Abstract
Aim
We still have limited understanding of the contingent and deterministic factors that have fostered the evolutionary success of some species lineages over others. We investigated how the interplay of intercontinental migration and key innovations promoted diversification of the genus Androsace.
Location
Mountain ranges and cold steppes of the Northern Hemisphere.
Methods
We reconstructed ancestral biogeographical ranges at regional and continental scales by means of a dispersal–extinction–cladogenesis analysis using dated Bayesian phylogenies and contrasting two migration scenarios. Based on diversification analyses under two frameworks, we tested the influence of life form on speciation rates and whether diversification has been diversity-dependent.
Results
We found that three radiations occurred in this genus, at different periods and on different continents, and that life form played a critical role in the history of Androsace. Short-lived ancestors first facilitated the expansion of the genus’ range from Asia to Europe, while cushions, which appeared independently in Asia and Europe, enhanced species diversification in alpine regions. One long-distance dispersal event from Europe to North America led to the diversification of the nested genus Douglasia. We found support for a model in which speciation of the North American–European clade is diversity-dependent and close to its carrying capacity, and that the diversification dynamics of the North American subclade are uncoupled from this and follow a pure birth process.
Main conclusions
The contingency of past biogeographical connections combined with the evolutionary determinism of convergent key innovations may have led to replicated radiations of Androsace in three mountain regions of the world. The repeated emergence of the cushion life form was a convergent key innovation that fostered radiation into alpine habitats. Given the large ecological similarity of Androsace species, allopatry may have been the main mode of speciation.
Keywords: Allopatry, Androsace, biogeography, cushion plants, diversification, evolutionary determinism, historical contingency, long-distance dispersal, speciation rates
INTRODUCTION
Discerning the processes that foster species diversification and shape spatial patterns of biodiversity is a major challenge in evolutionary biology (Schluter, 2000). Although recent studies have reported exceptional species radiations (e.g. Hughes & Eastwood, 2006; Valente et al., 2010), little is known about the driving forces of the evolutionary success (i.e. higher speciation rates and geographical expansion; Gould & Eldredge, 1977) of some clades while close relatives remain species-poor or geographically restricted. In particular, the relative influence of historical contingency (i.e. stochastic past events) versus deterministic processes (i.e. similar selective pressures leading to convergent evolution) in driving species radiations remains unclear (Schluter, 2000; Losos & Mahler, 2010). Evidence for the prevalence of determinism in nature is provided by the increasing number of replicated adaptive radiations and evolutionary convergences documented in various groups of plants and animals (Schluter, 2000; Vermeij, 2006). In such cases, different clades facing similar environmental conditions developed convergent key innovations, which provided the stimulus for increased species and niche diversification, e.g. toe pads in lizards (Larson & Losos, 1996), phytophagy in insects (Farrell, 1998), and pharyngeal jaws in fish (Mabuchi et al., 2007). However, rare events that have given rise to unprecedented ecological opportunities have been highlighted to show the importance of historical contingencies on organism diversification (Losos & Mahler, 2010). The most common type of contingent event might be long-distance dispersal (hereafter, LDD) (Nathan, 2006; Gillespie et al., 2012), a phenomenon best exemplified by organisms that have colonized remote oceanic islands and radiated in situ (e.g. Hawaiian violets; Ballard & Sytsma, 2000).
In comparison to islands, which have traditionally served as natural laboratories for evolutionary studies (Losos & Ricklefs, 2009), less is known about the history of continental clades because of their complex geographical and historical settings. However, some continental systems exhibit a certain degree of similarity with island systems in that they are delimited to identifiable and distinct geographical units. Among such systems, mountain ranges constitute networks of cold environmental islands (Ackerly, 2003) that can be seen as continental analogies of island archipelagos systems (Gehrke & Linder, 2009). Like oceanic islands that have different ages, mountain ranges with their own specific orogenic histories allow us to compare the tempo of species diversification of sister lineages. Recent studies on tropical mountain taxa have enhanced our understanding of adaptive radiations (e.g. Hughes & Eastwood, 2006; Särkinen et al., 2011). However, examples of evolutionary radiations in temperate mountain ranges are quite scarce (but see Emadzade & Hörandl, 2011), probably due to the fact that the extreme abiotic conditions in these regions led to lower rates of diversification than energy-rich environments.
Here, we attempt to understand the evolutionary success of rock-jasmines, i.e. the plant genus Androsace s.l. (sensu Martins et al., 2003). The case of Androsace is intriguing for two main reasons. First, the genus has successfully colonized most temperate and arctic–alpine regions of the Northern Hemisphere although it is mainly made up of narrow endemics with low dispersal abilities (Anderberg & Kelso, 1996). Second, Androsace species seem to have diversified in three major regions (Central Asia, Western Europe and Northern America) reaching a relatively high number of species within the genus (c. 110 species), most of them found in alpine habitats (see Fig. S1 in Appendix S1 in Supporting Information). This is somehow unexpected given the general observation that arctic–alpine ecosystems are species-poor (McCain & Grytnes, 2010) and have low productivity (Körner, 1999), which may limit the opportunities for diversification (e.g. O’Brien, 1998; Francis & Currie, 2003). Androsace displays a variety of life forms including annual, herbaceous perennials and cushions, i.e. slow-growing plants in compact form with very dense leaf canopy, characterized by extremely long individual lifetimes. Recently, Boucher et al. (2012) demonstrated that the cushion life form evolved independently in two separate clades as a key innovation that led to the occupancy of the extremely cold ‘alpine niches’. What remains puzzling and unexplored is how the genus Androsace has colonized most of the Northern Hemisphere, and which processes led to the pattern of increased species richness in alpine regions. In particular, it remains unknown whether the cushion life form fostered diversification in the genus and whether ecological forces have been regulating cladogenesis in different geographical areas.
Here, we address several questions raised by previous studies on Androsace. We explore how the various life forms, which have different climatic tolerances, longevities and dispersal abilities (due to variable seed weight) may have triggered the geographical spread and diversification of Androsace in different ways. To answer this, we analysed a comprehensive data set including geographical distributions, climatic preferences, morphological data and phylogenetic relationships for nearly two-thirds of all Androsace species. We reconstructed the historical biogeography of the genus and defined the most likely migration routes across the mountain ranges of the Northern Hemisphere. We then examined the tempo of diversification in three main geographical regions (Central Asia, Europe and North America) and tested the relative role of the cushion life form and climatic niche vicariance (i.e. divergence of sister species due to specialization to different climatic regimes) in the diversification of Androsace.
MATERIALS AND METHODS
Study group
Androsace s.l. comprises the (former) genera Androsace, Douglasia, Pomatosace and Vitaliana (Martins et al., 2003; Schneeweiss et al., 2004). Its highest species richness is located in the Himalayas and Hengduan Mountains (a plant endemism hotspot in Western China; López-Pujol et al., 2011). The three life forms found in Androsace tend to occupy different habitats: short-lived species (annual or biennial) mainly occur in cold steppes, rosette perennials occupy mesic subalpine and alpine meadows or open woodlands, and cushion species occur on alpine scree slopes or cliffs, sometimes at very high elevations (up to 3850 m in the French Alps; S. Lavergne, Laboratoire d’Écologie Alpine, pers. comm.). Cushions occupy the coldest niches; annuals and perennials are adapted to the driest and wettest environments, respectively (Boucher et al., 2012). Despite life-form differences, Androsace species have similar floral morphology, with relatively large white or pink homostylous flowers (except for Vitaliana primuliflora Bertol., which produces yellow, heterostylous flowers).
Biogeographical inference
Different life forms may have had varied importance in the geographical spread of the genus. Migration could have been fostered by annuals because of their lighter seeds and wider climatic tolerances. We tested two alternative biogeographical scenarios with the dispersal–extinction–cladogenesis (DEC) parametric method implemented in Lagrange (Ree & Smith, 2008), which is able to integrate temporal and dispersal inputs. This enables the comparison of biogeographical hypotheses based on their likelihood. As Lagrange takes branch lengths of the provided phylogeny into account and allows us to define the specific dispersal probabilities between geographical areas, this program enables the integration of temporal and dispersal variables into the comparison of alternative biogeographical hypotheses using likelihood.
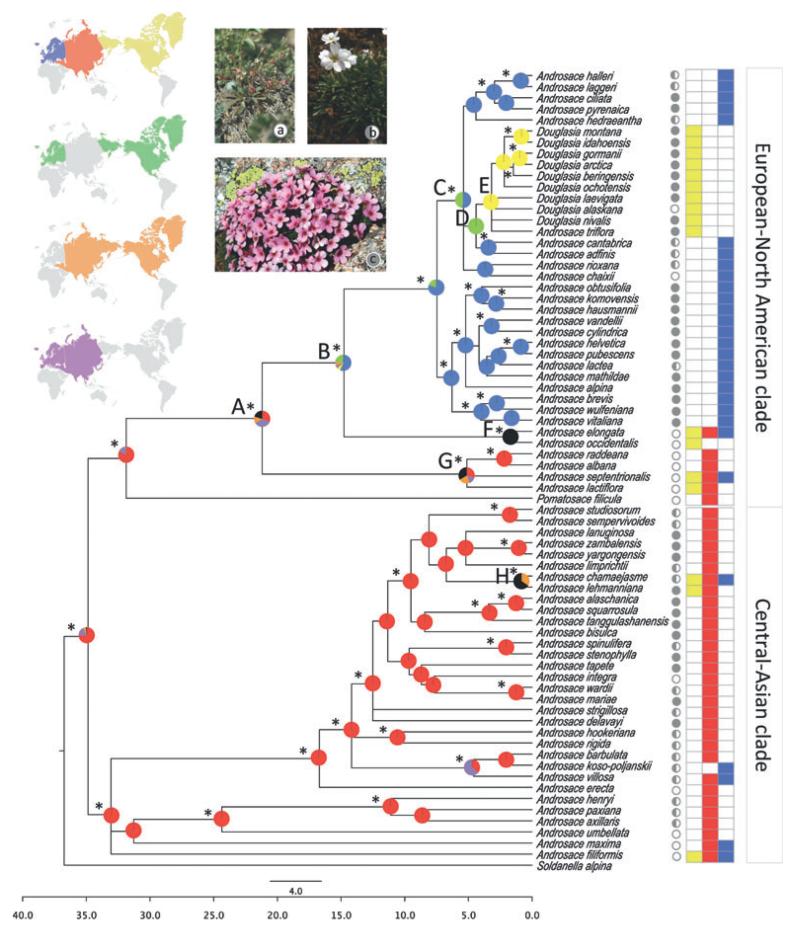
Biogeographical analyses were conducted on two spatial scales: continental and regional. At a continental level, we looked for broad patterns in the biogeographical reconstruction and tested which area delimitation (see Fig. 1, and Fig. S2 in Appendix S1) best suited Androsace species. At a regional level, we defined the following areas for which two or more species were endemic: (A) Iberian Peninsula (excluding the Pyrenees); (B) Pyrenees; (C) Alps and Apennines; (D) south-eastern Europe; (E) Caucasus; (G) Himalayas; (H) Tibetan Plateau; (F) Hengduan Mountains; (J) Eastern Asia region; (I) Asian Arctic region and Mongolian plateau; (M) North American Arctic region; (K) Cascade Range; (L) Central Rocky Mountains and Central North America (Fig. 2).
Figure 1. Continental-scale biogeographical reconstruction plotted on the Androsace consensus tree.
The time-scale at the bottom is in millions of years. World maps show the three areas defined for the continental analysis and all their possible combinations. Pie charts represent the relative probability of ancestral area reconstructed for each node averaged over the 100 trees, with colours corresponding to the areas highlighted on the world maps. Asterisks indicate nodes with a posterior probability ≥ 0.95. Black portions represent ancestral reconstructions spread on the three defined areas. On the right side of tip labels, circles indicate species’ life forms: grey circles represent cushions; half-filled circles indicate perennials; and empty circles are short-lived species. These three life forms are illustrated with one photograph each: (a) A. elongata L., annual (photo: S. Aubert/SAJF); (b) A. lactea L., perennial (photo: S. Aubert/SAJF); (c) A. laggeri Huet, cushion (photo: C. Roquet). Coloured boxes on the extreme right show current geographical distributions corresponding to the distribution map. Some nodes are labelled with a letter for text discussion.
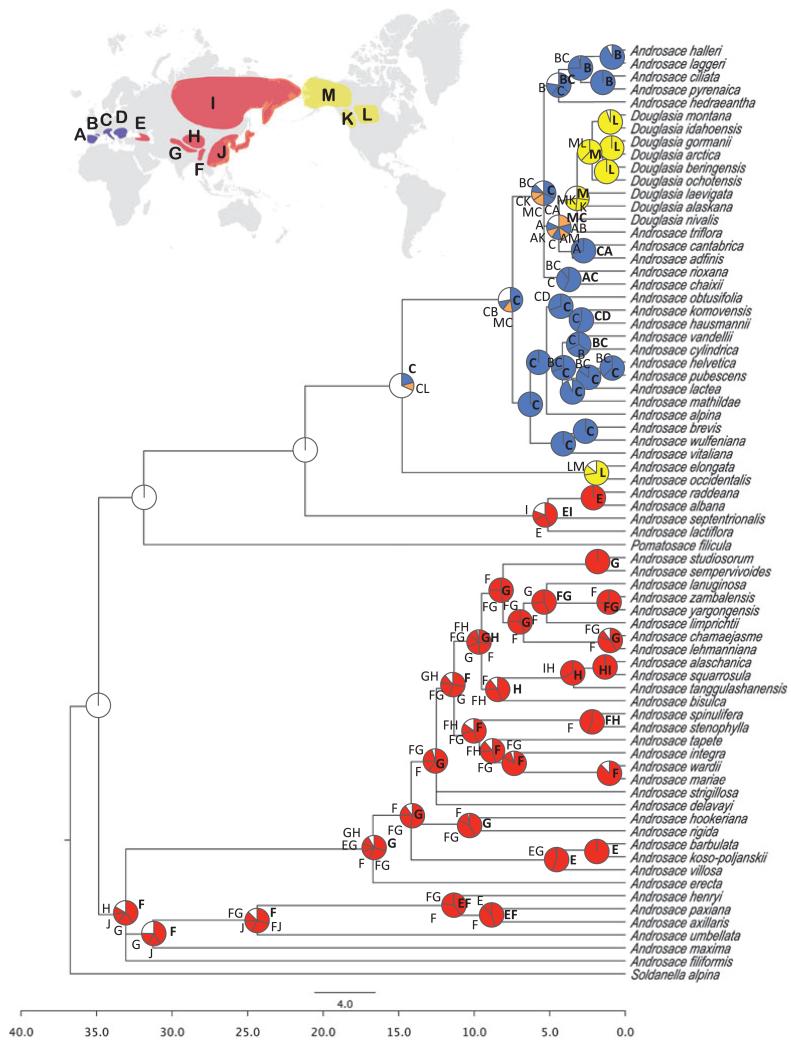
Figure 2. Regional-scale biogeographical reconstruction plotted on the Androsace consensus tree.
Pie charts represent the relative probability of ancestral area reconstructed for each node averaged over the 100 trees. Letters next to pie charts correspond to areas of distribution with codes as in the world map; bold letters correspond to the portion with a highest probability. Colours of the pie chart portions correspond to continental-scale areas as in Fig. 1; white portions represent reconstructions with a probability < 0.10.
Two biogeographical models were then compared at a regional level – a baseline model without dispersal constraints, and a stepping-stone model with higher dispersal probabilities between neighbouring areas than between non-neighbouring ones (Fig. S3 in Appendix S1). Neighbouring areas were defined as adjacent areas or areas that do not have another area or sea between them that could act as a barrier (except for Arctic Asia and Arctic North America, which were considered as neighbouring areas due to the lability of the Bering Strait; Hopkins, 1967; Wen, 1999). We also constrained the number of maximum areas of ancestral range to see whether this led to a better model.
All biogeographical analyses were run for 100 phylogenetic trees from Boucher et al. (2012). The phylogenetic trees included 72 species of the study group, thus covering nearly two-thirds of Androsace species (c. 110). These phylogenies were based on two DNA regions (ITS and trnL–F) and were built using Bayesian inference on MrBayes (Ronquist & Huelsenbeck, 2003), and run over 20 million generations, sampling one tree every 100 steps. A random set of 100 phylogenies from the posterior distribution of trees were dated with a Bayesian relaxed molecular clock (Yang, 1997; Kishino et al., 2001; Thorne & Kishino, 2002), which was calibrated with the divergence time range estimations of the Androsace lineage obtained by Yesson et al. (2009). Ancestral area reconstructions for each node were averaged in a consensus tree. When several reconstructions were obtained for a given node, they were weighted by their marginal probability at this given node. To summarize the results for each node (pie charts in Figs 1–2), only the trees where the node was present were used, thus yielding a ‘node-by-node reconstruction’ (Nylander et al., 2008).
Diversification analyses
The cushion life form has been identified as a key morphological innovation in Androsace that facilitated the occupation of colder alpine environments (Boucher et al., 2012). Macro-evolutionary theory predicts that this new life form would have increased diversification rates (either increasing speciation or decreasing extinction rates) due to the ecological opportunity it provided (Glor, 2010; Yoder et al., 2010). To test this prediction, we used the MuSSE (Multiple State Speciation Extinction) framework (Fitzjohn et al., 2009), which accounts for different rates of speciation and/or extinction depending on the state of a discrete trait, while simultaneously estimating the ancestral states of this trait using a likelihood approach. Life forms of extant species were classified into three categories – short-lived (annuals, biennials), perennials and cushions (see Boucher et al., 2012). Diversification methods that can deal with incomplete phylogenies require random undersampling, which was not the case here (all European and North American species are included, but only half of the Asian ones). Thus, we defined two monophyletic clades with a coherent geographical distribution and ran the analyses independently on both of them: the (mainly) Central Asian clade (Fig. 1); and the clade formed by 32 species distributed in Europe and North America (stemming from node B; see Fig. 1; hereafter ‘North American–European clade’). Four models of diversification were used: (1) a pure birth model with a single speciation rate (PB); (2) a birth–death model common to all species (BD); (3) a model with different speciation and extinction rates for each life form (BD-Form); and (4) a model with different speciation rates but null extinction for each life form (PB-Form). Given that a previous study showed that the ancestor of Androsace species was probably short-lived, and that it is very unlikely that short-lived species could directly evolve into cushion species (Boucher et al., 2012), we defined the root of the tree as short-lived in each clade and we did not allow any evolutionary jumps from short-lived to cushions. For the Central Asian clade, sampling was fixed at 57% of short-lived species, 25% of perennials and 44% of cushions, according to taxonomic knowledge (Nasir, 1984; Hu & Kelso, 1996).
Examining the tempo of diversification in a clade can provide insights into potential ecological regulation of cladogenesis. Declining diversification rates over time may suggest that a diversity limit was reached during diversification (Rabosky, 2009). Density-dependent diversification can be explicitly modelled using a logistic growth function for speciation rates and a clade’s upper diversity bound can be estimated (Rabosky & Lovette, 2008). This limit is frequently thought to be the result of niche partitioning between interacting members of the clade and is one of the main signatures of adaptive radiations (Losos & Mahler, 2010). However, such diversity limits could also arise as a consequence of decreasing range sizes following successive events of allopatric speciation (Rosenzweig, 1996). In the case of Androsace, the clades found in Central Asia, Europe and Northern America may be at different stages of diversification, because they have different ages and geographical contexts. To determine whether each of these clades has reached a diversity bound, we used the likelihood framework for density-dependent diversification implemented in the R package ddd (Etienne & Haegeman, 2012). In order to meet the hypothesis of random undersampling, we first analysed the Central Asian clade on its own by fitting four different models: (1) a pure birth model (PB); (2) a birth–death model (BD); (3) a density-dependent model with no extinction (DDL); and (4) a density-dependent model with extinction (DDL+E). We applied the same four models to the North American–European clade, and also tested the hypothesis that North American species underwent their own diversity dynamics by enabling a decoupling of the diversification of the North American subclade (stemming from node E, Fig. 1) versus the main clade (Etienne & Haegeman, 2012). This resulted in four additional models being fitted: (5) a model where European species experience density-dependence with no extinction but North American species follow a pure birth process (DDL/PB); (6) the same model with extinction in both clades (DDL+E/BD); (7) a model where both European and North American species experience density-dependence with no extinction but at different paces and with different carrying capacities (DDL/DDL); and finally (8) the same model with extinction (DDL+E/DDL+E).
All diversification analyses were run on 100 phylogenetic trees and alternative models were compared using the Akaike information criterion (AIC). In all density-dependent diversification models, the speciation rate was assumed to depend linearly on species richness, and the last 0.6 Myr (during which no branching occurs) were chopped off to avoid detecting a final negative rate shift due to a lack of species recognition (i.e. incipient species not detected in the phylogeny because of incomplete speciation; Egan & Crandall, 2008). Models without extinction were specified by fixing the extinction rate to zero, and models without density-dependence in the subclade (i.e. DDL/PB and DDL+E/BD) were approximated by giving a very large value to the carrying capacity (K = 5000).
Androsace species show low levels of sympatry and species belonging to the same life form are usually found in similar habitats. Thus, parapatric speciation due to climatic vicariance is unlikely. However, in order to discard the hypothesis that climatic vicariance may have played an important role in speciation events of Androsace, we performed a set of statistical analyses implemented in the software seeva (Struwe et al., 2011), and this showed that climatic vicariance played no role in the speciation of the genus. See Appendix S1 for a detailed description of the analyses performed and Appendix S2 for the results.
RESULTS
Biogeographical reconstruction
The continental and regional analyses led to congruent results for the main biogeographical patterns (Figs 1–2). At a continental level, the area delimitations with the highest likelihood were: (a) Europe; (b) North America and Arctic Asia; and (c) Asia excluding the Arctic (see Table S1 in Appendix S2). In the regional analyses, the stepping-stone model yielded a higher likelihood than the baseline model (Fig. S4 in Appendix S2). Restricting the ancestral range to two areas also gave higher likelihood values, which was congruent with the distribution range of most Androsace species, with most of them being restricted to one or two areas. Hence, we will only present the results obtained with these settings here.
The continental analysis suggested an Asian ancestor for Androsace (Fig. 1), followed by at least two LDD events: first, to Europe in the early–middle Miocene (nodes A and B; Fig. 1); secondly, to North America in the late Miocene–Pliocene (nodes C and D; Fig. 1). In addition, there were intercontinental dispersal events for three minor clades: (1) from Asia to other continents by the ancestor of A. chamaejasme Wulfen (Holarctic distribution) and A. lehmanniana Spreng. (present in Alaska and Asia); (2) from Asia to Europe by the ancestor of A. villosa L. (widespread in the mountains of Europe and Western Asia) and two western Asiatic species, A. barbulata Ovcz. and A. koso-poljanskii Ovcz.; and (3) from Europe to other continents by the ancestor of the North American endemic A. occidentalis Pursh and the widespread A. elongata L.
Results for the regional analyses suggested that the ancestors of most of European species originated from the Alps and Apennines, and subsequently expanded to the Pyrenees and south-eastern Europe mountain areas. Results for Douglasia and A. triflora Adans. (nested within Douglasia) showed an ancestor from the south-western European mountains that spread to North America through LDD. In the Asian clade, a high proportion of ancestors were distributed in the Himalayas and/or the Hengduan Range.
Diversification analysis
When trying to determine the influence of life forms on the diversification in Androsace, the model that fitted best in both clades was the PB-Form model where speciation rates were different for each life form but extinction was null (mean ΔAIC > 5.2 with all other models and in the two clades; see Table S3 in Appendix S2). Lineages with a cushion life form experienced significantly higher speciation rates than the other life forms in both clades (Table 1).
Table 1. Speciation rates (species per million years) estimated in the two main clades of Androsace using a pure birth model with different speciation rates but null extinction for the three life forms (PB-Form).
Means and standard deviations over the 100 trees are presented.
| Short-lived | Perennial | Cushions | |
|---|---|---|---|
| Central Asian clade | 0.037 ± 0.0003 | 0.140 ± 0.0212 | 0.282 ± 0.018 |
| North American–European clade | 0.052 ± 0.002 | 0.183 ± 0.0778 | 0.216 ± 0.0282 |
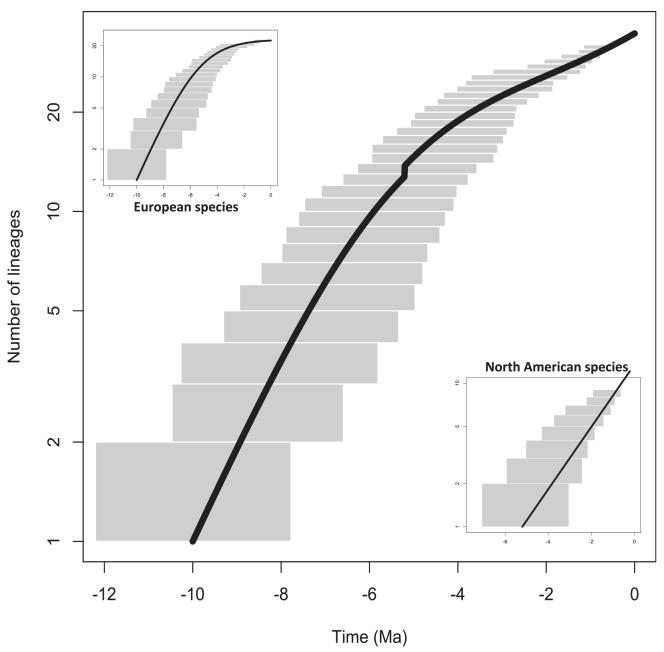
However, contrasting diversification patterns were found for different clades. All models had similar AIC scores in the Central Asian clade, but a pure birth model was slightly favoured over the others (Table 2). The constant speciation rate was estimated to be 0.124 ± 0.004 species Myr−1. A decoupling of diversity dynamics was detected in the North American–European clade, where the most likely model suggested diversity-dependent diversification in Europe and pure birth process in North America (Table 2; see also the line-age-through-time plot Fig. 3). In this model, European species experience diversity-dependence with an estimated initial speciation rate of 0.619 ± 0.075 species Myr−1 and a carrying capacity of 22.76 ± 1.12 species. This bound is extremely close to the current number of species in the European clade, which is 22. The North American species undergo their own diversity dynamics and follow a pure birth process with a speciation rate of 0.355 ± 0.071 species Myr−1.
Table 2. Performance of diversification models based on the Akaike information criterion (AIC) and parameter estimates (λ, speciation rate; μ, extinction rate; K, carrying capacity).
For each clade of Androsace, the model having the lowest mean AIC over the 100 trees is considered the best and other models are compared to it based on their ΔAIC (mean and standard deviation over the 100 trees are reported). Thus the best model has a ΔAIC of zero. Row names indicate the model types: PB, pure birth model; BD, birth–death model; DDL, density-dependent model with no extinction; DDL+E, density-dependent model with extinction. The last four models were only fitted to the North American (NA)–European (E) clade, with decoupling of diversification dynamics within the North American clade from the rest of the clade: DDL/PB, a model where European species undergo a density-dependence without extinction and North American species follow a pure-birth process; DDL+E/BD, the same model with extinction in both clades; DDL/DDL, a model where both European and North American species undergo density-dependence with no extinction, but at different paces and with different carrying-capacities; DDL+E/DDL+E, the same model with extinction.
| Central Asian clade (32 species) |
North American–European clade (32 species) |
|||||||
|---|---|---|---|---|---|---|---|---|
| ΔAIC | λ | μ | K | ΔAIC | λ | μ | K | |
| PB | 0 | 0.123 ± 0.004 | 0 | +∞ | 4.67 ± 0.91 | 0.238 ± 0.034 | 0 | +∞ |
| BD | 1.42 ± 0.27 | 0.172 ± 0.018 | 0.081 ± 0.023 | +∞ | 6.67 ± 0.91 | 0.238 ± 0.034 | 8.50 × 10−8 ± 8.50 × 10−7 | +∞ |
| DDL | 2.00 ± 0.00 | 0.123 ± 0.004 | 0 | 2.62 × 106 ± 2.12 × 106 | 2.48 ± 0.77 | 0.550 ± 0.061 | 0 | 37.0 ± 1.67 |
| DDL+E | 3.51 ± 0.96 | 2.00 ± 0.439 | 0.325 ± 0.027 | 70.14 ± 1.45 | 4.35 ± 0.95 | 0.723 ± 0.148 | 0.070 ± 0.035 | 35.5 ± 11.9 |
| DDL/PB | — | — | — | — | 0 | λE = 0.624 ± 0.075 | μE = 0 | KE = 22.7 ± 1.12 |
| λNA = 0.364 ± 0.071 | μNA = 0 | KNA = +∞ | ||||||
| DDL+E/BD | — | — | — | — | 3.86 ± 0.12 | λE = 0.738 ± 0.115 | μE = 0.039 ± 0.022 | KE = 22.0 ± 1.01 |
| λNA = 0.364 ± 0.071 | μNA = 1.84 × 10−5 ± 0.071 | KNA = +∞ | ||||||
| DDL/DDL | — | — | — | — | 0.81 ± 0.65 | λE = 0.628 ± 0.075 | μE = 0 | KE = 22.7 ± 1.12 |
| λNA = 0.868 ± 0.228 | μNA = 0 | KNA = 10.39 ± 1.55 | ||||||
| DDL+E/DDL+E | — | — | — | — | 4.23 ± 0.48 | λE = 0.743 ± 0.117 | μE = 0.041 ± 0.023 | KE = 22.7 ± 1.12 |
| λNA = 2.00 ± 0.824 | μNA = 0.230 ± 0.166 | KNA = 9.73 ± 3.13 | ||||||
Figure 3. Diversification of the whole North American–European clade (main plot), the European species only (upper left plot), and the North American subclade (lower right plot) of Androsace.
The grey area represents the upper and lower bounds of all lineage-through-time (LTT) plots obtained on the 100 trees. The black line shows the clade richness predicted by the most likely model with parameters estimated on the consensus tree.
DISCUSSION
Short-lived species may have promoted the geographical expansion of Androsace
Our biogeographical reconstruction suggests that Asia is the ancestral region of Androsace, as hypothesized earlier (Kress, 1965; Wang et al., 2004). The biogeographical history of the genus is characterized by two intercontinental dispersal events followed by diversification bursts: from Asia to Europe in the early–middle Miocene (nodes A/B; Fig. 1); and from Europe to North America in the late Miocene–Pliocene (nodes C/D, Fig. 1). One striking result is that almost all the ancestors that have expanded from one continent to another were probably short-lived species (nodes A, B, F, G, H; Fig. 1) according to the ancestral reconstruction inferred by Boucher et al. (2012). This is congruent with the contemporary observation that the large majority of Androsace species with widespread distributions are short-lived [A. elongata, A. erecta Maxim., A. filiformis Retz., A. lactiflora Kar. & Kir., A. maxima L., A. septentrionalis L. and A. umbellata (Lour.) Merr.]. The biogeographical expansion of Androsace has thus apparently been triggered by the high migration potential of annual species (e.g. Lavergne et al., 2012), which have rapid population growth (due to short generation times) and lighter seed mass (mean seed mass was 1 mg over 38 measured taxa, Table S4 in Appendix S2), and by the preference for open habitats of short-lived Androsace, which may provide better conditions for seed dispersal (Nathan et al., 2008).
Surprisingly, the ancestor that colonized North America from Europe was most probably a long-lived species (Boucher et al., 2012). The connection of the Douglasia clade, which has an amphi-Beringian distribution (i.e. it is found in the Arctic areas around the Bering Strait), with European ancestors was already suggested by Schneeweiss et al. (2004) although they did not explicitly test any particular biogeographical scenarios. Since the ancestor of Douglasia would have arrived during the Pliocene, the so-called North Atlantic land bridge was no longer available as a migration route (Tiffney, 1985; Milne & Abbot, 2002; Denk et al., 2010). Two plausible scenarios would be a LDD event over the Atlantic Ocean, or a gradual eastward migration through Asia followed by posterior extinction of Asian populations (Schneeweiss et al., 2004). However, LDD seems a more parsimonious explanation, being in line with the monophyly of the amphi-Beringian species and the increasing evidence for trans-oceanic LDD (de Queiroz, 2005). Androsace seeds have no specific dispersal adaptations (Anderberg & Kelso, 1996), but their small size may make them susceptible to rare LDD events by wind. Although our sampling is incomplete (several Asian species are lacking), the available morphological and karyological evidence suggests that species missing from our study would probably not fall within the North American–European clade (Anderberg & Kelso, 1996).
Cushion life form probably fostered speciation in Androsace
Previous studies have shown that the cushion life form is a key morphological innovation in Androsace (sensu Miller, 1949), which enabled the occupancy of alpine niches due to its dense canopy that buffers it from temperature variations (Boucher et al., 2012), but evidence was lacking that this trait also fostered species diversification. Here we have shown that cushions probably spurred diversification in the clades where they emerged, as expected with key innovations (Glor, 2010). This result may seem counterintuitive in terms of the extreme longevity of cushion species (up to several hundred years; e.g. Morris & Doak, 1998) and the type of environment in which these bursts of diversification occurred. Cushion species are associated with extremely cold environments (Boucher et al., 2012) that are among the coldest on Earth (Körner, 2011). This is contrary to the common expectation that short life history alone promotes diversification, and to theoretical studies suggesting that warmer environments favour speciation due to higher metabolic rates (Allen et al., 2006). However, it can be explained by the ecological opportunity (Simpson, 1953; Glor, 2010) that high alpine environments represented for Androsace ancestors. This opportunity was probably provided by: (1) active orogenic areas providing new physical environments; (2) progressive climate cooling (Zachos et al., 2008), resulting in the emergence of the alpine biome; and (3) the emergence of the cushion life-form, providing a morphological innovation that allowed new niches to be explored.
Androsace provides a case where the effects of ecological and biogeographical processes that promote diversification overcome any limits on diversification rates that arise from environmental stress. This example is, however, not the first of its kind. Several large radiations of pachycaulous plants in the alpine tropics have already been documented (Monasterio & Sarmiento, 1991; Knox & Palmer, 1995), some of them with exceptionally high speciation rates (more than 2 species Myr−1 on average in Lupinus; Hughes & Eastwood, 2006). All these alpine radiations support Schluter’s (2000) hypothesis that ecological opportunity not only increases the probability of ecological speciation, but can also spur diversification by increasing the opportunities for reproductive isolation. Indeed, by opening the way to alpine environments, cushion and pachycaulous life forms provided access to highly fragmented habitats (i.e. mountain tops), thereby increasing the chances of allopatric speciation.
Replicated radiations in distinct mountain ranges and evidence for geographical limitations on diversity
Focal analyses of the three radiations that occurred in Asia, Europe and North America lead to two diversification patterns. The radiation of Asian species shows evidence of constant speciation through time, which could indicate that this clade is still far from having reached any upper diversity limit in Central Asia. However, the fact that all diversification models had similar AIC scores indicates that the low sampling of our phylogenies for this clade probably reduces the statistical power of our analyses and prevents solid inferences on the tempo of speciation. At the same time, the radiation of the better-sampled clade of European species showed evidence for density-dependent speciation, a pattern consistent with other studies (e.g. Phillimore & Price, 2008; Rabosky & Lovette, 2008; Etienne et al., 2012). European Androsace are apparently close to their estimated carrying capacity and current speciation rates are very low, but the estimated speciation rate at the beginning of the radiation in Europe was relatively high for a group of plants in a temperate continental settings (Klak et al., 2004; Hughes & Eastwood, 2006). Conversely, the clade of North American species made up of all Douglasia plus A. triflora shows no signs of a slowdown in diversification.
Relatively high speciation rates at the beginning of the European and North American radiations may be explained by the fragmented landscape of the areas where Androsace is found. They may also be due to past climatic oscillations (Zachos et al., 2008), which have alternately connected and disconnected regions of suitable climate for Androsace species – a phenomenon known to promote speciation (Kadereit et al., 2004; Aguilée et al., 2011). The North American radiation could also have been promoted by Pliocene–Pleistocene climatic cooling: the beginning of the Douglasia diversification coincides with the onset of the Arctic ecosystem, c. 3 Ma (Matthews & Ovenden, 1990; Murray, 1995). It is interesting to note that, within the Arctic flora, the radiation of Douglasia is an exception, as most Arctic plant species have been shown to originate from non-arctic lineages (Hoffmann & Roser, 2009).
In the European clade, this initial phase of rapid radiation has been followed by a slowdown caused by density dependence. Such diversity bounds are usually thought to arise when ecological space is divided between coexisting species originating from the same radiation, progressively filling out the entire niche space (Rabosky, 2009). In the case of Androsace, this explanation is unlikely, because cushion species exhibit few ecological differences (either in their resource use, morphology or in their biotic interactions) and low levels of sympatry. Additional analyses of climatic vicariance indeed show that, besides the differences between life forms, partitioning of the climatic space has not played a major role in the diversification of Androsace (Appendix S2). Thus, the radiation of Androsace has apparently been little driven by adaptation to different ecological niches, and it should be considered more as an example of non-adaptive radiation (Gittenberger, 1991).
The observed density-dependence in European Androsace may probably be attributed to a progressive filling of the geographical space by species with similar ecological niches (Schluter, 2000; Rundell & Price, 2009), as previously documented in several non-adaptive radiations of alpine plants (Kadereit et al., 2004). The fact that current species richness in European Androsace almost reaches the estimated carrying capacity of this clade suggests that geographical filling might be complete in Europe. This agrees with the observation that Androsace species occur in all alpine mountain ranges of Southern Europe and that most European species have small ranges, thereby reducing the chances for allopatric speciation. On the other hand, given the much larger area available for Douglasia species in Alaska and the Rocky Mountains, it is logical that this young clade does not yet show strong signs of density-dependent regulation, and it is probably still in a phase of active diversification.
The fact that models with no extinction were favoured in all cases should not be interpreted as evidence for null extinction in Androsace. We cannot totally exclude the hypothesis that cushion species experienced reduced extinction rates (for instance, their extreme longevity could help to survive rapid environmental fluctuations) instead of having higher speciation rates. The difficulty of estimating extinction rates from phylogenies of extant taxa alone is a major area of concern in macroevolution (e.g. Purvis, 2008; Rabosky, 2010). Information from the fossil record could be useful for this purpose, but it is non-existent for Androsace and very scarce for Primulaceae. Alternatively, one could separately examine different extant clades in order to detect positive extinction rates in some clades that would have otherwise been masked by recently radiating lineages (e.g. Morlon et al., 2011). We did account for rate heterogeneity in Androsace in that we studied three clades separately, while allowing diversification rates to vary across life forms, but still failed to detect non-null extinction rates. Since it is possible that these subdivisions may still have blurred the signal of extinction in other clades, it was not possible to distinguish speciation rates from net diversification rates.
CONCLUSIONS
Androsace has several characteristics that could have doomed the genus to remain geographically restricted and species-poor. Most Androsace species have low dispersal abilities and occupy very harsh and disconnected habitats. However, in spite of these obvious limits to diversification, Androsace has expanded throughout the Northern Hemisphere and has experienced some periods of rapid diversification. Interestingly, two types of life form have played complementary roles in this success story. First, short-lived ancestors allowed range expansion throughout Eurasia thanks to their better dispersal abilities and their temperate climatic tolerance. Second, cushion species appeared independently at least twice and promoted diversification in the Himalayas, Europe and North America by enabling the colonization of high alpine niches, which in turn lead to allopatric speciation in fragmented alpine habitats. Although convergent evolution towards the same key innovation (i.e. cushion life form) in Europe and Asia reveals strong ecological and evolutionary determinism, important differences remain in the timing, tempo and rate of species radiations in Asia, Europe and North America. Perhaps the most striking contingency is the LDD event that occurred from Western Europe to North America, probably across the North Atlantic, and provoked the recent radiation of Douglasia species. The history of Androsace thus illustrates how deterministic and contingent events can contribute to a clade’s evolutionary success and advances our understanding of the origins of diversity in arctic and alpine ecosystems. Further research is needed to confirm the role of dispersal, habitat fragmentation and life form evolution on the diversification and distribution of alpine plants. This should enable us to understand why alpine ecosystems are relatively rich in spite of their harsh environmental conditions.
Supplementary Material
ACKNOWLEDGEMENTS
We thank H. Morlon and R. Fitzjohn for help with R code. R. Douzet and S. Aubert provided valuable insights into the history of alpine Androsace. A. Phillimore and two anonymous referees helped greatly in improving this manuscript. We also thank Version Originale for checking and correcting the English in this article. The research leading to these results received funding from the European Research Council under the European Community’s Seven Framework Programme FP7/2007-2013 Grant Agreement no. 281422 (TEEMBIO). We also acknowledge support from the French ANR EVORANGE (ANR-09-PEXT-011) project. C.R. was partly supported by a grant from Fundación Ramón Areces. The grant to F.B. was provided by the École Polytechnique.
BIOSKETCH
The authors of this article work in a research group called EMABIO (Evolution, Modelling and Analysis of Biodiversity), which is hosted by the French CNRS and the University of Grenoble. The authors are involved in the TEEMBIO ERC project. One of the main axes of this multidisciplinary project is to improve the understanding of how evolution shapes species ranges at micro- and macro-evolutionary scales.
REFERENCES
- Ackerly DD. Community assembly, niche conservatism, and adaptive evolution in changing environments. International Journal of Plant Sciences. 2003;164:S165–S184. [Google Scholar]
- Aguilée R, Lambert A, Claessen D. Ecological speciation in dynamic landscapes. Journal of Evolutionary Biology. 2011;24:2663–2677. doi: 10.1111/j.1420-9101.2011.02392.x. [DOI] [PubMed] [Google Scholar]
- Allen AP, Gillooly JF, Savage VM, Brown JH. Kinetic effects of temperature on rates of genetic divergence and speciation. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:9130–9135. doi: 10.1073/pnas.0603587103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderberg AA, Kelso S. Phylogenetic implications of endosperm cell wall morphology in Douglasia, Androsace, and Vitaliana (Primulaceae) Nordic Journal of Botany. 1996;16:481–486. [Google Scholar]
- Ballard HE, Jr, Sytsma KJ. Evolution and biogeography of the woody Hawaiian violets (Viola, Violaceae): Arctic origins, herbaceous ancestry and bird dispersal. Evolution. 2000;54:1521–1532. doi: 10.1111/j.0014-3820.2000.tb00698.x. [DOI] [PubMed] [Google Scholar]
- Boucher FC, Thuiller W, Roquet C, Douzet R, Aubert S, Alvarez N, Lavergne S. Reconstructing the origins of high-alpine niches and cushion life form in the genus Androsace s.l. (Primulaceae) Evolution. 2012;66:1255–1268. doi: 10.1111/j.1558-5646.2011.01483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denk T, Grímsson F, Zetter R. Episodic migration of oaks to Iceland: evidence for a North Atlantic “land bridge” in the latest Miocene. American Journal of Botany. 2010;97:276–287. doi: 10.3732/ajb.0900195. [DOI] [PubMed] [Google Scholar]
- Egan AN, Crandall KA. Divergence and diversification in North American Psoraleeae (Fabaceae) due to climate change. BMC Biology. 2008;6:55. doi: 10.1186/1741-7007-6-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emadzade K, Hörandl E. Northern Hemisphere origin, transoceanic dispersal, and diversification of Ranunculeae DC. (Ranunculaceae) in the Cenozoic. Journal of Biogeography. 2011;38:517–530. [Google Scholar]
- Etienne RS, Haegeman B. A conceptual and statistical framework for adaptive radiations with a key role for diversity dependence. The American Naturalist. 2012;180:E75–E89. doi: 10.1086/667574. [DOI] [PubMed] [Google Scholar]
- Etienne RS, Haegeman B, Stadler T, Aze T, Pearson PN, Purvis A, Phillimore AB. Diversity-dependence brings molecular phylogenies closer to agreement with the fossil record. Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences. 2012;279:1300–1309. doi: 10.1098/rspb.2011.1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell BD. “Inordinate fondness” explained: why are there so many beetles? Science. 1998;281:555–559. doi: 10.1126/science.281.5376.555. [DOI] [PubMed] [Google Scholar]
- Fitzjohn RG, Maddison WP, Otto SP. Estimating trait-dependent speciation and extinction rates from incompletely resolved phylogenies. Systematic Biology. 2009;58:595–611. doi: 10.1093/sysbio/syp067. [DOI] [PubMed] [Google Scholar]
- Francis AP, Currie DJ. A globally consistent richness–climate relationship for angiosperms. The American Naturalist. 2003;161:523–536. doi: 10.1086/368223. [DOI] [PubMed] [Google Scholar]
- Gehrke B, Linder HP. The scramble for Africa: pan-temperate elements on the African high mountains. Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences. 2009;276:2657–2665. doi: 10.1098/rspb.2009.0334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie RG, Baldwin BG, Waters JM, Fraser CI, Nikula R, Roderick GK. Long-distance dispersal: a framework for hypothesis testing. Trends in Ecology and Evolution. 2012;27:47–56. doi: 10.1016/j.tree.2011.08.009. [DOI] [PubMed] [Google Scholar]
- Gittenberger E. What about non-adaptive radiation? Biological Journal of the Linnean Society. 1991;43:263–272. [Google Scholar]
- Glor RE. Phylogenetic insights on adaptive radiation. Annual Review of Ecology, Evolution, and Systematics. 2010;41:251–270. [Google Scholar]
- Gould SJ, Eldredge N. Punctuated equilibria: the tempo and mode of evolution reconsidered. Paleobiology. 1977;3:115–151. [Google Scholar]
- Hoffmann MH, Roser M. Taxon recruitment of the arctic flora: an analysis of phylogenies. New Phytologist. 2009;182:774–780. doi: 10.1111/j.1469-8137.2009.02782.x. [DOI] [PubMed] [Google Scholar]
- Hopkins DM. The Bering land bridge. Stanford University Press; Palo Alto, CA: 1967. [Google Scholar]
- Hu CM, Kelso S. Primulaceae. In: Wu Z-Y, Raven PH, editors. Flora of China. Science Press; Beijing: 1996. pp. 118–119. [Google Scholar]
- Hughes C, Eastwood R. Island radiation on a continental scale: exceptional rates of plant diversification after uplift of the Andes. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:10334–10339. doi: 10.1073/pnas.0601928103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadereit JW, Griebeler EM, Comes HP. Quaternary diversification in European alpine plants: pattern and process. Philosophical Transactions of the Royal Society B: Biological Sciences. 2004;359:265–274. doi: 10.1098/rstb.2003.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishino H, Thorne JL, Bruno WJ. Performance of a divergence time estimation method under a probabilistic model of rate evolution. Molecular Biology and Evolution. 2001;18:352–361. doi: 10.1093/oxfordjournals.molbev.a003811. [DOI] [PubMed] [Google Scholar]
- Klak C, Reeves G, Hedderson T. Unmatched tempo of evolution in Southern African semi-desert ice plants. Nature. 2004;427:63–65. doi: 10.1038/nature02243. [DOI] [PubMed] [Google Scholar]
- Knox EB, Palmer JD. The origin of Dendrosenecio within the Senecioneae (Asteraceae) based on chloroplast DNA evidence. American Journal of Botany. 1995;82:1567–1573. [Google Scholar]
- Körner C. Alpine plant life: functional plant ecology of high mountain ecosystems. Springer; Berlin: 1999. [Google Scholar]
- Körner C. Coldest places on earth with angiosperm plant life. Alpine Botany. 2011;121:11–22. [Google Scholar]
- Kress A. Zur Zytotaxonomie der Androsace–Vitaliana–Douglasia–Verwandtschaft. Mitteilungen der Botanischen Staatssammlung, München. 1965;5:653–674. [Google Scholar]
- Larson A, Losos JB. Phylogenetic systematics of adaptation. In: Lauder G, Rose M, editors. Adaptation. Academic Press; New York: 1996. pp. 187–220. [Google Scholar]
- Lavergne S, Hampe A, Arroyo J. In and out of Africa: how did the Strait of Gibraltar affect plant species migration and local diversification? Journal of Biogeography. 2012;40:24–36. [Google Scholar]
- López-Pujol J, Zhang F-M, Sun H-Q, Ying T-S, Ge S. Centres of plant endemism in China: places for survival or for speciation? Journal of Biogeography. 2011;38:1267–1280. [Google Scholar]
- Losos JB, Mahler DL. Adaptive radiation: the interaction of ecological opportunity, adaptation, and speciation. In: Bell MA, Futuyma DJ, Eanes WF, Levinton JS, editors. Evolution since Darwin: the first 150 years. Sinauer Associates; Sunderland, MA: 2010. pp. 381–420. [Google Scholar]
- Losos JB, Ricklefs RE. Adaptation and diversification on islands. Nature. 2009;457:830–836. doi: 10.1038/nature07893. [DOI] [PubMed] [Google Scholar]
- Mabuchi K, Miya M, Azuma Y, Nishida M. Independent evolution of the specialized pharyngeal jaw apparatus in cichlid and labrid fishes. BMC Evolutionary Biology. 2007;7:10. doi: 10.1186/1471-2148-7-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins L, Oberprieler C, Hellwig FH. A phylo-genetic analysis of Primulaceae s.l. based on internal transcribed spacer (ITS) DNA sequence data. Plant Systematics and Evolution. 2003;237:75–85. [Google Scholar]
- Matthews JV, Jr, Ovenden LE. Late Tertiary plant macrofossils from localities in Arctic/Subarctic North America: a review of the data. Arctic. 1990;43:384–392. [Google Scholar]
- McCain CM, Grytnes J-A. Elevational gradients in species richness. In: Jonsson R, editor. Encyclopedia of Life Sciences. John Wiley & Sons; Chichester, UK: 2010. p. 10. doi:10.1002/9780470015902.a0022548. [Google Scholar]
- Miller AH. Some ecologic and morphologic considerations in the evolution of higher taxonomic categories. In: Mayr E, Schüz E, editors. Ornithologie als biologische Wissenschaft. Carl Winter; Heidelberg: 1949. pp. 84–88. [Google Scholar]
- Milne RI, Abbot RJ. The origin and evolution of Tertiary relict floras. Advances in Botanical Research. 2002;38:281–314. [Google Scholar]
- Monasterio M, Sarmiento L. Adaptive radiation of Espeletia in the cold Andean tropics. Trends in Ecology and Evolution. 1991;6:387–391. doi: 10.1016/0169-5347(91)90159-U. [DOI] [PubMed] [Google Scholar]
- Morlon H, Parsons TL, Plotkin JB. Reconciling molecular phylogenies with the fossil record. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:16327–16332. doi: 10.1073/pnas.1102543108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris WF, Doak DF. Life history of the long-lived gynodioecious cushion plant Silene acaulis (Caryophyllaceae), inferred from size-based population projection matrices. American Journal of Botany. 1998;85:784–793. [PubMed] [Google Scholar]
- Murray DF. Causes of arctic plant diversity: origin and evolution. In: Chapin FS, Körner C, editors. Arctic and alpine biodiversity: patterns, causes and ecosystem consequences. Springer; Heidelberg: 1995. pp. 21–32. [Google Scholar]
- Nasir YJ. In: Androsace. Flora of Pakistan. Nasir E, Ali SI, editors. University of Karachi; Karachi: 1984. p. 74. [Google Scholar]
- Nathan R. Long-distance dispersal of plants. Science. 2006;313:786–788. doi: 10.1126/science.1124975. [DOI] [PubMed] [Google Scholar]
- Nathan R, Schurr FM, Spiegel O, Steinitz O, Trakhtenbrot A, Tsoar A. Mechanisms of long-distance seed dispersal. Trends in Ecology and Evolution. 2008;23:638–647. doi: 10.1016/j.tree.2008.08.003. [DOI] [PubMed] [Google Scholar]
- Nylander JAA, Olsson U, Alström P, Sanmartín I. Accounting for phylogenetic uncertainty in biogeography: a Bayesian approach to dispersal–vicariance analysis of the thrushes (Aves: Turdus) Systematic Biology. 2008;57:257–268. doi: 10.1080/10635150802044003. [DOI] [PubMed] [Google Scholar]
- O’Brien EM. Water-energy dynamics, climate, and prediction of woody plant species richness: an interim general model. Journal of Biogeography. 1998;25:379–398. [Google Scholar]
- Phillimore AB, Price TD. Density-dependent cladogenesis in birds. PLoS Biology. 2008;6:e71. doi: 10.1371/journal.pbio.0060071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purvis A. Phylogenetic approaches to the study of extinction. Annual Review of Ecology, Evolution, and Systematics. 2008;39:301–319. [Google Scholar]
- de Queiroz A. The resurrection of oceanic dispersal in historical biogeography. Trends in Ecology and Evolution. 2005;20:68–73. doi: 10.1016/j.tree.2004.11.006. [DOI] [PubMed] [Google Scholar]
- Rabosky DL. Ecological limits and diversification rate: alternative paradigms to explain the variation in species richness among clades and regions. Ecology Letters. 2009;12:735–743. doi: 10.1111/j.1461-0248.2009.01333.x. [DOI] [PubMed] [Google Scholar]
- Rabosky DL. Extinction rates should not be estimated from molecular phylogenies. Evolution. 2010;64:1816–1824. doi: 10.1111/j.1558-5646.2009.00926.x. [DOI] [PubMed] [Google Scholar]
- Rabosky DL, Lovette IJ. Density-dependent diversification in North American wood warblers. Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences. 2008;275:2363–2371. doi: 10.1098/rspb.2008.0630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ree RH, Smith SA. Maximum likelihood inference of geographic range evolution by dispersal, local extinction, and cladogenesis. Systematic Biology. 2008;57:4–14. doi: 10.1080/10635150701883881. [DOI] [PubMed] [Google Scholar]
- Ronquist F, Huelsenbeck JP. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics. 2003;19:1572–1574. doi: 10.1093/bioinformatics/btg180. [DOI] [PubMed] [Google Scholar]
- Rosenzweig ML. Species diversity in space and time. Cambridge University Press; Cambridge, UK: 1996. [Google Scholar]
- Rundell RJ, Price TD. Adaptive radiation, nonadaptive radiation, ecological speciation and nonecological speciation. Trends in Ecology and Evolution. 2009;24:394–399. doi: 10.1016/j.tree.2009.02.007. [DOI] [PubMed] [Google Scholar]
- Särkinen TE, Marcelo-Peña JL, Yomona AD, Simon MF, Pennington RT, Hughes CE. Underestimated endemic species diversity in the dry inter-Andean valley of the Río Marañón, northern Peru: an example from Mimosa (Leguminosae: Mimosoideae) Taxon. 2011;60:139–150. [Google Scholar]
- Schluter D. The ecology of adaptive radiation. Oxford University Press; Oxford: 2000. [Google Scholar]
- Schneeweiss GM, Schönswetter P, Kelso S, Niklfeld H. Complex biogeographic patterns in Androsace (Primulaceae) and related genera: evidence from phylogenetic analyses of nuclear internal transcribed spacer and plastid trnL-F sequences. Systematic Biology. 2004;53:856–876. doi: 10.1080/10635150490522566. [DOI] [PubMed] [Google Scholar]
- Simpson GG. Evolution and geography: an essay on historical biogeography with special reference to mammals. Oregon State System of Higher Education; Eugene, OR: 1953. [Google Scholar]
- Struwe L, Smouse PE, Heiberg E, Haag S, Lathrop RG. Spatial evolutionary and ecological vicariance analysis (SEEVA), a novel approach to biogeography and speciation research, with an example from Brazilian Gentianaceae. Journal of Biogeography. 2011;38:1841–1854. [Google Scholar]
- Thorne JL, Kishino H. Divergence time and evolutionary rate estimation with multilocus data. Systematic Biology. 2002;51:689–702. doi: 10.1080/10635150290102456. [DOI] [PubMed] [Google Scholar]
- Tiffney BH. The Eocene North Atlantic land bridge: its importance in Tertiary and modern phytogeography of the Northern Hemisphere. Journal of the Arnold Arboretum. 1985;66:243–273. [Google Scholar]
- Valente LM, Savolainen V, Vargas P. Unparalleled rates of species diversification in Europe. Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences. 2010;277:1489–1496. doi: 10.1098/rspb.2009.2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeij GJ. Historical contingency and the purported uniqueness of evolutionary innovations. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:1804–1809. doi: 10.1073/pnas.0508724103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y-J, Li X-J, Hao G, Liu J-Q. Molecular phylogeny and biogeography of Androsace (Primulaceae) and the convergent evolution of cushion morphology. Acta Phytotaxonomica Sinica. 2004;42:481–499. [Google Scholar]
- Wen J. Evolution of eastern Asian and eastern North American disjunct distributions in flowering plants. Annual Review of Ecology and Systematics. 1999;30:421–455. [Google Scholar]
- Yang Z. PAML: a program package for phylogenetic analysis by maximum likelihood. Computer Applications in the Biosciences. 1997;13:555–556. doi: 10.1093/bioinformatics/13.5.555. [DOI] [PubMed] [Google Scholar]
- Yesson C, Toomey NH, Culham A. Cyclamen: time, sea and speciation biogeography using a temporally calibrated phylogeny. Journal of Biogeography. 2009;36:1234–1252. [Google Scholar]
- Yoder JB, Clancey E, Des Roches S, Eastman JM, Gentry L, Godsoe W, Hagey TJ, Jochimsen D, Oswald BP, Robertson J, Sarver BAJ, Schenk JJ, Spear SF, Harmon LJ. Ecological opportunity and the origin of adaptive radiations. Journal of Evolutionary Biology. 2010;23:1581–1596. doi: 10.1111/j.1420-9101.2010.02029.x. [DOI] [PubMed] [Google Scholar]
- Zachos JC, Dickens GR, Zeebe RE. An early Cenozoic perspective on greenhouse warming and carbon-cycle dynamics. Nature. 2008;451:279–283. doi: 10.1038/nature06588. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.