Summary
Sparse coding may be a general strategy of neural systems to augment memory capacity. In Drosophila, sparse odor coding by the Kenyon cells of the mushroom body is thought to generate a large number of precisely addressable locations for the storage of odor-specific memories. However, it remains untested how sparse coding relates to behavioral performance. Here we demonstrate that sparseness is controlled by a negative feedback circuit between Kenyon cells and the GABAergic anterior paired lateral (APL) neuron. Systematic activation and blockade of each leg of this feedback circuit show that Kenyon cells activate APL and APL inhibits Kenyon cells. Disrupting the Kenyon cell-APL feedback loop decreases the sparseness of Kenyon cell odor responses, increases inter-odor correlations, and prevents flies from learning to discriminate similar, but not dissimilar, odors. These results suggest that feedback inhibition suppresses Kenyon cell activity to maintain sparse, decorrelated odor coding and thus the odor-specificity of memories.
Introduction
To adapt to their environments, animals must learn which stimuli are associated with rewards or punishments and distinguish these reinforced stimuli from similar but irrelevant ones. One widely proposed mechanism for implementing stimulus-specific associative memories is sparse coding, in which only a few neurons out of a population respond to any given stimulus and each neuron responds to only a few stimuli out of all possible stimuli. Theoretical work has suggested that sparse coding increases the capacity of associative memory by reducing overlap between representations1–4. Experimentally, sparse representations of sensory information have been observed in many systems, including vision5, audition6, touch7, and olfaction8–12. However, despite the accumulating evidence for widespread sparse coding and theoretical arguments for its importance, a demonstration that sparse coding improves the stimulus-specificity of associative memory has been lacking. Addressing this gap experimentally must begin with an understanding of how sparse coding arises, a problem that also remains incompletely understood (but see refs. 8,13–16).
An attractive model for studying these questions is the Drosophila olfactory system. Projection neurons (PNs), the second-order neurons that carry olfactory information from the antennal lobe to the protocerebrum, encode odors broadly: many PNs respond to the majority of odor stimuli, and many odors elicit responses in most PNs, in both locust and Drosophila8,17. This odor code is dramatically sparsened at the third level of olfactory processing, the Kenyon cells of the mushroom body, where in both locust and Drosophila, only ~5–10% of Kenyon cells respond to any given odor, and Kenyon cells that do respond fire only a few spikes8,9,12,18. Notably, Kenyon cells are the major site of olfactory associative memory storage19. The sparseness of Kenyon cell activity may reduce overlap between odor representations and thereby help the animal retrieve distinct learned responses to similar odors13,14.
The low activity levels associated with sparse coding suggest a role for inhibition. Indeed, feedforward and feedback inhibitory motifs are common in sparsely responding sensory systems and have been widely suggested to underlie sparseness11,15,16,20,21. However, direct evidence that inhibition causes sparseness and that sparseness is behaviorally relevant is scant. The ability in Drosophila to combine optical imaging of neuronal population responses12,22, acute silencing23 and activation24,25 of genetically defined neurons, and behavioral assays for learned odor discrimination26,27 provides a unique opportunity to test the hypotheses that i) feedback inhibition underlies sparse odor coding in Kenyon cells, and ii) sparse odor coding improves the stimulus-specificity of associative memory.
Results
Feedback inhibition of Kenyon cell responses
To test whether Kenyon cell activity leads to negative feedback onto Kenyon cells themselves, we acutely blocked Kenyon cell synaptic output using temperature-sensitive shibire (shits1), a dominant-negative mutant of dynamin that interferes with synaptic vesicle re-endocytosis at the restrictive temperature23 (>30 °C). We took advantage of the fact that shits1 blocks synaptic transmission, not electrical activity, by co-expressing shits1 and the Ca2+ reporter GCaMP3 in Kenyon cells.
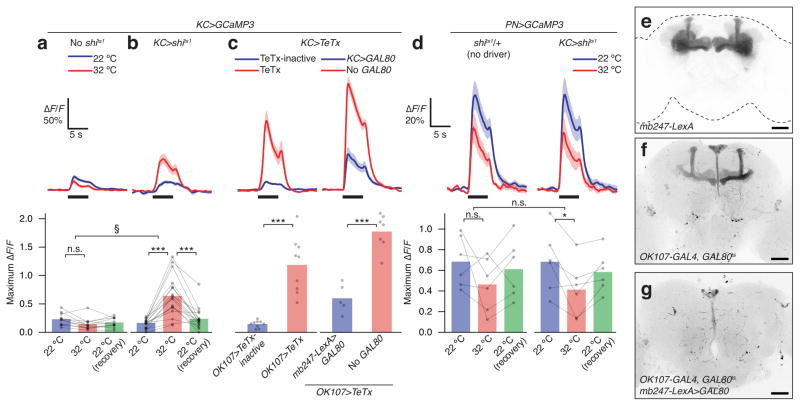
Control flies expressing only GCaMP3 under the control of mb247-LexA showed robust odor-evoked Ca2+ influx in the α lobe that did not change, or decreased slightly, at the restrictive 32 °C (Figs. 1a, 2). In contrast, flies expressing both GCaMP3 and shits1 in Kenyon cells exhibited greatly increased odor-evoked Ca2+ transients at 32 °C (Figs. 1b, 2). The odor response recovered to baseline upon return to 22 °C in most but not all cases, consistent with previous reports that recovery from shits1 inactivation is not always complete28. The significant temperature effect in flies expressing GCaMP3 and shits1 compared to flies expressing only GCaMP3 is unlikely to be caused by blocking neurons other than Kenyon cells because mb247-LexA shows little or no expression elsewhere (Fig. 1e).
Figure 1. Feedback inhibition of Kenyon cell responses by Kenyon cell output.
(a) Kenyon cells in control mb247-LexA>GCaMP3 flies show no temperature-dependent increase in odor-evoked Ca2+ influx in the α lobe. Black bars indicate 5-s pulses of ethyl acetate. Traces depict average ΔF/F; shading indicates s.e.m. n=11 [10] (number of brain hemispheres [number of flies]). (b) Kenyon cells in experimental mb247-LexA>GCaMP3,shits1 flies show a large temperature-dependent increase in odor-evoked Ca2+ influx in the α lobe. n=16 [15]. *** P<0.001, Friedman test with Dunn’s multiple comparisons test. § P<0.001, Mann-Whitney U-test, comparing ratios of odor-evoked Ca2+ influx at 32 °C vs. 22 °C between control and mb247-LexA>shits1 flies. (c) Left panels: OK107>TeTx flies (red, n=9) show a large increase in odor-evoked Ca2+ influx in the α lobe compared to OK107>TeTx-inactive flies (blue, n=9). Right panels: odor-evoked Ca2+ influx is higher in OK107>TeTx, mb247-LexA>GCaMP3 flies (blue, n=5) than in OK107>TeTx, mb247-LexA>GCaMP3,GAL80 flies (red, n=7). *** P<0.001, unpaired Welch t-test. (d) Odor-evoked Ca2+ influx in PNs innervating the calyx declines slightly with temperature in both NP225>GCaMP3, mb247-LexA>shits1 and NP225>GCaMP3, shits1/+ flies. * P<0.05, repeated-measures ANOVA with Geisser-Greenhouse correction and Holm-Sidak multiple comparisons test. n=6. Bracket between panels indicates that the ratio of odor-evoked Ca2+ influx at 32 °C vs. 22 °C does not differ significantly between panels (unpaired Welch t-test, P=0.60). (e–g) Representative maximum intensity projections of confocal image stacks showing expression patterns of: (e) mb247-LexA; (f) OK107, GAL80ts; (g) OK107, GAL80ts, mb247-LexA>Gal80. Scale bars, 50 μm. See Supplementary Table 1 for full genotypes.
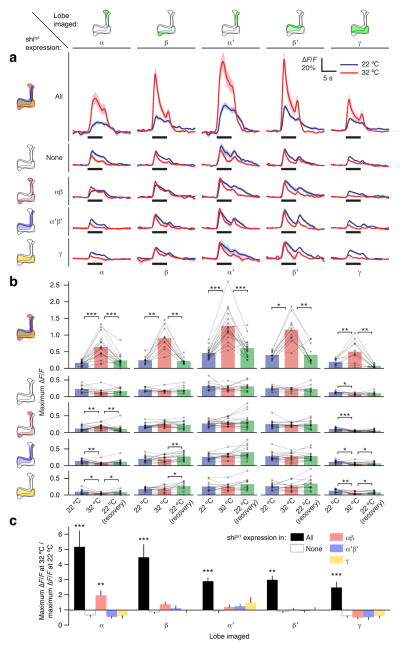
Figure 2. Feedback is from all Kenyon cells to all Kenyon cells.
(a) Impact of blocking output of different Kenyon cell populations (rows) on odor-evoked Ca2+ influx in different mushroom body lobes (columns). By row: All: blocking all Kenyon cells increases odor-evoked Ca2+ influx in all lobes of the mushroom body in mb247-LexA>GCaMP3,shits1 flies. None: raising the temperature has no effect on, or slightly decreases, odor responses in all lobes of control mb247-LexA>GCaMP3 flies. αβ: blocking αβ neurons slightly increases odor responses only in the α lobes of mb247-LexA>GCaMP3, c739>shits1 flies. α′β′: blocking α′β′ neurons does not affect odor responses in mb247-LexA>GCaMP3, R35B12>shits1 flies. γ: blocking γ neurons does not affect odor responses in mb247-LexA>GCaMP3, R64C08>shits1 flies. (b) Bar graphs summarizing data from (a). n, left to right, given as number of brain hemispheres [number of flies]: All: 16 [15], 11 [6], 17 [16], 11 [6], 11 [6]. None: 11 [10], 9 [7], 11 [10], 9 [7], 9 [7]. αβ: 16 [9], 15 [8], 16 [9], 15 [8], 15 [8]. α′β′: 13 [8] (all). γ: 11 [6] (all). * P<0.05, ** P<0.01, *** P<0.001, repeated-measures ANOVA with Geisser-Greenhouse correction and Holm-Sidak multiple comparisons test or Friedman test with Dunn’s multiple comparisons test, as appropriate. (c) Ratios of odor-evoked Ca2+ influx at 32 °C vs. 22 °C for data in (a–b). * P<0.05, ** P<0.01, *** P<0.001, Kruskal-Wallis ANOVA with post hoc Mann-Whitney U tests using Holm-Bonferroni multiple comparisons correction. Error bars show s.e.m. See Supplementary Table 1 for full genotypes.
To eliminate the possibility that shits1 inactivation affects synaptic integration by preventing membrane retrieval and thereby increasing membrane capacitance, we used tetanus toxin light chain (TeTx), which blocks vesicle exo- rather than endocytosis29. We targeted TeTx to Kenyon cells with the help of OK107-GAL4 and used tubP-GAL80ts to suppress transgene expression during development. Inactivation of the GAL80ts repressor by heating <1 day old flies to 31 °C for 16–24 h induced transgene expression in the pattern previously reported30 for OK107-GAL4 (Fig. 1f). Acute expression of TeTx led to increased odor-evoked Ca2+ influx relative to acute expression of a catalytically inactive toxin29 (Fig. 1c). The effect was abolished by mb247-LexA-driven expression of GAL80 (Fig. 1c), which subtracts Kenyon cells from the OK107-GAL4 pattern (Fig. 1g). Together, these results suggest that feedback inhibition suppresses Kenyon cell responses.
In Drosophila, Kenyon cells have been proposed to communicate with the antennal lobe via unidentified cholinergic neurons31, while in mammals, feedback from the olfactory cortex inhibits the mitral cells of the olfactory bulb32,33. We therefore examined whether the Kenyon cell-driven inhibitory feedback operates directly on Kenyon cells or an earlier stage of the olfactory pathway, by expressing GCaMP3 under NP225-GAL4 control in PNs. Odor-evoked responses of PNs innervating the mushroom body calyx did not increase after the removal of Kenyon cell output in PN>GCaMP3, KC>shits1 flies (Fig. 1d). Indeed, PN odor responses in both lexAop-shits1;mb247-LexA and lexAop-shits1/+ flies decreased slightly at the elevated temperature, but there was no difference in the magnitude of the decrease between the two groups (Fig. 1d). The small temperature effect is therefore unrelated to shits1-mediated blockade of Kenyon cells. These results indicate that feedback inhibition operates directly on the mushroom body.
Feedback is from all Kenyon cells to all Kenyon cells
Kenyon cells are subdivided into three main classes: γ neurons project to the horizontal lobes only, while the axons of αβ and α′β′ neurons bifurcate to form the α and α′ portions of the vertical lobes and the β and β′ portions of the horizontal lobes (Fig. 2). If feedback inhibition were strictly local or Kenyon cell class-specific, blocking output from one class would increase odor responses only in those cells. In contrast, if feedback were all-to-all, blockade of one class of Kenyon cells would have little effect because of compensatory drive from other Kenyon cells. To distinguish between these possibilities, we separately blocked the synaptic output of each main class of Kenyon cells, driving shits1 in αβ neurons using c739-GAL4, in α′β′ neurons using R35B12-GAL4, and in γ neurons using R64C08-GAL4 (Supplementary Fig. 1), while imaging odor responses in all lobes.
Blocking the output of all Kenyon cells in lexAop-shits1;mb247-LexA flies increased odor responses throughout the mushroom body (Fig. 2). In contrast, blocking only αβ Kenyon cells slightly elevated the odor responses of these cells but left those of other Kenyon cells unaltered; the increase of αβ responses, however, was minuscule compared to that observed in the same neurons after blocking output from all Kenyon cells (Fig. 2). Blocking only α′β′ or only γ neurons had no effect on odor responses in any lobe (Fig. 2). Similar results were seen with the α′β′ driver c305a-GAL4 and the γ drivers NP1131-GAL4, H24-GAL4, and 1471-GAL4 (data not shown). Because blocking output from all Kenyon cells is required to suppress inhibition in any lobe, feedback is in all likelihood all-to-all. The subtly different consequences of blocking αβ vs. α′β′ vs. γ neurons may simply reflect the differing sizes of the respective populations (about 1/2, 1/6 and 1/3 of all Kenyon cells30).
Kenyon cells activate APL
All-to-all feedback suggests that Kenyon cell output is integrated into a single inhibitory feedback signal, perhaps by a single neuron. In locust, a giant GABAergic neuron (GGN) present in a single copy per hemisphere provides negative feedback to Kenyon cells15. The GGN is most likely the locust analog of the Drosophila anterior paired lateral (APL) neuron. Each hemisphere of the Drosophila brain contains one APL neuron, which extends processes throughout the calyx, peduncle, and lobes of the mushroom body34,35. The APL neuron is GABAergic and responds to odors with depolarization and Ca2+ influx15,34. Kenyon cells express the GABAA receptor RDL, and overexpression of RDL reduces the amplitude of Kenyon cell odor-evoked Ca2+-influx, while knockdown of RDL by RNAi increases it36. As in locust15, where Kenyon cell spikes elicit excitatory postsynaptic potentials in the GGN, the APL neuron might thus form a negative feedback loop with Kenyon cells.
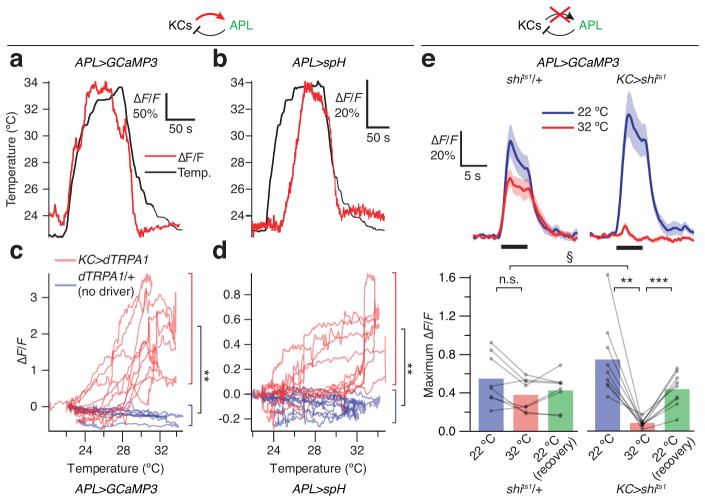
To probe for connectivity between APL and Kenyon cells, we expressed lexAop-dTRPA1 under mb247-LexA control in Kenyon cells and imaged calcium transients or synaptic vesicle release22 in APL, using UAS-GCaMP3 or UAS-synapto-pHluorin (spH) driven by GH146-GAL4. dTRPA1 encodes a cation channel whose conductance gates open at elevated temperatures (>25 °C), stimulating activity25. Thermal activation of Kenyon cells caused large increases in GCaMP3 and spH signals emitted by APL projections in the vertical mushroom body lobes (Fig. 3a,b). The signals were absent in flies carrying the lexAop-dTRPA1 responder but lacking the mb247-LexA driver transgene (Fig. 3c,d). These results, together with the anatomy of the APL neuron34,35, suggest that the APL odor response is driven by Kenyon cells. The response should therefore vanish when Kenyon cell output is blocked. Indeed, in flies expressing mb247-LexA-driven shits1, but not in controls, odor-evoked GCaMP3 responses were blocked at the restrictive temperature and restored at the permissive temperature (Fig. 3e). Although these data do not distinguish between a direct (monosynaptic) and an indirect connection between Kenyon cells and APL, they do identify Kenyon cells as the source of odor input to APL, thus delineating one leg of the negative feedback loop.
Figure 3. Kenyon cells activate APL.
(a,b) Thermal activation of Kenyon cells induces Ca2+ influx into APL in GH146>GCaMP3, mb247-LexA>dTRPA1 flies (a) and synaptic vesicle release from APL in GH146>spH, mb247-LexA>dTRPA1 flies (b). Red traces show ΔF/F; black trace show temperature. (c, d) ΔF/F of GCaMP3 (c) and spH (d) as functions of temperature; each trace represents one fly. Each red trace forms a loop: in mb247-LexA>dTRPA1 flies, ΔF/F rises as the fly is heated, and falls along a different trajectory as the fly is cooled (n=5). Heat does not induce Ca2+ influx in GH146>GCaMP3, dTRPA1/+ flies or vesicle fusion in GH146>spH, dTRPA1/+ flies (blue traces; GCaMP3: n=4; spH: n=5). ** P<0.01, unpaired Welch t-test, comparing the maximum ΔF/F (between 30 °C and the temperature maximum) between mb247-LexA>dTRPA1 and control flies. Mean ± s.e.m.: GCaMP3, mb247-LexA>dTRPA1, 1.90 ± 0.44; GCaMP3, dTRPA1/+, −0.27 ± 0.05; spH, mb247-LexA>dTRPA1, 0.68 ± 0.09; spH, dTRPA1/+, 0.06 ± 0.007. (e) Temperature block of transmission from Kenyon cells blocks odor-evoked APL responses in GH146>GCaMP3, mb247-LexA>shits1 flies (right) but not in GH146>GCaMP3, shits1/+ flies (left). *** P<0.001, repeated-measures ANOVA with Geisser-Greenhouse correction and Holm-Sidak multiple comparisons test. n=8, 9. § P<0.01, Mann-Whitney U test, comparing ratios of odor-evoked Ca2+ influx at 32 °C vs. 22 °C between control and mb247-LexA>shits1 flies. Black bars indicate 5 s pulses of ethyl acetate. Schematics on top indicate which neuron is imaged (green) and which connection is being manipulated (red arrow for dTRPA1 activation, red X for shits1 blockade). See Supplementary Table 1 for full genotypes.
APL inhibits Kenyon cells
While the odor-evoked Ca2+ transients in APL branches innervating the mushroom body might reflect postsynaptic activity only, the spH signal indicates that APL also forms presynaptic specializations in the lobes that release transmitter in response to odors. We therefore asked whether Kenyon cells themselves are the targets of APL inhibition. This question has been difficult to address because of a lack of clean genetic access to APL34,35. Previous studies observed phenotypes after RNAi knock-down of the GABA biosynthetic enzyme glutamic acid decarboxylase (GAD) using GH146-GAL4 or NP2631-GAL4, broad drivers that include APL34,37,38. However, in these approaches non-APL neurons may also be affected: GH146-GAL4 marks ~60% of PNs, ~6 of which are GABAergic39, while NP2631-GAL4 is similarly broad35. In addition, GADRNAi-based knockdown of GABA release is most likely incomplete, a problem that may be compounded by homeostatic adaptation or negative feedback (see below).
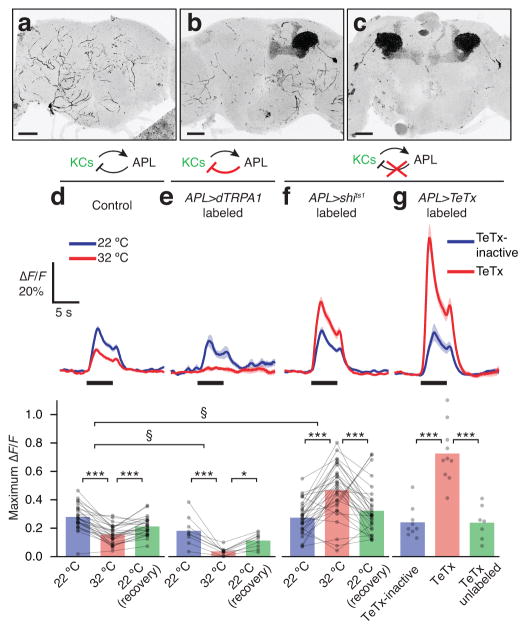
To achieve specific genetic access to APL, we intersected the expression domains of NP2631-GAL4 and GH146-Flp, using Flp-mediated recombination of FRT sites in a tubP-FRT-GAL80-FRT cassette (see Methods). Because the excision of GAL80 by Flp recombinase is stochastic, this strategy generated flies with neither, one, or both APL neurons labeled (Fig. 4a–c and Supplementary Fig. 2). Recombination events in APL were detected by immunolabeling or co-expression of fluorescent proteins. Importantly, we did not observe transgene expression in any neurons other than APL.
Figure 4. APL inhibits Kenyon cells.
(a–c) Stochastic transgene expression in neither (a), one (b), or both (c) APL neurons. Scale bars, 50 μm. (d–g) Impact of different APL manipulations on odor-evoked Ca2+ influx in the α lobe (see Supplementary Fig. 3 for all lobes). Black bars indicate 5 s pulses of ethyl acetate. (d) In control hemispheres of APL>shits1 flies where APL was unlabeled, odor-evoked Ca2+ influx in Kenyon cells declined slightly at 32 °C. (e) In hemispheres of APL>dTRPA1 flies where APL was labeled, odor-evoked Ca2+ influx in Kenyon cells was almost completely abolished at 32 °C. (f) In hemispheres of APL>shits1 flies where APL was labeled, odor-evoked Ca2+ influx in Kenyon cells increased greatly at 32 °C. (g) In APL-labeled hemispheres of APL>TeTx flies (red), odor-evoked Ca2+ influx in Kenyon cells was much higher than in APL-labeled hemispheres of APL>TeTx-inactive flies (blue) and APL-unlabeled hemispheres of APL>TeTx flies (green, lower panel). n, left to right, given as number of brain hemispheres [number of flies]: (d) n=24 [17.] (e) n=9 [5]. (f) n=30 [21]. (g) n=9 [7], 10 [8], 7 [6].* P<0.05, ** P<0.01, *** P<0.001, repeated-measures ANOVA with Geisser-Greenhouse correction and Holm-Sidak multiple comparisons test (d,e), Friedman test with Dunn’s multiple comparisons test (f), or one-way ANOVA with Tukey-Kramer post hoc test (g). § P<0.001, Mann-Whitney U test, comparing ratios of odor-evoked Ca2+ influx at 32 °C vs. 22 °C. See Supplementary Table 1 for full genotypes.
In imaging experiments on flies carrying NP2631-GAL4, tubP-FRT-GAL80-FRT, GH146-Flp, mb247-LexA, lexAop-GCaMP3, UAS-mCherry and UAS-shits1 transgenes (‘APL>shits1’ flies, see Supplementary Table 1), hemispheres in which APL was unlabeled served as controls (Fig. 4d, Supplementary Fig. 3a,e,i). As before (Fig. 1a), odor-evoked responses in the Kenyon cells of control hemispheres were slightly reduced at 32 °C. This decrease may reflect the temperature dependence of Ca2+ binding and/or subsequent conformational changes in GCaMP3, cellular Ca2+ dynamics, and/or Kenyon cell spike rates. In hemispheres where APL expressed dTRPA1, Kenyon cell odor responses were almost completely suppressed at 32 °C (Fig. 4e and Supplementary Fig. 3b,f,i), whereas in hemispheres where APL expressed shits1, Kenyon cell responses were greatly boosted at the elevated temperature (Fig. 4f and Supplementary Fig. 3c,g,i). Acute (16–24 h) expression of functional TeTx in APL also increased Kenyon cell odor responses compared to the expression of inactive TeTx, whereas control hemispheres always showed wild-type odor responses (Fig. 4 and Supplementary Fig. 3d,h,i). These findings confirm that the APL neuron inhibits Kenyon cells, thus completing the feedback loop.
Inhibition keeps Kenyon cell responses sparse and distinct
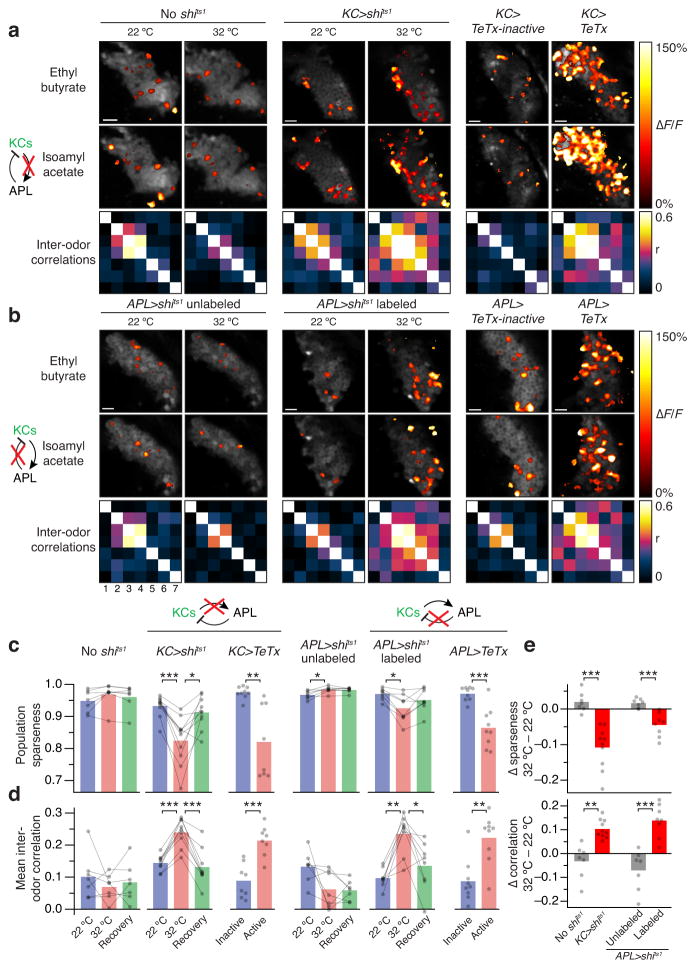
Blocking feedback inhibition on Kenyon cells may not only enhance individual responses but also augment the responsive Kenyon cell population. Although odor-specific spatial patterns of activity are visible in optical sections of the mushroom body lobes, the tightly bundled axons make it impossible to resolve the processes of individual cells. Therefore, to estimate the sizes of the responsive Kenyon cell populations, we imaged Kenyon cell somata during stimulation with a panel of 7 odors18,40,41 and generated activity maps of the responsive pixels (Fig. 5). In control flies expressing only mb247-LexA-driven GCaMP3, and in APL-unlabeled hemispheres of APL>shits1 flies, raising the temperature did not significantly alter the Kenyon cell odor response or slightly reduced it (Fig. 5a,b, ‘No shits1’ and ‘APL>shits1 unlabeled’). In contrast, when either Kenyon cell or APL synaptic output were blocked by shits1 or TeTx, Kenyon cell odor responses became much broader and more similar (Fig. 5a,b, ‘KC>shits1’, ‘KC>TeTx’, ‘APL>shits labeled1’ and ‘APL>TeTx’). As with α lobe responses, removing Kenyon cells from the OK107-GAL4 pattern with mb247-LexA-driven GAL80 eliminated the effect of OK107-GAL4-driven TeTx on sparseness and inter-odor similarity (Supplementary Fig. 4).
Figure 5. Inhibition keeps Kenyon cell responses sparse and distinct.
(a, b) Pseudocolored activity maps of odor responses in Kenyon cell somata, overlaid on grayscale images of baseline fluorescence. Color-coded matrices represent pairwise correlations between response maps to 7 odors: 1) ethyl acetate, 2) 3-octanol, 3) butyl acetate, 4) isoamyl acetate, 5) ethyl butyrate, 6) 2-pentanol, 7) 4-methylcyclohexanol. Scale bars, 10 μm. (a) Left: mb247-LexA>GCaMP3, at 22 °C and 32 °C. Center: mb247-LexA>GCaMP3,shits1, at 22 °C and 32 °C. Right: OK107>TeTx-inactive and OK107>TeTx. (b) Left: APL>shits1, APL unlabeled, at 22 °C and 32 °C. Center: APL>shits1, APL labeled, at 22 °C and 32 °C. Right: APL>TeTx-inactive and APL>TeTx, APL labeled. (c) Population sparseness in a and b decreases when Kenyon cell or APL synaptic output is blocked. (d) Mean inter-odor correlations in a and b increase when Kenyon cell or APL synaptic output is blocked. n, left to right, given as number of brain hemispheres [number of flies]: 7, 9, 9, 8, 7 [7], 8 [7], 9 [7], 9 [7]. Schematics indicate which neurons are being imaged (green) and which connection is being manipulated (red X indicates shits1 blockade). (e) Temperature-dependent changes in sparseness and correlation differ between mb247-LexA>GCaMP3 and mb247-LexA>GCaMP3,shits1 flies (left) and between APL-unlabeled and APL-labeled hemispheres of APL>shits1 flies (right). * P<0.05, ** P<0.01, *** P<0.001, unpaired Welch t-test or repeated-measures ANOVA with Geisser-Greenhouse correction and Holm-Sidak multiple comparisons test as appropriate (lines connecting dots indicate repeated measures); ‘APL>shits1 labeled’ in c used Friedman test with Dunn’s multiple comparisons test. See Supplementary Table 1 for full genotypes.
To quantify these effects, we measured the population sparseness5 of the response patterns and determined correlations between the representations of different odors (see Methods). To avoid bias caused by manual cell identification, we applied sparseness and correlation metrics to unsegmented activity maps. In the best case this method would replicate the results of manual cell identification (mathematically, sparseness and correlation remain the same if every element in each population is replicated an arbitrary number of times, as with each actual cell containing several pixels), and in the worst case it would merely add noise and thus be unlikely to create artificial effects.
In control flies, temperature did not significantly alter the population sparseness or inter-odor correlations of activity maps, except that APL>shits1 unlabeled hemispheres showed increased sparseness at 32 °C (Fig. 5c,d), consistent with the previously observed decline in vertical lobe responses at the elevated temperature (Fig. 4d). In contrast, population sparseness decreased and inter-odor correlations increased when either Kenyon cell or APL synaptic output were blocked by shits1 or TeTx (Fig. 5c–e).
Inhibition enables learned discrimination of similar odors
Having established a mechanism that contributes to the sparseness of Kenyon cell odor responses, we were now in a position to test the role of sparse coding in odor-specific memory. We conjectured that broadening Kenyon cell odor responses by blocking APL would impair learned discrimination of similar, but not dissimilar, odors. Previous studies have manipulated inhibition in the periphery (the antennal lobe or olfactory bulb) and attributed impaired odor discrimination to a loss of synchrony or contrast among PN or mitral cell signals, respectively42,43. None of these studies has perturbed the sparseness of central odor representations in a directed manner.
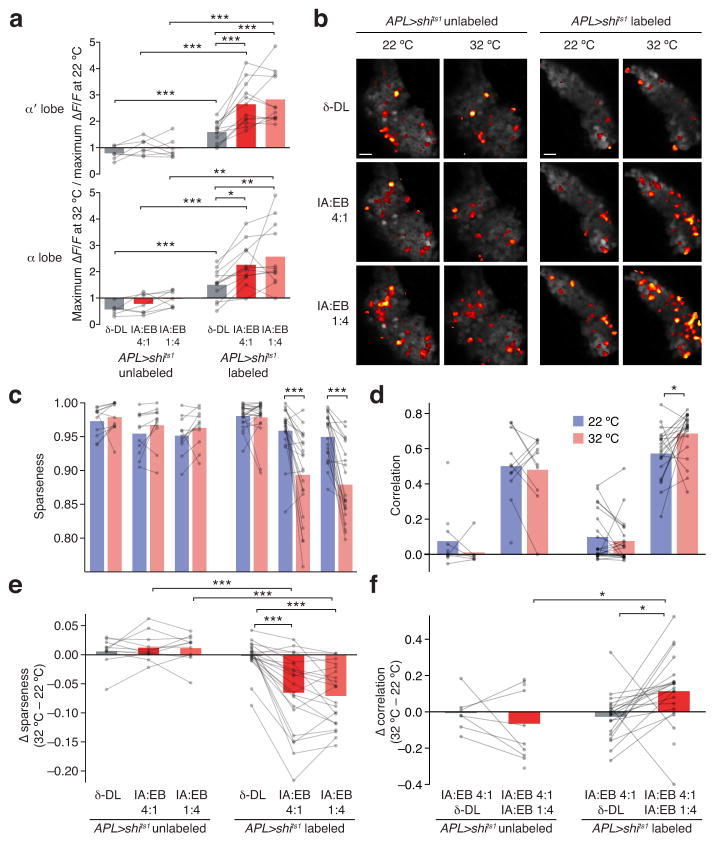
Similar odor pairs for behavioral tests consisted of binary mixtures of isoamyl acetate (IA) and ethyl butyrate (EB) with component ratios of 1:4 and 4:1. To find an odor that would be well-separated from an IA:EB mixture, we consulted a database40 of odor responses in olfactory receptor neurons (ORNs) and chose the odor that elicited the least overall ORN activity, δ-decalactone (δ-DL). Whereas IA and EB evoked a total of 2,030 and 1,860 spikes/s in the 24 ORN types studied40, respectively, δ-DL elicited only 286 spikes/s. Although ORN activity is transformed by the antennal lobe into PN activity with less variance in overall firing across odors17,44, applying this transformation computationally44 suggests that δ-DL elicits a low overall response compared to IA and EB also at PN level (937, 1,238, and 1,310 spikes/s, respectively). In addition, while IA and EB generate correlated ORN and PN activity (r=0.47 and 0.55, respectively), responses for δ-DL are uncorrelated to those for IA and EB (ORN: r=0.05, −0.07; PN: r=−0.05, −0.04, respectively). Predicted PN responses to δ-DL are also sparser than those to IA or EB (population sparseness=0.50 vs. 0.42 and 0.35, respectively). Because modeled PN inputs representing δ-DL are naturally sparse and uncorrelated with those of IA and EB, we surmised that there would still be little overlap between Kenyon cell representations of δ-DL and an IA:EB mixture even with APL blocked.
To verify that this was the case, we imaged Kenyon cell responses to δ-DL, IA:EB 1:4 and IA:EB 4:1. For all three odors, as in Figure 3, blocking APL increased Kenyon cell responses in the α and α′ lobes (Supplementary Fig. 5). The increase was greater for the IA:EB mixtures than for δ-DL (Fig. 6a), supporting the idea that inhibitory feedback is driven by overall Kenyon cell activity (Fig. 2). Similarly, blocking APL broadened the responses of Kenyon cell somata to the IA:EB mixtures, but not to δ-DL (Fig. 6b). Quantitatively, raising the temperature did not affect sparseness or correlations to any of the three odors in hemispheres where APL was unlabeled. In hemispheres where the APL neuron expressed shits1, raising the temperature did not affect the sparseness of δ-DL responses or δ-DL vs. IA:EB 4:1 correlations, but it did decrease the sparseness of responses to IA:EB mixtures and increase their correlations (Fig. 6c–f). Blocking APL synaptic output thus compromises sparse coding and interferes with the decorrelation of Kenyon cell responses to IA:EB mixtures, which have relatively broad PN inputs, but not to δ-DL, which has relatively sparse PN inputs.
Figure 6. APL sparsens and decorrelates Kenyon cell responses.
(a) Ratios of odor-evoked Ca2+ influx at 32 °C vs. 22 °C in the α′ (top) and α (bottom) lobes, for δ-decalactone (δ-DL), isoamyl acetate:ethyl butyrate (IA:EB) 1:4, and IA:EB 4:1, in hemispheres where the APL neuron was unlabeled (n=6–7 [5]) or labeled (n=12 [8]) with shits1. n given as number of brain hemispheres [number of flies]. See Supplementary Fig. 4 for original ΔF/F traces. * P<0.05, ** P<0.01, *** P<0.001, Friedman test with Dunn’s multiple comparisons test (top) or repeated-measures ANOVA with Geisser-Greenhouse correction and Holm-Sidak multiple comparisons test (bottom) for paired data; Bonferroni-corrected unpaired Welch t-test or Mann-Whitney U test for unpaired data, as appropriate. (b) Activity maps of odor responses in Kenyon cell bodies. Scale bars, 10 μm. (c) Population sparseness of activity maps in response to δ-DL, IA:EB 1:4, and IA:EB 4:1, at 22 °C (blue) and 32 °C (red). Blocking APL decreases sparseness only for IA:EB mixtures. (d) Correlations between activity maps for IA:EB 4:1 vs. δ-DL and IA:EB 1:4, at 22 °C (blue) and 32 °C (red). Blocking APL increases correlations only between IA:EB mixtures. (c–d) * P<0.05, *** P<0.001, Wilcoxon signed-rank test. (e) Temperature-dependent decrease in sparseness is greater for IA:EB mixtures than for δ-DL within APL-labeled hemisphere of APL>shits1 flies, and greater for IA:EB mixtures in APL-labeled than APL-unlabeled hemispheres of APL>shits1 flies. *** P<0.001, Mann-Whitney U test (labeled vs. unlabeled) or Friedman test with Dunn’s multiple comparisons test (comparisons between odors). (f) Temperature-dependent increase in correlation is greater for IA:EB mixtures than for IA:EB 4:1 vs. δ-DL within APL-labeled hemispheres of APL>shits1 flies (n=20 [13]), and greater for IA:EB mixtures in APL-labeled than APL-unlabeled hemispheres of APL>shits1 flies (n=10 [9]). * P<0.05, unpaired Welch t-test (between samples) and paired t-test (within samples).
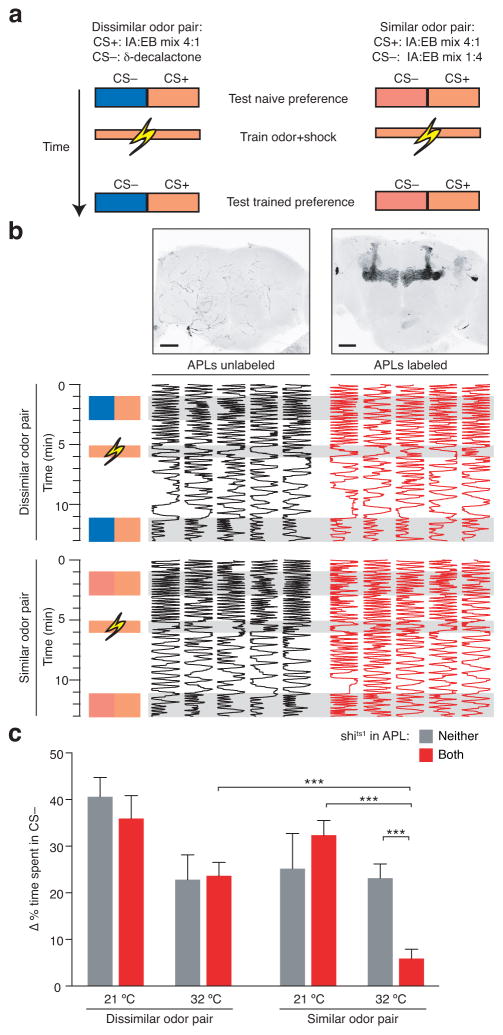
To test whether the broadening of Kenyon cell odor representations would impair learned odor discrimination, we used an individual-fly variant27 of the classical T-maze task26. The ability to quantify individual behavior was essential for this analysis, as the stochastic nature of our genetic manipulation required that the performance of each of 694 flies bearing NP2631-GAL4, tubP-FRT-GAL80-FRT, GH146-Flp, UAS-shits1 and UAS-CD8-GFP transgenes be related to the occurrence of recombination events in APL. To determine whether recombination had taken place in both, one, or neither of these neurons (see Fig. 4a–c), each fly was dissected after the behavioral measurements to see which APL neurons expressed GFP and, therefore, shits1. Flies with both APL neurons labeled constituted the experimental group and flies with neither APL neuron labeled served as controls. All experimental flies thus had the same genotype aside from the stochastic excision of GAL80 and subsequent expression of shits1 in the two APL neurons.
Odor discrimination was measured in single-fly chambers that were perfused from each direction with clean or odor-infused air, so that the amount of time spent on each side provided a read-out of preference27. Flies were first presented with a choice between two odors, one on each side, to benchmark their naïve preferences, then with one odor paired with electric shock (CS+), and after 5 minutes, with another choice between the original two odors. Anticipating that the behavioral effects of blocking APL might be subtle, we tried to make the learned discrimination more difficult by not presenting the non-reinforced odor (CS−) during training, taking into account previous data showing that discrimination of two similar odors is more difficult45 and performance on the classical T-maze is lower26 when the non-reinforced odor (CS−) is not encountered during training. Learning was measured as the difference in the proportion of time spent on the side with the unpunished odor (the CS−) before and after training. For learned discrimination of dissimilar odors, the CS+ was IA:EB 4:1 and the CS− was δ-DL, while for learned discrimination of similar odors, the CS+ was IA:EB 4:1 and the CS− was IA:EB 1:4 (Fig. 7a,b). Average untrained preferences between CS+ and CS− were close to 50:50 and did not vary significantly across groups (average untrained time in CS+ was between 47% and 53%; P=0.14, Kruskal-Wallis ANOVA).
Figure 7. Feedback inhibition facilitates learned discrimination of similar, but not dissimilar, odors.
(a) Schematic of training paradigm. See text for details. (b) Individual odor preferences before and after training. Fly position within the chamber (horizontal dimension) is plotted against time (vertical dimension). Maximum intensity projections of confocal image stacks show example APL>shits1,GFP brains with none or both APL neurons labeled. Scale bars, 50 μm. (c) Performance of APL>shits1,GFP flies sorted according to whether neither or both APL neurons were labeled. Scores are plotted as change in the proportion of time spent in CS− after training. n, left to right, given as number of flies [number of experiments]: 23 [6], 26 [6], 16 [7], 44 [7], 18 [8], 55 [8], 32 [9], 51 [9]. ** P<0.01, *** P<0.001, Kruskal-Wallis ANOVA with Holm-Bonferroni correction for post hoc tests, testing only pairs of data points with one variable changed (task, temperature, or APL labeling). P<0.01, 3-way ANOVA for interaction of task, temperature, and APL labeling. P<0.005, 2-way ANOVA for interaction of genotype and temperature for discrimination of similar odors. P<0.01, 2-way ANOVA for interaction of task and APL labeling at 32 °C. P<0.05, 2-way ANOVA for interaction of task and temperature for flies with both APL neurons labeled. Other 2-way ANOVAs did not reveal significant interactions. Error bars show s.e.m.
We predicted that blocking APL synaptic output would not prevent learned discrimination of δ-DL and IA:EB 4:1 because it did not increase the correlation between the Kenyon cell representations of these odors or decrease the sparseness of the δ-DL representation (Fig. 6d). Indeed, on this task, flies with both APL neurons labeled with shits1 performed the same as flies with neither APL neuron labeled, at both 21 °C and 32 °C (Fig. 7b,c). Although performance was lower overall at 32 °C, the lack of interaction between temperature and APL labeling shows that this effect is unrelated to shits1-mediated APL blockade (2-way ANOVA: no interaction, P=0.53; main effect of temperature, P<0.001). Similarly, blocking APL synaptic output did not significantly affect learned discrimination of the dissimilar odors 3-octanol (OCT) and 4-methylcyclohexanol (MCH) (Supplementary Fig. 6a). We conclude that blocking APL does not impair olfactory associative learning per se, at least at the timescale of 5-min memory.
In contrast, when flies had to discriminate IA:EB 1:4 from IA:EB 4:1, odors whose Kenyon cell representations became less sparse and more correlated when APL was blocked (Fig. 6), animals with both APL neurons labeled with shits1 were impaired compared to animals with neither APL neuron labeled, at 32 °C but not at 21 °C (Fig. 7b,c; 2-way ANOVA, significant interaction between temperature and APL labeling, P=0.0012). Flies with only one APL neuron labeled showed a marginal, but not statistically significant, impairment in discriminating IA:EB 1:4 and IA:EB 4:1 (Supplementary Fig. 6b). A 3-way ANOVA revealed a significant interaction between odor pair similarity, temperature, and shits1 expression in APL (P=0.009). Together, these results suggest that sparse coding in Kenyon cells improves learned odor discrimination by reducing overlap between the representations of similar odors.
Partial effect of APL-specific RNAi of GABA biosynthesis
Because the APL neuron is GABAergic, interference with GABA biosynthesis might cause similar effects as blocking synaptic output. Indeed, RNAi-mediated knockdown of GAD expression in APL, using GH146-GAL4, has been reported to increase Kenyon cell odor responses16 and improve learning34 of MCH and OCT. However, in preliminary experiments, we did not observe any impairment in learned discrimination of IA:EB 1:4 vs. IA:EB 4:1 in flies expressing GADRNAi driven by GH146-GAL4 (data not shown).
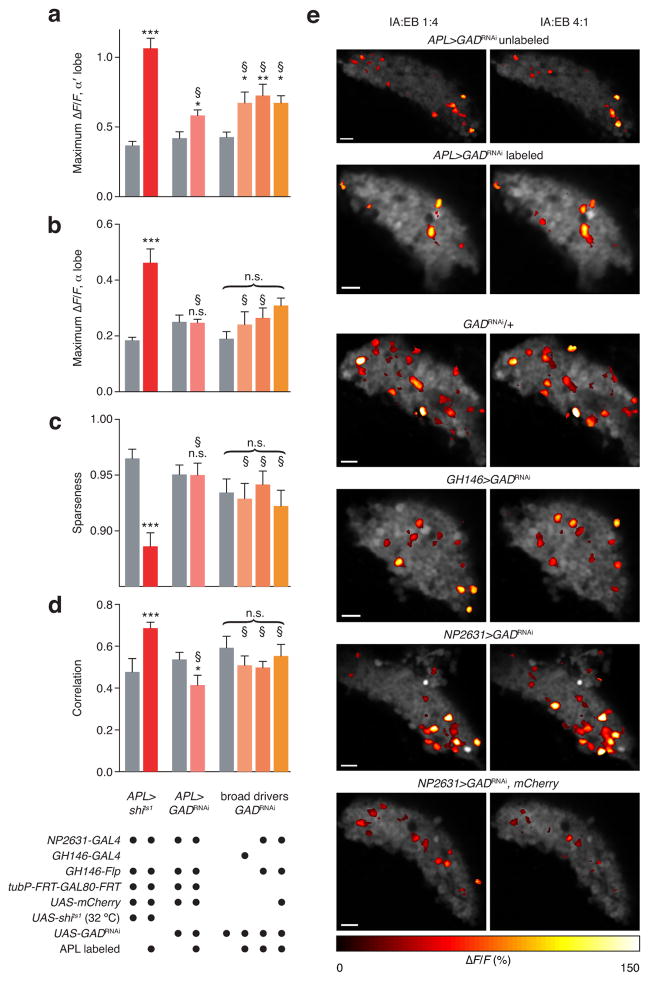
We suspected that knockdown of GABA signaling might be incomplete. RNAi rarely results in a complete knockout of the target gene46, and compensating homeostasis can partially negate the effect of a knockdown. Negative feedback systems such as the Kenyon cell–APL circuit may be especially robust to partial perturbations, as any reduced inhibition of Kenyon cells would increase the excitatory drive to APL, which might offset the partial depletion of GABA. GH146>GADRNAi and NP2631>GADRNAi reduce GABA immunoreactivity in the APL neuron34,38, but as immunohistochemistry is not necessarily linear, the degree of knockdown is unclear. Therefore, we directly compared the effects of GADRNAi and shits1 in APL on Kenyon cell responses to IA:EB 1:4 and IA:EB 4:1.
We expressed GADRNAi specifically in the APL neuron using our intersectional strategy. Compared to APL-unlabeled hemispheres, Kenyon cell odor responses in APL-labeled hemispheres were modestly higher in the α′ lobe (Fig. 8a), but not the α lobe (Fig. 8b). In both lobes, the effect of APL>GADRNAi was significantly smaller than the effect of APL>shits1 (2-way ANOVA, P<0.001). In Kenyon cell somata, APL>GADRNAi had no effect on population sparseness and slightly decreased the correlation between responses to IA:EB 1:4 and IA:EB 4:1. (Fig. 8c–e)
Figure 8. Partial effect of APL-specific RNAi of GABA biosynthesis.
(a–d) See grid at bottom for full genotypes. (a,b) α′ (a) and α lobe (b) responses to IA:EB mixtures (averages of responses to 1:4 and 4:1). n, left to right, given as number of brain hemispheres [number of flies]: 7 [5], 12 [8], 11 [10], 15 [12], 7, 6, 6, 6. (c,d) Population sparseness (c) and correlations of cell body responses to IA:EB mixtures (averages of responses to 1:4 and 4:1). n, left to right, given as number of brain hemispheres [number of flies]: 11 [9] (10 [9] in (d), 21 [14], 11 [10], 15 [12], 10, 10, 6, 6. (e) Sample activity maps of cell body responses analyzed in panels c and d. Compare to Fig. 5b. Scale bars, 10 μm. * P<0.05, ** P<0.01, *** P<0.001 significant difference between colored bars and relevant controls (gray bars), by unpaired Welch t-test for APL>shits1 and APL>GADRNAi (Mann-Whitney U test for APL>shits1 in d), and by one-way ANOVA and Dunnett’s multiple comparisons test for GH146-GAL4 and NP2631-GAL4 driving GADRNAi. § P<0.05 significant difference between effects of GADRNAi and APL>shits1 by 2-way ANOVA. Error bars show s.e.m.
We also compared this modest effect with the effects of the previously published16,34,38 manipulations GH146>GADRNAi and NP2631>GADRNAi. To ensure that the co-expression of UAS-mCherry with GADRNAi in our APL-specific labeling strategy did not lessen the efficacy of GADRNAi by titrating GAL4, we also tested NP2631>GADRNAi, UAS-mCherry flies. All three manipulations slightly increased α′, but not α, lobe responses to IA:EB 1:4 and IA:EB 4:1 relative to control GADRNAi/+ flies, but had no effect on population sparseness or correlation. Again, the effects of GADRNAi were significantly smaller than the effect of APL>shits1. Similar results were seen in vertical lobe odor responses to ethyl acetate and cell body responses to the panel of odors used in Figure 5 (Supplementary Fig. 7). There was a modest, though not statistically significant, increase in mean inter-odor correlation with GH146>GADRNAi and NP2631>GADRNAi, which was smaller than the increase in correlation seen with APL>shits1. Across all conditions, we thus find that APL-specific blockade of synaptic output with shits1 is a significantly more stringent perturbation than GH146>GADRNAi or NP2631>GADRNAi.
Discussion
Theoretical work has long predicted that sparse coding increases memory capacity by reducing overlap between representations of similar stimuli1–4,13, but experimental evidence tying sparse coding to behavior has been lacking. We present here the first such evidence by showing i) that the APL neuron sparsens and decorrelates Kenyon cell responses and ii) that disrupting sparse, decorrelated odor coding by blocking APL impairs learned discrimination of similar, but not dissimilar, odors.
In Drosophila, associative memories are thought to be written to Kenyon cell output synapses when a Kenyon cell is activated by odor at the same time as a reward or punishment induces release of neuromodulators such as dopamine onto the Kenyon cell19,27. The input odor, in the form of its PN activity pattern, specifies the memory address (i.e. the Kenyon cells whose synapses are to be modified) and induces the retrieval of the memory by activating the Kenyon cells whose synapses were modified during training19,27. If odor coding is too broad (i.e. too many Kenyon cells are activated by each odor) the likelihood is increased of unwanted overlap, in which an irrelevant odor activates enough Kenyon cells storing the memory that the conditioned response is inappropriately triggered. Our results support this model by showing that, when the loss of APL feedback decreases sparseness (Figs. 5 and 6), learned discrimination of similar odors is impaired (Fig. 7). Sparse coding is important only insofar as it decorrelates odor representations: even if the sparseness of the CS+ representation is reduced, learned discrimination is unaffected if the correlation between the CS+ and CS− representations remains low, as with the dissimilar odors IA:EB 4:1 and δ-DL. Consistent with this conclusion, the greater the overlap between the Kenyon cell representations of two odors, the greater the difficulty wild-type flies have in learning to discriminate the two odors41. This model therefore generates the testable prediction that blocking APL synaptic output will differentially affect learned discrimination of other similar and dissimilar odor pairs.
It remains formally possible that the defect in learned odor discrimination is caused by effects of blocking APL synaptic output that are unrelated to sparse coding. The only way to prove definitively that sparseness is the mediating factor would be to induce memory formation and retrieval by activating pairs of arbitrary subpopulations of Kenyon cells with varying degrees of sparseness and similarity, an experiment beyond current technological capabilities. That said, the manipulation used here—acutely blocking the output of a single neuron, which innervates only the mushroom body—is remarkably specific. In addition, blocking APL synaptic output did not affect learning of a dissimilar odor pair, arguing against a general learning defect. The most plausible explanation of the data is therefore that the effect of APL on learned discrimination occurs via its role in enforcing sparse coding.
Our results provide new perspectives on previous behavioral findings about APL. Learned discrimination of the dissimilar odors MCH and OCT at 3 hours after training involves both gap junctions between the dorsal paired medial (DPM) neuron and the APL neuron47 and APL synaptic output during consolidation35. Inhibition by APL was suggested to maintain the odor-specificity of memory during consolidation in a mechanism involving recurrent activity between DPM and α′/β′ neurons35. The proposed APL–DPM–α′/β′ loop is unlikely to play a role in our experiments, because DPM output is not required for short-term memory of most odors48. However, our results are nevertheless connected: the importance of the sparsening effects of APL for discrimination of similar odors in short-term memory, shown here, may extend to learned discrimination of dissimilar odors at longer time scales, when recurrent activity loops may spiral out of control without feedback inhibition.
It has also been found that reducing GABA synthesis in APL by RNAi increases Kenyon cell odor responses and can improve learning16,34. The apparent discrepancy can be explained by the partial effect on Kenyon cell odor responses of APL>GADRNAi relative to APL>shits1 (Fig. 8): a modest increase in Kenyon cell activity may improve memory retrieval by increasing Kenyon cell output while not overly compromising sparseness or discrimination, whereas the large increase in Kenyon cell activity by APL>shits1 overwhelms the Kenyon cell population’s ability to represent similar odors separately, thereby impairing discrimination. Consistent with this notion, APL>GADRNAi manipulations do not affect the sparseness of, or correlation between, Kenyon cell representations of MCH and OCT, the odors used in the previous studies showing improved learning in GH146>GADRNAi flies (Supplementary Fig. 8). GADRNAi in APL can also prevent olfactory reversal learning37, most likely because the initial memory is too strong. Finally, APL responses to the CS+ decline after aversive training34, possibly due to synaptic depression between APL and Kenyon cells that respond to the CS+. However, this trace is most likely dispensable for short-term memory, because flies with both APL neurons blocked can still learn to discriminate dissimilar odors (Fig. 7).
Why is APL feedback inhibition required to maintain sparse coding? Kenyon cells respond sparsely in part because they act as coincidence detectors, requiring inputs from multiple PNs to spike8,18. However, the remarkably robust sparse coding in Kenyon cells12 has been difficult to explain by computational modeling using only thresholded summation. Sparse coding in modeled Kenyon cells lies in a narrow, unstable band between silence and indiscriminate firing15, and thresholded summation of simulated PN odor responses results in significant detection failures14. Only adding global inhibition provides the modeled Kenyon cells with the flexibility to respond sparsely to a wide range of odors14,15. Indeed, locust GGN odor responses increase with stimulus intensity15,49, suggesting that feedback inhibition scales with input to stabilize sparseness. Consistent with this scenario, we found that the small difference in Kenyon cell sparseness in control conditions between the narrow odor δ-DL and the broad IA:EB mixtures was greatly increased when APL feedback was blocked (Fig. 6). Computational studies have modeled inhibitory regulation of Kenyon cell sparseness using both feedback15 and feedforward14,50 inhibition, but feedback appears to be the main mechanism49, perhaps because the fragility of sparse coding requires the error-canceling logic of feedback. The presence of both recurrent inhibition and sparse odor coding in the mammalian olfactory cortex10,11,20 suggests that inhibitory cortical interneurons may play a similar role to the Drosophila APL neuron in olfactory discrimination.
Online Methods
Fly strains
The following transgenic strains of Drosophila melanogaster were used: UAS-shits1 (refs. 23,51), mb247-LexA::VP16 (ref. 35), lexAop-GAL80 (ref. 52), OK107-GAL4 (ref. 53), tubP-GAL80ts (ref. 54), UAS-TeTx and UAS-TeTx-inactive (H233V, H237V) (ref. 29), UAS-synapto-pHluorin (refs. 22,55), UAS-dTRPA1 (ref. 25), lexAop-dTRPA1 (ref. 56), lexAop-CD2::RFP (ref. 57), NP225-GAL4 (ref. 58) and NP2631-GAL4 (Kyoto Drosophila Genetic Resource Center), c739-GAL4 (ref. 30), R35B12-GAL4 and R64C08-GAL4 (ref. 59), tubP-FRT-GAL80-FRT (refs. 60,61), GH146-Flp (ref. 62), mb247-dsRed (ref. 63), UAS-mCherry::CAAX (ref. 64), and UAS-CD8::GFP. Expression cassettes encoding GCaMP3 (ref. 65) and shits1 fused to UAS or lexAop regulatory sequences were targeted to attP2 and attP16 landing sites or inserted randomly, respectively (Genetic Services, Inc.).
Flies were cultivated on cornmeal agar under a 12-h light/12-h dark cycle at 25 °C unless they expressed temperature-sensitive gene products (shits1, GAL80ts, dTRPA1); in these cases the experimental animals and all relevant controls were grown at 18°C. Flies carrying tubP-GAL80ts were raised at 18 °C and placed at 31 °C for 16–24 h <1 day after eclosion. All experiments were performed on male and female flies aged 1–7 days.
Behavior
Learned odor discrimination was analyzed in clear polycarbonate chambers (length 50 mm, width 5 mm, height 1.3 mm) incorporating printed circuit boards (PCBs) with 1 mm electrodes and 1 mm electrode gaps as floors and ceilings27. Solid-state relays (Fairchild HSR312L) connected the PCBs to a 60 V source. For electric shock reinforcement, the relays were activated for 1.25 s at a repetition rate of 0.2 Hz during a 1 min odor presentation27.
Flow-controlled (2.7 l/min; CMOSens Performance Line, Sensirion), filtered, and humidified carrier air was mixed with flow-controlled odor streams (0.3 l/min) drawn through vials filled with 10−2 dilutions of odorant in mineral oil. IA:EB 1:4 and 4:1 were 2 × 10−3: 8 × 10−3 and vice versa. The air/odor streams were split between 20 chambers, yielding a flow rate of 0.15 l/min per half-chamber. A stack of 20 chambers was backlit by 940 nm LEDs (TSAL6100, Vishay) and imaged by a Stingray F080B CCD camera (Allied Vision Technologies) equipped with a Computar M1614 lens. The apparatus was operated in a temperature-controlled incubator (Sanyo MIR-154) maintained at 21 or 32 °C, as indicated. To impose a synaptic transmission block23 with shits1, experimental and control animals were transferred to the restrictive temperature of 32 °C for 15 min before the start of a behavioral experiment and maintained at the elevated temperature throughout. Flies were individually recovered into food vials and their brains were dissected to score for recombination events in APL (see Structural Imaging). Behavioral experiments were performed during the day (9 am – 8 pm).
A virtual instrument written in LabVIEW 2009 (National Instruments) extracted fly position data from video images and controlled the delivery of odors and electric shocks27. Experimental data were analyzed offline in MATLAB 2012a (The MathWorks). The amount of time a fly spent in each half of the chamber was scored during the initial naïve test and the final post-training test, and the learned discrimination was calculated as % time in CS− after training – % time in CS− before training. Flies making <2 entries into the choice zone during odor presentation were excluded from analysis.
Functional Imaging
Cuticle and trachea in a small window overlying the mushroom body were surgically removed, and the exposed brain was superfused with carbogenated solution (95% O2, 5% CO2) containing 5 mM TES, 103 mM NaCl, 3 mM KCl, 1.5 mM CaCl2, 4 mM MgCl2, 26 mM NaHCO3, 1 mM NaH2PO4, 8 mM trehalose, and 10 mM glucose, pH 7.3. Heating for shits1 and dTRPA1 experiments was provided by a TC-10 temperature controller (NPI) and an HPT-2 in-line perfusion heater (ALA). For dTRPA1 experiments, the temperature at the fly was measured with a TS-200 miniature temperature sensor (NPI) and a USB-1208FS DAQ device (Measurement Computing) at 30 Hz; temperature traces were smoothed over 20 frames by a moving average filter to remove digitization artifacts. For shits1 experiments, flies were held at 32 °C for at least 15 minutes and provided with 10–15 odor pulses before imaging to deplete the synaptic vesicle pool. Both hemispheres were imaged where possible in APL-specific labeling experiments with shits1, dTRPA1, TeTx, or GADRNAi, counting unlabeled hemispheres as controls; brains were dissected after each experiment to score for recombination events in APL. Non-responsive or damaged brains were excluded from analysis.
Odors at 10−2 dilution were delivered66 by switching mass-flow controlled air/odor streams (CMOSens Performance Line, Sensirion) with a custom-built solenoid valve system (The Lee Company). An odor tube ~5 mm in diameter was positioned ~1 cm from the fly’s head. The flow rate at the fly was 0.5 l/min.
Fly brains were imaged using two-photon microscopy22,66. Fluorescence was excited with 140 fs pulses of light centered at 910 nm (Chameleon Ultra II, Coherent). The excitation laser was attenuated with the help of a Pockels cell (Conoptics 302RM) and coupled to the scan engine of a Movable Objective Microscope (Sutter Instruments) equipped with a Zeiss 20×, 1.0 NA W-Plan-Apochromat objective. Emitted photons were separated from excitation light by a series of dichromatic mirrors and dielectric and colored glass filters and detected by GaAsP photomultiplier tubes (Hamamatsu Photonics H10770PA-40 SEL). Photomultiplier currents were amplified (Laser Components HCA-4M-500K-C) and passed through a custom-designed integrator circuit to maximize the signal-to-noise ratio. The microscope was controlled through MPScope 2.0 via a PCI-6110 DAQ board (National Instruments).
Images were converted to Analyze format and motion-corrected by maximizing the pixel-by-pixel correlation between a reference frame and each frame in the time series. ΔF/F traces were calculated in ImageJ using manually-drawn ROIs for the background and brain structure of interest. Traces were smoothed by a moving average over 5 frames and linearly interpolated to a frame time of 0.09 s in Igor Pro to allow averaging of movies with different frame rates. To match ΔF/F with temperature for dTRPA1 experiments, the smoothed temperature trace was linearly interpolated to the frame rate of the ΔF/F trace.
Activity maps were generated in MATLAB after smoothing with a Gaussian filter and background subtraction. A baseline fluorescence image was calculated as the average over the pre-stimulus interval. Frames in which the brain moved in the axial direction were automatically discarded by correlating each frame to the baseline image and discarding it if the correlation fell below a threshold value, which was manually selected for each brain by noting the constant high correlation value when the brain was stationary and sudden drops in correlation when the brain moved. For this motion elimination, pixel values were capped to prevent bona fide odor responses from causing changes in correlation values. For each pixel, the difference between mean intensity during the stimulus and the mean baseline fluorescence (ΔF) was calculated. If ΔF of a pixel was less than twice the standard deviation (σ) of the intensity of that pixel during the pre-stimulus interval, the pixel was considered unresponsive. (A 2σ threshold corresponds to the top ~5% of a normal distribution.) Activity maps were smoothed with a Gaussian filter for display purposes, but not for further similarity and sparseness analyses.
Inter-odor similarity was calculated in MATLAB by first aligning the activity maps of each odor response by maximizing the inter-odor correlations of baseline fluorescence, and then converting image matrices of the activity maps of each odor response into linear vectors and calculating the Pearson correlation coefficients between each “odor vector”. A threshold for baseline fluorescence was applied as a mask to the activity map to exclude pixels with no baseline GCaMP3 signal. Population sparseness was calculated for activity maps using the equation5,67:
For rare maps where no pixel had a ΔF/F greater than the 2σ threshold, sparseness was set to 1.0 and correlations involving that map were not calculated.
Statistical analysis
Statistical analyses were performed in Prism 6 (GraphPad) and R 2.14.2 (http://www.r-project.org). Data were tested for normality and homogeneity of variances before being analyzed with parametric (t-test, ANOVA) or non-parametric tests (Mann-Whitney, Friedman, Kruskal-Wallis), as appropriate. Random assignment to experimental groups was not used. In general, no statistical tests were done to predetermine sample size. However, where a conclusion relied on the absence of a significant effect (Fig. 1d and Supplementary Fig. 6a), a power analysis was performed to confirm that the sample size was sufficient to detect an effect of the expected size. The experimenter was blind to which APL neurons were labeled before post-experimental dissection (Figs. 4–8) but not otherwise.
Structural Imaging
Brains were dissected and fixed in 4% (w/v) paraformaldehyde in PBS (1.86 mM NaH2PO4, 8.41 mM Na2HPO4, 175 mM NaCl) for 20–60 min at room temperature under vacuum. Samples were washed for 3×10 min with PBS containing 0.1% (v/v) Triton-X100 (PBT) and twice in PBS before mounting in Vectashield (Vector Labs). Images were collected on a Leica TCS SP5 confocal microscope and processed in Fiji.
APL expression of shits1, dTRPA1, and GADRNAi was scored by widefield imaging of mCherry (for functional imaging experiments) or GFP (for behavioral experiments) in unfixed brains mounted in PBS. APL expression of TeTx and TeTx-inactive was detected by immunostainings using rabbit anti-TeTx antibody (POL 016, Statens Serum Institut, 1:100) and goat anti-rabbit Alexa 546 conjugate (Jackson ImmunoResearch, 1:800). Primary and secondary antisera were applied for 2 days in PBT at 4 °C. The dsRed driven by the 3XP3 promoter in the GH146-Flp insertion62 was not expressed in the mushroom body and therefore did not interfere with the detection of mCherry or TeTx in APL.
Supplementary Material
(a,b) Maximum intensity z-projections of confocal image stacks. (a) γ Kenyon cells are labeled in R64C08-GAL4/UAS-CD8::GFP flies. (b) α′β′ Kenyon cells are labeled in R35B12-GAL4/UAS-CD8::GFP flies. Scale bars, 50 μm. Representative images selected from at least 3 dissected brains.
(a) Anterior view of GFP expression in NP2631-GAL4, GH146-Flp/tubP-FRT-GAL80-FRT,UAS-CD8::GFP; UAS-shits1/+ fly with both APL neurons labeled. (b) Anterior view of GFP expression in NP2631-GAL4, GH146-Flp/UAS-CD8::GFP; tubP-FRT-GAL80-FRT/+ fly with both APL neurons labeled. Scale bars, 50 μm. Representative images selected from 10 (a) and 5 (b) confocal stacks and nearly 700 brains dissected (see Fig. 6).
(a–d) Impact of different APL manipulations (rows) on odor-evoked Ca2+ influx in different mushroom body lobes (columns). Black bars indicate 5 s pulses of ethyl acetate. (a) In control hemispheres of APL>shits1 flies where APL was unlabeled, odor-evoked Ca2+ influx in Kenyon cells declined slightly at 32 °C. (b) In hemispheres of APL>dTRPA1 flies where APL was labeled, odor-evoked Ca2+ influx in Kenyon cells was almost completely abolished at 32 °C. (c) In hemispheres of APL>shits1 flies where APL was labeled, odor-evoked Ca2+ influx in Kenyon cells increased greatly at 32 °C. (d) In APL-labeled hemispheres of APL>TeTx flies (red), odor-evoked Ca2+ influx in Kenyon cells was much higher than in APL-labeled hemispheres of APL>TeTx-inactive flies (blue). (e–h) Bar graphs summarizing maximum ΔF/F from (a–d) and including data after recovery to 22 °C after heating (e–g) and hemispheres from APL>TeTx flies where APL was unlabeled (green bars, h). n, left to right, given as number of brain hemispheres [number of flies]: (e) n=24 [17] (α and α′); 7 [6] (β, β′, γ) (f) n=9 [5], 8 [5]. (g) n=30 [21] (α and α′); 15 [10] (β, β′, γ). (h) n=9 [7], 10 [8], 7 [6] (α and α′); 10 [6], 7 [6] (β, β′, γ). (i) Ratios of odor-evoked Ca2+ influx at 32 °C vs. 22 °C, corresponding to panels e–h. Error bars show s.e.m. * P<0.05, ** P<0.01, *** P<0.001, repeated-measures ANOVA with Geisser-Greenhouse correction and Holm-Sidak multiple comparisons test (e,f – α, g – α′, β, β′, γ), Friedman test with Dunn’s multiple comparisons test (f – α′, g – α), one-way ANOVA with Tukey-Kramer post hoc test (h – α, α′), unpaired Welch t-test (h,i – β, β′, γ) or Mann-Whitney U test (i – α, α′). See Supplementary Table 1 for full genotypes.
(a) Activity maps of odor responses in OK107>TeTx, mb247-LexA>GCaMP3,GAL80 flies (left, n = 7 [5]) and OK107>TeTx, mb247-LexA>GCaMP3 (right, n = 6 [6]). Color-coded correlation matrices as in Fig. 5a,b. Scale bars, 10 μm. n given as number of hemispheres [number of flies]. (b, c) Removing Kenyon cell expression with mb247-LexA>GAL80 increases the population sparseness (b) and decreases the inter-odor correlations (c) of activity maps of odor responses in flies carrying OK107>TeTx. * P < 0.05, unpaired Welch t-test. See Supplementary Table 1 for full genotypes.
ΔF/F traces and maximum ΔF/F data corresponding to the ratios in Fig. 6a. Shading on traces indicates s.e.m. Black bars indicate 5-s pulses of δ-DL, IA:EB 1:4, or IA:EB 4:1 as labeled. * P < 0.05, ** P < 0.01, *** P < 0.001 by repeated-measures ANOVA with Geisser-Greenhouse correction and Holm-Sidak multiple comparisons test. n = 12 [8] (APL>shits1 labeled), n = 6–7 [5] (APL>shits1 unlabeled). n given as number of hemispheres [number of flies].
(a) Learned odor discrimination of 3-octanol (OCT) vs. 4-methylcyclohexanol (MCH). Protocol was as in Fig. 7a except the CS+ was OCT and the CS− was MCH. n, left to right, given as number of flies [number of experiments]: 29 [6], 14 [6], 19 [6], 41 [6], 25 [6], 18 [6], 16 [6], 32 [6]. Kruskal-Wallis ANOVA reveals no significant differences between the 8 groups (P = 0.09). 2-way ANOVA reveals a main effect of temperature (P < 0.05) but no significant interaction between genotype and temperature across flies with neither, left, right, or both APL neurons labeled (P = 0.52) or comparing flies with neither or both APL neurons labeled (P = 0.82). (b) Data as in Fig. 7c, adding single-APL-labeled flies. CS+ was IA:EB 4:1 and CS− was δ-DL (‘dissimilar’) or IA:EB 1:4 (‘similar’). At 32 °C, flies with only the left or right APL labeled by shits1 are somewhat impaired on learned discrimination of similar odors compared to flies with neither APL labeled, but the difference is not statistically significant. n, left to right, given as number of flies [number of experiments]: 23 [6], 28 [6], 19 [6], 26 [6], 16 [7], 32 [7], 34 [7], 44 [7], 18 [8], 30 [8], 22 [8], 55 [8], 32 [9], 33 [9], 37 [9], 51 [9]. * P < 0.05, ** P < 0.01, *** P < 0.001 by Kruskal-Wallis ANOVA with Holm-Bonferroni correction for post hoc tests, testing only pairs of data points with one variable changed (task, temperature, and APL labeling). P < 0.05, 3-way ANOVA for interaction of task, temperature, and APL labeling. P < 0.01, 2-way ANOVA for interaction of genotype and temperature for discrimination of similar odors. P < 0.05, 2-way ANOVA for interaction of task and APL labeling at 32 °C. P < 0.05, 2-way ANOVA for interaction of task and temperature for flies with both APL neurons or the left APL neuron labeled. Other 2-way ANOVAs did not reveal any significant interactions. Error bars show s.e.m.
(a–d) See grid at bottom for full genotypes. (a) α′ lobe responses to ethyl acetate. (b) α lobe responses to ethyl acetate. (a,b) n, left to right, given as number of brain hemispheres [number of flies]: 25 [17], 30 [21], 9 [7], 10 [8], 21 [19], 22 [19], 10, 11, 6, 6. n given as number of hemispheres [number of flies]. (c) Mean population sparseness of cell body responses to the panel of odors used in Fig. 5. (d) Mean inter-odor correlations between cell body responses for the panel of odors used in Fig. 5. (c,d) n, left to right, given as number of brain hemispheres [number of flies]: 7 [7], 8 [7], 9 [7], 9 [7], 8 [8], 10 [8], 10, 11, 5, 5. (e) Sample activity maps of cell body responses analyzed in panels c and d. Compare to Fig. 5a,b. Scale bars, 10 μm. Color grids represent pairwise correlations as in Fig. 5: 1) ethyl acetate, 2) 3-octanol, 3) butyl acetate, 4) isoamyl acetate, 5) ethyl butyrate, 6) 2-pentanol, 7) 4-methylcyclohexanol. * P < 0.05, ** P < 0.01, *** P < 0.001 significant difference between colored bars and the relevant controls (gray bars), by unpaired Welch t-test for APL>shits1 and APL>GADRNAi and by Kruskal-Wallis ANOVA and Dunn’s multiple comparisons test for GH146 and NP2631 driving GADRNAi. § P < 0.05 significant difference between effect of GADRNAi and effect of APL>shits1 by 2-way ANOVA. † P < 0.05 significant difference between effect of manipulation marked with † and effect of APL>TeTx by 2-way ANOVA. # P < 0.05 significant difference between effect of manipulation marked with # and effect of APL>GADRNAi by 2-way ANOVA. Error bars show s.e.m.
(a–c) See grid at bottom for full genotypes. (a) Population sparseness of cell body responses to 4-methylcyclohexanol (MCH). Statistical comparisons, left to right: Mann-Whitney U test (P = 0.094), unpaired Welch t-test (P = 0.50), one-way ANOVA (P = 0.48). (b) Population sparseness of cell body responses to 3-octanol (OCT). Statistical comparisons: Mann-Whitney U test (P = 0.054), unpaired Welch t-test (P = 0.49), Kruskal-Wallis ANOVA (P = 0.41). (c) Correlation between cell body responses to MCH and OCT. Statistical comparisons: unpaired Welch t-test (P = 0.10), Mann-Whitney U test (P = 0.75), Kruskal-Wallis ANOVA (P = 0.34). n, left to right, given as number of brain hemispheres [number of flies]: 7 [7], 8 [7], 8 [8], 10 [8], 10, 11, 5, 5. Error bars show s.e.m.
Acknowledgments
We thank Moshe Parnas and Shamik DasGupta for discussions; Yan Tan, Robert Roorda, Clifford Talbot, and Jessica Beevers for technical assistance; Loren Looger for a plasmid encoding GCaMP3; and Stephen Goodwin, Chi-Hon Lee, Liqun Luo, Kristin Scott, Scott Waddell, the Bloomington Stock Center, the Kyoto Drosophila Genetic Resource Center, and the Vienna Drosophila RNAi Center for fly strains. This work was supported by grants from the Wellcome Trust, the Gatsby Charitable Foundation, the Medical Research Council, the National Institutes of Health, and the Oxford Martin School (G.M.), a Sir Henry Wellcome Postdoctoral Fellowship (A.C.L.) and a Wellcome Trust OXION studentship (A.B.).
Footnotes
Author Contributions
A.C.L. and G.M. designed the study, analyzed the results, and wrote the paper. A.C.L. performed all behavioral and imaging experiments. A.B. assisted with behavioral experiments and dissections, A. de C. with dissections. T.L. provided lexAop-shits1 and mb247-LexA flies.
Competing Financial Interests
The authors declare no competing financial interests.
References
- 1.Kanerva P. Sparse distributed memory. MIT Press; 1988. [Google Scholar]
- 2.Marr D. A theory of cerebellar cortex. J Physiol (Lond) 1969;202:437–470. doi: 10.1113/jphysiol.1969.sp008820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Albus JS. A theory of cerebellar function. Math Biosci. 1971;10:25–61. [Google Scholar]
- 4.Treves A, Rolls ET. What determines the capacity of autoassociative memories in the brain? Network. 1991;2:371–397. [Google Scholar]
- 5.Vinje WE, Gallant JL. Sparse coding and decorrelation in primary visual cortex during natural vision. Science. 2000;287:1273–1276. doi: 10.1126/science.287.5456.1273. [DOI] [PubMed] [Google Scholar]
- 6.Hromádka T, Deweese MR, Zador AM. Sparse representation of sounds in the unanesthetized auditory cortex. PLoS Biol. 2008;6:e16. doi: 10.1371/journal.pbio.0060016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crochet S, Poulet JFA, Kremer Y, Petersen CCH. Synaptic mechanisms underlying sparse coding of active touch. Neuron. 2011;69:1160–1175. doi: 10.1016/j.neuron.2011.02.022. [DOI] [PubMed] [Google Scholar]
- 8.Perez-Orive J, et al. Oscillations and sparsening of odor representations in the mushroom body. Science. 2002;297:359–65. doi: 10.1126/science.1070502. [DOI] [PubMed] [Google Scholar]
- 9.Ito I, Ong RCY, Raman B, Stopfer M. Sparse odor representation and olfactory learning. Nat Neurosci. 2008;11:1177–1184. doi: 10.1038/nn.2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stettler DD, Axel R. Representations of odor in the piriform cortex. Neuron. 2009;63:854–864. doi: 10.1016/j.neuron.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 11.Poo C, Isaacson JS. Odor representations in olfactory cortex: ‘sparse’ coding, global inhibition, and oscillations. Neuron. 2009;62:850–861. doi: 10.1016/j.neuron.2009.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Honegger KS, Campbell RAA, Turner GC. Cellular-resolution population imaging reveals robust sparse coding in the Drosophila mushroom body. J Neurosci. 2011;31:11772–11785. doi: 10.1523/JNEUROSCI.1099-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jortner RA, Farivar SS, Laurent G. A simple connectivity scheme for sparse coding in an olfactory system. J Neurosci. 2007;27:1659–1669. doi: 10.1523/JNEUROSCI.4171-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luo SX, Axel R, Abbott LF. Generating sparse and selective third-order responses in the olfactory system of the fly. Proc Natl Acad Sci USA. 2010;107:10713–10718. doi: 10.1073/pnas.1005635107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Papadopoulou M, Cassenaer S, Nowotny T, Laurent G. Normalization for sparse encoding of odors by a wide-field interneuron. Science. 2011;332:721–725. doi: 10.1126/science.1201835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lei Z, Chen K, Li H, Liu H, Guo A. The GABA system regulates the sparse coding of odors in the mushroom bodies of Drosophila. Biochem Biophys Res Comm. 2013;436:35–40. doi: 10.1016/j.bbrc.2013.05.036. [DOI] [PubMed] [Google Scholar]
- 17.Bhandawat V, Olsen SR, Gouwens NW, Schlief ML, Wilson RI. Sensory processing in the Drosophila antennal lobe increases reliability and separability of ensemble odor representations. Nat Neurosci. 2007;10:1474–1482. doi: 10.1038/nn1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Turner GC, Bazhenov M, Laurent G. Olfactory representations by Drosophila mushroom body neurons. J Neurophysiol. 2008;99:734–746. doi: 10.1152/jn.01283.2007. [DOI] [PubMed] [Google Scholar]
- 19.Heisenberg M. Mushroom body memoir: from maps to models. Nat Rev Neurosci. 2003;4:266–275. doi: 10.1038/nrn1074. [DOI] [PubMed] [Google Scholar]
- 20.Franks KM, et al. Recurrent circuitry dynamically shapes the activation of piriform cortex. Neuron. 2011;72:49–56. doi: 10.1016/j.neuron.2011.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kapfer C, Glickfeld LL, Atallah BV, Scanziani M. Supralinear increase of recurrent inhibition during sparse activity in the somatosensory cortex. Nat Neurosci. 2007;10:743–753. doi: 10.1038/nn1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ng M, et al. Transmission of olfactory information between three populations of neurons in the antennal lobe of the fly. Neuron. 2002;36:463–474. doi: 10.1016/s0896-6273(02)00975-3. [DOI] [PubMed] [Google Scholar]
- 23.Kitamoto T. Conditional modification of behavior in Drosophila by targeted expression of a temperature-sensitive shibire allele in defined neurons. J Neurobiol. 2001;47:81–92. doi: 10.1002/neu.1018. [DOI] [PubMed] [Google Scholar]
- 24.Lima SQ, Miesenböck G. Remote control of behavior through genetically targeted photostimulation of neurons. Cell. 2005;121:141–152. doi: 10.1016/j.cell.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 25.Hamada FN, et al. An internal thermal sensor controlling temperature preference in Drosophila. Nature. 2008;454:217–220. doi: 10.1038/nature07001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tully T, Quinn WG. Classical conditioning and retention in normal and mutant Drosophila melanogaster. J Comp Physiol (A) 1985;157:263–277. doi: 10.1007/BF01350033. [DOI] [PubMed] [Google Scholar]
- 27.Claridge-Chang A, et al. Writing memories with light-addressable reinforcement circuitry. Cell. 2009;139:405–415. doi: 10.1016/j.cell.2009.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thum AS, et al. Differential potencies of effector genes in adult Drosophila. J Comp Neurol. 2006;498:194–203. doi: 10.1002/cne.21022. [DOI] [PubMed] [Google Scholar]
- 29.Sweeney ST, Broadie K, Keane J, Niemann H, O’Kane CJ. Targeted expression of tetanus toxin light chain in Drosophila specifically eliminates synaptic transmission and causes behavioral defects. Neuron. 1995;14:341–351. doi: 10.1016/0896-6273(95)90290-2. [DOI] [PubMed] [Google Scholar]
- 30.Aso Y, et al. The mushroom body of adult Drosophila characterized by GAL4 drivers. J Neurogenet. 2009;23:156–172. doi: 10.1080/01677060802471718. [DOI] [PubMed] [Google Scholar]
- 31.Hu A, Zhang W, Wang Z. Functional feedback from mushroom bodies to antennal lobes in the Drosophila olfactory pathway. Proc Natl Acad Sci USA. 2010;107:10262–10267. doi: 10.1073/pnas.0914912107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Markopoulos F, Rokni D, Gire DH, Murthy VN. Functional properties of cortical feedback projections to the olfactory bulb. Neuron. 2012;76:1175–1188. doi: 10.1016/j.neuron.2012.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boyd AM, Sturgill JF, Poo C, Isaacson JS. Cortical feedback control of olfactory bulb circuits. Neuron. 2012;76:1161–1174. doi: 10.1016/j.neuron.2012.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu X, Davis RL. The GABAergic anterior paired lateral neuron suppresses and is suppressed by olfactory learning. Nat Neurosci. 2009;12:53–59. doi: 10.1038/nn.2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pitman JL, et al. A pair of inhibitory neurons are required to sustain labile memory in the Drosophila mushroom body. Curr Biol. 2011;21:855–861. doi: 10.1016/j.cub.2011.03.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu X, Krause WC, Davis RL. GABAA receptor RDL inhibits Drosophila olfactory associative learning. Neuron. 2007;56:1090–1102. doi: 10.1016/j.neuron.2007.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu Y, Ren Q, Li H, Guo A. The GABAergic anterior paired lateral neurons facilitate olfactory reversal learning in Drosophila. Learn Mem. 2012;19:478–486. doi: 10.1101/lm.025726.112. [DOI] [PubMed] [Google Scholar]
- 38.Ren Q, Li H, Wu Y, Ren J, Guo A. A GABAergic inhibitory neural circuit regulates visual reversal learning in Drosophila. J Neurosci. 2012;32:11524–11538. doi: 10.1523/JNEUROSCI.0827-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wilson RI, Laurent G. Role of GABAergic inhibition in shaping odor-evoked spatiotemporal patterns in the Drosophila antennal lobe. J Neurosci. 2005;25:9069–9079. doi: 10.1523/JNEUROSCI.2070-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hallem EA, Carlson JR. Coding of odors by a receptor repertoire. Cell. 2006;125:143–160. doi: 10.1016/j.cell.2006.01.050. [DOI] [PubMed] [Google Scholar]
- 41.Campbell RAA, et al. Imaging a population code for odor identity in the Drosophila mushroom body. J Neurosci. 2013;33:10568–10581. doi: 10.1523/JNEUROSCI.0682-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stopfer M, Bhagavan S, Smith BH, Laurent G. Impaired odour discrimination on desynchronization of odour-encoding neural assemblies. Nature. 1997;390:70–74. doi: 10.1038/36335. [DOI] [PubMed] [Google Scholar]
- 43.Abraham NM, et al. Synaptic inhibition in the olfactory bulb accelerates odor discrimination in mice. Neuron. 2010;65:399–411. doi: 10.1016/j.neuron.2010.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Olsen SR, Bhandawat V, Wilson RI. Divisive normalization in olfactory population codes. Neuron. 2010;66:287–299. doi: 10.1016/j.neuron.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mishra D, Louis M, Gerber B. Adaptive adjustment of the generalization-discrimination balance in larval Drosophila. J Neurogenet. 2010;24:168–175. doi: 10.3109/01677063.2010.498066. [DOI] [PubMed] [Google Scholar]
- 46.Dietzl G, et al. A genome-wide transgenic RNAi library for conditional gene inactivation in Drosophila. Nature. 2007;448:151–156. doi: 10.1038/nature05954. [DOI] [PubMed] [Google Scholar]
- 47.Wu CL, et al. Heterotypic gap junctions between two neurons in the drosophila brain are critical for memory. Curr Biol. 2011;21:848–854. doi: 10.1016/j.cub.2011.02.041. [DOI] [PubMed] [Google Scholar]
- 48.Keene AC, et al. Diverse odor-conditioned memories require uniquely timed dorsal paired medial neuron output. Neuron. 2004;44:521–533. doi: 10.1016/j.neuron.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 49.Gupta N, Stopfer M. Functional analysis of a higher olfactory center, the lateral horn. J Neurosci. 2012;32:8138–8148. doi: 10.1523/JNEUROSCI.1066-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Assisi C, Stopfer M, Laurent G, Bazhenov M. Adaptive regulation of sparseness by feedforward inhibition. Nat Neurosci. 2007;10:1176–1184. doi: 10.1038/nn1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pfeiffer BD, Truman JW, Rubin GM. Using translational enhancers to increase transgene expression in Drosophila. Proc Natl Acad Sci USA. 2012;109:6626–6631. doi: 10.1073/pnas.1204520109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thistle R, Cameron P, Ghorayshi A, Dennison L, Scott K. Contact chemoreceptors mediate male-male repulsion and male-female attraction during Drosophila courtship. Cell. 2012;149:1140–1151. doi: 10.1016/j.cell.2012.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Connolly JB, et al. Associative learning disrupted by impaired Gs signaling in Drosophila mushroom bodies. Science. 1996;274:2104–2107. doi: 10.1126/science.274.5295.2104. [DOI] [PubMed] [Google Scholar]
- 54.McGuire SE, Le PT, Osborn AJ, Matsumoto K, Davis RL. Spatiotemporal rescue of memory dysfunction in Drosophila. Science. 2003;302:1765–1768. doi: 10.1126/science.1089035. [DOI] [PubMed] [Google Scholar]
- 55.Miesenböck G, De Angelis DA, Rothman JE. Visualizing secretion and synaptic transmission with pH-sensitive green fluorescent proteins. Nature. 1998;394:192–195. doi: 10.1038/28190. [DOI] [PubMed] [Google Scholar]
- 56.Burke CJ, et al. Layered reward signalling through octopamine and dopamine in Drosophila. Nature. 2012;492:433–437. doi: 10.1038/nature11614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Glia instruct developmental neuronal remodeling through TGF-β signaling. 2011;14:821–823. doi: 10.1038/nn.2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Thum AS, Jenett A, Ito K, Heisenberg M, Tanimoto H. Multiple memory traces for olfactory reward learning in Drosophila. J Neurosci. 2007;27:11132–11138. doi: 10.1523/JNEUROSCI.2712-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jenett A, et al. A GAL4-driver line resource for Drosophila neurobiology. Cell Rep. 2012;2:991–1001. doi: 10.1016/j.celrep.2012.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gao S, et al. The neural substrate of spectral preference in Drosophila. Neuron. 2008;60:328–342. doi: 10.1016/j.neuron.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gordon MD, Scott K. Motor control in a Drosophila taste circuit. Neuron. 2009;61:373–384. doi: 10.1016/j.neuron.2008.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hong W, et al. Leucine-rich repeat transmembrane proteins instruct discrete dendrite targeting in an olfactory map. Nat Neurosci. 2009;12:1542–1550. doi: 10.1038/nn.2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Riemensperger T, Völler T, Stock P, Buchner E, Fiala A. Punishment prediction by dopaminergic neurons in Drosophila. Curr Biol. 2005;15:1953–1960. doi: 10.1016/j.cub.2005.09.042. [DOI] [PubMed] [Google Scholar]
- 64.Kakihara K, Shinmyozu K, Kato K, Wada H, Hayashi S. Conversion of plasma membrane topology during epithelial tube connection requires Arf-like 3 small GTPase in Drosophila. Mech Dev. 2008;125:325–336. doi: 10.1016/j.mod.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 65.Tian L, et al. Imaging neural activity in worms, flies and mice with improved GCaMP calcium indicators. Nat Methods. 2009;6:875–881. doi: 10.1038/nmeth.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shang Y, Claridge-Chang A, Sjulson L, Pypaert M, Miesenböck G. Excitatory local circuits and their implications for olfactory processing in the fly antennal lobe. Cell. 2007;128:601–612. doi: 10.1016/j.cell.2006.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Willmore B, Tolhurst DJ. Characterizing the sparseness of neural codes. Network. 2001;12:255–270. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(a,b) Maximum intensity z-projections of confocal image stacks. (a) γ Kenyon cells are labeled in R64C08-GAL4/UAS-CD8::GFP flies. (b) α′β′ Kenyon cells are labeled in R35B12-GAL4/UAS-CD8::GFP flies. Scale bars, 50 μm. Representative images selected from at least 3 dissected brains.
(a) Anterior view of GFP expression in NP2631-GAL4, GH146-Flp/tubP-FRT-GAL80-FRT,UAS-CD8::GFP; UAS-shits1/+ fly with both APL neurons labeled. (b) Anterior view of GFP expression in NP2631-GAL4, GH146-Flp/UAS-CD8::GFP; tubP-FRT-GAL80-FRT/+ fly with both APL neurons labeled. Scale bars, 50 μm. Representative images selected from 10 (a) and 5 (b) confocal stacks and nearly 700 brains dissected (see Fig. 6).
(a–d) Impact of different APL manipulations (rows) on odor-evoked Ca2+ influx in different mushroom body lobes (columns). Black bars indicate 5 s pulses of ethyl acetate. (a) In control hemispheres of APL>shits1 flies where APL was unlabeled, odor-evoked Ca2+ influx in Kenyon cells declined slightly at 32 °C. (b) In hemispheres of APL>dTRPA1 flies where APL was labeled, odor-evoked Ca2+ influx in Kenyon cells was almost completely abolished at 32 °C. (c) In hemispheres of APL>shits1 flies where APL was labeled, odor-evoked Ca2+ influx in Kenyon cells increased greatly at 32 °C. (d) In APL-labeled hemispheres of APL>TeTx flies (red), odor-evoked Ca2+ influx in Kenyon cells was much higher than in APL-labeled hemispheres of APL>TeTx-inactive flies (blue). (e–h) Bar graphs summarizing maximum ΔF/F from (a–d) and including data after recovery to 22 °C after heating (e–g) and hemispheres from APL>TeTx flies where APL was unlabeled (green bars, h). n, left to right, given as number of brain hemispheres [number of flies]: (e) n=24 [17] (α and α′); 7 [6] (β, β′, γ) (f) n=9 [5], 8 [5]. (g) n=30 [21] (α and α′); 15 [10] (β, β′, γ). (h) n=9 [7], 10 [8], 7 [6] (α and α′); 10 [6], 7 [6] (β, β′, γ). (i) Ratios of odor-evoked Ca2+ influx at 32 °C vs. 22 °C, corresponding to panels e–h. Error bars show s.e.m. * P<0.05, ** P<0.01, *** P<0.001, repeated-measures ANOVA with Geisser-Greenhouse correction and Holm-Sidak multiple comparisons test (e,f – α, g – α′, β, β′, γ), Friedman test with Dunn’s multiple comparisons test (f – α′, g – α), one-way ANOVA with Tukey-Kramer post hoc test (h – α, α′), unpaired Welch t-test (h,i – β, β′, γ) or Mann-Whitney U test (i – α, α′). See Supplementary Table 1 for full genotypes.
(a) Activity maps of odor responses in OK107>TeTx, mb247-LexA>GCaMP3,GAL80 flies (left, n = 7 [5]) and OK107>TeTx, mb247-LexA>GCaMP3 (right, n = 6 [6]). Color-coded correlation matrices as in Fig. 5a,b. Scale bars, 10 μm. n given as number of hemispheres [number of flies]. (b, c) Removing Kenyon cell expression with mb247-LexA>GAL80 increases the population sparseness (b) and decreases the inter-odor correlations (c) of activity maps of odor responses in flies carrying OK107>TeTx. * P < 0.05, unpaired Welch t-test. See Supplementary Table 1 for full genotypes.
ΔF/F traces and maximum ΔF/F data corresponding to the ratios in Fig. 6a. Shading on traces indicates s.e.m. Black bars indicate 5-s pulses of δ-DL, IA:EB 1:4, or IA:EB 4:1 as labeled. * P < 0.05, ** P < 0.01, *** P < 0.001 by repeated-measures ANOVA with Geisser-Greenhouse correction and Holm-Sidak multiple comparisons test. n = 12 [8] (APL>shits1 labeled), n = 6–7 [5] (APL>shits1 unlabeled). n given as number of hemispheres [number of flies].
(a) Learned odor discrimination of 3-octanol (OCT) vs. 4-methylcyclohexanol (MCH). Protocol was as in Fig. 7a except the CS+ was OCT and the CS− was MCH. n, left to right, given as number of flies [number of experiments]: 29 [6], 14 [6], 19 [6], 41 [6], 25 [6], 18 [6], 16 [6], 32 [6]. Kruskal-Wallis ANOVA reveals no significant differences between the 8 groups (P = 0.09). 2-way ANOVA reveals a main effect of temperature (P < 0.05) but no significant interaction between genotype and temperature across flies with neither, left, right, or both APL neurons labeled (P = 0.52) or comparing flies with neither or both APL neurons labeled (P = 0.82). (b) Data as in Fig. 7c, adding single-APL-labeled flies. CS+ was IA:EB 4:1 and CS− was δ-DL (‘dissimilar’) or IA:EB 1:4 (‘similar’). At 32 °C, flies with only the left or right APL labeled by shits1 are somewhat impaired on learned discrimination of similar odors compared to flies with neither APL labeled, but the difference is not statistically significant. n, left to right, given as number of flies [number of experiments]: 23 [6], 28 [6], 19 [6], 26 [6], 16 [7], 32 [7], 34 [7], 44 [7], 18 [8], 30 [8], 22 [8], 55 [8], 32 [9], 33 [9], 37 [9], 51 [9]. * P < 0.05, ** P < 0.01, *** P < 0.001 by Kruskal-Wallis ANOVA with Holm-Bonferroni correction for post hoc tests, testing only pairs of data points with one variable changed (task, temperature, and APL labeling). P < 0.05, 3-way ANOVA for interaction of task, temperature, and APL labeling. P < 0.01, 2-way ANOVA for interaction of genotype and temperature for discrimination of similar odors. P < 0.05, 2-way ANOVA for interaction of task and APL labeling at 32 °C. P < 0.05, 2-way ANOVA for interaction of task and temperature for flies with both APL neurons or the left APL neuron labeled. Other 2-way ANOVAs did not reveal any significant interactions. Error bars show s.e.m.
(a–d) See grid at bottom for full genotypes. (a) α′ lobe responses to ethyl acetate. (b) α lobe responses to ethyl acetate. (a,b) n, left to right, given as number of brain hemispheres [number of flies]: 25 [17], 30 [21], 9 [7], 10 [8], 21 [19], 22 [19], 10, 11, 6, 6. n given as number of hemispheres [number of flies]. (c) Mean population sparseness of cell body responses to the panel of odors used in Fig. 5. (d) Mean inter-odor correlations between cell body responses for the panel of odors used in Fig. 5. (c,d) n, left to right, given as number of brain hemispheres [number of flies]: 7 [7], 8 [7], 9 [7], 9 [7], 8 [8], 10 [8], 10, 11, 5, 5. (e) Sample activity maps of cell body responses analyzed in panels c and d. Compare to Fig. 5a,b. Scale bars, 10 μm. Color grids represent pairwise correlations as in Fig. 5: 1) ethyl acetate, 2) 3-octanol, 3) butyl acetate, 4) isoamyl acetate, 5) ethyl butyrate, 6) 2-pentanol, 7) 4-methylcyclohexanol. * P < 0.05, ** P < 0.01, *** P < 0.001 significant difference between colored bars and the relevant controls (gray bars), by unpaired Welch t-test for APL>shits1 and APL>GADRNAi and by Kruskal-Wallis ANOVA and Dunn’s multiple comparisons test for GH146 and NP2631 driving GADRNAi. § P < 0.05 significant difference between effect of GADRNAi and effect of APL>shits1 by 2-way ANOVA. † P < 0.05 significant difference between effect of manipulation marked with † and effect of APL>TeTx by 2-way ANOVA. # P < 0.05 significant difference between effect of manipulation marked with # and effect of APL>GADRNAi by 2-way ANOVA. Error bars show s.e.m.
(a–c) See grid at bottom for full genotypes. (a) Population sparseness of cell body responses to 4-methylcyclohexanol (MCH). Statistical comparisons, left to right: Mann-Whitney U test (P = 0.094), unpaired Welch t-test (P = 0.50), one-way ANOVA (P = 0.48). (b) Population sparseness of cell body responses to 3-octanol (OCT). Statistical comparisons: Mann-Whitney U test (P = 0.054), unpaired Welch t-test (P = 0.49), Kruskal-Wallis ANOVA (P = 0.41). (c) Correlation between cell body responses to MCH and OCT. Statistical comparisons: unpaired Welch t-test (P = 0.10), Mann-Whitney U test (P = 0.75), Kruskal-Wallis ANOVA (P = 0.34). n, left to right, given as number of brain hemispheres [number of flies]: 7 [7], 8 [7], 8 [8], 10 [8], 10, 11, 5, 5. Error bars show s.e.m.