Abstract
Heterocellular electrotonic coupling between cardiac myocytes and non-excitable connective tissue cells has been a long-established and well-researched fact in vitro. Whether or not such coupling exists in vivo has been a matter of considerable debate. This paper reviews the development of experimental insight and conceptual views on this topic, describes evidence in favour of and against the presence of such coupling in native myocardium, and identifies directions for further study needed to resolve the riddle, perhaps less so in terms of principal presence which has been demonstrated, but undoubtedly in terms of extent, regulation, patho-physiological context, and actual relevance of cardiac myocyte–non-myocyte coupling in vivo. This article is part of a Special Issue entitled "Myocyte-Fibroblast Signalling in Myocardium."
Keywords: Heart, Electrophysiology, Connective tissue, Gap junction, Fibrosis, Scar
Highlights
-
•
Electrical coupling of cardiomyocytes and fibroblasts is well-established in vitro
-
•
Whether such hetero-cellular coupling exists in vivo has been a matter of debate
-
•
We review the development of experimental and conceptual insight into the topic
-
•
Conclusion 1: hetero-cellular coupling in heart tissue has been shown in principle
-
•
Conclusion 2: extent, regulation, context, and relevance remain to be established
1. Heterocellular coupling between cardiac cells in vivo?
When we consider the cellular basis of cardiac function, we tend to focus on myocytes, ‘forgetting’ the seemingly silent majority of electrically non-excitable cells. These include endothelial, fat, immune and stem cells, but the largest sub-population is formed by connective tissue cells (fibroblasts). Non-excitable does not mean ‘not exciting’, however, as these cells are crucial for structural, biochemical, and electro-mechanical integrity of the heart [1–3].
1.1. ‘The’ cardiac fibroblast?
Until the 1990s, cardiac fibroblasts were largely considered to be of limited relevance beyond structural support (a bit like the traditional view on neuroglia). That changed with the discovery of fibroblast-mediated signalling two decades ago, and led to a step-increase in the number of publications on cardiac fibroblasts from tens or fewer to hundreds per year (roughly 99% of all cardiac fibroblast papers currently listed on Web of Science have been published after 1990). As a result of this surge, fibroblasts are now accepted as key contributors to development, adaptation, and disease-related remodelling of the heart (e.g. [4–7]). At the same time, we are still far from having comprehensive insight into the roles of connective tissue in the heart. In part, this is caused by the fact that ‘the’ cardiac fibroblast does not exist.
Cardiac fibroblasts originate from at least three progenitor populations. Firstly, fibroblasts arise from endocardium via epithelial-to-mesenchymal transformation [8]. Secondly, retroviral lineage tracing showed that fibroblasts also originate from the pro-epicardium, a group of embryonic progenitor cells known to give rise to the epicardium [9]. Thirdly, cardiac fibroblasts derive from bone marrow in normal development and as a contributor to the homeostatic maintenance of adult myocardium [10–12], but also during post-injury scar formation [13]. Fibroblasts therefore constitute a heterogeneous and dynamic population of cells, whose origin, regulation, and function in health [14] and disease [15], including their role in repair, impact on cardiomyocyte proliferation [16] and, possibly, their transformation into cardiac muscle cells [17], pose exciting challenges of high clinical relevance. In addition to heterogeneity related to their origin, cardiac fibroblasts respond to activation, such as during myocardial infarction, thorough phenotype transformation into myofibroblasts, which are regarded by some as a distinct cell type altogether (for recent reviews see [18–21]).
This review is focussed on exploring the presence, in vivo, of electrophysiologically relevant heterocellular connections between cardiac myocytes and connective tissue cells. Given that (i) the developmental origin and (ii) the precise state of cell-activation are not usually known or reported, and since (iii) neither of these aspects is of primary concern for exploring the principal question as to whether or not there is in vivo heterocellular coupling in the heart between the excitable muscle and the non-excitable connective tissue cells, we will refer to the latter as ‘fibroblasts’, aware of the inherent limitations of such abbreviated terminology.
1.2. Properties of fibroblasts in tissue and in a dish
For roughly half a century, the presence of electrotonic coupling between cardiac fibroblasts and myocytes has been a well-established fact in vitro. Since the mid-1960s, the synchronization of spontaneous contractile activity in isolated cardiomyocytes, interconnected solely by fibroblasts, has been noted and characterised using time-lapse microscopy, microelectrode recordings, and dye transfer studies [22–24]. More recently, using linearly structured cell cultures [25–28], optical mapping of voltage sensitive dyes has shown that fibroblast inserts can electrotonically bridge gaps between groups of myocytes that are up to 300 μm apart [29].
This ability of fibroblasts to act as long-distance conductors benefits from their high membrane resistance and relatively low capacitance [30]. When considering numerical parameter ranges for fibroblast electrophysiological properties, it is imperative to appreciate pronounced differences between cells in vivo and in vitro (even prior to electrophysiological remodelling of fibroblasts in cell culture [31]).
Cardiac fibroblasts in vivo form large sheet-like extensions, often with additional irregular folds, and elongated cytoplasmic processes [32,33] (not just in mammals; see on-line movie S1 of [34] with data from fish). Careful electron microscopy (EM) based reconstruction of an individual fibroblast in rabbit sino-atrial node revealed that it formed a membrane juxtaposition with a neighbouring myocyte covering 720 μm2 [33]. As this reconstruction excluded some of the more distant fibroblast extensions, total surface area of this cell will have been 1500 μm2, or more.
In contrast, fibroblasts, freshly-isolated from healthy myocardium, are rounded cells with initial diameters of about 7–9 μm [31,35,36]. EM data showed that they lack not only the typical membrane extensions, but also folds or membrane invaginations that otherwise could increase surface area [36]. Therefore, considering their near-spherical cell shapes, one can calculate the surface membrane area of freshly isolated fibroblasts as 150–250 μm2, an order of magnitude less than in tissue. A probable reason for this discrepancy is the fact that cardiac cell isolation protocols tend to involve a combination of enzymatic digestion (to destroy connective tissue bonds) and mechanical agitation (to disturb tissue integrity), with the effect that surviving fibroblasts are likely to be the truncated non-myocyte fragments that contain a nucleus [37].
Currently, there is no data on the cell size distribution of fibroblasts in vivo, so we don't know how typical a surface area of 1500 μm2 is for fibroblasts in the heart. As a ball-park value, though, it is in keeping with the observation that fibroblast membrane resistances in situ (0.5–1 GΩ [38,39]) are generally about an order of magnitude lower than in freshly isolated cells [40,41]. It stands to reason that fibroblast membrane capacitances in vivo (hard to quantify by direct electrophysiological means in these extended and mutually interconnected cells) exceed those of freshly isolated cells (typically 6–10 pF in fibroblasts isolated from healthy myocardium [31,41]). Even if that were by an order of magnitude as well, which is not inconceivable, it would still render fibroblast capacitances small compared to cardiac myocytes (values for ventricular cardiomyocytes, isolated from healthy tissue, range from about 150 pF in rabbit and ferret to 300 pF in rat [42]).
Fibroblasts have a relatively depolarised membrane potential (usually between - 10 and - 50 mV; in tissue at the less negative end of this range), whether recorded using sharp electrodes in situ [38,39], or using single [43,44] and dual [38,40] patch clamp in vitro. While precise membrane potential measurements with these direct (but invasive) electrophysiological techniques are challenging in individual cells when cell- and seal-resistances are in a similar ball-park, the above potential range is also evident from observations based on an indirect assessment of biological reporter systems (e.g. by monitoring fibroblast effects on cultured cardiomyocytes [45–48]). Therefore, in addition to potentially supporting conduction, cardiac fibroblasts can depolarize resting, electrotonically connected cardiac myocytes [38–40,45]. Quantitative computational modelling since the mid-1990s has suggested that this could modulate pacemaker rate and alter excitability of working cardiomyocytes [38,49] (for the recent state-of-the-art in cardiac myocyte–fibroblast interaction modelling, see [50,51]), and this has been corroborated in experimental studies using myocyte–fibroblast co-culture systems (e.g. [45–48]).
1.3. Scenarios of fibroblast electrophysiological integration
Electrical coupling of cardiac cells is generally thought to be mediated by gap junctions [52,53], although the possibility of direct cytosolic links through ‘tunnelling nanotubes’ has recently come to light [54]. Electrotonic effects of non-excitable cells on cultured cardiomyocytes can indeed be modulated by altering connexin expression [55].
Loss or gain of Connexin43 (Cx43) function in fibroblasts can be achieved by genetic approaches [56]. While Cx43 knockdown or knockout provides useful experimental tools for studies of electrical effects of fibroblasts on myocytes in vitro, this approach may entail complexities that confound matters in vivo. For example, reduced Cx43 expression appears to up-regulate fibrosis [57] and may be associated with mesenchymal tumour formation in the heart [58]. In contrast, fibroblasts that are genetically engineered to overexpress Cx43 appear to have more benign effects on cultured cardiomyocyte electrophysiology, potentially offering an anti-arrhythmogenic ‘electrotonic buffer’ [44]. These and other observations have stimulated research into the potential of turning fibroblasts from a bystander in, or even culprit of, cardiovascular disease, into a therapeutic target. Indeed, cell-therapeutic approaches using fibroblasts transfected to overexpress potassium channels have been used to reduce cardiac automaticity and prolong ventricular refractoriness in vivo [59].
Based on the topology of fibroblast–myocyte interactions, three hypothetical types of functionally-relevant coupling effects of fibroblasts on the electrophysiology of the intact heart can be proposed [60]. First, ‘zero-sided coupling’, i.e. fibroblasts not interacting with myocytes, will separate myocardial cells and/or layers, acting as an electrical insulator, as indeed will be the effect of the a-cellular part of cardiac connective tissue. This option is most in keeping with traditionally-held views, such as pointed out as early as 1906 by Keith and Flack in their original description of the human atrio-ventricular node [61]. Second, ‘single-sided coupling’ refers to fibroblasts connected to groups of myocytes that are themselves well-connected electrically. Here, electrotonic load, or buffer effects would dominate. Third, ‘double-sided coupling’ refers to fibroblast-connections that interlink myocytes that are not, or less directly, coupled electrically. These links are not necessarily afforded just by single cells, since cardiac fibroblasts are interconnected by homotypic gap junctions [33,62] that can support fibroblast–fibroblast electrotonic coupling in situ, as confirmed by transmission of hyperpolarizing impulses between fibroblasts, 40 μm apart, studied using double-barrelled microelectrodes in the atria of frog and rat [63,64], and via dye coupling studies in rabbit [65]. Double-sided coupling could allow cardiac fibroblasts to form conducting pathways of electrophysiological relevance at the tissue and organ level. If confirmed, this case would be of particular interest, as it would offer novel, therapeutically relevant, insight into post-ablation and post-infarction electrophysiological behaviour.
This being said, the presence and extent of fibroblast–myocyte electrotonic coupling in native myocardium (i.e. including observations in vivo and ex vivo, as long they pertain to cells in a morphologically intact tissue environment) remain controversial, forming the topic of this review. We will first re-cap the reasons for which one would doubt the presence of heterocellular coupling in vivo (‘Contra’), highlight why at least some of these doubts are not without limitations either (‘Contra-Contra’), review what is known in support of the presence of heterocellular coupling in the heart (‘Pro’), and finish by summarizing what should be done to resolve the riddle (‘Ergo’).
2. Contra
There are (at least) three common objections to the view that non-myocytes may be electrically coupled to muscle cells in native cardiac tissue.
2.1. Heterocellular gap junctions are not normally seen in histological studies
Generations of histologists have studied cardiac tissue. Intercalated discs at the juncture of cardiac myocytes were identified, using EM, in the early 1950s (e.g. [66]). In the mid-1960s, these disks were proposed to contain structures that link the cytosol of neighbouring cardiomyocytes [67] by bridging the gap at the junction of cells [68]. This suggestion was confirmed in the 1970s by purification of gap-junction plaques [69], identification in the 1980s of the first single gap-junctional protein capable of linking neighbouring cells via formation of inter-cellular ion channels [70], and followed up by detailed characterisation of multiple connexins (Cx), including the major isoform found in the heart, Cx43 [71]. This triggered significant interest in the role of cardiac gap junctions, including links to other cellular structures (such as the cytoskeleton [72]). Towards the end of the 1980s, Cx research received a significant boost from the introduction of immunological techniques to identify presence and location of Cx in various tissues of the body, including heart [73–77]. During the 1990s, Cx distribution studies opened up a treasure chest of insight into cardiac cell–cell interactions (for a selection of reviews from that time, see [78–90]) — yet none of the studies suggested a presence of heterocellular junctions between myocytes and non-myocytes in native cardiac tissue.
2.2. Heterocellular junctions are exceedingly rare, even when specifically chased
Following up on early reports on heterocellular coupling in vitro [22–24,52], and after detailed electrophysiological characterisation of heterocellular gap-junctional channels connecting freshly isolated or cultured cell pairs [30,40], the team of Jongsma set out to specifically search for gap junctions between myocytes and fibroblasts in native heart tissue. In a painstaking, serial sectioning based EM study of adult rabbit sino-atrial node, covering a total area of 7.7 mm2, they observed just one gap-junction like contact between a fibroblast and a myocyte [33]. In contrast, small gap junctions between fibroblast pairs were common, while the analysed tissue volume was estimated to have contained ~ 104 gap junctions between cardiac myocytes [33]. Thus, even targeted EM studies did not support the notion of a regular presence of heterocellular gap junctions in the heart.
2.3. Ockham's Razor: the heart works without invoking heterocellular coupling
William of Ockham, early 14th century English scholar, stated that, if there are multiple possible explanations of a phenomenon, the simplest of them should be preferred. Cardiac electrophysiology can generally be explained, even in quantitative computational models [91–102], based solely on considering electrotonic coupling of myocytes. This shifts the burden of proof: if there is no need to invoke heterocellular coupling between myocytes and non-myocytes, one should not do so (never change a winning team).
3. Contra–Contra
3.1. Connexin-based coupling is possible in the absence of detectable gap junctions
Gap junctions, in particular between cardiac myocytes, lend themselves to relatively easy identification. Fibroblast gap junctions are significantly smaller [33], and thus are more easily overlooked. One should further note that early immuno-histochemical characterisation of connexin distribution in cardiac tissue did not tend to include any form of cell labelling. In this setting, intercalated discs between myocytes are a prominent feature, while the small ‘speckles’ of fluorescence, indicative of fibroblast-based gap junctions, could easily be regarded as background noise [103].
What's more, the absence of immuno-histochemically detectable gap junctions in confocal microscopy studies is not necessarily synonymous with a lack of connexin-based cell coupling, as shown for example in native arterial smooth muscle [104,105]. Unless super-resolution optical techniques are used, fluorescently labelled Cx will be detected only if spatially-clustered, involving at least 80–120 channels [106] (of note, smaller arrays, containing 15–20 Cx-channels, are regularly present in rat ventricular myocardium, as confirmed by EM [106]). Computer modelling studies suggest that as few as 10–30 connexin channels would provide sufficient coupling for electrophysiologically relevant effects of cardiac fibroblasts on myocytes [38]. Possible sites for such coupling could be finger-like extensions of cardiac fibroblasts into the cardiomyocyte basement membrane [32] — points of contact that may be more frequent in living (Fig. 1A), rather than the fixed and dehydrated tissue used in most histological analyses.
Fig. 1.
Fibroblast–myocyte interrelations in native cardiac tissue. A: Confocal laser scanning microscopy images of rabbit sino-atrial node live tissue, following diffusion-loading of the CellTracker dye CMFDA. Groups of sino-atrial node myocytes (centrally located ‘large’ uniform structures) are intermingled with cardiac fibroblasts (smaller cells with numerous fine processes towards the myocytes, see white arrows). Outline view left: 158 × 158 μm; detail view right: 30 × 30 μm (corresponds to the area near the arrow at the right edge of the outline). B: Extended structures, resembling membrane nanotubes (see white arrows), in adult mouse ventricular tissue. Confocal micrographs of cardiac tissue, labelled for WGA (membranes), vimentin (fibroblasts) and α-actinin (myocytes). Scale bar: 10 μm. From [32] in (A), and [54] in (B); with permission.
3.2. Connexin-based coupling may not be necessary for heterocellular cell junctions
So-called ‘tunneling nanotubes’ [107], membranous channels that are 50–200 nm wide and that can link cells over distances up to 300 μm in vivo [108], provide an alternative substrate for electrotonic coupling between different cell types. These structures may either form connexin-free direct cytosolic links [109], or involve Cx-coupling at the point of contact of two semi-tubes. The latter could occur ‘anywhere’ along the nanotubular connection, i.e. somewhere between apparently separate cells. Punctate immuno-labelling (in the absence of a cytosolic dye) would, in this setting, probably be regarded as background unrelated to intercellular connections [107]. Evidence for the presence of nanotube coupling between cardiac fibroblasts and myocytes has been shown in vitro and in vivo (Fig. 1B; [54]), although their relevance for cardiac electrophysiological integration remains to be established.
3.3. Ockham's Razor: special cases
The notion of being able to explain cardiac electrophysiology, based solely on homocellular myocyte coupling, is not without limitations. For example, atrial ablation lines (scars, created to interrupt uncontrolled excitation waves) often become ‘electrically transparent’ with time, due to re-emergence of functional conduction pathways [110]. While this may be due to incomplete lesioning, 10–20% of heart transplant recipients also show electrical coupling across the (unquestionably continuous) separation between donor and recipient tissue [111]. Aberrant electrical coupling can arise also across suture lines after operations to fix cardiac birth defects [112]. In all these cases, electrical conduction crosses scar tissue. Possible explanations include direct ‘touch-and-go’ interaction of surviving myocytes either side of a cut, de novo generation of cardiomyocytes that link the two tissue edges, or electrical conduction via non-myocardial cells such as fibroblasts [62]. Based on the histological appearance of post-transplantation scar tissue [113], the last option may offer the most straightforward explanation and, hence, be in keeping with Ockham.
4. Pro
4.1. Electrotonic fibroblast–myocyte coupling in native tissue
Fibroblasts in native heart tissue at rest have a membrane potential of - 10 to - 20 mV when electrically isolated from other cells [38]. They are electrically non-excitable (though subsequent to long-term cell culture, expression of the voltage-gated sodium channel Nav1.5 has been reported in human atrial fibroblasts [114]). Fibroblasts are excellent ‘followers’ of an electrotonically provided waveform. Thus, if electrically coupled to a cardiomyocyte, the fibroblast's membrane potential will show a myocyte-like action potential shape, albeit with a slowed upstroke and reduced amplitude (as illustrated in double whole-cell patch clamp experiments [40]). The ability of fibroblasts to passively convey action potentials, together with their high membrane resistance that supports ‘low-loss’ long-range signal conduction, makes fibroblasts a tantalising potential conduit for electrotonic signal transmission.
Unfortunately, this very behaviour also makes it nearly impossible to confidently explore cardiac fibroblast electrical integration in situ, using classic electrophysiological techniques [38]. While fibroblasts that are electrically isolated from cardiac muscle cells can be identified by their weakly polarised membrane potential and mechanically-induced polarizations in the rhythm of tissue contraction, those fibroblasts that are well-coupled (and, hence, at the centre of interest here) are indistinguishable from poorly impaled cardiomyocytes (Fig. 2; [38]). Nonetheless, indirect support for heterocellular connections in weakly coupled fibroblasts has been found (Fig. 2, centre; note that upon electro-mechanical uncoupling of the preparation, the mechanically induced component ‘a’ recedes, while ‘b’ and ‘c’ remain).
Fig. 2.

Membrane potential pairs (top and bottom), recorded simultaneously in spontaneously beating adult rat atrium using double-barrelled floating microelectrodes (tip-to-tip distance 40 μm) from putative fibroblasts (F, top) and cardiomyocytes (M, bottom). Left: F, electrically not connected to surrounding cardiomyocytes, showing a relatively depolarized membrane potential (here about - 20 mV) and the contraction-induced membrane potential change typical for these cells (label ‘a’). Middle: in some cases, presumably when weakly coupled to adjacent myocytes, F display an additional, probably electrotonically transmitted membrane depolarization (labelled ‘b’) while the myocyte membrane potential is positive to the diastolic potential in F, and a ‘capacitative’ spike (labelled ‘c’) believed to be caused by the synchronous depolarization of myocytes in the proximity of F. Right: recording of a potential waveform that would be commensurate with an F that is well-coupled to adjacent cardiomyocytes, where the electrotonically transmitted component mimics an action potential waveform of reduced amplitude and upstroke velocity (here on top of the atrial contraction-induced mechano-sensitive peak; note different potential scales for first two F potentials). Adapted from [38]; with permission.
4.2. Connexins at fibroblast–myocyte contact sites in cardiac tissue
Immuno-histochemical labelling studies for a range of cardiac connexins, combined with identification of cells on either side of connexin signals, suggest that Cx-localization at the point of contact between cardiac fibroblasts and myocytes is far from uncommon, both in healthy (Fig. 3 [62]) and post-infarct tissue (Fig. 4 [115]).
Fig. 3.
Connexin location relative to cardiac myocytes and connective tissue cells in native myocardium of healthy rabbit. Triple immunolabeling for myocytes (M; red: antimyomesin), fibroblasts (F; blue: antivimentin), and Cx43 or Cx40 (top and bottom rows, respectively; green: anti-Cx43/anti-Cx40) in ventricle, atrium, and atrioventricular node (AVN). Vertical arrows: homotypic myocyte contacts; horizontal arrows: homotypic non-myocyte connections; slated arrows: Cx at heterotypic cell contacts. Side-panels show 2.55 × zoomed views of the areas highlighted by dashed squares in the main images. Note significantly smaller size of Cx labels at homo- and heterotypic contact sites that involve non-myocytes. Scale bars: 20 μm. From [62], with permission.
Fig. 4.
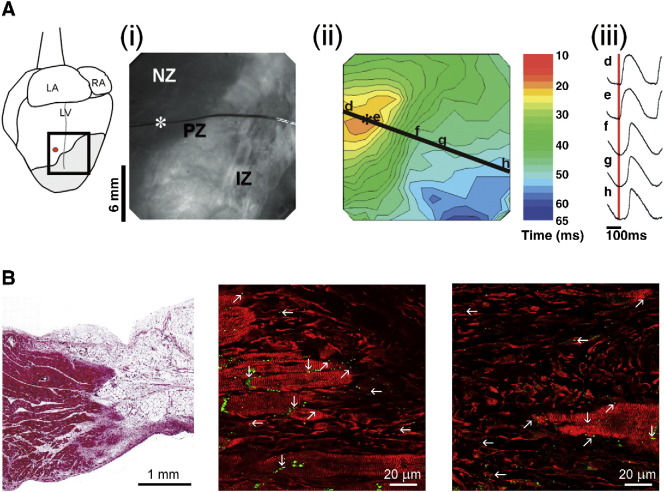
Propagation of electrical activation into ventricular scar tissue. A: Epicardially recorded optical maps of trans-membrane potential changes (dye used: RH237) in Langendorff-perfused rabbit isolated heart, 8 weeks after induction of transmural left-ventricular infraction (see B, left panel). Electrical pacing (at site identified in the schematic on the left by a red dot, and in the photograph (i) of the infract area by a white asterisk) in the normal zone (NZ) gives rise to a conduced wave of electrical excitation, which is delayed by about 10 ms at the peri-infarct before invading the infarct zone (PZ, IZ; respectively); see crowding and thinning of activation isochrones (ii). Normalised trans-membrane potential shapes, recorded at the locations labelled d–h, are shown in (iii). Signals from inside the scar tissue (i.e. at sites g and h) persisted even after chemical ablation of surviving endocardial muscle layers (panel B). LA: left atrium, RA: right atrium; LV: left ventricle; red vertical line in (iii): time of pacing stimulus. B: Histological substrate of scar tissue in rabbit (left, 8 weeks post-infarct, used in the experimental studies shown in A) and sheep (border zone at 1 week post infract [middle]; infarct zone 2 weeks post-infarct [right]). The panel on the left (trichrome staining) illustrates the transmural nature of infarcts used in the optical mapping studies, including the presence of islands of surviving myocytes in the infarct zone, and a surviving endocardial tissue rim (lower edge of section) that was ablated in part of the study. The images in the centre and at the right (triple immuno-labelling for Cx43 [green], myomesin to identify myocytes by their striation, and vimentin to label fibroblasts [both red]) highlights Cx43 distribution, including presence at heterocellular contact sites at the infract border (middle) and in central zone myocyte islands. Vertical arrows: homotypic myocyte contacts; horizontal arrows: homotypic non-myocyte connections; slated arrows: Cx43 at heterotypic cell contacts. From [116] in (A) and from [117,115,1], respectively, in (B); with permission.
Of course, presence of a protein ‘in the right place’ does not confirm its functionality. Direct evidence for heterocellular coupling in native tissue has thus far been obtained only in rabbit sino-atrial node, where Lucifer yellow dye transfer between myocytes and fibroblasts was observed [65].
Clearly, further investigation is needed to explore developmental dynamics, anatomical distribution, physiological regulation, and response to disease progression or treatment attempts, but proof-of-concept for fibroblast–myocyte coupling in vivo has been established. A conceptual approach to myocardial function that excluded the possibility of heterocellular coupling would, therefore, be unnecessarily restricted.
4.3. Optical mapping of conduction inside scar tissue
Given the principal difficulty of distinguishing between trans-membrane potential waveforms of (active) cardiac myocytes and (passive) fibroblast-followers in normal heart tissue, cardiac scars offer an interesting alternative study subject, because the ratio of electrically active and passive cells is shifted significantly towards predominance of the latter. A fine example of such work is the 2007 study of Walker et al., investigating fully-transmural infarcts in adult rabbit left ventricle by optical mapping of voltage-sensitive dye signals (Fig. 4A). They observed propagation of cardiac excitation waves into scar tissue, even after chemical ablation of any surviving sub-endocardial muscle layers (Fig. 4B), performed to exclude possible contributions from these deeper myocyte-sheets to epicardially mapped potentials; [116]). The signals from within the scar resembled ventricular action potentials, albeit with slowed upstroke and reduced amplitude — very much in keeping with previous observations in neonatal rat fibroblast–myocyte cell pairs [40] and adult rat atrial tissue [38].
Subsequent work by other investigators confirmed that excitation waves can indeed invade and pass through cardiac scar tissue [118,119]. These studies further showed that the myocyte-like action potentials inside four-week old ventricular scar tissue in rabbit ventricle are not accompanied by significant cyclic changes in intracellular free calcium concentration [118]. As calcium transients are a signature activity of cardiomyocytes, the most straightforward explanation again is that non-myocytes passively display much of the electrical signals conducted inside scars [117], perhaps electrically integrating surviving myocyte islands that could act as active ‘repeater stations’ that maintain or boost electrical signal amplitude.
5. Ergo
The short response to the initial question “Fibroblast–myocyte electrotonic coupling: does it occur in native cardiac tissue?” is: ‘yes’. However, as will be clear from the above, this raises many more questions than it answers. Some of the aspects, relevant for further study, are summarised below.
5.1. Need targeted assessment of dynamic heterocellular interactions
Confirmation, in native myocardium, of functional fibroblast–myocyte coupling at the cellular level has been obtained thus far in healthy atrial tissue of a single species only [65]. Whether heterocellular interactions in vivo change during development, disease, and/or in response to therapeutic interventions, and whether they differ between species and/or cardiac tissue regions, is unknown. Given the presence of fractional differences in fibroblast origin [8–13], as well as disease-induced fibroblast phenotype transitions [120], and mechanical modulation of Cx coupling [121], it is reasonable to expect differences in heterocellular interactions of fibroblasts in diseased tissue, compared to control [122].
Equally unconfirmed is the functional relevance of heterocellular coupling in in vivo. For example, fibroblasts show cardiac contraction-related fluctuations in membrane potential [38], probably mediated by stretch-activated ion channels [123–125] and changes in calcium handling [126]. This may be functionally relevant beyond electrophysiology, integrating cardiac electrical and mechanical activity [127]. Another area of possible functional importance is related to the observation that, during myocardial infarction, fibroblasts increasingly express functional KATP channels in scar and border zone tissue [128]. This could be of relevance for arrhythmia prevention and treatment, and lends support to the suggestion that (pre-/post-)conditioning needs to consider effects on the whole heart, not just the cardiomyocytes [129].
In addition, it is important to realise that not all scars are created equal. Oxygen starvation during myocardial infarction, for example, will affect preferentially the metabolically more active cardiomyocytes, so that locally-surviving cells (with a bias towards non-myocytes) will contribute to scar tissue composition. In contrast, ablation by excess local energy delivery will destroy cells irrespective of their nature (but potentially dependent on their proximity to circulating normothermic ‘coolant’ in coronary vessels), so that the vast majority of cells forming the post-ablation scar will invade from intra- or extra-cardiac sources outside the ablation focus. Yet another setting is observed at surgical suture lines, where the scar is built de novo, in the absence even of any pre-existing extracellular matrix. Further variances in electrophysiological properties of atrial vs. ventricular fibroblasts [130] may contribute to dissimilar functional behaviours displayed by different cardiac scars, for example after atrial ablation or ventricular infarction. And last, but not least, scar tissue is highly dynamic, containing a range of non-myocyte cell- and phenotypes whose relative quantities, and Cx expression patterns [115], change over time with scar maturation.
Scars, of course, are but the tip of the iceberg and it will be important to develop an understanding of the roles that fibroblasts may have as electro-mechanical signalling hubs in normal, and diffusely or patchy fibrotic tissue. This will require a better understanding of whole-heart histo-anatomical features, benefitting from imaging-based 3D reconstruction and biophysically-detailed simulation [131–134], an area that has made significant progress in recent years in terms of inclusion of connective tissue effects on arrhythmogenesis [100,135–137] and defibrillation [138,139]. It is vital, though, not to fall victim of the ‘plausibility trap’ [140]: simply because modelling and reality ‘agree’ with one another does not mean that mechanisms invoked in a theoretical model are crucial, or indeed even involved at all. Key to progress is validation, and this requires development and application of novel experimental tools.
5.2. Need better tools
It is evident that, on the one hand, fibroblast–myocyte interactions in cardiac cell cultures overestimate Cx-coupling while, on the other hand, native ‘bulk’ tissue studies are associated with significant challenges in terms of electrophysiological identification and characterisation of fibroblast activity and coupling. This calls for intermediate level biological model systems, such as thin sections of live cardiac tissue. Cardiac tissue slices were originally established and used as an experimental model for biochemical and pharmacological research (e.g. [141–143], reviewed in [144]). By now, they have been employed in electrophysiology research, using extra- and intracellular potential recordings [145] and optical mapping techniques, including dual-dye (calcium and voltage) studies [146]. They have been tested for a range of cardiac tissue sources, from neonatal and adult rat to human [147]. Heart tissue slices, usually cut at a thickness of 300–400 μm to prevent oxygen starvation, allow prolonged maintenance of biological activity [145,148]. They lend themselves to improved correlation of observed electrophysiological signals to underlying histological substrates, both because they are thin enough for visual inspection, and since local signals are less affected by more distant tissue (e.g. far-field potentials for contact recordings, or depth-effects of photon scattering in optical mapping of bulk tissue [149]).
In addition to the use of pseudo two-dimensional yet organotypic models for observation, optical tools for targeted interference offer another exciting route to further progress. This has been exquisitely illustrated by Entcheva and colleagues, who transfected non-excitable cells with a genetically encoded light-sensitive ion channel (channelrhodopsin-2), which they used to pace electrotonically connected cardiomyocytes in vitro [150]. It would be interesting to combine studies like this with the in vivo engraftment of Cx43-expressing non-excitable cells in scar tissue, which has been found to strongly reduce infarct-related arrhythmias in mice [151]. In any case, cell-type specific targeting of optogenetic probes will open up significant potential for novel insight into the multicellular nature of cardiac electrical function.
5.3. Need a broader vision of cell coupling in the integrated heart
The need for a broader conceptual approach to heterocellular interactions is evident also from the observation that gap junctions are sites not only of electrical, but also mechanical integration [152]. Mechanical interaction of fibroblasts and myocytes could not be illustrated more vividly than in the five-day time-lapse movie accompanying Driesens' beautiful in vitro report on partial cell fusion of rat cardiomyocytes and fibroblasts [153]. The targeted contacting and mechanical pull of fibroblasts on myocytes may give rise to mechano-electric feedback effects of the former on the latter [154], and/or serve as a guide for fibroblast cell migration [155]. In addition, it illustrates the highly dynamic nature of the heterocellular contact points (Fig. 1) between cardiac fibroblasts and myocytes (and serves as a note of caution regarding apparent cell-type transitions, in particular in vitro, given the presence of cell fusion events).
In addition, the ‘perinexus’ [156], a specialised region surrounding the connexin-dominated part of cell junctions, may support non-electrotonic ephaptic coupling. Presence of this type of cell interaction has been proposed in the 1980s [157], and it has recently seen a revival in terms of interest and insight [158,159].
Finally, when considering the heart as a hetero-cellular organ, we should not stop at myocytes and fibroblasts. Electrophysiological effects of nerve, immune and fat cells, vascular smooth muscle, or endothelium (to name but a few), clearly desire a better understanding as well [2,3].
6. Heterocellular coupling between cardiac cells in vivo!
There is a growing array of new experimental tools at our disposal to explore heterocellular coupling in native cardiac tissue. In coming years it is likely that we will see these tools provide unexpected advances in our understanding of the electrical interplay between the multiple cell types that make up the heart. In particular, it is anticipated that there will be definitive answers on where and when myocytes and non-myocytes are capable of electrophysiologically-relevant coupling in vivo, and what the context and the relevance are of such interactions. Answers here will provide new insight into normal cardiac function and into mechanisms of disease development. Perhaps more importantly, an understanding of the nature and dynamics of heterocellular electrical communication in the heart could give rise to novel or improved therapeutic approaches. This may range from amendments to established procedures, such as atrial ablation, to the vision of modifying cardiac scar properties in a targeted manner to reduce arrhythmogenesis following infarction.
The key question to which we should move on, therefore, is: Fibroblast–myocyte electrical coupling: does it matter in native cardiac tissue?
Disclosures
RGG has modest equity in FirstString Research Inc., a company testing Cx43-based therapeutics for wound healing (co-founder, scientific advisory board member).
Acknowledgements
PK is a Senior Fellow of the British Heart Foundation and work in his lab is supported by an Advanced Grant from the European Research Council and the UK Biotechnology and Biological Sciences Research Council. RGG acknowledges support from NIH grants (RO1 HL56728, R01 DE019355). We thank Dr's Thomas K Borg (MUSC), Roger R Markwald (MUSC), and T Alexander Quinn (Dalhousie University) for helpful discussions on the topic.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivative Works License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
References
- 1.Camelliti P., Borg T.K., Kohl P. Structural and functional characterisation of cardiac fibroblasts. Cardiovasc Res. 2005;65:40–51. doi: 10.1016/j.cardiores.2004.08.020. [DOI] [PubMed] [Google Scholar]
- 2.Bowers S.L., Borg T.K., Baudino T.A. The dynamics of fibroblast-myocyte-capillary interactions in the heart. Ann N Y Acad Sci. 2010;1188:143–152. doi: 10.1111/j.1749-6632.2009.05094.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fujiu K., Nagai R. Contributions of cardiomyocyte-cardiac fibroblast-immune cell interactions in heart failure development. Basic Res Cardiol. 2013;108:357. doi: 10.1007/s00395-013-0357-x. [DOI] [PubMed] [Google Scholar]
- 4.Zhang P., Su J., Mende U. Cross talk between cardiac myocytes and fibroblasts: from multiscale investigative approaches to mechanisms and functional consequences. Am J Physiol Heart Circ Physiol. 2012;303:H1385–H1396. doi: 10.1152/ajpheart.01167.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yamazaki K.G., Gonzalez E., Zambon A.C. Crosstalk between the renin-angiotensin system and the advance glycation end product axis in the heart: role of the cardiac fibroblast. J Cardiovasc Transl Res. 2012;5:805–813. doi: 10.1007/s12265-012-9405-4. [DOI] [PubMed] [Google Scholar]
- 6.Martin M.L., Blaxall B.C. Cardiac intercellular communication: are myocytes and fibroblasts fair-weather friends? J Cardiovasc Transl Res. 2012;5:768–782. doi: 10.1007/s12265-012-9404-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lajiness J.D., Conway S.J. The dynamic role of cardiac fibroblasts in development and disease. J Cardiovasc Transl Res. 2012;5:739–748. doi: 10.1007/s12265-012-9394-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eisenberg L.M., Markwald R.R. Molecular regulation of atrioventricular valvuloseptal morphogenesis. Circ Res. 1995;77:1–6. doi: 10.1161/01.res.77.1.1. [DOI] [PubMed] [Google Scholar]
- 9.Mikawa T., Gourdie R.G. Pericardial mesoderm generates a population of coronary smooth muscle cells migrating into the heart along with ingrowth of the epicardial organ. Dev Biol. 1996;174:221–232. doi: 10.1006/dbio.1996.0068. [DOI] [PubMed] [Google Scholar]
- 10.Visconti R.P., Ebihara Y., LaRue A.C., Fleming P.A., McQuinn T.C., Masuya M. An in vivo analysis of hematopoietic stem cell potential: hematopoietic origin of cardiac valve interstitial cells. Circ Res. 2006;98:690–696. doi: 10.1161/01.RES.0000207384.81818.d4. [DOI] [PubMed] [Google Scholar]
- 11.Mollmann H., Nef H.M., Kostin S., von Kalle C., Pilz I., Weber M. Bone marrow-derived cells contribute to infarct remodelling. Cardiovasc Res. 2006;71:661–671. doi: 10.1016/j.cardiores.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 12.Hajdu Z., Romeo S.J., Fleming P.A., Markwald R.R., Visconti R.P., Drake C.J. Recruitment of bone marrow-derived valve interstitial cells is a normal homeostatic process. J Mol Cell Cardiol. 2011;51:955–965. doi: 10.1016/j.yjmcc.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Norris RA, Gourdie RG, O'Quinn MP, Markwald RR. Periostin inhibitory compositions for myocardial regeneration (patent). USA 2011.
- 14.Krenning G., Zeisberg E.M., Kalluri R. The origin of fibroblasts and mechanism of cardiac fibrosis. J Cell Physiol. 2010;225:631–637. doi: 10.1002/jcp.22322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Norris R.A., Borg T.K., Butcher J.T., Baudino T.A., Banerjee I., Markwald R.R. Neonatal and adult cardiovascular pathophysiological remodeling and repair: developmental role of periostin. Ann N Y Acad Sci. 2008;1123:30–40. doi: 10.1196/annals.1420.005. [DOI] [PubMed] [Google Scholar]
- 16.Ieda M., Tsuchihashi T., Ivey K.N., Ross R.S., Hong T.T., Shaw R.M. Cardiac fibroblasts regulate myocardial proliferation through beta1 integrin signaling. Dev Cell. 2009;16:233–244. doi: 10.1016/j.devcel.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ieda M., Fu J.D., Delgado-Olguin P., Vedantham V., Hayashi Y., Bruneau B.G. Direct reprogramming of fibroblasts into functional cardiomyocytes by defined factors. Cell. 2010;142:375–386. doi: 10.1016/j.cell.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lajiness J.D., Conway S.J. Origin, development, and differentiation of cardiac fibroblasts. J Mol Cell Cardiol. 2014;70:2–8. doi: 10.1016/j.yjmcc.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Davis J., Molkentin J.D. Myofibroblasts: trust your heart and let fate decide. J Mol Cell Cardiol. 2014;70:9–18. doi: 10.1016/j.yjmcc.2013.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Turner N.A., Porter K.E. Function and fate of myofibroblasts after myocardial infarction. Fibrogenesis Tissue Repair. 2013;6:5. doi: 10.1186/1755-1536-6-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weber K.T., Sun Y., Bhattacharya S.K., Ahokas R.A., Gerling I.C. Myofibroblast-mediated mechanisms of pathological remodelling of the heart. Nat Rev Cardiol. 2013;10:15–26. doi: 10.1038/nrcardio.2012.158. [DOI] [PubMed] [Google Scholar]
- 22.Mark G.E., Strasser F.F. Pacemaker activity and mitosis in cultures of newborn rat heart ventricle cells. Exp Cell Res. 1966;44:217–233. doi: 10.1016/0014-4827(66)90427-7. [DOI] [PubMed] [Google Scholar]
- 23.Goshima K., Tonomura Y. Synchronized beating of embryonic mouse myocardial cells mediated by FL cells in monolayer culture. Exp Cell Res. 1969;56:387–392. doi: 10.1016/0014-4827(69)90029-9. [DOI] [PubMed] [Google Scholar]
- 24.Hyde A., Blondel B., Matter A., Cheneval J.P., Filloux B., Girardier L. Homo- and hetero-cellular junctions in cell cultures: an electrophysiological and morphological study. In: Akert K., Waser P.G., editors. Mechanisms of synaptic transmission. Elsevier; Amsterdam: 1969. pp. 283–311. [DOI] [PubMed] [Google Scholar]
- 25.Lieberman M., Sawanobori T., Kootsey J.M., Johnson E.A. A synthetic strand of cardiac muscle: its passive electrical properties. J Gen Physiol. 1975;65:527–550. doi: 10.1085/jgp.65.4.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fast V.G., Kleber A.G. Microscopic conduction in cultured strands of neonatal rat heart cells measured with voltage-sensitive dyes. Circ Res. 1993;73:914–925. doi: 10.1161/01.res.73.5.914. [DOI] [PubMed] [Google Scholar]
- 27.Camelliti P., McCulloch A.D., Kohl P. Microstructured cocultures of cardiac myocytes and fibroblasts: a two-dimensional in vitro model of cardiac tissue. Microsc Microanal. 2005;11:249–259. doi: 10.1017/S1431927605050506. [DOI] [PubMed] [Google Scholar]
- 28.Camelliti P., Gallagher J.O., Kohl P., McCulloch A.D. Micropatterned cell cultures on elastic membranes as an in vitro model of myocardium. Nat Protoc. 2006;1:1379–1391. doi: 10.1038/nprot.2006.203. [DOI] [PubMed] [Google Scholar]
- 29.Gaudesius G., Miragoli M., Thomas S.P., Rohr S. Coupling of cardiac electrical activity over extended distances by fibroblasts of cardiac origin. Circ Res. 2003;93:421–428. doi: 10.1161/01.RES.0000089258.40661.0C. [DOI] [PubMed] [Google Scholar]
- 30.Rook M.B., Jongsma H.J., de Jonge B. Single channel currents of homo- and heterologous gap junctions between cardiac fibroblasts and myocytes. Pflugers Arch. 1989;414:95–98. doi: 10.1007/BF00585633. [DOI] [PubMed] [Google Scholar]
- 31.Dawson K., Wu C.T., Qi X.Y., Nattel S. Congestive heart failure effects on atrial fibroblast phenotype: differences between freshly-isolated and cultured cells. PLoS ONE. 2012;7:e52032. doi: 10.1371/journal.pone.0052032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kohl P., Hunter P., Noble D. Stretch-induced changes in heart rate and rhythm: clinical observations, experiments and mathematical models. Prog Biophys Mol Biol. 1999;71:91–138. doi: 10.1016/s0079-6107(98)00038-8. [DOI] [PubMed] [Google Scholar]
- 33.Demaziere A.M.G.L., Vanginneken A.C.G., Wilders R., Jongsma H.J., Bouman L.N. Spatial and functional-relationship between myocytes and fibroblasts in the rabbit sinoatrial node. J Mol Cell Cardiol. 1992;24:567–578. doi: 10.1016/0022-2828(92)91041-3. [DOI] [PubMed] [Google Scholar]
- 34.Lafontant P.J., Behzad A.R., Brown E., Landry P., Hu N., Burns A.R. Cardiac myocyte diversity and a fibroblast network in the junctional region of the zebrafish heart revealed by transmission and serial block-face scanning electron microscopy. PLoS ONE. 2013;8:e72388. doi: 10.1371/journal.pone.0072388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nikolskaya A., Sharma V. Cell culture models and methods. In: Sigg D.C., Iaizzo P.A., Xiao Y.-F., He B., editors. Cardiac electrophysiology methods and models. Springer; 2010. pp. 213–235. [Google Scholar]
- 36.Rook M.B., Jongsma H., Van Ginneken A.C. Properties of single gap junctional channels between isolated neonatal rat heart cells. Am J Physiol Heart Circ Physiol. 1988;255:H770–H782. doi: 10.1152/ajpheart.1988.255.4.H770. [DOI] [PubMed] [Google Scholar]
- 37.Yue L., Xie J., Nattel S. Molecular determinants of cardiac fibroblast electrical function and therapeutic implications for atrial fibrillation. Cardiovasc Res. 2011;89:744–753. doi: 10.1093/cvr/cvq329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kohl P., Kamkin A.G., Kiseleva I.S., Noble D. Mechanosensitive fibroblasts in the sino-atrial node region of rat heart: interaction with cardiomyocytes and possible role. Exp Physiol. 1994;79:943–956. doi: 10.1113/expphysiol.1994.sp003819. [DOI] [PubMed] [Google Scholar]
- 39.Kamkin A., Kiseleva I., Wagner K.D., Pylaev A., Leiterer K.P., Theres H. A possible role for atrial fibroblasts in postinfarction bradycardia. Am J Physiol Heart Circ Physiol. 2002;282:H842–H849. doi: 10.1152/ajpheart.00240.2001. [DOI] [PubMed] [Google Scholar]
- 40.Rook M.B., Vanginneken A.C.G., Dejonge B., Elaoumari A., Gros D., Jongsma H.J. Differences in gap junction channels between cardiac myocytes, fibroblasts, and heterologous pairs. Am J Physiol Cell Physiol. 1992;263:C959–C977. doi: 10.1152/ajpcell.1992.263.5.C959. [DOI] [PubMed] [Google Scholar]
- 41.Chilton L., Ohya S., Freed D., George E., Drobic V., Shibukawa Y. K + currents regulate the resting membrane potential, proliferation, and contractile responses in ventricular fibroblasts and myofibroblasts. Am J Physiol Heart Circ Physiol. 2005;288:H2931–H2939. doi: 10.1152/ajpheart.01220.2004. [DOI] [PubMed] [Google Scholar]
- 42.Satoh H., Delbridge L.M., Blatter L.A., Bers D.M. Surface:volume relationship in cardiac myocytes studied with confocal microscopy and membrane capacitance measurements: species-dependence and developmental effects. Biophys J. 1996;70:1494–1504. doi: 10.1016/S0006-3495(96)79711-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.El Chemaly A., Guinamard R., Demion M., Fares N., Jebara V., Faivre J.F. A voltage-activated proton current in human cardiac fibroblasts. Biochem Biophys Res Commun. 2006;340:512–516. doi: 10.1016/j.bbrc.2005.12.038. [DOI] [PubMed] [Google Scholar]
- 44.Hou L.Q., Hu B., Jalife J. Genetically engineered excitable cardiac myofibroblasts coupled to cardiomyocytes rescue normal propagation and reduce arrhythmia complexity in heterocellular monolayers. PLoS One. 2013:8. doi: 10.1371/journal.pone.0055400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miragoli M., Salvarani N., Rohr S. Myofibroblasts induce ectopic activity in cardiac tissue. Circ Res. 2007;101:755–758. doi: 10.1161/CIRCRESAHA.107.160549. [DOI] [PubMed] [Google Scholar]
- 46.Fahrenbach J.P., Mejia-Alvarez R., Banach K. The relevance of non-excitable cells for cardiac pacemaker function. J Physiol Lond. 2007;585:565–578. doi: 10.1113/jphysiol.2007.144121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jacquemet V., Henriquez C.S. Loading effect of fibroblast-myocyte coupling on resting potential, impulse propagation, and repolarization: insights from a microstructure model. Am J Physiol Heart Circ Physiol. 2008;294:H2040–H2052. doi: 10.1152/ajpheart.01298.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kizana E., Ginn S.L., Smyth C.M., Boyd A., Thomas S.P., Allen D.G. Fibroblasts modulate cardiomyocyte excitability: implications for cardiac gene therapy. Gene Ther. 2006;13:1611–1615. doi: 10.1038/sj.gt.3302813. [DOI] [PubMed] [Google Scholar]
- 49.Maleckar M.M., Greenstein J.L., Giles W.R., Trayanova N.A. Electrotonic coupling between human atrial myocytes and fibroblasts alters myocyte excitability and repolarization. Biophys J. 2009;97:2179–2190. doi: 10.1016/j.bpj.2009.07.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McDowell K.S., Vadakkumpadan F., Blake R., Blauer J., Plank G., Macleod R.S. Mechanistic inquiry into the role of tissue remodeling in fibrotic lesions in human atrial fibrillation. Biophys J. 2013;104:2764–2773. doi: 10.1016/j.bpj.2013.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nayak A.R., Shajahan T.K., Panfilov A.V., Pandit R. Spiral-wave dynamics in a mathematical model of human ventricular tissue with myocytes and fibroblasts. PLoS One. 2013;8:e72950. doi: 10.1371/journal.pone.0072950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Burt J.M., Frank J.S., Berns M.W. Permeability and structural studies of heart cell gap junctions under normal and altered ionic conditions. J Membr Biol. 1982;68:227–238. doi: 10.1007/BF01872267. [DOI] [PubMed] [Google Scholar]
- 53.Chilton L., Giles W.R., Smith G.L. Evidence of intercellular coupling between co-cultured adult rabbit ventricular myocytes and myofibroblasts. J Physiol Lond. 2007;583:225–236. doi: 10.1113/jphysiol.2007.135038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.He K.M., Shi X.L., Zhang X.J., Dang S., Ma X.W., Liu F. Long-distance intercellular connectivity between cardiomyocytes and cardiofibroblasts mediated by membrane nanotubes. Cardiovasc Res. 2011;92:39–47. doi: 10.1093/cvr/cvr189. [DOI] [PubMed] [Google Scholar]
- 55.McSpadden L.C., Kirkton R.D., Bursac N. Electrotonic loading of anisotropic cardiac monolayers by unexcitable cells depends on connexin type and expression level. Am J Physiol Cell Physiol. 2009;297:C339–C351. doi: 10.1152/ajpcell.00024.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Askar S.F., Bingen B.O., Swildens J., Ypey D.L., van der Laarse A., Atsma D.E. Connexin43 silencing in myofibroblasts prevents arrhythmias in myocardial cultures: role of maximal diastolic potential. Cardiovasc Res. 2012;93:434–444. doi: 10.1093/cvr/cvr351. [DOI] [PubMed] [Google Scholar]
- 57.Jansen J.A., van Veen T.A.B.., de Jong S., van der Nagel R., van Stuijvenberg L., Driessen H. Reduced Cx43 expression triggers increased fibrosis due to enhanced fibroblast activity. Circ Arrhythm Electrophysiol. 2012;5:380–390. doi: 10.1161/CIRCEP.111.966580. [DOI] [PubMed] [Google Scholar]
- 58.Spath C., Schlegel F., Leontyev S., Mohr F.W., Dhein S. Inverse relationship between tumor proliferation markers and connexin expression in a malignant cardiac tumor originating from mesenchymal stem cell engineered tissue in a rat in vivo model. Front Pharmacol. 2013;4:42. doi: 10.3389/fphar.2013.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yankelson L., Feld Y., Bressler-Stramer T., Itzhaki I., Huber I., Gepstein A. Cell therapy for modification of the myocardial electrophysiological substrate. Circulation. 2008;117:720–731. doi: 10.1161/CIRCULATIONAHA.106.671776. [DOI] [PubMed] [Google Scholar]
- 60.Kohl P., Camelliti P. Cardiac myocyte-nonmyocyte electrotonic coupling: implications for ventricular arrhythmogenesis. Heart Rhythm. 2007;4:233–235. doi: 10.1016/j.hrthm.2006.10.014. [DOI] [PubMed] [Google Scholar]
- 61.Keith A., Flack M.W. The auriculo-ventricular bundle of the human heart. 1906. Ann Noninvasive Electrocardiol. 2004;9:400–409. doi: 10.1111/j.1542-474X.2004.94003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kohl P., Camelliti P. Fibroblast-myocyte connections in the heart. Heart Rhythm. 2012;9:461–464. doi: 10.1016/j.hrthm.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 63.Kohl P., Kamkin A.G., Kiseleva I.S., Streubel T. Mechanosensitive cells in the atrium of frog-heart. Exp Physiol. 1992;77:213–216. doi: 10.1113/expphysiol.1992.sp003576. [DOI] [PubMed] [Google Scholar]
- 64.Kamkin A., Kiseleva I., Lozinsky I., Scholz H. Electrical interaction of mechanosensitive fibroblasts and myocytes in the heart. Basic Res Cardiol. 2005;100:337–345. doi: 10.1007/s00395-005-0529-4. [DOI] [PubMed] [Google Scholar]
- 65.Camelliti P., Green C.R., LeGrice I., Kohl P. Fibroblast network in rabbit sinoatrial node: structural and functional identification of homogeneous and heterogeneous cell coupling. Circ Res. 2004;94:828–835. doi: 10.1161/01.RES.0000122382.19400.14. [DOI] [PubMed] [Google Scholar]
- 66.Vanbreemen V.L. Intercalated discs in heart muscle studied with the electron microscope. Anat Rec. 1953;117:49–63. doi: 10.1002/ar.1091170106. [DOI] [PubMed] [Google Scholar]
- 67.Barr L., Dewey M.M., Berger W. Propagation of action potentials and the structure of the nexus in cardiac muscle. J Gen Physiol. 1965;48:797–823. doi: 10.1085/jgp.48.5.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Revel J.P., Karnovsky M.J. Hexagonal array of subunits in intercellular junctions of the mouse heart and liver. J Cell Biol. 1967;33:C7–C12. doi: 10.1083/jcb.33.3.c7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Goodenough D.A., Stoeckenius W. The isolation of mouse hepatocyte gap junctions. Preliminary chemical characterization and x-ray diffraction. J Cell Biol. 1972;54:646–656. doi: 10.1083/jcb.54.3.646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Young J.D.E., Cohn Z.A., Gilula N.B. Functional assembly of gap junction conductance in lipid bilayers — demonstration that the major 27-Kd protein forms the junctional channel. Cell. 1987;48:733–743. doi: 10.1016/0092-8674(87)90071-7. [DOI] [PubMed] [Google Scholar]
- 71.Beyer E.C., Paul D.L., Goodenough D.A. Connexin43 — a protein from rat-heart homologous to a gap junction protein from liver. J Cell Biol. 1987;105:2621–2629. doi: 10.1083/jcb.105.6.2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Green C.R., Severs N.J. Connexon rearrangement in cardiac gap-junctions — evidence for cytoskeletal control. Cell Tissue Res. 1984;237:185–186. doi: 10.1007/BF00229215. [DOI] [PubMed] [Google Scholar]
- 73.Dupont E., Elaoumari A., Roustiausevere S., Briand J.P., Gros D. Immunological characterization of rat cardiac gap-junctions — presence of common antigenic determinants in heart of other vertebrate species and in various organs. J Membr Biol. 1988;104:119–128. doi: 10.1007/BF01870924. [DOI] [PubMed] [Google Scholar]
- 74.Beyer E.C., Kistler J., Paul D.L., Goodenough D.A. Antisera directed against connexin43 peptides react with a 43-kD protein localized to gap junctions in myocardium and other tissues. J Cell Biol. 1989;108:595–605. doi: 10.1083/jcb.108.2.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Luke R.A., Beyer E.C., Hoyt R.H., Saffitz J.E. Quantitative analysis of intercellular connections by immunohistochemistry of the cardiac gap junction protein connexin43. Circ Res. 1989;65:1450–1457. doi: 10.1161/01.res.65.5.1450. [DOI] [PubMed] [Google Scholar]
- 76.Zhang J.T., Nicholson B.J. Sequence and tissue distribution of a second protein of hepatic gap junctions, Cx26, as deduced from its cDNA. J Cell Biol. 1989;109:3391–3401. doi: 10.1083/jcb.109.6.3391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gourdie R.G., Harfst E., Severs N.J., Green C.R. Cardiac gap junctions in rat ventricle: localization using site-directed antibodies and laser scanning confocal microscopy. Cardioscience. 1990;1:75–82. [PubMed] [Google Scholar]
- 78.Severs N.J., Gourdie R.G., Harfst E., Peters N.S., Green C.R. Intercellular junctions and the application of microscopical techniques: the cardiac gap junction as a case model. J Microsc. 1993;169:299–328. doi: 10.1111/j.1365-2818.1993.tb03308.x. [DOI] [PubMed] [Google Scholar]
- 79.Peters N.S., Green C.R., Poolewilson P.A., Severs N.J. Cardiac arrhythmogenesis and the gap junction. J Mol Cell Cardiol. 1995;27:37–44. doi: 10.1016/s0022-2828(08)80005-3. [DOI] [PubMed] [Google Scholar]
- 80.Gourdie R.G. A map of the heart — gap-junctions, connexin diversity and retroviral studies of conduction myocyte lineage. Clin Sci. 1995;88:257–262. doi: 10.1042/cs0880257. [DOI] [PubMed] [Google Scholar]
- 81.Saffitz J.E., Davis L.M., Darrow B.J., Kanter H.L., Laing J.G., Beyer E.C. The molecular basis of anisotropy — role of gap-junctions. J Cardiovasc Electrophysiol. 1995;6:498–510. doi: 10.1111/j.1540-8167.1995.tb00423.x. [DOI] [PubMed] [Google Scholar]
- 82.Gros D.B., Jongsma H.J. Connexins in mammalian heart function. Bioessays. 1996;18:719–730. doi: 10.1002/bies.950180907. [DOI] [PubMed] [Google Scholar]
- 83.Morley G.E., EkVitorin J.F., Taffet S.M., Delmar M. Structure of connexin43 and its regulation by pH(i) J Cardiovasc Electrophysiol. 1997;8:939–951. doi: 10.1111/j.1540-8167.1997.tb00856.x. [DOI] [PubMed] [Google Scholar]
- 84.Saffitz J.E., Yamada K.A. Do alterations in intercellular coupling play a role in cardiac contractile dysfunction? Circulation. 1998;97:630–632. doi: 10.1161/01.cir.97.7.630. [DOI] [PubMed] [Google Scholar]
- 85.Ya J., Erdtsieck-Ernste E.B.H.W., de Boer P.A.J., van Kempen M.J.A., Jongsma H., Gros D. Heart defects in connexin43-deficient mice. Circ Res. 1998;82:360–366. doi: 10.1161/01.res.82.3.360. [DOI] [PubMed] [Google Scholar]
- 86.Dhein S. Gap junction channels in the cardiovascular system: pharmacological and physiological modulation. Trends Pharmacol Sci. 1998;19:229–241. doi: 10.1016/s0165-6147(98)01192-4. [DOI] [PubMed] [Google Scholar]
- 87.Yamasaki H., Krutovskikh V., Mesnil M., Tanaka T., Zaidan-Dagli M.L., Omori Y. Role of connexin (gap junction) genes in cell growth control and carcinogenesis. C R Acad Sci III Sci Vie Life Sci. 1999;322:151–159. doi: 10.1016/s0764-4469(99)80038-9. [DOI] [PubMed] [Google Scholar]
- 88.Unger V.M., Kumar N.M., Gilula N.B., Yeager M. Electron cryo-crystallography of a recombinant cardiac gap junction channel. Novartis Found Symp. 1999;219:22–37. doi: 10.1002/9780470515587.ch3. [DOI] [PubMed] [Google Scholar]
- 89.de Mello W.C. Cell coupling and impulse propagation in the failing heart. J Cardiovasc Electrophysiol. 1999;10:1409–1420. doi: 10.1111/j.1540-8167.1999.tb00197.x. [DOI] [PubMed] [Google Scholar]
- 90.Jalife J., Morley G.E., Vaidya D. Connexins and impulse propagation in the mouse heart. J Cardiovasc Electrophysiol. 1999;10:1649–1663. doi: 10.1111/j.1540-8167.1999.tb00230.x. [DOI] [PubMed] [Google Scholar]
- 91.Hunter P.J., Kohl P., Noble D. Integrative models of the heart: achievements and limitations. Philos Trans R Soc Lond A. 2001;359:1049–1054. [Google Scholar]
- 92.Austin T.M., Hooks D.A., Hunter P.J., Nickerson D.P., Pullan A.J., Sands G.B. Modeling cardiac electrical activity at the cell and tissue levels. Ann N Y Acad Sci. 2006;1080:334–347. doi: 10.1196/annals.1380.025. [DOI] [PubMed] [Google Scholar]
- 93.Trayanova N.A., Constantino J., Gurev V. Models of stretch-activated ventricular arrhythmias. J Electrocardiol. 2010;43:479–485. doi: 10.1016/j.jelectrocard.2010.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rodriguez B., Burrage K., Gavaghan D., Grau V., Kohl P., Noble D. The systems biology approach to drug development: application to toxicity assessment of cardiac drugs. Clin Pharmacol Ther. 2010;88:130–134. doi: 10.1038/clpt.2010.95. [DOI] [PubMed] [Google Scholar]
- 95.Bers D.M., Grandi E. Human atrial fibrillation: insights from computational electrophysiological models. Trends Cardiovasc Med. 2011;21:145–150. doi: 10.1016/j.tcm.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Campbell S.G., McCulloch A.D. Multi-scale computational models of familial hypertrophic cardiomyopathy: genotype to phenotype. J R Soc Interface. 2011;8:1550–1561. doi: 10.1098/rsif.2011.0184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Clayton R.H., Bernus O., Cherry E.M., Dierckx H., Fenton F.H., Mirabella L. Models of cardiac tissue electrophysiology: progress, challenges and open questions. Prog Biophys Mol Biol. 2011;104:22–48. doi: 10.1016/j.pbiomolbio.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 98.Roberts B.N., Yang P.C., Behrens S.B., Moreno J.D., Clancy C.E. Computational approaches to understand cardiac electrophysiology and arrhythmias. Am J Physiol Heart Circ Physiol. 2012;303:H766–H783. doi: 10.1152/ajpheart.01081.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Smaill B.H., Zhao J.C., Trew M.L. Three-dimensional impulse propagation in myocardium: arrhythmogenic mechanisms at the tissue level. Circ Res. 2013;112:834–848. doi: 10.1161/CIRCRESAHA.111.300157. [DOI] [PubMed] [Google Scholar]
- 100.Quinn T.A., Kohl P. Combining wet and dry research: experience with model development for cardiac mechano-electric structure-function studies. Cardiovasc Res. 2013;97:601–611. doi: 10.1093/cvr/cvt003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Niederer S.A., Land S., Omholt S.W., Smith N.P. Interpreting genetic effects through models of cardiac electromechanics. Am J Physiol Heart Circ Physiol. 2012;303:H1294–H1303. doi: 10.1152/ajpheart.00121.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Potse M. Mathematical modeling and simulation of ventricular activation sequences: implications for cardiac resynchronization therapy. J Cardiovasc Transl Res. 2012;5:146–158. doi: 10.1007/s12265-011-9343-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Camelliti P., Green C.R., Kohl P. Structural and functional coupling of cardiac myocytes and fibroblasts. Adv Cardiol. 2006;42:132–149. doi: 10.1159/000092566. [DOI] [PubMed] [Google Scholar]
- 104.Beny J.L., Connat J.L. An electron-microscopic study of smooth-muscle cell dye coupling in the pig coronary arteries — role of gap-junctions. Circ Res. 1992;70:49–55. doi: 10.1161/01.res.70.1.49. [DOI] [PubMed] [Google Scholar]
- 105.Yamamoto Y., Klemm M.F., Edwards F.R., Suzuki H. Intercellular electrical communication among smooth muscle and endothelial cells in guinea-pig mesenteric arterioles. J Physiol Lond. 2001;535:181–195. doi: 10.1111/j.1469-7793.2001.00181.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Green C.R., Peters N.S., Gourdie R.G., Rothery S., Severs N.J. Validation of immunohistochemical quantification in confocal scanning laser microscopy: a comparative assessment of gap junction size with confocal and ultrastructural techniques. J Histochem Cytochem. 1993;41:1339–1349. doi: 10.1177/41.9.8354875. [DOI] [PubMed] [Google Scholar]
- 107.Rustom A., Saffrich R., Markovic I., Walther P., Gerdes H.H. Nanotubular highways for intercellular organelle transport. Science. 2004;303:1007–1010. doi: 10.1126/science.1093133. [DOI] [PubMed] [Google Scholar]
- 108.Chinnery H.R., Pearlman E., McMenamin P.G. Cutting edge: membrane nanotubes in vivo: a feature of MHC class II + cells in the mouse cornea. J Immunol. 2008;180:5779–5783. doi: 10.4049/jimmunol.180.9.5779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Davis D.M., Sowinski S. Membrane nanotubes: dynamic long-distance connections between animal cells. Nat Rev Mol Cell Biol. 2008;9:431–436. doi: 10.1038/nrm2399. [DOI] [PubMed] [Google Scholar]
- 110.Pratola C., Baldo E., Notarstefano P., Toselli T., Ferrari R. Radiofrequency ablation of atrial fibrillation: is the persistence of all intraprocedural targets necessary for long-term maintenance of sinus rhythm? Circulation. 2008;117:136–143. doi: 10.1161/CIRCULATIONAHA.106.678789. [DOI] [PubMed] [Google Scholar]
- 111.Lefroy D.C., Fang J.C., Stevenson L.W., Hartley L.H., Friedman P.L., Stevenson W.G. Recipient-to-donor atrioatrial conduction after orthotopic heart transplantation: surface electrocardiographic features and estimated prevalence. Am J Cardiol. 1998;82:444–450. doi: 10.1016/s0002-9149(98)00359-2. [DOI] [PubMed] [Google Scholar]
- 112.Hager A., Zrenner B., Brodherr-Heberlein S., Steinbauer-Rosenthal I., Schreieck J., Hess J. Congenital and surgically acquired Wolff-Parkinson-White syndrome in patients with tricuspid atresia. J Thorac Cardiovasc Surg. 2005;130:48–53. doi: 10.1016/j.jtcvs.2004.11.017. [DOI] [PubMed] [Google Scholar]
- 113.Birnie D., Green M.S., Veinot J.P., Tang A.S., Davies R.A. Interatrial conduction of atrial tachycardia in heart transplant recipients: potential pathophysiology. J Heart Lung Transplant. 2000;19:1007–1010. doi: 10.1016/s1053-2498(00)00152-2. [DOI] [PubMed] [Google Scholar]
- 114.Chatelier A., Mercier A., Tremblier B., Theriault O., Moubarak M., Benamer N. A distinct de novo expression of Nav1.5 sodium channels in human atrial fibroblasts differentiated into myofibroblasts. J Physiol Lond. 2012;590:4307–4319. doi: 10.1113/jphysiol.2012.233593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Camelliti P., Devlin G.P., Matthews K.G., Kohl P., Green C.R. Spatially and temporally distinct expression of fibroblast connexins after sheep ventricular infarction. Cardiovasc Res. 2004;62:415–425. doi: 10.1016/j.cardiores.2004.01.027. [DOI] [PubMed] [Google Scholar]
- 116.Walker N.L., Burton F.L., Kettlewell S., Smith G.L., Cobbe S.M. Mapping of epicardial activation in a rabbit model of chronic myocardial infarction: response to atrial, endocardial, and epicardial pacing. J Cardiovasc Electrophysiol. 2007;18:862–868. doi: 10.1111/j.1540-8167.2007.00858.x. [DOI] [PubMed] [Google Scholar]
- 117.Kohl P., Camelliti P., Burton F.L., Smith G.L. Electrical coupling of fibroblasts and myocytes: relevance for cardiac propagation. J Electrocardiol. 2005;38:45–50. doi: 10.1016/j.jelectrocard.2005.06.096. [DOI] [PubMed] [Google Scholar]
- 118.Saba S., Mathier M.A., Mehdi H., Liu T., Choi B.R., London B. Dual-dye optical mapping after myocardial infarction: does the site of ventricular stimulation alter the properties of electrical propagation? J Cardiovasc Electrophysiol. 2008;19:197–202. doi: 10.1111/j.1540-8167.2007.00998.x. [DOI] [PubMed] [Google Scholar]
- 119.Ripplinger C.M., Lou Q., Li W., Hadley J., Efimov I.R. Panoramic imaging reveals basic mechanisms of induction and termination of ventricular tachycardia in rabbit heart with chronic infarction: implications for low-voltage cardioversion. Heart Rhythm. 2009;6:87–97. doi: 10.1016/j.hrthm.2008.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Daskalopoulos E.P., Janssen B.J.A., Blankesteijn W.M. Myofibroblasts in the infarct area: concepts and challenges. Microsc Microanal. 2012;18:35–49. doi: 10.1017/S143192761101227X. [DOI] [PubMed] [Google Scholar]
- 121.Salameh A., Dhein S. Effects of mechanical forces and stretch on intercellular gap junction coupling. Biochim Biophys Acta Biomembr. 1828;2013:147–156. doi: 10.1016/j.bbamem.2011.12.030. [DOI] [PubMed] [Google Scholar]
- 122.Vasquez C., Mohandas P., Louie K.L., Benamer N., Bapat A.C., Morley G.E. Enhanced fibroblast-myocyte interactions in response to cardiac injury. Circ Res. 2010;107:1011–1020. doi: 10.1161/CIRCRESAHA.110.227421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kamkin A., Kiseleva I., Isenberg G. Ion selectivity of stretch-activated cation currents in mouse ventricular myocytes. Pflugers Arch. 2003;446:220–231. doi: 10.1007/s00424-003-1018-y. [DOI] [PubMed] [Google Scholar]
- 124.Kamkin A., Kirischuk S., Kiseleva I. Single mechano-gated channels activated by mechanical deformation of acutely isolated cardiac fibroblasts from rats. Acta Physiol. 2010;199:277–292. doi: 10.1111/j.1748-1716.2010.02086.x. [DOI] [PubMed] [Google Scholar]
- 125.Yue Z., Zhang Y., Xie J., Jiang J., Yue L. Transient receptor potential (TRP) channels and cardiac fibrosis. Curr Top Med Chem. 2013;13:270–282. doi: 10.2174/1568026611313030005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kiseleva I., Kamkin A., Kohl P., Lab M.J. Calcium and mechanically induced potentials in fibroblasts of rat atrium. Cardiovasc Res. 1996;32:98–111. [PubMed] [Google Scholar]
- 127.Kohl P., Sachs F. Mechanoelectric feedback in cardiac cells. Philos Trans R Soc Lond A. 2001;359:1173–1185. [Google Scholar]
- 128.Benamer N., Vasquez C., Mahoney V.M., Steinhardt M.J., Coetzee W.A., Morley G.E. Fibroblast KATP currents modulate myocyte electrophysiology in infarcted hearts. Am J Physiol Heart Circ Physiol. 2013;304:H1231–H1239. doi: 10.1152/ajpheart.00878.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Bell R.M., Yellon D.M. Conditioning the whole heart—not just the cardiomyocyte. J Mol Cell Cardiol. 2012;53:24–32. doi: 10.1016/j.yjmcc.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 130.Burstein B., Libby E., Calderone A., Nattel S. Differential behaviors of atrial versus ventricular fibroblasts: a potential role for platelet-derived growth factor in atrial-ventricular remodeling differences. Circulation. 2008;117:1630–1641. doi: 10.1161/CIRCULATIONAHA.107.748053. [DOI] [PubMed] [Google Scholar]
- 131.Vadakkumpadan F., Arevalo H., Prassl A.J., Chen J., Kickinger F., Kohl P. Image-based models of cardiac structure in health and disease. Wiley Interdiscip Rev Syst Biol Med. 2010;2:489–506. doi: 10.1002/wsbm.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Weiss J.N., Nivala M., Garfinkel A., Qu Z. Alternans and arrhythmias: from cell to heart. Circ Res. 2011;108:98–112. doi: 10.1161/CIRCRESAHA.110.223586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Dossel O., Krueger M.W., Weber F.M., Wilhelms M., Seemann G. Computational modeling of the human atrial anatomy and electrophysiology. Med Biol Eng Comput. 2012;50:773–799. doi: 10.1007/s11517-012-0924-6. [DOI] [PubMed] [Google Scholar]
- 134.Niederer S.A., Smith N.P. At the heart of computational modelling. J Physiol Lond. 2012;590:1331–1338. doi: 10.1113/jphysiol.2011.225045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Xie Y., Garfinkel A., Camelliti P., Kohl P., Weiss J.N., Qu Z. Effects of fibroblast-myocyte coupling on cardiac conduction and vulnerability to reentry: a computational study. Heart Rhythm. 2009;6:1641–1649. doi: 10.1016/j.hrthm.2009.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.McDowell K.S., Arevalo H.J., Maleckar M.M., Trayanova N.A. Susceptibility to arrhythmia in the infarcted heart depends on myofibroblast density. Biophys J. 2011;101:1307–1315. doi: 10.1016/j.bpj.2011.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Rutherford S.L., Trew M.L., Sands G.B., LeGrice I.J., Smaill B.H. High-resolution 3-dimensional reconstruction of the infarct border zone: impact of structural remodeling on electrical activation. Circ Res. 2012;111:301–311. doi: 10.1161/CIRCRESAHA.111.260943. [DOI] [PubMed] [Google Scholar]
- 138.Li W., Ripplinger C.M., Lou Q., Efimov I.R. Multiple monophasic shocks improve electrotherapy of ventricular tachycardia in a rabbit model of chronic infarction. Heart Rhythm. 2009;6:1020–1027. doi: 10.1016/j.hrthm.2009.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Rantner L.J., Arevalo H.J., Constantino J.L., Efimov I.R., Plank G., Trayanova N.A. Three-dimensional mechanisms of increased vulnerability to electric shocks in myocardial infarction: altered virtual electrode polarizations and conduction delay in the peri-infarct zone. J Physiol Lond. 2012;590:4537–4551. doi: 10.1113/jphysiol.2012.229088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Quinn T.A., Kohl P. Systems biology of the heart: hype or hope? Ann N Y Acad Sci. 2011;1245:40–43. doi: 10.1111/j.1749-6632.2011.06327.x. [DOI] [PubMed] [Google Scholar]
- 141.Claycomb W.C. Biochemical aspects of cardiac muscle differentiation. Possible control of deoxyribonucleic acid synthesis and cell differentiation by adrenergic innervation and cyclic adenosine 3':5'-monophosphate. J Biol Chem. 1976;251:6082–6089. [PubMed] [Google Scholar]
- 142.Katano Y., Akera T., Temma K., Kennedy R.H. Enhanced ouabain sensitivity of the heart and myocardial sodium pump in aged rats. Eur J Pharmacol. 1984;105:95–103. doi: 10.1016/0014-2999(84)90652-6. [DOI] [PubMed] [Google Scholar]
- 143.Yasuhara S., Takaki M., Kikuta A., Ito H., Suga H. Myocardial VO2 of mechanically unloaded contraction of rat ventricular slices measured by a new approach. Am J Physiol Heart Circ Physiol. 1996;270:H1063–H1070. doi: 10.1152/ajpheart.1996.270.3.H1063. [DOI] [PubMed] [Google Scholar]
- 144.DeBoer T., Camelliti P., Ravens U., Kohl P. Myocardial tissue slices: organotypic pseudo-twodimensional models for cardiac research & development. Future Cardiol. 2009;5:425–430. doi: 10.2217/fca.09.32. [DOI] [PubMed] [Google Scholar]
- 145.Bussek A., Schmidt M., Bauriedl J., Ravens U., Wettwer E., Lohmann H. Cardiac tissue slices with prolonged survival for in vitro drug safety screening. J Pharmacol Toxicol Methods. 2012;66:145–151. doi: 10.1016/j.vascn.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 146.Lee P., Klos M., Bollensdorff C., Hou L., Ewart P., Kamp T.J. Simultaneous voltage and calcium mapping of genetically purified human induced pluripotent stem cell-derived cardiac myocyte monolayers. Circ Res. 2012;110:1556–1563. doi: 10.1161/CIRCRESAHA.111.262535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Camelliti P., Al-Saud S.A., Smolenski R.T., Al-Ayoubi S., Bussek A., Wettwer E. Adult human heart slices are a multicellular system suitable for electrophysiological and pharmacological studies. J Mol Cell Cardiol. 2011;51:390–398. doi: 10.1016/j.yjmcc.2011.06.018. [DOI] [PubMed] [Google Scholar]
- 148.Bussek A., Wettwer E., Christ T., Lohmann H., Camelliti P., Ravens U. Tissue slices from adult mammalian hearts as a model for pharmacological drug testing. Cell Physiol Biochem. 2009;24:527–536. doi: 10.1159/000257528. [DOI] [PubMed] [Google Scholar]
- 149.Bishop M.J., Gavaghan D.J., Trayanova N.A., Rodriguez B. Photon scattering effects in optical mapping of propagation and arrhythmogenesis in the heart. J Electrocardiol. 2007;40:S75–S80. doi: 10.1016/j.jelectrocard.2007.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Jia Z., Valiunas V., Lu Z., Bien H., Liu H., Wang H.Z. Stimulating cardiac muscle by light: cardiac optogenetics by cell delivery. Circ Arrhythm Electrophysiol. 2011;4:753–760. doi: 10.1161/CIRCEP.111.964247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Roell W., Lewalter T., Sasse P., Tallini Y.N., Choi B.R., Breitbach M. Engraftment of connexin 43-expressing cells prevents post-infarct arrhythmia. Nature. 2007;450:819–824. doi: 10.1038/nature06321. [DOI] [PubMed] [Google Scholar]
- 152.Noorman M., van der Heyden M.A.G., van Veen T.A.B.., Cox M.G.P.J., Hauer R.N.W., de Bakker J.M.T. Cardiac cell-cell junctions in health and disease: electrical versus mechanical coupling. J Mol Cell Cardiol. 2009;47:23–31. doi: 10.1016/j.yjmcc.2009.03.016. [DOI] [PubMed] [Google Scholar]
- 153.Driesen R.B., Dispersyn G.D., Verheyen F.K., van den Eijnde S.M., Hofstra L., Thone F. Partial cell fusion: a newly recognized type of communication between dedifferentiating cardiomyocytes and fibroblasts. Cardiovasc Res. 2005;68:37–46. doi: 10.1016/j.cardiores.2005.05.020. [DOI] [PubMed] [Google Scholar]
- 154.Thompson S.A., Copeland C.R., Reich D.H., Tung L. Mechanical coupling between myofibroblasts and cardiomyocytes slows electric conduction in fibrotic cell monolayers. Circulation. 2011;123:2083–2093. doi: 10.1161/CIRCULATIONAHA.110.015057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Plotnikov S.V., Waterman C.M. Guiding cell migration by tugging. Curr Opin Cell Biol. 2013;25:619–626. doi: 10.1016/j.ceb.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Rhett J.M., Veeraraghavan R., Poelzing S., Gourdie R.G. The perinexus: sign-post on the path to a new model of cardiac conduction? Trends Cardiovasc Med. 2013;23:222–228. doi: 10.1016/j.tcm.2012.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Suenson M. Ephaptic impulse transmission between ventricular myocardial cells in vitro. Acta Physiol Scand. 1984;120:445–455. doi: 10.1111/j.1748-1716.1984.tb07405.x. [DOI] [PubMed] [Google Scholar]
- 158.Mori Y., Fishman G.I., Peskin C.S. Ephaptic conduction in a cardiac strand model with 3D electrodiffusion. Proc Natl Acad Sci U S A. 2008;105:6463–6468. doi: 10.1073/pnas.0801089105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Lin J., Keener J.P. Modeling electrical activity of myocardial cells incorporating the effects of ephaptic coupling. Proc Natl Acad Sci U S A. 2010;107:20935–20940. doi: 10.1073/pnas.1010154107. [DOI] [PMC free article] [PubMed] [Google Scholar]