Abstract
The diagnosis of multiple primary malignancies (MPMs) in a patient has been reported rather frequently during the past decade. Here we present two cases with three synchronous primary malignant tumors. The first patient is a 66-year-old male with synchronous colorectal cancer, renal cell carcinoma (RCC) and non-small cell lung cancer (NSCLC). The second patient is a 64-year-old female with breast cancer, transitional cell carcinoma of the ureter and endometrial cancer. MPMs seem to be diagnosed in a higher incidence than that predicted only by the influence of hazard and, whenever found, they raise questions regarding not only possible common etiologic factors or same pathogenetic mechanisms but also they cause a lot of troubles to both clinicians and patients because the therapeutic options usually become limited.
Keywords: Multiple, primary, malignancies, synchronous, metachronous
Introduction
The diagnosis of multiple primary malignancies (MPMs) in a patient has been reported rather frequently during the past decade. Multiple mechanisms have been implicated in the pathogenesis of this entity, including hereditary, immune and environmental factors such as chemicals, viruses, chemotherapeutic regimens and ionizing radiation (1,2). Furthermore, increased survival of cancer patients, the growing life expectancy and the development of improved diagnostic techniques, have all contributed to the increased frequency of MPMs (2).
Here we present two patients with three primary malignant tumors. The first case describes a patient with synchronous colorectal cancer, renal cell carcinoma (RCC) and non-small cell lung cancer (NSCLC), and the second is a case of synchronous breast, urothelial cell and endometrial cancer (3,4).
Case report
Case 1
The first patient was a 66-year-old Greek male with no prior medical history, except for smoking (20 cigarettes per day/40 years) and mild long standing hypertension, which was left untreated. His family history was negative for malignancy. He visited Department of Oncology because of a recent diagnosis of a grade 2, mucous producing adenocarcinoma in the ileocecal valve. Chest X-rays, blood tests and tumor markers were all normal. Furthermore, abdominal ultrasound was normal as well except for a right renal cyst. The patient underwent a partial colectomy and pathology report confirmed the diagnosis. Additionally, 3 out of 14 lymph nodes were reported positive for tumor infiltration (stage 3 according to Dukes Classification). However, the patient refused to undertake adjuvant chemotherapy. Post surgical reassessment with whole body computed tomography (CT) scan revealed nodular lesions mostly eminent in the left lower lobe of the lung with a longest diameter of 2.8 cm (Figure 1A). Additionally, an abdominal CT scan showed a renal cyst (7 cm) with mixed characteristics in the right kidney (Figure 1B). The patient underwent a positron emission tomography (PET) scan that revealed increased rates of absorption of 18F-fludeoxyglucose (18F-FDG) in the above lesions. In addition, it showed a new focus of hypermetabolic state at the splenic curve of the large bowel. Further evaluation with magnetic resonance imaging (MRI) of the upper abdomen revealed that the cystic mass of the right kidney was likely to be a cystic renal cell cancer. Colonoscopy was negative for disease relapse, but two polyps were found and resected. Two months later, the patient underwent a left lung sublobar resection and the obtained lesions were found to be lung adenocarcinoma (NSCLC). After four consecutive months, the patient also underwent a subtotal nephrectomy that revealed a stage T1a RCC. After three months, while on surveillance, the CT scan of the chest showed a hilar mass in the right lung that was attributed to a relapse of the lung adenocarcinoma. The patient received only external beam radiation therapy with disease partial response. At present, two years after the diagnosis of three different primary cancers, the patient is feeling well and the follow up shows no signs of disease progression (Figure 2).
Figure 1.
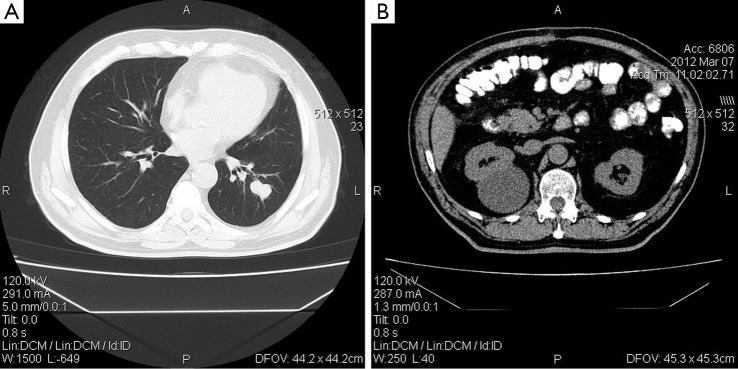
CT scan of a patient with synchronous lung cancer (A), cystic hypernephroma (B) and surgically treated colon cancer (B).
Figure 2.
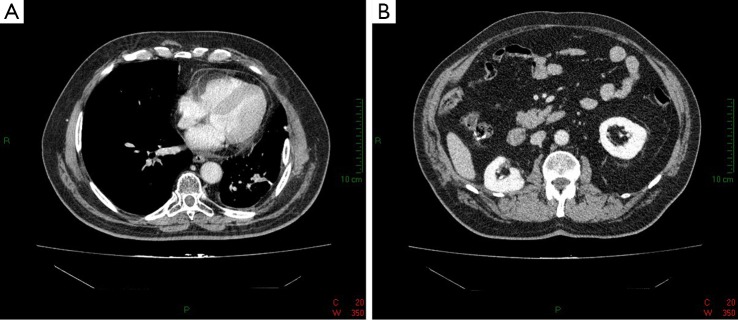
CT scan of the first patient after right subtotal lobectomy (A) and subtotal nephrectomy (B).
Case 2
The second case refers to a 64-year-old Greek woman with a past medical history of chronic obstructive pulmonary disease (COPD) due to heavy smoking, diabetes mellitus, hyperuricemia and dyslipidemia. During self examination, she palpated a lump in her left breast and underwent a mammography that revealed an inoperable locally advanced tumor [Breast Imaging Reporting and Data System (BI-RADS) 5]. A biopsy was performed and revealed invasive ductal carcinoma. The tumor was negative for estrogen and progesterone receptors (ER/PR) and positive for human epidermal growth factor receptor 2 (HER2). CT scan of the chest showed a 1.3 cm nodule in the middle lobe of the right lung, which was considered as metastatic disease (Figure 3A). Bone scan revealed also diffuse skeletal metastases. Moreover, an abdominal CT scan revealed distention and dilation of both renal pelvis and calyces of the left kidney and thickened endometrial wall (Figure 3B,C).
Figure 3.
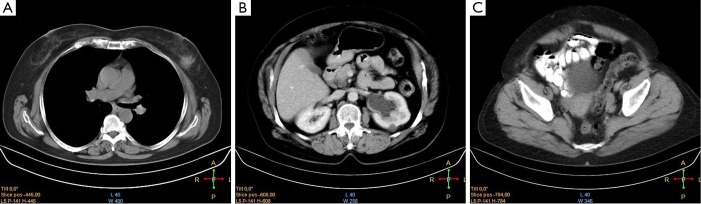
CT scan of a patient with synchronous breast (A), transitional cell (B), and endometrial cancers (C).
MRI urography showed that the obstruction was at the lower part of the left ureter. Ureteroscopy was performed and a polypoid mass was resected. Diagnosis was transitional cell cancer (stage T1).
Additionally, the patient underwent endometrial curettage and adenocarcinoma with high degree of differentiation of endometrial origin was revealed. Subsequently, the patient received 6 cycles of chemotherapy with paclitaxel/carboplatin followed by administration of trastuzumab and zoledronic acid. Imaging studies performed shortly after showed that there were no signs of disease progression regarding the breast cancer, or in the internal genital organs of the patient and anywhere else in the abdomen (Figure 4). A year after the completion of chemotherapy, the patient is feeling well and shows no evidence of disease progression.
Figure 4.
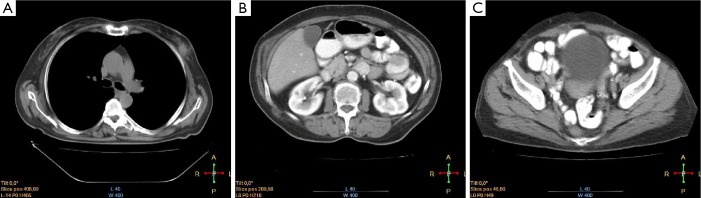
CT scan of the chest (A), abdomen (B) and pelvis (C) of the second patient after resection of a ureteral mass and administration of six cycles of carboplatin/paclitaxel.
Discussion
Three criteria must be fulfilled so as to characterize MPMs: (I) each tumor must be distinct from each other; (II) each tumor must present definite features of malignancy; and (III) the possibility that the one is a metastasis of another must be ruled out (5,6). They are divided basically into two large categories depending on the time of diagnosis of each tumor. If the tumors are diagnosed simultaneously or within a 6-month interval, they are called synchronous. If the interval is longer, the tumors are called metachronous (5,6).
Despite its increasing rates, MPMs remain rare. Occurrence ranges between 0.7% and 11.7% (7). Population-based studies have attempted to estimate the incidence of a second or third malignancy in an individual that had already been diagnosed with cancer. Schoenberg et al. found that patients with cancer had 1.29 times the risk of developing a new malignancy compared with those that were never diagnosed (8). The risk is slightly greater for women in respect to metachronous tumors but synchronous lesions slightly favor men compared with women (2).
The study of MPMs may provide useful information not only for clinical purposes, but it can also provide clues for the etiology and the management of cancer. They can generally be categorized into three major groups depending on the main etiologic factor. The first group includes treatment related neoplasms, the second includes syndromic cases, and the third includes neoplasms that may share common etiologic factors, such as genetic predisposition or same environmental factors (9). Additionally, two or more cancers can also be a result of pure chance (10).
Although lungs are the most common site for renal cell cancer metastasis, in our first case the two malignancies were separate entities. Despite their coexistence is rare, they share some common etiologic factors such as smoking and asbestos exposure. Our patient was a smoker, which is a well documented risk factor not only for RCC and lung adenocarcinoma, but also for colorectal cancer. Furthermore, the fact that RCC was found inside a cystic focus and surveillance colonoscopy revealed two colonic polyps, indicates an additional genetic predisposition in this patient.
In the second case, the coexistence of breast and endometrial cancer possibly reflects the fact that certain hormonal factors act upon both the breast and uterus and this can predispose to the development of cancer on both organs. Furthermore, environmental and metabolic factors are present in this patient, such as unhealthy lifestyle, diabetes and obesity that can further play a part. Moreover, smoking can also contribute to the development of urothelial cancer. The same embryological origin of both urothelial and endometrial tissues can constitute an additional factor.
MPMs, whenever found, raise questions regarding not only possible common etiologic factors and pathogenetic mechanisms, but above all, they cause a lot of troubles to both clinicians and patients because the therapeutic options usually become limited (2,11). In the first case, the combination of colon cancer, RCC and NSCLC was treated with surgical and radiotherapeutic interventions. During the course of his disease, the plan was to receive a combination chemotherapy consisting of paclitaxel plus carboplatin but the patient denied because of socioeconomic and family problems. In the second case, the combination of urothelial, endometrial and advanced breast cancer was treated with the combination of paclitaxel/carboplatin and trastuzumab with zoledronic acid, plus the surgical resection of the lesion in the lower part of the left ureter.
In conclusion, MPMs seem to be diagnosed in a higher incidence than that predicted only by the influence of hazard. The study of these cases can provide useful information regarding the development of effective screening and surveillance protocols, with the goal to treat patients effectively.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- 1.Warren S, Gates O.Multiple primary malignant tumors: survey of the literature and a statistical study. Am J Cancer 1932;16:1358-414 [Google Scholar]
- 2.Spratt JS, Jr, Hoag MG. Incidence of multiple primary cancers per man-year of follow up: 20-year review from the Ellis Fischel State Cancer Hospital. Ann Surg 1966;164:775-84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fante R, Roncucci L, Di Gregorio C, et al. Frequency and clinical features of multiple tumors of the large bowel in the general population and in patients with hereditary colorectal carcinoma. Cancer 1996;77:2013-21 [DOI] [PubMed] [Google Scholar]
- 4.Beisland C, Talleraas O, Bakke A, et al. Multiple primary malignancies in patients with renal cell carcinoma: a national population-based cohort study. BJU Int 2006;97:698-702 [DOI] [PubMed] [Google Scholar]
- 5.Aydiner A, Karadeniz A, Uygun K, et al. Multiple primary neoplasms at a single institution: differences between synchronous and metachronous neoplasms. Am J Clin Oncol 2000;23:364-70 [DOI] [PubMed] [Google Scholar]
- 6.Derwinger K, Gustavsson B.A study of aspects on gender and prognosis in synchronous colorectal cancer. Clin Med Insights Oncol 2011;5:259-64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coleman MP. Multiple primary malignant neoplasms in England and Wales, 1971-1981. Yale J Biol Med 1986;59:517-31 [PMC free article] [PubMed] [Google Scholar]
- 8.Schoenberg BS. Multiple primary malignant neoplasms. The Connecticut experience, 1935-1964. Recent Results Cancer Res 1977;58:1-173 [PubMed] [Google Scholar]
- 9.Takalkar U, Asegaonkar BN, Kodlikeri P, et al. An elderly woman with triple primary metachronous malignancy: A case report and review of literature. Int J Surg Case Rep 2013;4:593-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wood ME, Vogel V, Ng A, et al. Second malignant neoplasms: Assessment and strategies for risk reduction. J Clin Oncol 2012;30:3734-45 [DOI] [PubMed] [Google Scholar]
- 11.Jung EJ, Lee JH, Jeon K, et al. Treatment outcomes for patients with synchronous multiple primary non-small cell lung cancer. Lung Cancer 2011;73:237-42 [DOI] [PubMed] [Google Scholar]


