Abstract
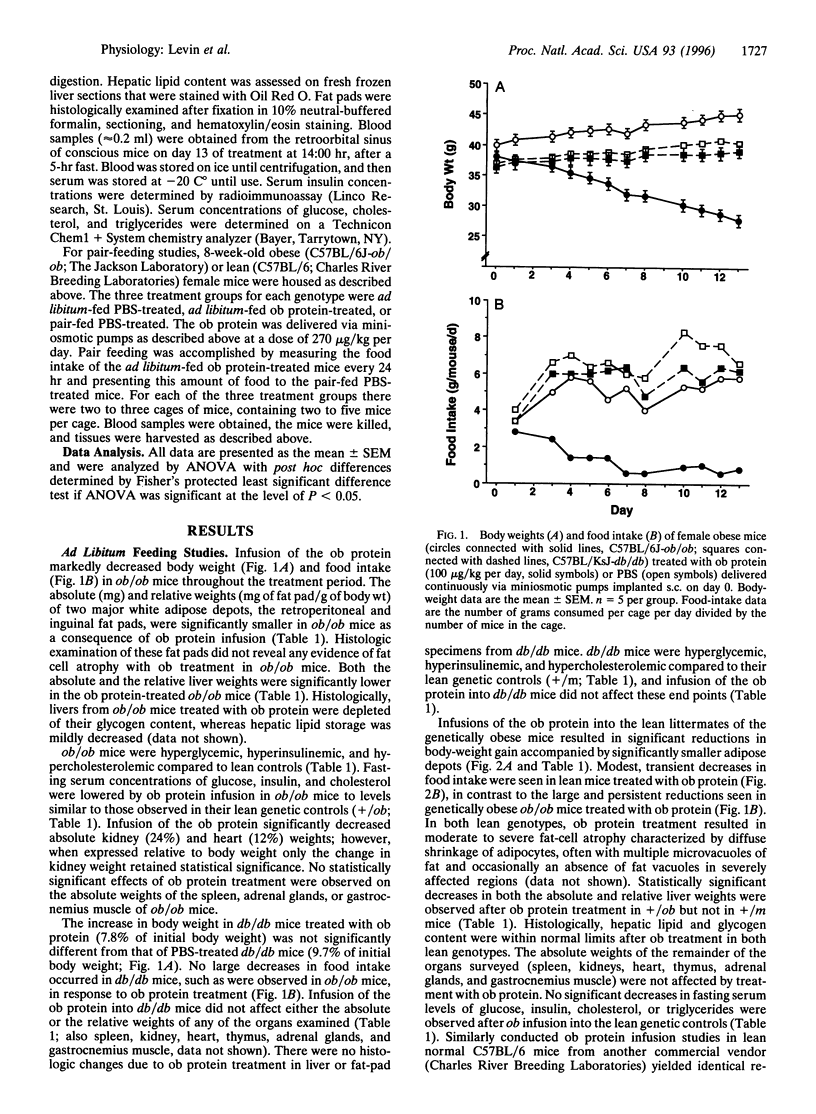
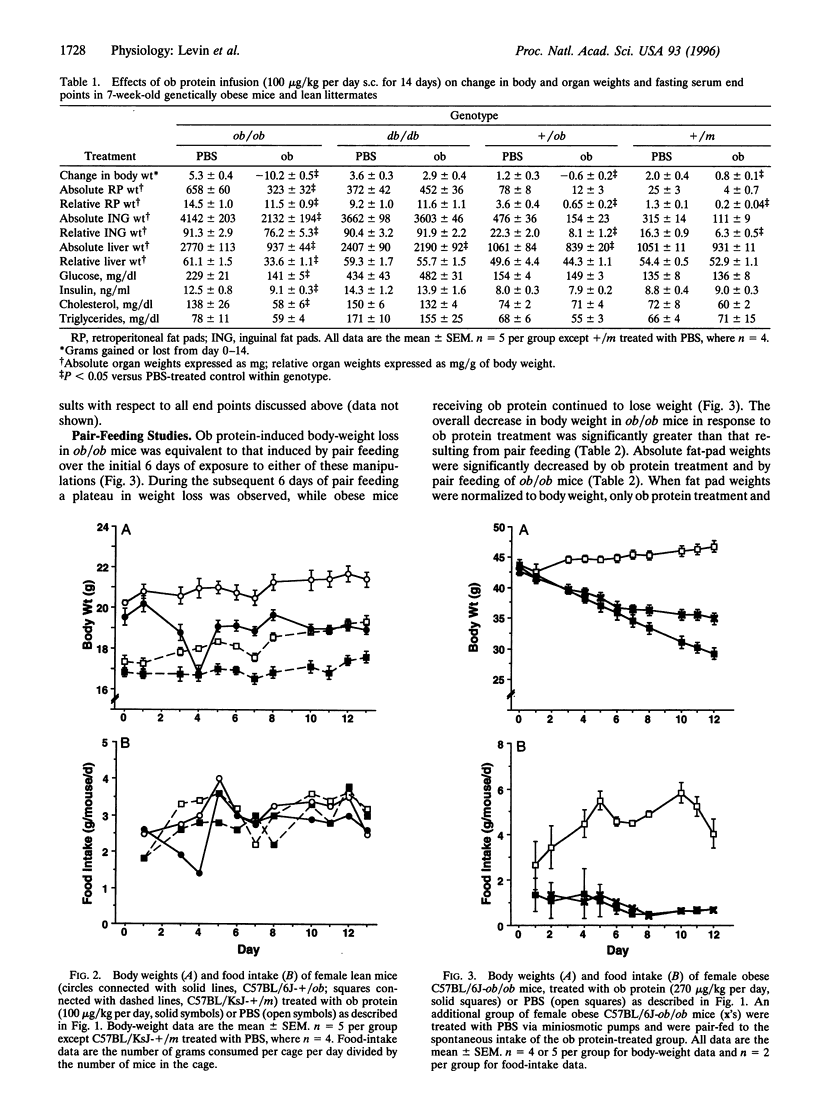
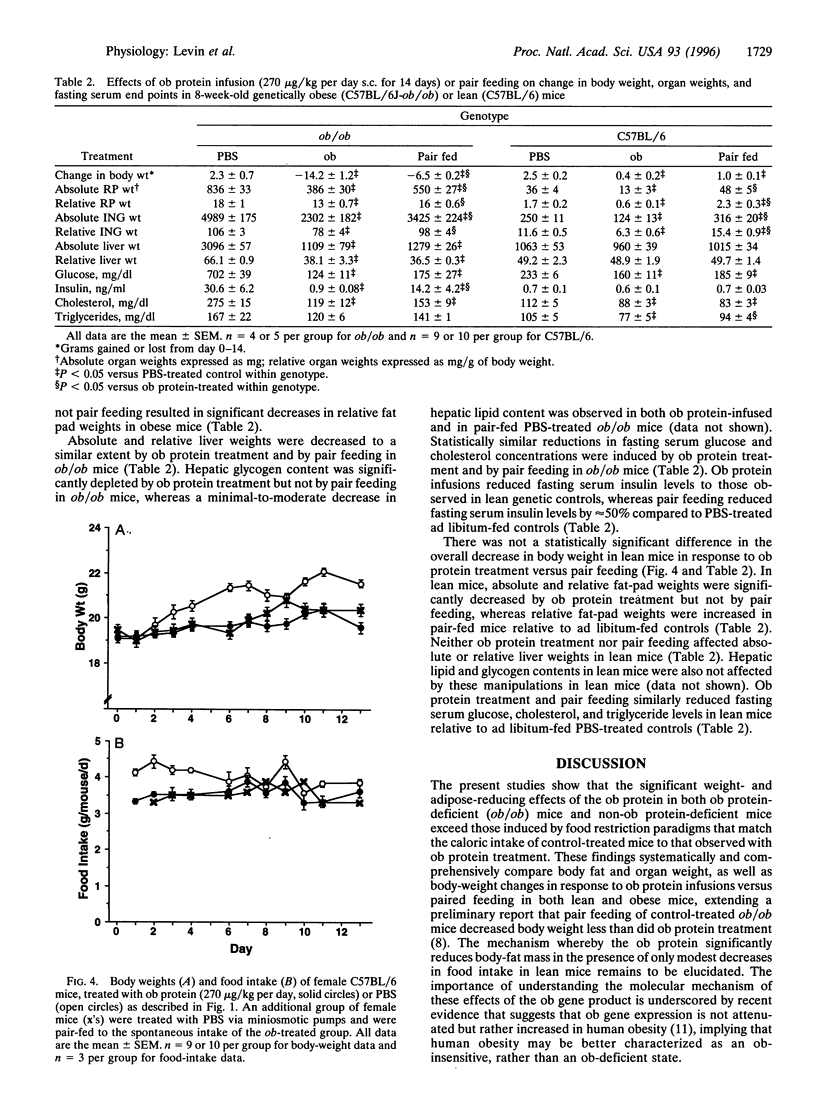
The effects of recombinantly produced ob protein were compared to those of food restriction in normal lean and genetically obese mice. Ob protein infusion into ob/ob mice resulted in large decreases in body and fat-depot weight and food intake that persisted throughout the study. Smaller decreases in body and fat-depot weights were observed in vehicle-treated ob/ob mice that were fed the same amount of food as that consumed by ob protein-treated ob/ob mice (pair feeding). In lean mice, ob protein infusion significantly decreased body and fat-depot weights, while decreasing food intake to a much lesser extent than in ob/ob mice. Pair feeding of lean vehicle-treated mice to the intake of ob protein-treated mice did not reduce body fat-depot weights. The potent weight-, adipose-, and appetite-reducing effects exerted by the ob protein in ob protein-deficient mice (ob/ob) confirm hypotheses generated from early parabiotic studies that suggested the existence of a circulating satiety factor of adipose origin. Pair-feeding studies provide compelling evidence that the ob protein exerts adipose-reducing effects in excess of those induced by reductions in food intake.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Campfield L. A., Smith F. J., Guisez Y., Devos R., Burn P. Recombinant mouse OB protein: evidence for a peripheral signal linking adiposity and central neural networks. Science. 1995 Jul 28;269(5223):546–549. doi: 10.1126/science.7624778. [DOI] [PubMed] [Google Scholar]
- Chang C. N., Rey M., Bochner B., Heyneker H., Gray G. High-level secretion of human growth hormone by Escherichia coli. Gene. 1987;55(2-3):189–196. doi: 10.1016/0378-1119(87)90279-4. [DOI] [PubMed] [Google Scholar]
- Colditz G. A. Economic costs of obesity. Am J Clin Nutr. 1992 Feb;55(2 Suppl):503S–507S. doi: 10.1093/ajcn/55.2.503s. [DOI] [PubMed] [Google Scholar]
- Coleman D. L. Effects of parabiosis of obese with diabetes and normal mice. Diabetologia. 1973 Aug;9(4):294–298. doi: 10.1007/BF01221857. [DOI] [PubMed] [Google Scholar]
- Coleman D. L., Hummel K. P. Effects of parabiosis of normal with genetically diabetic mice. Am J Physiol. 1969 Nov;217(5):1298–1304. doi: 10.1152/ajplegacy.1969.217.5.1298. [DOI] [PubMed] [Google Scholar]
- Considine R. V., Considine E. L., Williams C. J., Nyce M. R., Magosin S. A., Bauer T. L., Rosato E. L., Colberg J., Caro J. F. Evidence against either a premature stop codon or the absence of obese gene mRNA in human obesity. J Clin Invest. 1995 Jun;95(6):2986–2988. doi: 10.1172/JCI118007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halaas J. L., Gajiwala K. S., Maffei M., Cohen S. L., Chait B. T., Rabinowitz D., Lallone R. L., Burley S. K., Friedman J. M. Weight-reducing effects of the plasma protein encoded by the obese gene. Science. 1995 Jul 28;269(5223):543–546. doi: 10.1126/science.7624777. [DOI] [PubMed] [Google Scholar]
- Kuczmarski R. J., Flegal K. M., Campbell S. M., Johnson C. L. Increasing prevalence of overweight among US adults. The National Health and Nutrition Examination Surveys, 1960 to 1991. JAMA. 1994 Jul 20;272(3):205–211. doi: 10.1001/jama.272.3.205. [DOI] [PubMed] [Google Scholar]
- Maffei M., Fei H., Lee G. H., Dani C., Leroy P., Zhang Y., Proenca R., Negrel R., Ailhaud G., Friedman J. M. Increased expression in adipocytes of ob RNA in mice with lesions of the hypothalamus and with mutations at the db locus. Proc Natl Acad Sci U S A. 1995 Jul 18;92(15):6957–6960. doi: 10.1073/pnas.92.15.6957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelleymounter M. A., Cullen M. J., Baker M. B., Hecht R., Winters D., Boone T., Collins F. Effects of the obese gene product on body weight regulation in ob/ob mice. Science. 1995 Jul 28;269(5223):540–543. doi: 10.1126/science.7624776. [DOI] [PubMed] [Google Scholar]
- Pi-Sunyer F. X. Medical hazards of obesity. Ann Intern Med. 1993 Oct 1;119(7 Pt 2):655–660. doi: 10.7326/0003-4819-119-7_part_2-199310011-00006. [DOI] [PubMed] [Google Scholar]
- Rink T. J. Genetics. In search of a satiety factor. Nature. 1994 Dec 1;372(6505):406–407. doi: 10.1038/372406a0. [DOI] [PubMed] [Google Scholar]
- Stephens T. W., Basinski M., Bristow P. K., Bue-Valleskey J. M., Burgett S. G., Craft L., Hale J., Hoffmann J., Hsiung H. M., Kriauciunas A. The role of neuropeptide Y in the antiobesity action of the obese gene product. Nature. 1995 Oct 12;377(6549):530–532. doi: 10.1038/377530a0. [DOI] [PubMed] [Google Scholar]
- Tartaglia L. A., Dembski M., Weng X., Deng N., Culpepper J., Devos R., Richards G. J., Campfield L. A., Clark F. T., Deeds J. Identification and expression cloning of a leptin receptor, OB-R. Cell. 1995 Dec 29;83(7):1263–1271. doi: 10.1016/0092-8674(95)90151-5. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Proenca R., Maffei M., Barone M., Leopold L., Friedman J. M. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994 Dec 1;372(6505):425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]



