Abstract
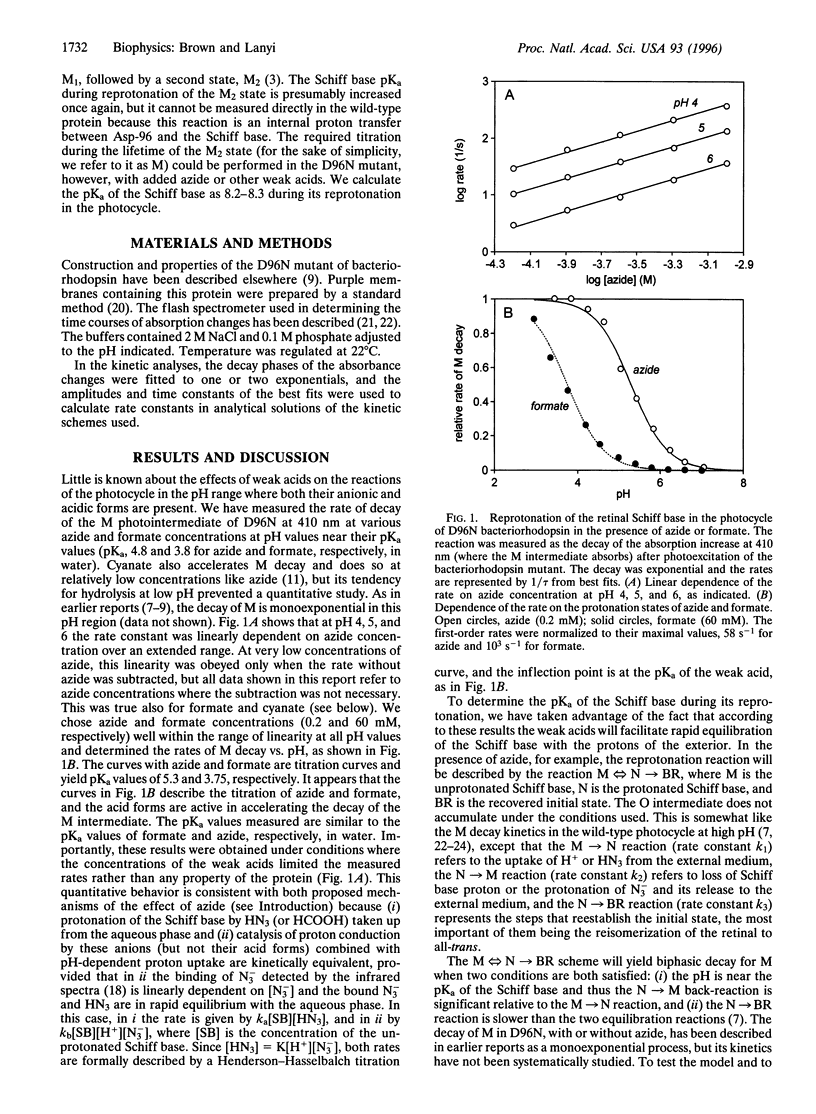
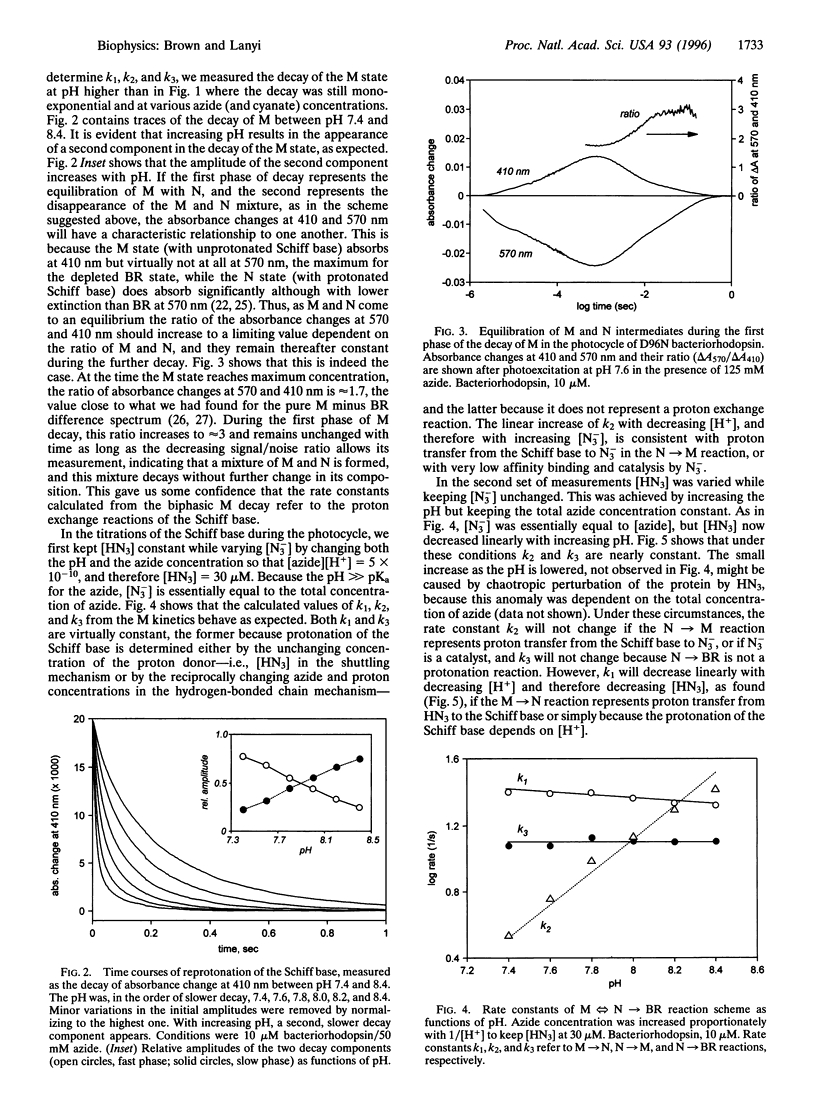
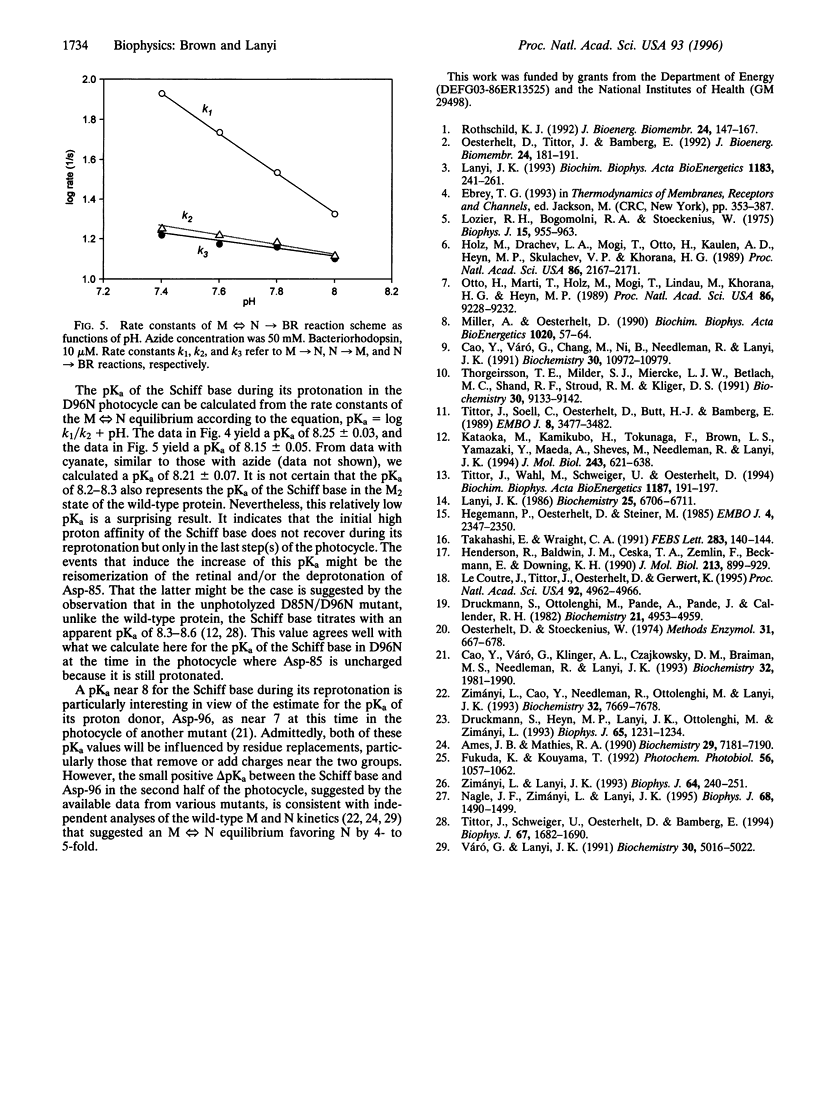
Reprotonation of the transiently deprotonated retinal Schiff base in the bacteriorhodopsin photocycle is greatly slowed when the proton donor Asp-96 is removed with site-specific mutagenesis, but its rate is restored upon adding azide or other weak acids such as formate and cyanate. As expected, between pH 3 and 7 the rate of Schiff base protonation in the photocycle of the D96N mutant correlates with the concentrations of the acid forms of these agents. Dissection of the rates in the biexponential reprotonation kinetics of the Schiff base between pH 7 and 9 yielded calculated rate constants for the protonation equilibrium. Their dependencies on pH and azide or cyanate concentrations are consistent with both earlier suggested mechanisms: (i) azide and other weak acids may function as proton carriers in the protonation equilibrium of the Schiff base, or (ii) the binding of their anionic forms may catalyze proton conduction to and from the Schiff base. The measured rate constants allow the calculation of the pKa of the Schiff base during its reprotonation in the photocycle of D96N. It is 8.2-8.3, a value much below the pKa determined earlier in unphotolyzed bacteriorhodopsin.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames J. B., Mathies R. A. The role of back-reactions and proton uptake during the N----O transition in bacteriorhodopsin's photocycle: a kinetic resonance Raman study. Biochemistry. 1990 Aug 7;29(31):7181–7190. doi: 10.1021/bi00483a005. [DOI] [PubMed] [Google Scholar]
- Cao Y., Váró G., Chang M., Ni B. F., Needleman R., Lanyi J. K. Water is required for proton transfer from aspartate-96 to the bacteriorhodopsin Schiff base. Biochemistry. 1991 Nov 12;30(45):10972–10979. doi: 10.1021/bi00109a023. [DOI] [PubMed] [Google Scholar]
- Cao Y., Váró G., Klinger A. L., Czajkowsky D. M., Braiman M. S., Needleman R., Lanyi J. K. Proton transfer from Asp-96 to the bacteriorhodopsin Schiff base is caused by a decrease of the pKa of Asp-96 which follows a protein backbone conformational change. Biochemistry. 1993 Mar 2;32(8):1981–1990. doi: 10.1021/bi00059a015. [DOI] [PubMed] [Google Scholar]
- Druckmann S., Heyn M. P., Lanyi J. K., Ottolenghi M., Zimanyi L. Thermal equilibration between the M and N intermediates in the photocycle of bacteriorhodopsin. Biophys J. 1993 Sep;65(3):1231–1234. doi: 10.1016/S0006-3495(93)81166-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Druckmann S., Ottolenghi M., Pande A., Pande J., Callender R. H. Acid-base equilibrium of the Schiff base in bacteriorhodopsin. Biochemistry. 1982 Sep 28;21(20):4953–4959. doi: 10.1021/bi00263a019. [DOI] [PubMed] [Google Scholar]
- Hegemann P., Oesterbelt D., Steiner M. The photocycle of the chloride pump halorhodopsin. I: Azide-catalyzed deprotonation of the chromophore is a side reaction of photocycle intermediates inactivating the pump. EMBO J. 1985 Sep;4(9):2347–2350. doi: 10.1002/j.1460-2075.1985.tb03937.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson R., Baldwin J. M., Ceska T. A., Zemlin F., Beckmann E., Downing K. H. Model for the structure of bacteriorhodopsin based on high-resolution electron cryo-microscopy. J Mol Biol. 1990 Jun 20;213(4):899–929. doi: 10.1016/S0022-2836(05)80271-2. [DOI] [PubMed] [Google Scholar]
- Holz M., Drachev L. A., Mogi T., Otto H., Kaulen A. D., Heyn M. P., Skulachev V. P., Khorana H. G. Replacement of aspartic acid-96 by asparagine in bacteriorhodopsin slows both the decay of the M intermediate and the associated proton movement. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2167–2171. doi: 10.1073/pnas.86.7.2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kataoka M., Kamikubo H., Tokunaga F., Brown L. S., Yamazaki Y., Maeda A., Sheves M., Needleman R., Lanyi J. K. Energy coupling in an ion pump. The reprotonation switch of bacteriorhodopsin. J Mol Biol. 1994 Nov 4;243(4):621–638. doi: 10.1016/0022-2836(94)90037-x. [DOI] [PubMed] [Google Scholar]
- Lanyi J. K. Mechanism of base-catalyzed Schiff base deprotonation in halorhodopsin. Biochemistry. 1986 Oct 21;25(21):6706–6711. doi: 10.1021/bi00369a057. [DOI] [PubMed] [Google Scholar]
- Lanyi J. K. Proton translocation mechanism and energetics in the light-driven pump bacteriorhodopsin. Biochim Biophys Acta. 1993 Dec 7;1183(2):241–261. doi: 10.1016/0005-2728(93)90226-6. [DOI] [PubMed] [Google Scholar]
- Le Coutre J., Tittor J., Oesterhelt D., Gerwert K. Experimental evidence for hydrogen-bonded network proton transfer in bacteriorhodopsin shown by Fourier-transform infrared spectroscopy using azide as catalyst. Proc Natl Acad Sci U S A. 1995 May 23;92(11):4962–4966. doi: 10.1073/pnas.92.11.4962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozier R. H., Bogomolni R. A., Stoeckenius W. Bacteriorhodopsin: a light-driven proton pump in Halobacterium Halobium. Biophys J. 1975 Sep;15(9):955–962. doi: 10.1016/S0006-3495(75)85875-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagle J. F., Zimanyi L., Lanyi J. K. Testing BR photocycle kinetics. Biophys J. 1995 Apr;68(4):1490–1499. doi: 10.1016/S0006-3495(95)80321-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oesterhelt D., Stoeckenius W. Isolation of the cell membrane of Halobacterium halobium and its fractionation into red and purple membrane. Methods Enzymol. 1974;31:667–678. doi: 10.1016/0076-6879(74)31072-5. [DOI] [PubMed] [Google Scholar]
- Oesterhelt D., Tittor J., Bamberg E. A unifying concept for ion translocation by retinal proteins. J Bioenerg Biomembr. 1992 Apr;24(2):181–191. doi: 10.1007/BF00762676. [DOI] [PubMed] [Google Scholar]
- Otto H., Marti T., Holz M., Mogi T., Lindau M., Khorana H. G., Heyn M. P. Aspartic acid-96 is the internal proton donor in the reprotonation of the Schiff base of bacteriorhodopsin. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9228–9232. doi: 10.1073/pnas.86.23.9228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothschild K. J. FTIR difference spectroscopy of bacteriorhodopsin: toward a molecular model. J Bioenerg Biomembr. 1992 Apr;24(2):147–167. doi: 10.1007/BF00762674. [DOI] [PubMed] [Google Scholar]
- Takahashi E., Wraight C. A. Small weak acids stimulate proton transfer events in site-directed mutants of the two ionizable residues, GluL212 and AspL213, in the QB-binding site of Rhodobacter sphaeroides reaction center. FEBS Lett. 1991 May 20;283(1):140–144. doi: 10.1016/0014-5793(91)80572-k. [DOI] [PubMed] [Google Scholar]
- Thorgeirsson T. E., Milder S. J., Miercke L. J., Betlach M. C., Shand R. F., Stroud R. M., Kliger D. S. Effects of Asp-96----Asn, Asp-85----Asn, and Arg-82----Gln single-site substitutions on the photocycle of bacteriorhodopsin. Biochemistry. 1991 Sep 24;30(38):9133–9142. doi: 10.1021/bi00102a003. [DOI] [PubMed] [Google Scholar]
- Tittor J., Schweiger U., Oesterhelt D., Bamberg E. Inversion of proton translocation in bacteriorhodopsin mutants D85N, D85T, and D85,96N. Biophys J. 1994 Oct;67(4):1682–1690. doi: 10.1016/S0006-3495(94)80642-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tittor J., Soell C., Oesterhelt D., Butt H. J., Bamberg E. A defective proton pump, point-mutated bacteriorhodopsin Asp96----Asn is fully reactivated by azide. EMBO J. 1989 Nov;8(11):3477–3482. doi: 10.1002/j.1460-2075.1989.tb08512.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Váró G., Lanyi J. K. Thermodynamics and energy coupling in the bacteriorhodopsin photocycle. Biochemistry. 1991 May 21;30(20):5016–5022. doi: 10.1021/bi00234a025. [DOI] [PubMed] [Google Scholar]
- Zimányi L., Cao Y., Needleman R., Ottolenghi M., Lanyi J. K. Pathway of proton uptake in the bacteriorhodopsin photocycle. Biochemistry. 1993 Aug 3;32(30):7669–7678. doi: 10.1021/bi00081a010. [DOI] [PubMed] [Google Scholar]
- Zimányi L., Lanyi J. K. Deriving the intermediate spectra and photocycle kinetics from time-resolved difference spectra of bacteriorhodopsin. The simpler case of the recombinant D96N protein. Biophys J. 1993 Jan;64(1):240–251. doi: 10.1016/S0006-3495(93)81360-2. [DOI] [PMC free article] [PubMed] [Google Scholar]



