Abstract
IMPORTANCE
The latest generation of benchtop DNA sequencing platforms can provide an accurate whole-genome sequence (WGS) for a broad range of bacteria in less than a day. These could be used to more effectively contain the spread of multidrug-resistant pathogens.
OBJECTIVE
To compare WGS with standard clinical microbiology practice for the investigation of nosocomial outbreaks caused by multidrug-resistant bacteria, the identification of genetic determinants of antimicrobial resistance, and typing of other clinically important pathogens.
DESIGN, SETTING, AND PARTICIPANTS
A laboratory-based study of hospital inpatients with a range of bacterial infections at Cambridge University Hospitals NHS Foundation Trust, a secondary and tertiary referral center in England, comparing WGS with standard diagnostic microbiology using stored bacterial isolates and clinical information.
MAIN OUTCOMES AND MEASURES
Specimens were taken and processed as part of routine clinical care, and cultured isolates stored and referred for additional reference laboratory testing as necessary. Isolates underwent DNA extraction and library preparation prior to sequencing on the Illumina MiSeq platform. Bioinformatic analyses were performed by persons blinded to the clinical, epidemiologic, and antimicrobial susceptibility data.
RESULTS
We investigated 2 putative nosocomial outbreaks, one caused by vancomycin-resistant Enterococcus faecium and the other by carbapenem-resistant Enterobacter cloacae; WGS accurately discriminated between outbreak and nonoutbreak isolates and was superior to conventional typing methods. We compared WGS with standard methods for the identification of the mechanism of carbapenem resistance in a range of gram-negative bacteria (Acinetobacter baumannii, E cloacae, Escherichia coli, and Klebsiella pneumoniae). This demonstrated concordance between phenotypic and genotypic results, and the ability to determine whether resistance was attributable to the presence of carbapenemases or other resistance mechanisms. Whole-genome sequencing was used to recapitulate reference laboratory typing of clinical isolates of Neisseria meningitidis and to provide extended phylogenetic analyses of these.
CONCLUSIONS AND RELEVANCE
The speed, accuracy, and depth of information provided by WGS platforms to confirm or refute outbreaks in hospitals and the community, and to accurately define transmission of multidrug-resistant and other organisms, represents an important advance.
Antimicrobial resistance poses a grave threat to human health.1 Efforts to control the emergence and spread of drug-resistant organisms depend on rapid detection and effective treatment of cases combined with epidemiologic investigations and public health interventions to prevent ongoing transmission. Laboratory support for these activities begins with the detection, identification, and antimicrobial susceptibility testing of a pathogen, which are usually performed in a local diagnostic microbiology laboratory. For a minority of organisms, further identification, extended antimicrobial susceptibility testing, and/or confirmation of resistance mechanisms is required, which are commonly performed by reference laboratories. Such laboratories also perform molecular typing to assist epidemiologic investigations and public health surveillance, although current typing methods often lack sufficient resolution to allow accurate inferences regarding transmission events.2-11 Ideally, surveillance should be a dynamic process in which the flow of relevant information is monitored for a range of purposes, including the emergence of clinically significant drug resistance and detection of outbreaks.8 Currently, this information is gathered retrospectively, usually in response to an outbreak, and can fail to have an impact on disease control, as demonstrated by the recent Escherichia coli O104:H4 outbreak in Germany.12
The latest generation of benchtop DNA sequencing platforms can provide an accurate whole-genome sequence (WGS) for a broad range of bacteria in less than a day. There is increasing evidence that use of these techniques could enhance the provision of diagnostic and public health microbiologic analyses and support efforts to understand and contain the spread of microbial pathogens, including multidrug-resistant organisms. Herein, we compare WGS with standard clinical microbiology practice in England through a series of case studies that demonstrate the potential utility of WGS for the management and control of infectious diseases.
Methods
Study Setting and Participants
Cambridge University Hospitals NHS Foundation Trust (CUH) is an 1100-bed secondary and tertiary referral center in England. Patients at CUH with a range of bacterial infections were eligible for inclusion in the study. Written informed consent was not required because patients received routine clinical care, and there were no additional specimens collected or study-specific interventions. Ethical approval was not required because the study was conducted as part of surveillance and infection control management. Research and development (R&D) approval for WGS was granted by the R&D Department at CUH.
Laboratory Methods
Clinical specimens were collected during routine clinical care and processed and stored at the Clinical Microbiology and Public Health Laboratory, Cambridge. Antimicrobial susceptibility testing was performed using disk diffusion techniques.13 Isolates were referred to a reference laboratory for additional testing, as required. Relevant reference laboratory testing is described in the eMethods in the Supplement. Isolates used in this study were recultured from frozen stocks maintained at −80°C and assigned anonymous identification numbers. DNA was extracted using the QIAamp DNA Mini Kit (Qiagen). DNA fragment libraries were prepared using the Nextera Kit (Illumina Inc), and 150-bp paired end DNA sequences were determined using the Illumina MiSeq (Illumina Inc), as previously described.7 Genome sequence data were analyzed at the Wellcome Trust Sanger Institute by an investigator who was blinded to all clinical, epidemiologic, and antimicrobial susceptibility data. Additional methodologic details are provided in eMethods in the Supplement. Genome sequence data have been deposited in the European Nucleotide Archive (eTable 1 in the Supplement).
Results
Carbapenem-Resistant Enterobacter cloacae Outbreak Investigation
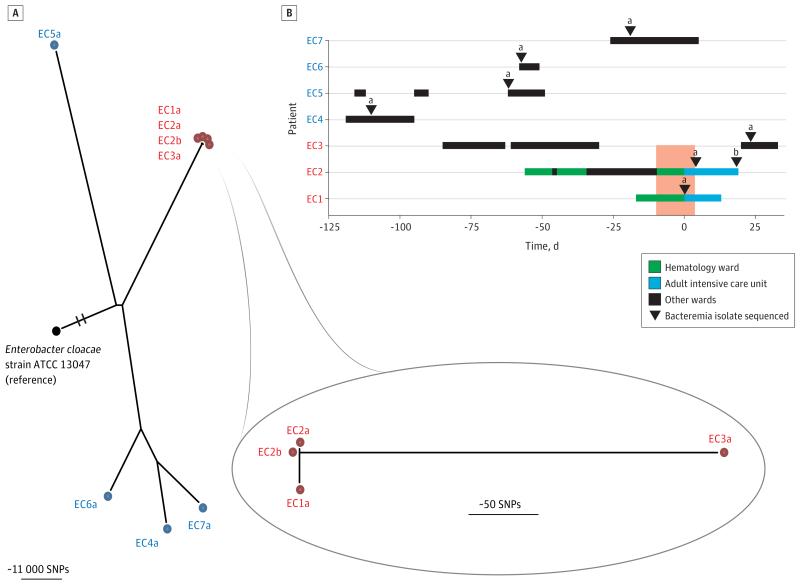
Enterobacter cloacae is a nosocomial pathogen that can inactivate third-generation cephalosporins through the overproduction of a chromosomally encoded AmpC β-lactamase enzyme,14 which has led to the use of carbapenems for E cloacae infections. Therapeutic options have become further limited by the emergence of acquired carbapenemase-producing strains of Enterobacteriaceae, a global public health concern.15 A suspected outbreak of carbapenem-resistant E cloacae was investigated at CUH,16 the timeline for which is illustrated in Figure 1. A patient (EC1) was admitted to the hematology unit for autologous stem cell transplantation. Nine days after transplantation, the patient developed neutropenic sepsis with multiorgan failure and was transferred to the intensive care unit (ICU). Blood cultures grew E cloacae (EC1a) resistant to ertapenem, meropenem, gentamicin, and ciprofloxacin and susceptible to amikacin and colistin. Despite treatment with amikacin and subsequent negative blood cultures, the patient died on day 12 of ICU admission. A second hematology patient (EC2), a previous allogeneic bone marrow transplant recipient, was admitted to the ICU on the same day as patient EC1. Four days later, this patient developed bacteremia with E cloacae (EC2a) that had the same antibiotic susceptibility pattern (antibiogram) as EC1a. Subsequent blood cultures from patient EC2 remained positive, growing a mixture of E cloacae (EC2b) and vancomycin-resistant Enterococcus faecium (VREF) despite treatment with tigecycline and amikacin. The patient died on day 18 of ICU admission. Both patients were nursed in separate side rooms on the hematology ward and ICU. Twenty-three days after the index case bacteremia, E cloacae (EC3a) was isolated from the urine of a renal transplant recipient (EC3) on the renal transplant ward. The antibiogram was the same as those of EC1a, EC2a, and EC2b with the exception of ciprofloxacin, to which EC3a was susceptible. All 3 isolates were sent to the reference laboratory shortly after isolation, where they were found to produce the IMP-1 metallo-carbapenemase enzyme and to be indistinguishable by pulsed-field gel electrophoresis (PFGE) typing.16 Screening of patients for rectal carriage of carbapenem-resistant E cloacae was instigated in the ICU and hematology and renal wards, but no further cases were detected.
Figure 1. Enterobacter cloacae Outbreak Investigation.
A, Phylogenetic tree based on whole-genome sequencing analysis. The whole genome sequence of E cloacae ATCC 13047 was used as the mapping reference. Putative outbreak isolate genomes are shown in red, controls in blue, and the reference isolate in black. The reference genome branch is truncated for clarity. The inset shows a close-up of the suspected outbreak isolates in which the EC1a genome was used as the mapping reference. We concluded that patients EC1 (the index case) and EC2 were involved in the outbreak, but that patient EC3 was unlikely to have been associated with a transmission event from patient EC1 or EC2. SNP indicates single-nucleotide polymorphism. B, Epidemiologic map of 7 patients with E cloacae bacteremia. EC1, EC2, and EC3 (in red) represent patients involved in an infection control investigation, and EC4 through EC7 (in blue) represent patients not linked to the putative outbreak. Day 0 denotes the day of bacteremia in the index case (EC1). One isolate from each patient was sequenced (denoted by the lowercase letter a), with the exception of patient EC2 who had 2 isolates sequenced, a and b (ie, EC2a and EC2b, from blood samples taken 14 days apart). The vertical dark pink block indicates when EC1 and EC2 were both on the same ward.
We investigated this putative outbreak by sequencing the 4 E cloacae isolates (EC1a, EC2a, EC2b, EC3a) from the 3 patients. We also sequenced 4 E cloacae bacteremia isolates (EC4a, EC5a, EC6a, EC7a) from 4 patients admitted to unrelated wards prior to the outbreak, which were susceptible to the carbapenem drugs and acted as controls (Figure 1B). Sequence data were initially analyzed to identify single-nucleotide polymorphisms (SNPs) relative to a reference genome (E cloacae ATCC13047),17 from which a phylogenetic tree was reconstructed (Figure 1A). This showed that the putative outbreak isolates were more closely related to each other than to the control isolates, and that each of the control isolates was distantly related, which is consistent with the epidemiologic information.
This analysis was problematic, however, since the long branch lengths of this tree (>400 000 SNPs between each isolate and the reference) precluded further analysis within the suspected outbreak cluster because SNP identification by mapping to a divergent reference underreports variation and reduces resolution. To address this, the genome of the earliest isolate (EC1a) was assembled de novo and used as the reference for read mapping. The resulting phylogeny showed that EC1a, EC2a, and EC2b were separated from one another by less than 22 SNPs, whereas EC3a was distinctly positioned at a distance greater than 150 SNPs (Figure 1A, inset). These phylogenetic relationships indicated that EC1a and EC2a/b were closely related and, coupled with the epidemiologic data, can be interpreted to indicate that transmission was likely between patients EC1 and EC2. An alternative explanation is that both acquired their isolate from a third patient who acted as a common source, although there was no epidemiologic evidence to support this.
Although EC3a was genetically distant from EC1a and EC2a, we explored whether it was still possible that EC3a was related to the outbreak. The introduction of a gene fragment from another bacterium through homologous recombination can introduce numerous mutations in a single event and can result in a raised SNP number between 2 otherwise highly related isolates. This did not appear to be the case, since manual inspection of the EC3a genome failed to demonstrate the presence of SNPs in high-density clusters. An alternative mechanism for a higher rate of mutation is a defect in the DNA proofreading mismatch repair pathway, but inspection of SNPs within the relevant pathway (mutS, mutL, mutY) revealed no evidence of a hypermutator genotype for EC3a. We propose, therefore, that the SNP differences were indicative of true phylogenetic distance. This suggests that despite indistinguishable PFGE and IMP types reported by the reference laboratory, patient EC3 was unlikely to have been associated with a transmission event involving patient EC1 or EC2. However, a more in-depth understanding of the population structure of E cloacae is required to interpret these genetic distances with greater confidence, particularly in light of the possibility of horizontal transfer of the IMP metallo-carbapenemase, which can be carried on a plasmid.18
VREF Outbreak Investigation
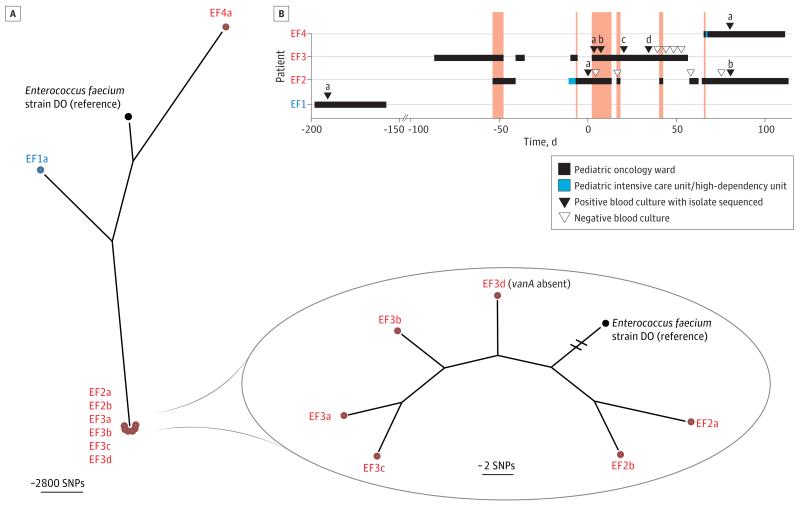
Vancomycin-resistant E faecium is an important nosocomial pathogen with a global distribution19 and is clinically important because infections caused by vancomycin-resistant enterococci are more difficult to treat than those caused by vancomycin-susceptible enterococci. A cluster of VREF bacteremias occurred in 3 children with hematologic malignancies (EF2, EF3, and EF4) undergoing treatment in the pediatric oncology ward at CUH over a period of 11 weeks. A timeline for these patients is shown in Figure 2. Patient EF2 was the first case in the cluster and was successfully treated for VREF bacteremia caused by bacterial isolate EF2a before developing a second infection (EF2b) 11 weeks later.
Figure 2. Enterococcus faecium Outbreak Investigation.
A, Phylogenetic tree based on whole-genome sequencing analysis. The whole genome sequence of E faecium DO was used as the mapping reference. The genomes of putative outbreak isolates are shown in red, the control in blue, and reference isolate in black. The inset shows a close-up of the suspected outbreak isolates in which the reference genome branch is truncated for clarity. We concluded that patient EF4 was excluded from the outbreak involving patients EF2 and EF3. SNP indicates single-nucleotide polymorphism. B, Epidemiologic map of 4 patients with E faecium bacteremia. EF2, EF3, and EF4 (in red) represent patients involved in an infection control investigation, and EF1 (in blue) represents a patient with bacteremia on the same ward but not linked to the putative outbreak. Day 0 denotes the day of first bacteremia in EF2. The 8 sequenced isolates from 4 patients are labeled a, b, c, and d. The vertical dark pink blocks indicate when patients EF2, EF3, and/or EF4 were present on the same ward at the same time. With the exception of EF3d, all isolates were vancomycin resistant.
The second case was patient EF3 who had VREF (EF3a) isolated from a blood culture taken 3 days after the first episode in patient EF2. Patient EF3 had a poor response to antimicrobial therapy and had a further 2 blood cultures that were positive for VREF in the next 17 days (EF3b, and EF3c) followed by a blood culture positive for vancomycin-susceptible E faecium (EF3d) 14 days after isolate EF3c was isolated.
The third case was patient EF4, who had VREF (EF4a) isolated from a blood culture on the same day as the second episode in patient EF2. The overlap in time and place between patients EF2, EF3, and EF4 suggested that transmission of VREF may have occurred, and the investigating infection control team referred 6 isolates (EF2b, EF3a, EF3b, EF3c, EF3d, and EF4a) in 2 batches to the reference laboratory for typing by PFGE. Results received 10 to 14 days after isolate referral revealed that 5 isolates (EF2b, EF3a, EF3b, EF3c, and EF3d) were the same PFGE type (CAMPBEC-6) but that EF4a was distinct.
We sequenced the 7 E faecium isolates from these 3 patients (EF2a/b, EF3a/b/c/d, and EF4a) together with a VREF isolate (EF1a) from a blood culture taken from a fourth unrelated child (EF1) who was cared for on the same ward and developed VREF bacteremia 7 months before the suspected outbreak. Sequence data were analyzed to identify SNPs relative to a reference genome (E faecium strain DO),20 from which a phylogenetic tree was reconstructed (Figure 2A). The 6 isolates from patients EF2 and EF3 formed a closely related cluster, whereas isolates from patients EF1 and EF4 occupied distinct branches of the tree. This confirmed the reference laboratory results that the isolate from patient EF4 was unrelated to those from patients EF2 and EF3, despite the overlap in time and place between EF2 and EF4.
Closer inspection of the outbreak cluster (Figure 2A, inset) revealed that isolates from patients EF2 and EF3 did not fall into distinct populations. Notably, this included the vancomycin-susceptible EF3d, which was found to lack the vancomycin resistance gene (vanA). Furthermore, the phylogenetic position of the 4 isolates from patient EF3 did not reflect the time of isolation, suggesting that these isolates represented sampling from a larger underlying population. Because of this apparent “cloud of diversity,” it was not possible to determine whether the second episode of VREF bacteremia in patient EF2 (EF2b) was due to relapse by the same isolate (EF2a), or reinfection from patient EF3. Higher density of sampling within individuals and greater representation of closely related isolates will be required to fully understand the extent of genetic diversity within a given individual.
Identification of the Mechanism of Carbapenem Resistance in Gram-Negative Bacteria
Standard, inexpensive assays for antimicrobial susceptibility testing (eg, disk diffusion or automated liquid culture assay) are usually able to detect carbapenem resistance but cannot reliably distinguish carbapenemase producers from other resistance mechanisms (eg, extended-spectrum β-lactamase [ESBL] or AmpC production in combination with outer-membrane porin loss) that are considered less likely to be horizontally transmitted between bacteria.21 Instead, results of phenotypic testing (most often by reference laboratories) using a comprehensive panel of antibiotics including inhibitor combinations are used to infer the likely resistance mechanism. When carbapenemase production is suspected, targeted multiplex polymerase chain reaction assays are used to confirm the genetic identity of the resistance determinant.22 We identified 5 carbapenem-resistant gram-negative bacterial isolates in our laboratory that were sent to the reference laboratory for further characterization: Acinetobacter baumannii (strain AB223), E cloacae (EC1a from the outbreak described herein and EC302), E coli (Eco216), and Klebsiella pneumoniae (KP652).
We performed WGS and bioinformatic analyses in which sequence data were mapped against an assembly of reference resistance genes as described in the eMethods in the Supplement and found that only 2 of the 5 isolates harbored carbapenemases (Table 1): OXA-23 in the A baumannii isolate and IMP-1 in the E cloacae isolate (EC1a). The E coli and K pneumoniae isolates both contained the epidemic CTX-M-15 ESBL coupled with loss of function of an outer-membrane porin, which together are associated with ertapenem resistance.23 The fifth isolate was an E cloacae that lacked an outer-membrane porin but did not have an acquired β-lactamase, indicating hyperproduction of the chromosomal AmpC, as predicted by the reference laboratory. This was not subjected to further bioinformatic analysis because the genetic basis of this phenotype is complex (ie, upregulation of AmpC is not caused by easily identifiable loss-of-function mutations via nonsense mutations, frameshifts, or insertion elements, as is the case for porin mutants24).
Table 1. Comparison ofWGS and Reference Laboratory Testing of Carbapenem-Resistant Gram-Negative Bacteria.
| Organism | Isolate No. |
Phenotypic Resistance to Carbapenems and Third-Generation Cephalosporins |
Attributable Resistance Mechanism According to Reference Laboratorya |
Dominant Resistance Mechanism Based on WGSb |
|---|---|---|---|---|
| Acinetobacter baumannii | AB223 | MEM, IPMc | OXA-23 carbapenemase | OXA-23 carbapenemase |
| Enterobacter cloacae | EC1ad | ETP, MEM, IPM, CTX, CAZ | IMP-1 carbapenemase | IMP-1 carbapenemase |
| E cloacae | EC302 | ETP, CTX, CAZ | No carbapenemase genes detected. AmpC activity present |
No carbapenemase genes detected. OmpF porin loss |
| Klebsiella pneumoniae | KP652 | ETP, CTX, CAZ | No carbapenemase genes detected. ESBL activity consistent with CTX-M. ETP resistance consistent with porin loss |
No carbapenemase genes detected. CTX-M-15 ESBL with OmpK36 porin loss |
| Escherichia coli | Eco216 | ETP, CTX, CAZ | No carbapenemase genes detected. ESBL activity present. ETP resistance consistent with porin loss |
No carbapenemase genes detected. CTX-M-15 ESBL with OmpF porin loss |
Abbreviations: CAZ, ceftazidime; CTX, cefotaxime; ESBL, extended-spectrum β-lactamase; IPM, imipenem; ETP, ertapenem; MEM, meropenem;WGS, whole-genome sequencing.
For a more comprehensive list see eMethods and eTable 2 in the Supplement.
Ertapenem has no activity against A baumannii; no EUCAST (European Committee on Antimicrobial Susceptibility Testing) susceptibility breakpoints available for cefotaxime and ceftazidime.
Isolate from patient EC1 described in E cloacae outbreak.
The results obtained by WGS were concordant with the reference laboratory results with respect to the detection of carbapenemase and ESBL genes and porin loss (Table 1). Whole-genome sequencing yielded additional information by identifying the precise subtype of the resistance gene in question and providing a list of resistance determinants beyond the dominant determinants implicated in carbapenem resistance (eg, the SHV-12 ESBL in EC1a was phenotypically masked by the presence of IMP; see eTable 2 in the Supplement). The complexity of the genetic basis of AmpC hyperproduction and the risk that WGS could miss a novel resistance mechanism highlights the importance of concurrent phenotypic susceptibility testing. Nonetheless, WGS performed in a local laboratory would provide rapid results that could inform infection control decisions in real time. The genomic data can also be used for epidemiologic tracking to investigate onward transmission, as illustrated by the E cloacae outbreak described above.
Typing of Neisseria meningitidis
Meningococcal disease is a global public health problem, affecting between 500 000 and 1.2 million people per year.25 Typing of Neisseria meningitidis isolated from patients with meningococcal disease informs outbreak investigations and national surveillance programs. N meningitidis has been classified into 13 serogroups based on the immunogenicity of the polysaccharide capsule, although 6 serogroups (A, B, C, W135, X, and Y) cause almost all cases of life-threatening disease.26 Further classification schemes have been developed using serologic or DNA-based approaches to detect variants in 4 distinct surface proteins (PorA, PorB, FetA, FHbp) and the surface lipooligosaccharide, each with differing resolving power and frequency of utilization.27 Multilocus sequence typing (MLST) has more recently been accepted as the gold standard for epidemiologic typing and has been used to define the main hypervirulent lineages.28
Seven patients with microbiologically confirmed meningococcal disease were admitted to CUH over a period of 2 years, 4 with meningococcal septicemia and 3 with meningitis plus septicemia (Table 2). Four of these patients presented within 30 days of each other. Contact tracing and antimicrobial prophylaxis of close contacts was carried out by Public Health England, but no epidemiologic links were made between these cases. One isolate from each of the 7 cases (6 from blood culture and 1 from cerebrospinal fluid) were sent to the reference laboratory at the time of isolation for additional testing (serogroup, serotype, and serosubtype) (Table 2).
Table 2. Summary of Clinical Features, and Reference Laboratory, and Whole Genome Sequencing Results for Neisseria meningitidis Isolates.
| Reference Laboratory Results |
WGS Results |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Patient | Clinical Features | Serogroupa | Serotypeb | Serosubtypec | Serogroup | porB | porA d | fetA e | fHbp e |
| NM1 | Meningitis, septicemia | Y | NT | 5-1/2-2/36-2 | Y | 243f | 5-1/2-2 | 5-8 | 25 |
|
| |||||||||
| NM2 | Septicemia | B | 4 | 7-2/4/37 | B | 303g | 7-2/4 | 3-28 | 4 |
|
| |||||||||
| NM3 | Meningitis, septicemia | B | NT | 22/9/35-1 | B | 198 | 22/9 | 3-3 | 19 |
|
| |||||||||
| NM4 | Septicemia | B | 1 | 7-1/1/35-1 | B | 46 | 7-1/1 | 5-28 | 13 |
|
| |||||||||
| NM5 | Septicemia | B | 4 | 12-1/16-65/37-1 | B | 42 | 12-1/16-65 | 1-72/1-5g | 4 |
|
| |||||||||
| NM6 | Meningitis, septicemia | W135 | NT | 5/2/36-2 | W135 | 2 | 5/2 | 4-1 | 16 |
|
| |||||||||
| NM7 | Septicemia | W135 | NT | 18-1/3/38 | W135 | 2 | 18-1/3 | 4-1 | 16 |
Abbreviations: NT, nontypable; PCR, polymerase chain reaction; WGS, whole-genome sequencing.
Identification of capsular polysaccharide antigens by serologic reaction or PCR assays.
Identification of class 2/3 (PorB) outer membrane proteins by dot-blot enzyme-linked immunosorbent assay using monoclonal antibodies.
Identification of class 1 (PorA) outer membrane proteins by PCR and DNA sequencing.
The reference laboratory serosubtype is based on sequencing of 3 variable regions of porA, but the Neisseria BIGSdb database (http://pubmlst.org/neisseria/) does not report on variable region 3.
Molecular subtyping based on fetA and fHbp genes is not routinely performed by reference laboratory.
Closest hit with gaps.
Sequence assembly broken; gene split onto 2 continuous regions of DNA sequence.
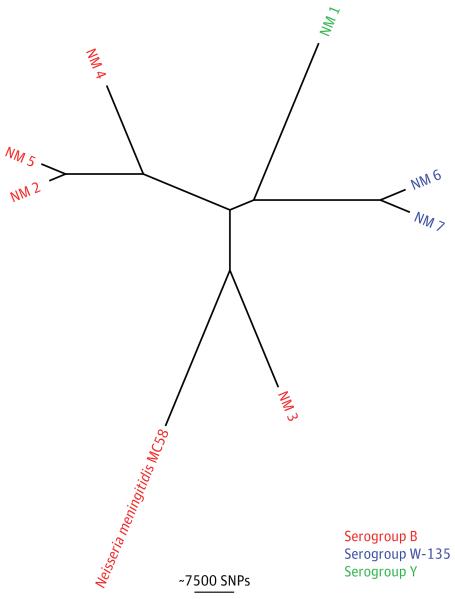
We performed WGS and bioinformatic analysis of the 7 isolates with the aim of recapitulating and extending the reference laboratory testing. The PubMLST Neisseria BIGSdb database (http://pubmlst.org/neisseria/) was used to type the PorA variable region (VR1 and VR2), porB allele, FetA variable region, and fHbp allele (Table 2). This confirms previous reports that sequence data can be used to extract and analyze a specific gene subset.29,30 The capsular type was derived from the sequence data by comparison with a pseudomolecule consisting of csa, csb, csc, csw, and csy genes (see eMethods in the Supplement), which demonstrated concordance with the reference laboratory results (Table 2 and eFigure 1 in the Supplement). These single-locus analyses were extended by using the WGS data to reconstruct a phylogenetic tree based on SNPs compared with the reference genome N meningitidis MC58 (Figure 3). The large phylogenetic distances between the N meningitidis isolates in this study did not support any link between these patients, which is consistent with the epidemiologic data.
Figure 3. Neisseria meningitidis Phylogenetic Analysis.
Phylogenetic tree based on whole-genome sequencing analysis. The whole genome sequence of N meningitidis MC58 was used as the mapping reference. Isolates are color-coded based on the serogroup, which was derived from the sequence data. Phylogenetic distances between isolates do not support a link between any of these cases, which is consistent with the findings of an epidemiologic investigation. SNP indicates single-nucleotide polymorphism.
Discussion
The structure of diagnostic microbiology services is a historical compromise between clinical need, cost, and technological advances. Local or regional microbiology laboratories can offer tests that cover most clinically relevant questions, but the questions addressed in this study required more specialized tests. Because the pathogens or antibiotic resistance classes in our study are relatively rare in England, the costs for re-agents, equipment, and staff to run the associated specialized tests are prohibitive for local laboratories.31 In contrast, the cost per sample is reduced at the reference laboratory by collecting samples from across the country, but this is at the expense of turnaround time. As a result, many reference tests are not provided with sufficient speed to inform individual patient treatment or contemporary infection control decisions, and their value is limited to retrospective epidemiology and surveillance.32,33
Whole-genome sequencing offers a potential solution to this problem in routine diagnostic laboratories once sequence analysis has become fully automated.8 Sequencing is a common process regardless of the organism, and multiple rare (as well as common) pathogens could be sequenced simultaneously in a single sequencing run. This means that WGS could allow economies of scale at local or regional laboratories while providing equivalent or superior-quality information and reducing turnaround times. Furthermore, a single WGS can often replace several existing tests carried out on the same isolate at the reference laboratory (eg, we used the same data set to investigate the mechanism of carbapenem resistance in E cloacae as well as onward transmission). Moreover, regular updates of analysis software tools and reference databases would allow local laboratories to respond to new threats (eg, the discovery of a novel carbapenemase34), equipping diagnostic services with an unprecedented degree of flexibility.8
A transition to the use of WGS will not make reference laboratories obsolete but instead will provide the capability to monitor in real time the spread of pathogens across a country and beyond.8 This would allow public health micro-biology to become more proactive as opposed to reactive by identifying unusual isolates earlier, which could then be requested and studied by reference laboratories to gain vital insights before they cause major outbreaks.8 To make such a transition, it will first be necessary to understand the population structure of significant pathogenic species and lineages, both on a national scale and within an individual patient and specimen. This will provide the necessary context to the analysis of WGS data, the need for which was highlighted by the E cloacae and E faecium outbreaks detailed in the present study. Moreover, improvements in sample preparation techniques to enable WGS directly from single colonies from primary isolation plates are called for to eliminate the need to subculture pathogens to obtain sufficient DNA for sequencing.8,35,36 Automated sequence interpretation tools will also be required to achieve rapid turnaround times and dissemination of the technology to nonexpert settings.8 Prospective studies are also required to understand the cost vs benefit for individual patient management and public health.
Supplementary Material
Acknowledgments
Conflict of Interest Disclosures: Dr Ellington was funded by Bruker Daltonics to attend a conference. Dr Török has received speaker honoraria and book royalties from Oxford University Press and funding for travel and accommodation from Illumina Inc. Dr Brown is a consultant for Astellas Pharma Ltd and Discuva Ltd. Dr Smith is an employee and shareholder of Illumina Inc. Dr Parkhill has received funding for travel and accommodation from Pacific Biosciences Inc. and Illumina Inc. Dr Peacock is a consultant for Pfizer Inc.
Funding/Support: This work was supported by grants from the UKCRC Translational Infection Research Initiative and the Medical Research Council (G1000803) with contributions to the grant from the Biotechnology and Biological Sciences Research Council, the National Institute for Health Research on behalf of the Department of Health, and the Chief Scientist Office of the Scottish Government Health Directorate (Peacock); the Wellcome Trust (098051) awarded to the Wellcome Trust Sanger Institute; Public Health England (Peacock); and the NIHR Cambridge Biomedical Research Centre (Cartwright, Török, Bentley, Peacock).
Footnotes
Supplemental content at jamainternalmedicine.com
REFERENCES
- 1.Spellberg B, Bartlett JG, Gilbert DN. The future of antibiotics and resistance. N Engl J Med. 2013;368(4):299–302. doi: 10.1056/NEJMp1215093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hall BG, Ehrlich GD, Hu FZ. Pan-genome analysis provides much higher strain typing resolution than multilocus sequence typing. Microbiology. 2010;156(Pt 4):1060–1068. doi: 10.1099/mic.0.035188-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hiller NL, Ahmed A, Powell E, et al. Generation of genic diversity among Streptococcus pneumoniae strains via horizontal gene transfer during a chronic polyclonal pediatric infection. PLoS Pathog. 2010;6(9):e1001108. doi: 10.1371/journal.ppat.1001108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gardy JL, Johnston JC, Ho Sui SJ, et al. Whole-genome sequencing and social-network analysis of a tuberculosis outbreak. N Engl J Med. 2011;364(8):730–739. doi: 10.1056/NEJMoa1003176. [DOI] [PubMed] [Google Scholar]
- 5.Didelot X, Bowden R, Wilson DJ, Peto TE, Crook DW. Transforming clinical microbiology with bacterial genome sequencing. Nat Rev Genet. 2012;13(9):601–612. doi: 10.1038/nrg3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hu FZ, Eutsey R, Ahmed A, et al. In vivo capsular switch in Streptococcus pneumoniae: analysis by whole genome sequencing. PLoS One. 2012;7(11):e47983. doi: 10.1371/journal.pone.0047983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Köser CU, Holden MT, Ellington MJ, et al. Rapid whole-genome sequencing for investigation of a neonatal MRSA outbreak. N Engl J Med. 2012;366(24):2267–2275. doi: 10.1056/NEJMoa1109910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Köser CU, Ellington MJ, Cartwright EJ, et al. Routine use of microbial whole genome sequencing in diagnostic and public health microbiology. PLoS Pathog. 2012;8(8):e1002824. doi: 10.1371/journal.ppat.1002824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McAdam PR, Templeton KE, Edwards GF, et al. Molecular tracing of the emergence, adaptation, and transmission of hospital-associated methicillin-resistant Staphylococcus aureus. Proc Natl Acad Sci U S A. 2012;109(23):9107–9112. doi: 10.1073/pnas.1202869109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Young BC, Golubchik T, Batty EM, et al. Evolutionary dynamics of Staphylococcus aureus during progression from carriage to disease. Proc Natl Acad Sci U S A. 2012;109(12):4550–4555. doi: 10.1073/pnas.1113219109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harris SR, Cartwright EJ, Török ME, et al. Whole-genome sequencing for analysis of an outbreak of meticillin-resistant Staphylococcus aureus: a descriptive study. Lancet Infect Dis. 2013;13(2):130–136. doi: 10.1016/S1473-3099(12)70268-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rohde H, Qin J, Cui Y, et al. E. coli O104:H4 Genome Analysis Crowd-Sourcing Consortium. Open-source genomic analysis of Shiga-toxin-producing E. coli O104:H4. N Engl J Med. 2011;365(8):718–724. doi: 10.1056/NEJMoa1107643. [DOI] [PubMed] [Google Scholar]
- 13.British Society of Antimicrobial Chemotherapy [Accessed May 14, 2013];BSAC Methods for Antimicrobial Susceptibility Testing. 2012 May; http://bsac.org.uk/
- 14.Jacoby GA. AmpC beta-lactamases. Clin Microbiol Rev. 2009;22(1):161–182. doi: 10.1128/CMR.00036-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Walsh TR. Emerging carbapenemases: a global perspective. Int J Antimicrob Agents. 2010;36(suppl 3):S8–S14. doi: 10.1016/S0924-8579(10)70004-2. [DOI] [PubMed] [Google Scholar]
- 16.Shet V, Gouliouris T, Brown NM, Turton JF, Zhang J, Woodford N. IMP metallo-β-lactamase-producing clinical isolates of Enterobacter cloacae in the UK. J Antimicrob Chemother. 2011;66(6):1408–1409. doi: 10.1093/jac/dkr078. [DOI] [PubMed] [Google Scholar]
- 17.Ren Y, Ren Y, Zhou Z, et al. Complete genome sequence of Enterobacter cloacae subsp. cloacae type strain ATCC 13047. J Bacteriol. 2010;192(9):2463–2464. doi: 10.1128/JB.00067-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mathers AJ, Cox HL, Kitchel B, et al. Molecular dissection of an outbreak of carbapenem-resistant Enterobacteriaceae reveals intergenus KPC carbapenemase transmission through a promiscuous plasmid. MBio. 2011;2(6):e00204–e00211. doi: 10.1128/mBio.00204-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Willems RJ, van Schaik W. Transition of Enterococcus faecium from commensal organism to nosocomial pathogen. Future Microbiol. 2009;4(9):1125–1135. doi: 10.2217/fmb.09.82. [DOI] [PubMed] [Google Scholar]
- 20.Qin X, Galloway-Peña JR, Sillanpaa J, et al. Complete genome sequence of Enterococcus faecium strain TX16 and comparative genomic analysis of Enterococcus faecium genomes. BMC Microbiol. 2012;12(1):135. doi: 10.1186/1471-2180-12-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Health Protection Agency [Accessed March 17, 2013];Advice on carbapenemase producers: recognition, infection control and treatment. http://www.hpa.org.uk/webc/HPAwebFile/HPAweb_C/1294740725984
- 22.Ellington MJ, Kistler J, Livermore DM, Woodford N. Multiplex PCR for rapid detection of genes encoding acquired metallo-β-lactamases. J Antimicrob Chemother. 2007;59(2):321–322. doi: 10.1093/jac/dkl481. [DOI] [PubMed] [Google Scholar]
- 23.Doumith M, Ellington MJ, Livermore DM, Woodford N. Molecular mechanisms disrupting porin expression in ertapenem-resistant Klebsiella and Enterobacter spp. clinical isolates from the UK. J Antimicrob Chemother. 2009;63(4):659–667. doi: 10.1093/jac/dkp029. [DOI] [PubMed] [Google Scholar]
- 24.Hanson ND, Sanders CC. Regulation of inducible AmpC beta-lactamase expression among Enterobacteriaceae. Curr Pharm Des. 1999;5(11):881–894. [PubMed] [Google Scholar]
- 25.Chang Q, Tzeng YL, Stephens DS. Meningococcal disease: changes in epidemiology and prevention. Clin Epidemiol. 2012;4:237–245. doi: 10.2147/CLEP.S28410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stephens DS, Greenwood B, Brandtzaeg P. Epidemic meningitis, meningococcaemia, and Neisseria meningitidis. Lancet. 2007;369(9580):2196–2210. doi: 10.1016/S0140-6736(07)61016-2. [DOI] [PubMed] [Google Scholar]
- 27.Stephens DS. Biology and pathogenesis of the evolutionarily successful, obligate human bacterium Neisseria meningitidis. Vaccine. 2009;27(suppl 2):B71–B77. doi: 10.1016/j.vaccine.2009.04.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maiden MC, Bygraves JA, Feil E, et al. Multilocus sequence typing: a portable approach to the identification of clones within populations of pathogenic microorganisms. Proc Natl Acad Sci U S A. 1998;95(6):3140–3145. doi: 10.1073/pnas.95.6.3140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vogel U, Szczepanowski R, Claus H, Jünemann S, Prior K, Harmsen D. Ion torrent personal genome machine sequencing for genomic typing of Neisseria meningitidis for rapid determination of multiple layers of typing information. J Clin Microbiol. 2012;50(6):1889–1894. doi: 10.1128/JCM.00038-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jolley KA, Hill DM, Bratcher HB, et al. Resolution of a meningococcal disease outbreak from whole-genome sequence data with rapid web-based analysis methods. J Clin Microbiol. 2012;50(9):3046–3053. doi: 10.1128/JCM.01312-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mosammaparast N, McAdam AJ, Nolte FS. Molecular testing for infectious diseases should be done in the clinical microbiology laboratory. J Clin Microbiol. 2012;50(6):1836–1840. doi: 10.1128/JCM.00488-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Health Protection Agency [Accessed March 17, 2013];Meningococcal reference unit user manual. 2012 Apr; http://www.hpa.org.uk/webc/HPAwebFile/HPAweb_C/1194947367872
- 33.Health Protection Agency [Accessed March 17, 2013];Department of healthcare associated infection and antibiotic resistance user manual (effective date: 13.02.13) http://www.hpa.org.uk/webc/HPAwebFile/HPAweb_C/1194947406124
- 34.Kumarasamy KK, Toleman MA, Walsh TR, et al. Emergence of a new antibiotic resistance mechanism in India, Pakistan, and the UK: a molecular, biological, and epidemiological study. Lancet Infect Dis. 2010;10(9):597–602. doi: 10.1016/S1473-3099(10)70143-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Adey A, Morrison HG, Asan, et al. Rapid, low-input, low-bias construction of shotgun fragment libraries by high-density in vitro transposition. Genome Biol. 2010;11(12):R119. doi: 10.1186/gb-2010-11-12-r119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Eyre DW, Golubchik T, Gerdon NC, et al. A pilot study of rapid benchtop sequencing of Staphylococcus aureus and Clostridium difficile for outbreak detection and surveillance. BMJ Open. 2012;2(3):e001124. doi: 10.1136/bmjopen-2012-001124. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.