facilities.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 3.Cancer Research UK. Ten-year predicted survival estimates were provided by the Cancer Research UK Cancer Survival Group, London School of Hygiene and Tropical Medicine on request, December [Internet] Oxford: Cancer Research UK; 2012. [cited 2014 Feb 20]. Available from: http://www.cancerresearchuk.org/cancer-info/cancerstats/types/lung/survival/lung-cancer-survival-statistics#source2. [Google Scholar]
- 4.Haura EB. Treatment of advanced non-small-cell lung cancer: a review of current randomized clinical trials and an examination of emerging therapies. Cancer Control. 2001;8:326–336. doi: 10.1177/107327480100800404. [DOI] [PubMed] [Google Scholar]
- 5.Ries LA, Eisner MP, Kosary CL, et al. SEER cancer statistics review, 1975-2000 [Internet] Bethesda (MD): National Cancer Institute; 2003. [cited 2003 Apr 29]. Available from: http://seer.cancer.gov/csr/1975_2000. [Google Scholar]
- 6.Jung KW, Park S, Kong HJ, et al. Cancer statistics in Korea: incidence, mortality and survival in 2006-2007. J Korean Med Sci. 2010;25:1113–1121. doi: 10.3346/jkms.2010.25.8.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jung KW, Won YJ, Park S, et al. Cancer statistics in Korea: incidence, mortality and survival in 2005. J Korean Med Sci. 2009;24:995–1003. doi: 10.3346/jkms.2009.24.6.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jin G, Jeon HS, Lee EB, et al. EML4-ALK fusion gene in Korean non-small cell lung cancer. J Korean Med Sci. 2012;27:228–230. doi: 10.3346/jkms.2012.27.2.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wakelee HA, Bernardo P, Johnson DH, Schiller JH. Changes in the natural history of nonsmall cell lung cancer (NSCLC): comparison of outcomes and characteristics in patients with advanced NSCLC entered in Eastern Cooperative Oncology Group trials before and after 1990. Cancer. 2006;106:2208–2217. doi: 10.1002/cncr.21869. [DOI] [PubMed] [Google Scholar]
- 10.Yang P, Allen MS, Aubry MC, et al. Clinical features of 5,628 primary lung cancer patients: experience at Mayo Clinic from 1997 to 2003. Chest. 2005;128:452–462. doi: 10.1378/chest.128.1.452. [DOI] [PubMed] [Google Scholar]
- 11.Govindan R, Page N, Morgensztern D, et al. Changing epidemiology of small-cell lung cancer in the United States over the last 30 years: analysis of the surveillance, epidemiologic, and end results database. J Clin Oncol. 2006;24:4539–4544. doi: 10.1200/JCO.2005.04.4859. [DOI] [PubMed] [Google Scholar]
- 12.Non-small Cell Lung Cancer Collaborative Group. Chemotherapy in non-small cell lung cancer: a meta-analysis using updated data on individual patients from 52 randomised clinical trials. BMJ. 1995;311:899–909. [PMC free article] [PubMed] [Google Scholar]
- 13.Natale RB. Gemcitabine-based doublets for advanced nonsmall-cell lung cancer: beyond gemcitabine/cisplatin. Clin Lung Cancer. 2002;3(Suppl 1):S10–S16. doi: 10.3816/clc.2002.s.002. [DOI] [PubMed] [Google Scholar]
- 14.Schiller JH, Harrington D, Belani CP, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med. 2002;346:92–98. doi: 10.1056/NEJMoa011954. [DOI] [PubMed] [Google Scholar]
- 15.Hanna N, Shepherd FA, Fossella FV, et al. Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. J Clin Oncol. 2004;22:1589–1597. doi: 10.1200/JCO.2004.08.163. [DOI] [PubMed] [Google Scholar]
- 16.Roy M, Luo YH, Ye M, Liu J. Nonsmall cell lung cancer therapy: insight into multitargeted small-molecule growth factor receptor inhibitors. Biomed Res Int. 2013;2013:964743. doi: 10.1155/2013/964743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Engelman JA, Zejnullahu K, Mitsudomi T, et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science. 2007;316:1039–1043. doi: 10.1126/science.1141478. [DOI] [PubMed] [Google Scholar]
- 18.Ganti AK, West WW, Zhen W. Current concepts in the management of small cell lung cancer. Indian J Med Res. 2013;137:1043–1051. [PMC free article] [PubMed] [Google Scholar]
- 19.Hildebrandt B, Wust P, Ahlers O, et al. The cellular and molecular basis of hyperthermia. Crit Rev Oncol Hematol. 2002;43:33–56. doi: 10.1016/s1040-8428(01)00179-2. [DOI] [PubMed] [Google Scholar]
- 20.Jiang Z, Yan W, Ming J, Yu Y. Docetaxel weekly regimen in conjunction with RF hyperthermia for pretreated locally advanced non-small cell lung cancer: a preliminary study. BMC Cancer. 2007;7:189. doi: 10.1186/1471-2407-7-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shen H, Li XD, Wu CP, Yin YM, Wang RS, Shu YQ. The regimen of gemcitabine and cisplatin combined with radio frequency hyperthermia for advanced non-small cell lung cancer: a phase II study. Int J Hyperthermia. 2011;27:27–32. doi: 10.3109/02656736.2010.500645. [DOI] [PubMed] [Google Scholar]
- 22.Moon SD, Ohguri T, Imada H, et al. Definitive radiotherapy plus regional hyperthermia with or without chemotherapy for superior sulcus tumors: a 20-year, single center experience. Lung Cancer. 2011;71:338–343. doi: 10.1016/j.lungcan.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 23.Sumiyoshi K, Strebel FR, Rowe RW, Bull JM. The effect of whole-body hyperthermia combined with 'metronomic' chemotherapy on rat mammary adenocarcinoma metastases. Int J Hyperthermia. 2003;19:103–118. doi: 10.1080/0265673021000017091. [DOI] [PubMed] [Google Scholar]
- 24.Hermisson M, Weller M. Hyperthermia enhanced chemosensitivity of human malignant glioma cells. Anticancer Res. 2000;20:1819–1823. [PubMed] [Google Scholar]
- 25.Hiraoka M, Masunaga S, Nishimura Y, et al. Regional hyperthermia combined with radiotherapy in the treatment of lung cancers. Int J Radiat Oncol Biol Phys. 1992;22:1009–1014. doi: 10.1016/0360-3016(92)90800-w. [DOI] [PubMed] [Google Scholar]
- 26.Imada H, Nomoto S, Tomimatsu A, et al. Local control of nonsmall cell lung cancer by radiotherapy combined with high-power hyperthermia using 8 MHz RF capacitive heating device. Jpn J Hyperthermic Oncol. 1999;15:19–24. [Google Scholar]
- 27.Karasawa K, Muta N, Nakagawa K, et al. Thermoradiotherapy in the treatment of locally advanced nonsmall cell lung cancer. Int J Radiat Oncol Biol Phys. 1994;30:1171–1177. doi: 10.1016/0360-3016(94)90325-5. [DOI] [PubMed] [Google Scholar]
- 28.Sakurai H, Hayakawa K, Mitsuhashi N, et al. Effect of hyperthermia combined with external radiation therapy in primary non-small cell lung cancer with direct bony invasion. Int J Hyperthermia. 2002;18:472–483. doi: 10.1080/02656730210146917. [DOI] [PubMed] [Google Scholar]
- 29.Sakao S, Takiguchi Y, Nemoto K, et al. Thermoradiotherapy for local control of chest wall invasion in patients with advanced non-small cell lung cancer. Int J Clin Oncol. 2002;7:343–348. doi: 10.1007/s101470200052. [DOI] [PubMed] [Google Scholar]
- 30.Hettinga JV, Lemstra W, Meijer C, et al. Hyperthermic potentiation of cisplatin toxicity in a human small cell lung carcinoma cell line and a cisplatin resistant subline. Int J Hyperthermia. 1994;10:795–805. doi: 10.3109/02656739409012372. [DOI] [PubMed] [Google Scholar]
- 31.Higashiyama M, Doi O, Kodama K, Yokouchi H. Intrathoracic chemothermotherapy following panpleuropneumonectomy for pleural dissemination of invasive thymoma. Chest. 1994;105:1884–1885. doi: 10.1378/chest.105.6.1884. [DOI] [PubMed] [Google Scholar]
- 32.Doi O, Kodama K, Higashiyama M, Kuriyama K, Tateishi R. Postoperative chemothermotherapy fo locally advanced lung cancer with carcinomatous pleuritis. In: Matsuda T, editor. Cancer treatment by hyperthermia, radiation, and drugs. London: Taylor & Francis; 1993. pp. 338–352. [Google Scholar]
- 33.Yang H, Jiang G, Fu X, Liao J. Radiotherapy and hyperthermia for NSCLC. Chicago: American Society of Clinical Oncology; 2005. ASCO Annual Meeting: no. 7289. [Google Scholar]
- 34.Kodama K, Doi O, Higashiyama M, Yokouchi H, Tatsuta M. Long-term results of postoperative intrathoracic chemo-thermotherapy for lung cancer with pleural dissemination. Cancer. 1993;72:426–431. doi: 10.1002/1097-0142(19930715)72:2<426::aid-cncr2820720218>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 35.Yang H, Jiang G, Fu X, et al. Radiotherapy and hyperthermia for NSCLC. Chicago: American Society of Clinical Oncology; 2005. ASCO Annual Meeting: no. 7289. [Google Scholar]
- 36.Vertrees RA, Das GC, Popov VL, et al. Synergistic interaction of hyperthermia and Gemcitabine in lung cancer. Cancer Biol Ther. 2005;4:1144–1153. doi: 10.4161/cbt.4.10.2074. [DOI] [PubMed] [Google Scholar]
- 37.Shinn KS, Choi IB, Kim IA, Choi BO, Jang JY, Kay CS. Thermoradiotherapy in the treatment of locally advanced nonsmall cell lung cancer. J Korean Soc Ther Radiol. 1996;14:115–122. [Google Scholar]
- 38.Vasanthan A, Mitsumori M, Park JH, et al. Regional hyperthermia combined with radiotherapy for uterine cervical cancers: a multi-institutional prospective randomized trial of the international atomic energy agency. Int J Radiat Oncol Biol Phys. 2005;61:145–153. doi: 10.1016/j.ijrobp.2004.04.057. [DOI] [PubMed] [Google Scholar]
- 39.Mitsumori M, Zeng ZF, Oliynychenko P, et al. Regional hyperthermia combined with radiotherapy for locally advanced non-small cell lung cancers: a multi-institutional prospective randomized trial of the International Atomic Energy Agency. Int J Clin Oncol. 2007;12:192–198. doi: 10.1007/s10147-006-0647-5. [DOI] [PubMed] [Google Scholar]
- 40.Yokoyama M, Nitta S. Systemic hyperthermia to patients with advanced pulmonary cancer. In: Matsuda T, editor. Cancer treatment by hyperthermia, radiation and drugs. London: Taylor & Francis; 1993. pp. 376–381. [Google Scholar]
- 41.Chhajed PN, Tamm M. Radiofrequency heat ablation for lung tumors: potential applications. Med Sci Monit. 2003;9:ED5–ED7. [PubMed] [Google Scholar]
- 42.Okuma T, Matsuoka T, Yamamoto A, et al. Factors contributing to cavitation after CT-guided percutaneous radiofrequency ablation for lung tumors. J Vasc Interv Radiol. 2007;18:399–404. doi: 10.1016/j.jvir.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 43.Weigel C, Kirsch M, Mensel B, Nerger U, Hosten N. Percutaneous laser-induced thermotherapy of lung metastases: experience gained during 4 years. Radiologe. 2004;44:700–707. doi: 10.1007/s00117-004-1083-z. [DOI] [PubMed] [Google Scholar]
- 44.Matsuzaki Y, Edagawa M, Shimizu T, et al. Intrapleural hyperthermic perfusion with chemotherapy increases apoptosis in malignant pleuritis. Ann Thorac Surg. 2004;78:1769–1772. doi: 10.1016/j.athoracsur.2004.05.025. [DOI] [PubMed] [Google Scholar]
- 45.Sekins KM, Leeper DB, Hoffman JK, et al. Feasibility of lung cancer hyperthermia using breathable perfluorochemical (PFC) liquids. Part II: Ultrasound hyperthermia. Int J Hyperthermia. 2004;20:278–299. doi: 10.1080/02656730310001605528. [DOI] [PubMed] [Google Scholar]
- 46.Szasz A, Szasz N, Szasz O. Oncothermia: principles and practices. London: Springer Verlag; 2010. [Google Scholar]
- 47.Szasz A, Morita T. Heat therapy in oncology: oncothermia, new paradigm in hyperthermia. Tokyo: Nippon Gyorosha; 2012. [Google Scholar]
- 48.Andocs G, Szasz O, Szasz A. Oncothermia treatment of cancer: from the laboratory to clinic. Electromagn Biol Med. 2009;28:148–165. doi: 10.1080/15368370902724633. [DOI] [PubMed] [Google Scholar]
- 49.Szasz O. Burden of oncothermia: why is it special? Conference Papers in Medicine: vol. 2013. Cairo: Hindawi Publishing Co.; 2013. [Google Scholar]
- 50.Szasz A. Physical background and technical realization of hyperthermia. In: Baronzio GF, Hager ED, editors. Locoregional radiofrequency-, perfusional- and wholebody-hyperthermia in cancer treatment: new clinical aspects. New York: Springer; 2006. pp. 27–59. [Google Scholar]
- 51.Szasz A. Challenges and solutions in oncological hyperthermia. Thermal Med. 2013;29:1–23. [Google Scholar]
- 52.Szasz A, Szasz N, Szasz O. Local hyperthermia in oncology. In: Huilgol N, editor. Hyperthermia. Rijeka: InTech; 2013. [Google Scholar]
- 53.Andocs G, Renner H, Balogh L, Fonyad L, Jakab C, Szasz A. Strong synergy of heat and modulated electromagnetic field in tumor cell killing. Strahlenther Onkol. 2009;185:120–126. doi: 10.1007/s00066-009-1903-1. [DOI] [PubMed] [Google Scholar]
- 54.Szasz O. Conference Papers in Medicine: vol. 2013. Cairo: Hindawi Publishing Co.; 2013. Essentials of oncothermia. [Google Scholar]
- 55.Szasz A. Electromagnetic effects of nanoscale range. In: Shimizu T, Kondo T, editors. Cellular response to physical stress and therapeutic application. New York: Nova Biomedical; 2013. [Google Scholar]
- 56.Szasz O, Andocs G, Meggyeshazi N. Modulation effect in oncothermia. Conference Papers in Medicine: vol. 2013. Cairo: Hindawi Publishing Co.; 2013. [Google Scholar]
- 57.Szasz O, Szasz N, Meggyeshazi N. Oncothermia as personalized treatment option. Conference Papers in Medicine: vol. 2013. Cairo: Hindawi Publishing Co.; 2013. [Google Scholar]
- 58.Meggyeshazi N, Andocs G, Krenacs T. Programmed cell death induced by modulated electrohyperthermia. Conference Papers in Medicine: vol. 2013. Cairo: Hindawi Publishing Co.; 2013. [Google Scholar]
- 59.Meggyeshazi N, Andocs G, Balogh L, et al. DNA fragmentation and caspase-independent programmed cell death by modulated electrohyperthermia. Strahlenther Onkol. 2014 Feb 22; doi: 10.1007/s00066-014-0617-1. [Epub] [DOI] [PubMed] [Google Scholar]
- 60.Elmore S. Apoptosis: a review of programmed cell death. Toxicol Pathol. 2007;35:495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Meggyeshazi N, Krenacs T, Andocs G, Szasz O. Oncothermia in laboratory; Proceedings of the 32nd Conference of the International Clinical Hyperthermia Society; 2013 Nov 8-10; Guangzhou, China. [place unknown]: International Clinical Hyperthermia Society; 2013. [Google Scholar]
- 62.Andocs G. Complex structure in oncothermia research and innovation; Proceedings of the International Hyperthermia Symposium; 2014 Feb 15; Jeju, Korea. [place unknown]: International Clinical Hyperthermia Society; 2014. [Google Scholar]
- 63.Andocs G, Meggyeshazi N, Okamoto Y, Balogh L, Szasz O. Bystander effect of oncothermia. Conference Papers in Medicine: vol. 2013. Cairo: Hindawi Publishing Co.; 2013. [Google Scholar]
- 64.Ahmed K, Zaidi SF. Treating cancer with heat: hyperthermia as promising strategy to enhance apoptosis. J Pak Med Assoc. 2013;63:504–508. [PubMed] [Google Scholar]
- 65.Hall RD, Gray JE, Chiappori AA. Beyond the standard of care: a review of novel immunotherapy trials for the treatment of lung cancer. Cancer Control. 2013;20:22–31. doi: 10.1177/107327481302000105. [DOI] [PubMed] [Google Scholar]
- 66.Kibble A, D'Souza P. Spotlight on... non-small-cell lung cancer: a new era in personalized care. New York: Thomson Reuters; 2013. [Google Scholar]
- 67.Ahn JM, Cho JY. Current serum lung cancer biomarkers. J Mol Biomark Diagn. 2013;S4:2. [Google Scholar]
- 68.Haam SJ, Kim GD, Cho SH, Lee DY. Clinical effectiveness of tumor markers (CEA, NSE, Cyfra 21-1) in completely resected non-small cell lung cancer. J Lung Cancer. 2006;5:75–83. [Google Scholar]
- 69.Oyama T, Osaki T, Baba T, et al. Molecular genetic tumor markers in non-small cell lung cancer. Anticancer Res. 2005;25:1193–1196. [PubMed] [Google Scholar]
- 70.Stieber P, Hatz R, Holdenrieder S, et al. National Academy of Clinical Biochemistry guidelines for the use of tumor markers in lung cancer, section 3P. Basel: Roche Diagnostics; 2008. [Google Scholar]
- 71.Jeung TS, Ma SY, Yu S, Lim S. Cases that respond to oncothermia monotherapy. Conference Papers in Medicine: vol. 2013. Cairo: Hindawi Publishing Co.; 2013. [Google Scholar]
- 72.Rubovszky G, Nagy T, Godeny M, Szasz A, Lang I. Successful treatment of solitary bone metastasis of non-small cell lung cancer with bevacizumab and hyperthermia. Pathol Oncol Res. 2013;19:119–122. doi: 10.1007/s12253-012-9551-7. [DOI] [PubMed] [Google Scholar]
- 73.Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355:2542–2550. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- 74.Reck M, von Pawel J, Zatloukal P, et al. Phase III trial of cisplatin plus gemcitabine with either placebo or bevacizumab as first-line therapy for nonsquamous non-small-cell lung cancer: AVAil. J Clin Oncol. 2009;27:1227–1234. doi: 10.1200/JCO.2007.14.5466. [DOI] [PubMed] [Google Scholar]
- 75.Laskin J, Crino L, Tsai C. MO19390 (SAIL): first-line bevacizumab-based therapy in advanced non-small cell lung cancer (NSCLC): outcome by chemotherapy regimen [abstract] J Thorac Oncol. 2009;4(Suppl 1):S359. [Google Scholar]
- 76.Hirsh V. Emerging safety data for bevacizumab in advanced non-small-cell lung cancer. Clin Lung Cancer. 2008;9(Suppl 2):S62–S70. doi: 10.3816/CLC.2008.s.010. [DOI] [PubMed] [Google Scholar]
- 77.Crino L, Mezger J, Griesinger F, et al. MO19390 (SAIL): safety and efficacy of first-line bevacizumab (Bv)-based therapy in advanced non-small cell lung cancer (NSCLC) [abstract] Proc Am Soc Clin Oncol. 2009;27:417S. [Google Scholar]
- 78.Fischbach N, Spigel D, Brahmer J On Behalf of the ARIES Investigators. Preliminary safety and effectiveness of bevacizumab (BV) based treatment in subpopulations of patients (pts) with non-small cell lung cancer (NSCLC) from the ARIES study: a bevacizumab (BV) treatment observational cohort study (OCS) [abstract] Proc Am Soc Clin Oncol. 2009;27:416S. [Google Scholar]
- 79.Mezger J, von Pawel J, Reck M. Bevacizumab (Bv) single-agent maintenance following Bv-based chemotherapy in patients with advanced non-small cell lung cancer (NSCLC): results from an exploratory analysis of the AVAiL study [abstract] Proc Am Soc Clin Oncol. 2009;27:e19001. [Google Scholar]
- 80.Miller V, O'Connor P, Soh C On Behalf of the ATLAS Investigators. A randomized, double-blind, placebo-controlled, phase IIIb trial (ATLAS) comparing bevacizumab (B) therapy with or without erlotinib (E) after completion of chemotherapy with B for first-line treatment of locally advanced, recurrent, or metastatic non-small cell lung cancer (NSCLC) [abstract] Proc Am Soc Clin Oncol. 2009;27:799S. [Google Scholar]
- 81.Yoon SM, Lee JS. Case of abscopal effect with metastatic non-small-cell lung cancer. Oncothermia J. 2012;5:53–57. [Google Scholar]
- 82.Min SG. Clinically complete remission of a disseminated non-small-cell lung cancer (NSCLC) case with oncothermia and concurrent 5th-line chemotherapy; Poster of the 31st International Clinical Hyperthermia Society Conference; 2012 Oct 12-14; Budapest, Hungary. [place unknown]: International Clinical Hyperthermia Society; 2012. [Google Scholar]
- 83.Polo V, Besse B. Maintenance strategies in stage IV non-small-cell lung cancer (NSCLC): in which patients, with which drugs? Ann Oncol. 2013 Dec 18; doi: 10.1093/annonc/mdt529. [Epub] [DOI] [PubMed] [Google Scholar]
- 84.Lee DY, Park JS, Leem CY, et al. The outcomes of oncothermia with chemotherapy for far advanced lung cancer (3 cases of adenocarcinoma); Proceedings of the 32nd Conference of the International Clinical Hyperthermia Society; 2013 Nov 8-10; Guangzhou, China. [place unknown]: International Clinical Hyperthermia Society; 2013. [Google Scholar]
- 85.Lee Y. Oncothermia application for various malignant diseases. Conference Papers in Medicine: vol. 2013. Cairo: Hindawi Publishing Co.; 2013. [Google Scholar]
- 86.Kim JH, Kim DR. Mixed response to TS-1 and oncothermia in an esophageal cancer patient with lung metastases: case report; Poster at the 32nd Conference of the International Clinical Hyperthermia Society; 2013 Nov 8-10; Guangzhou, China. [place unknown]: International Clinical Hyperthermia Society; 2013. [Google Scholar]
- 87.Lee CG. Definitive concurrent radio-oncothermia with or without chemotherapy in locally advanced non-small cell lung cancer: early clinical experience; Proceedings of the International Hyperthermia Symposium; 2014 Feb 15; Jeju, Korea. [place unknown]: International Clinical Hyperthermia Society; 2014. [Google Scholar]
- 88.Zhao C. Evaluation the effect of local hyperthermia on late stage lung cancer patients; Proceedings of the 32nd Annual Conference of International Clinical Hyperthermia Society; 2013 Nov 12-14; Guangzhou, China. [place unknown]: International Clinical Hyperthermia Society; 2013. [Google Scholar]
- 89.Lee DY, Haam SJ, Kim TH, et al. Oncothermia with chemotherapy in the patients with small-cell lung cancer. Conference Papers in Medicine: vol. 2013. Cairo: Hindawi Publishing Co.; 2013. [Google Scholar]
- 90.Dani A, Varkonyi A, Osvath M, Szasz A. Treatment of non-small-lung-cancer by electro-hyperthermia. Strahlenter Onko. 2004;180:20. [Google Scholar]
- 91.Dani A, Varkonyi A, Nyiro I, Osvath M. Clinical experience of electro-hyperthermia for advanced lung-tumors; Proceedings of the European Society of Hyperthermic Oncology Conference; 2003 Jun 4-7; Munich, Germany. Rotterdam: European Society of Hyperthermic Oncology; 2003. [Google Scholar]
- 92.Hager ED, Krautgartner IH, Popa C, Hohmann D, Dziambor H. Deep hyperthermia with short waves of patients with advanced stage lung cancer, hyperthermia in clinical practice; XXII Meeting of International Clinical Hyperthermia Society; [place unknown]: International Clinical Hyperthermia Society; 1999. [Google Scholar]
- 93.Dani A, Varkonyi A, Magyar T, Szasz A. Clinical study for advanced non-small-cell lung-cancer treated by oncothermia. Forum Hyperthermie; 2010. [Google Scholar]