Abstract
Background
Atrial fibrillation (AF) is a cause of stroke, and undertreatment with anticoagulants is a persistent issue despite their effectiveness.
Aim
To increase the proportion of people with AF treated appropriately using anticoagulants, and reduce inappropriate antiplatelet therapy.
Design of study
Cross-sectional analysis.
Setting
Electronic patient health records on 4604 patients with AF obtained from general practices in three inner London primary care trusts between April 2011 and 2013.
Method
The Anticoagulant Programme East London (APEL) sought to achieve its aims through an intervention with three components: altering professional beliefs using new clinical guidance and related education; facilitating change using computer software to support clinical decisions and patient review optimising anticoagulation; motivating change through evaluative feedback showing individual practice performance relative to peers.
Results
From April 2011 to April 2013, the proportion of people with CHA2DS2-VASc ≥1 on anticoagulants increased from 52.6% to 59.8% (trend difference P<0.001). The proportion of people with CHA2DS2-VASc ≥1 on aspirin declined from 37.7% to 30.3% (trend difference P<0.001). Comparing the 2 years before the intervention with the 2 years after, numbers of new people on the AF register almost doubled from 108 to 204.
Conclusions
The APEL programme supports improvement in clinical managing AF by a combined programme of education around agreed guidance, computer aids to facilitate decision-making and patient review and feedback of locally identifiable results. If replicated nationally over 3 years, such a programme could result in approximately 1600 fewer strokes every year.
Keywords: anticoagulants, atrial fibrillation, clinical decision support systems, patterns, prescribing, primary health care, stroke
INTRODUCTION
Atrial fibrillation (AF) is diagnosed in one million people in the UK. Prevalence increases from 0.7% at 55–59 years to 18% >85 years of age, with variation by ethnic group.1,2 A 74-year-old man with AF and no other risk factors has a one in five chance of a stroke and a 40% chance of either a heart attack or stroke within 10 years, estimated using contemporary UK data.3,4 Diabetes and hypertension increase this risk to over 75%. AF accounts for one in eight strokes at all ages and one in three >80 years.5,6
In 2006, the National Institute for Health and Care Excellence (NICE) recommended anticoagulation with warfarin for patients with a CHADS2 score of ≥2, either anticoagulant or antiplatelet treatment for a score of 1, and antiplatelet agents or no treatment for a 0 score.7 Recent European guidelines recommend anticoagulation in patients who score ≥1 with the more accurate CHA2DS2-VASc score; that is anyone age ≥65 years or with at least one risk factor.5,8 For those with a CHA2DS2-VASc score of 0 — people aged <65 years with no other risk factors — no antithrombotic medication is recommended.5 Aspirin is no longer considered an acceptable antithrombotic agent for routine use in AF as it does not effectively reduce stroke and at older ages bleeding may result in net harm.9–11 In 2011, new oral anticoagulant agents (NOAC) were licensed in the UK and supported by NICE as a cost-effective treatment option. These currently include dabigatran, rivaroxaban, and apixaban.12
The problem
Despite the effectiveness of oral anticoagulants in AF, only 50–60% of patients received warfarin or newer anticoagulants.13–15 Fewer older patients with AF are prescribed anticoagulants, and anticoagulant use is poorly related to stroke risk.2,16 The slope of improvement in anticoagulant use has been static for more than 10 years, improving by 1% per annum to only 50% in 2012.14,17,18 Undertreatment at older ages persists in more recent studies.
Three main reasons have been proposed for underuse of anticoagulation; professional therapeutic caution about the balance of risks to benefit, and in particular uncertainty about the role of aspirin; poor quality organisational process including risk stratification; and patient complexity and non-adherence to therapy.6,19,20,21 Revision of current NICE guidance recommending antiplatelet agents is likely to advise against routine antiplatelet use and include new oral anticoagulant agents (NOACs).5,7 But translating up-to-date evidence into a step change in improved anticoagulation in primary care will require three main drivers for change. These are supported by theories of behaviour change and practical exemplars.22–26 They comprise altering professional beliefs using guidelines and education;26,27 making changes in treatment easier for staff and patients with computer decision support and patient recall tools;28 and motivating practices by publishing comparative performance.29,30 Financial incentives may further improve performance31 but were not used in the current study.
How this fits in
Over the last 10 years, the increase in the proportion of people with atrial fibrillation on anticoagulants has been slow at around 1% per year, and only 50% were receiving anticoagulant treatment in 2012. This study aimed at increasing the rate of improvement through implementation of a multiple component intervention in primary care. Anticoagulation rates improved by 3.5% per year after the intervention. If replicated nationally over 3 years, it is estimated that an increase of 10% in the proportion of people on anticoagulants (from 50% to 60%), would result in approximately 1600 fewer strokes every year.
This study describes an intervention using these three components to change clinical practice in all 143 general practices in east London, which serve a population of 800 000 people in three geographically coterminous primary care trusts (PCTs). The study aimed at increasing the proportion of people with AF treated appropriately with anticoagulants and reduce those inappropriately on antiplatelet therapy.
METHOD
Setting
The study was located in three coterminous inner east London boroughs of Tower Hamlets, Newham, and City and Hackney, and their PCTs, serving the population of 800 000 registered with 143 general practices in the area. The population has some of the highest levels of social deprivation in the UK and half are from ethnic minority groups including large South Asian and African–Caribbean communities.
Eligibility criteria and intervention
The programme of improvement was designed and coordinated by the Clinical Effectiveness Group (CEG) funded by the three participating PCTs and based at Queen Mary University of London. Patients were eligible if they had a diagnostic code of atrial fibrillation as specified in the Quality and Outcomes Framework (QOF).32 The Anticoagulation Programme East London (APEL) was started in April 2011. It aimed at influencing improvement in antithrombotic treatment of AF by altering professional beliefs, influencing clinical processes, and increasing motivation. Changing professional beliefs required provision of new summary clinical guidelines with associated peer education. The new guidance was developed with local stakeholders (GPs, consultants, and prescribing advisers), published by the CEG and sent to all participating practices and made available online. The guidance was also accessible in the routinely used GP clinical record, such that a single ‘click’ on the data entry template opened up the guidance or decision support algorithms.33 Peer education consisted of a multidisciplinary primary care team meeting in each of the three localities to discuss the evidence and implementation of improvement in AF anticoagulation.
The new guidance was supported with software tools to improve the process of decision making and implementation of care pathways for reviewing and assessing patients with AF and changes in their treatment. Computer software included the APEL tool, which was installed on all practice computers with instruction, to provide an accessible summary of treatment status of individual patients with AF and overall practice performance. This included relevant details such as comorbidities, palliative care, housebound, serious mental illness, alcohol use, falls, bleeding, non-steroidal anti-inflammatory drugs, and the CHA2DS2-VASc, CHADS2 and HAS-BLED scores. Standard data entry templates were used by all practices to ensure fidelity and completeness of coding. ‘Pop-up’ on-screen computer prompts were used by some practices as reminders if patients were on aspirin or no treatment. In April 2011, a baseline audit was circulated to practices that showed the proportion of patients with AF on anticoagulants and aspirin so that comparisons could be made between identifiable practices in the locality. Financial incentives may further improve performance34 but were not used in the current study.
Data collection
Anonymised data was extracted in May 2013 for 6-monthly periods in each of the 4 years commencing 1 April 2008 to 1 April 2013. The anonymised data were extracted from the electronic patient health record in all patients with AF in all general practices with accessible data in the three east London PCTs Newham, Tower Hamlets, and City and Hackney. These data were extracted using EMIS Web on secure N3 terminals. CHA2DS2-VASc, CHADS2 scores were complete on all patients. Prescribing was defined by at least one prescription in the preceding 6 months. The prescribing categories were mutually exclusive: warfarin alone or in combination with an antiplatelet agent; antiplatelet agents (aspirin and/or clopidogrel) did not include warfarin. Any prior comorbidity was recorded and blood pressure or other indices were the latest recorded values. CHA2DS2-VASc was calculated from the available data on all patients.
Outcomes
No exclusions were made from this cohort of people with AF. The primary outcome was proportion of patients with AF and CHA2DS2-VASc score ≥1 on anticoagulants. The secondary outcome was the proportion on antiplatelet agents.
Analysis
The study conformed to the STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) study design recommendations.34 The funnel plot was compiled using a spreadsheet from the Association of Public Health Observatories.35 All analyses were undertaken using Stata (version 10), and logistic regression was used to test whether the difference in the annual rate of increase before and after the intervention differed significantly from 0.
RESULTS
Data were available from 139/143 practices in the three inner London Boroughs of Newham, City and Hackney, and Tower Hamlets. Four practices could not contribute because they used a different computer system.
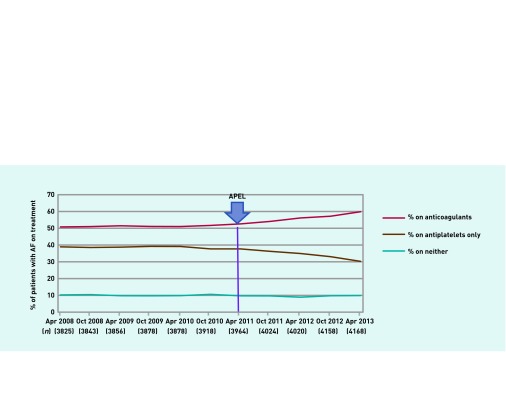
In the period before the intervention April 2008 to April 2011, in all three PCTs combined, the proportion of people with CHA2DS2-VASc ≥1 on an anticoagulant increased slowly from 50.8% (1943/3825) to 52.6% (2085/3964), a non-significant increase of 2.2% in 3 years; test to determine whether the increase differed from zero, P = 0.184.
After the intervention which started in April 2011, the proportion of people on anticoagulants increased from 52.6% (2085/3964) in April 2011 to 59.8% (2492/4168) by April 2013, an increase of 7.2% in 2 years. The difference in slope of the trends was 1.63; 95% confidence interval (CI) = 1.32 to 1.94, P<0.001. In people aged ≥80 years or over, anticoagulant prescription 2009 to 2013 increased by 9.0% (from 45.3% to 54.3%), whereas for people <80 years it increased by 6.6% (from 52.6% to 59.2%). These differences in increase between age groups were not significant (P = 0.598).
The proportion of people with CHA2DS2-VASc ≥1 on antiplatelets showed little change in the period 2008 to 2011, 38.8% to 37.7% (P = 0.458). From April 2011 there was a decline in antiplatelet use from 37.7% to 30.3%; a reduction of 7.4%. The difference in trend slope was −1.68 (95% CI = −2.21 to −1.16, P<0.001).
The proportion of people with AF on neither anticoagulants nor antiplatelet agents was 10.3% in April 2008 and 9.9% in April 2013, and did not change significantly (Figure 1).
Figure 1.
Anticoagulant and antiplatelet use in patients with AF with CHAD2S2-VASC ≥1.
These trends in anticoagulation in people with CHA2DS2-VASc ≥1 showed similarly significant improvement (P<0.001) in all three PCTs. City and Hackney 53.5% (748/1398) to 60.0% (882/147), difference 6.5%; Tower Hamlets 53.9% (622/1155) to 60.9% (745/1224), difference 7.0%; and Newham 50.7% (715/1411) to 58.9% (867/1473), difference 8.1%.
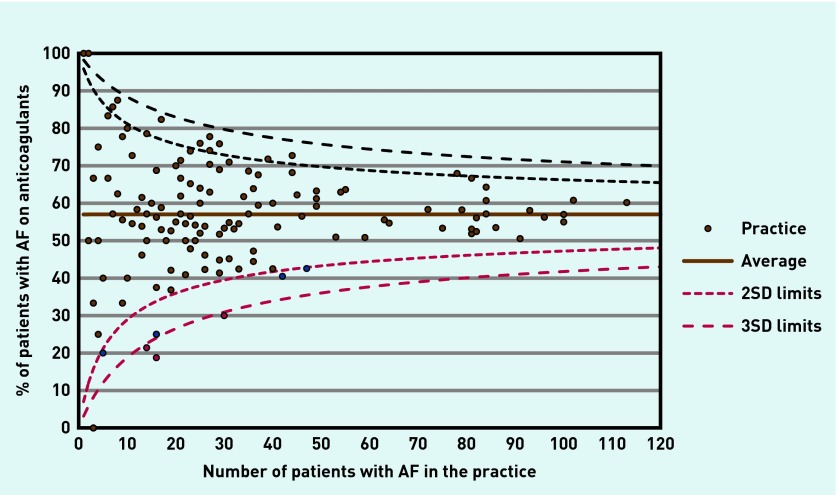
Individual practices showed substantial variation in prescribing of anticoagulants. Small numbers in small practices result in wide random variation. Figure 2 shows a funnel plot of the AF register size against proportion on anticoagulants in each practice, with 2 and 3 SD limits. Most practices fall within the 2 SD limit with six of 139 practices outside the lower 2 SD limit, and three outside the lower 3 SD limit. Of the 139 practices, 10.8% (15/139) prescribed 40% or fewer patients with anticoagulants and 16.5% (23/139) prescribed 70% or more anticoagulants. Total AF register size increased by 108 in the 2 years prior to the intervention and almost doubled to 204 in the 2 years after the intervention.
Figure 2.
Funnel plot: proportion on anticoagulants by east London practices.
DISCUSSION
Summary
The present study showed a threefold increase in the rate of improvement in anticoagulation in people with CHA2DS2-VASc ≥1 on anticoagulation increasing from 52.6% to 59.8%; 7.2% over 2 years in comparison with an increase of 2.2% over the preceding 3 years. Considerable variation persisted, much of it accountable by random variation in smaller practices. One in 10 practices had <40% or fewer patients with AF on anticoagulants, and one in six achieved ≥70% on anticoagulation. Overdispersion, with more than expected numbers of practices outside the lower 95% confidence limits indicate that systematic factors need to be considered, and that there is scope for further improvement in all practices to achieve ≥80% on anticoagulants.
Strengths and limitations
This study covers three entire local health economies in some of the most socially-diverse and disadvantaged boroughs in the UK, indicating the feasibility of improvement even in challenging local circumstances. No patients were excluded from this study.
The interventions — guidance, education, software enhancements, and evaluative feedback — are potentially available within every CCG and are generalisable. The initial involvement and cooperation of all stakeholders — haematologists, cardiologists, community and hospital prescribing advisers, PCT managers, GPs and their practice teams — were important components of the intervention, requiring a series of meetings and consultations to ensure that the strategy and content are agreeable to all participants. Since the end of the programme, financial incentives have been added to anticoagulation targets to support further improvement in two of the CCGs.
Since 2007 there have been national initiatives to improve anticoagulation, including financial incentives as part of the QOF and GRASP-AF, which made little impact in the period 2008–2011. The promotion of antiplatelet agents including aspirin, in line with the 2006 NICE guidance, may also have hindered improvements in anticoagulation rates before 2011. It was not possible to access data from a control primary care trust for the period under consideration. Therefore, it is, not possible to establish the extent to which changes in QOF 2012, when anticoagulation was separated from aspirin as a payment target, and the changed clinical consensus on aspirin in 2012,5 contributed to changes in 2012/13. The changes started, however, at the time of the intervention a year earlier.
Anticoagulant monitoring also improved. In Tower Hamlets and City and Hackney from 2009, and Newham from 2010, local practice- and pharmacy-based monitoring was established, with local community schemes for 30–60% of people on anticoagulants. This may have assisted the take-up and acceptability of anticoagulation with warfarin. Local prescribing advisers in Tower Hamlets have also included anticoagulant prescribing in incentive schemes since 2012.
Almost all practices in inner east London use a single web-enabled computer system (EMIS), which permits access to standard data entry templates, prompts, software tools such as the APEL tool, and evaluative reporting, and this was of benefit to this project. This functionality is replicable across all computer systems but may take more time to implement where CCGs use a diversity of systems. The CEG coordinated the programme with all three CCGs, providing leadership, stakeholder engagement, and a coherent plan including evaluation and this may be a ‘local’ factor in improvement. Clinical leads in each CCG were active local ‘champions’ promoting clear objectives and actions.
The intervention was complex and limited to only 2 years. It is difficult to estimate which components were responsible for the improvement observed and whether the rate of improvement will be sustained.
Comparison with existing literature
The UK General Practice Research Database showed an increase in the proportion on anticoagulants in patients aged ≥80 years from 25% in 2000 to 37% in 2009, and from 54% to 55% in those aged 60–69 years in this period.2,19
Attempts since 2007 by the NHS Improvement programme using the GRASP-AF software tool to improve anticoagulation had limited success.36 About one-quarter of practices in England UK participated, and anticoagulation increased from 49.3% to 54.7% over the period 2009–2012. In 2012, however, 36% remained on antiplatelet therapy alone and 11% on neither.37 Since 2007, the QOF financially incentivised antiplatelet and anticoagulant treatment, although until 2013 the impact on anticoagulation could not be disaggregated.38 Despite these initiatives, improvement remained static at 1% per annum; the proportions of people with CHADS2 ≥2 on anticoagulants were 49.7% in 2007 and 53% in 2010.14 The most recent national data 2012/13 from the QOF show that 65.1% of patients with AF received anticoagulants.39
Implications for research and practice
Checking and validation of people with AF is likely to have identified miscoding or resolved AF, particularly in those on no treatment or antiplatelet agents. The ‘cleaning’ of AF registers to remove patients who have left or been miscoded may have further contributed to denominator accuracy, increasing the proportion of the target group on anticoagulants, and this may be responsible for some of the observed effect. Despite culling of incorrect entries, new AF diagnoses almost doubled and was a wider consequence of the focus on AF management.
Anticoagulation is only one aspect of the patient pathway for AF from ascertainment to treatment, and raised blood pressure control, smoking, and statins also make a substantial impact on cardiovascular event reduction. Better access to monitoring and support for patient information and their continuing treatment can also improve patient experience and quality of life. Improvement in the current undertreatment of AF, however, is likely to have the single greatest impact and even in older patients with complex needs, ≥80% can be anticoagulated.40
Wide discrepancy in anticoagulation rates between practices remain with considerable room for further improvement. The 2012/13 QOF showed that although 29% of GP practices in London attained ≥70% on anticoagulants without exception, one-fifth had <55% of patients on anticoagulants. CCG averages in London ranged from 56.2% in Islington to 71.2% in Sutton, with City and Hackney 69.7%, Tower Hamlets 64.7%, and Newham 69.7%.23
This study demonstrates that increasing anticoagulation by 3.5% per annum to 10% within 3 years and a comparable reduction in antiplatelets agents is a feasible task. To indicate the likely benefit of this improvement, assumptions were made about the stroke risk in those patients newly started on anticoagulants. Anticoagulation reduces the risk of stroke by 64% compared with placebo.41 According to the BAFTA trial, 50% lower incidence of stroke and severe haemorrhagic episodes could be expected in patients on warfarin compared with aspirin.11 If the clinical improvement observed in the study area was applied to all 1 million people with AF known to GP practices in the UK, (assuming an annual risk of stroke of 4% in people ≥75 years, 2% 65–74 years, and 1% <65 years), then a reduction of stroke by approximately 1600 and heavy bleeding by 200 every year could be expected. Increased ascertainment of new cases of AF as a result of the programme and reduction of inappropriate dual use of aspirin with anticoagulants could further reduce stroke and bleeding. Reducing stroke nationally by 1600 per annum amounts to six per CCG at a 5-year healthcare cost of £150 000.42 This would be offset by a 1-year cost of anticoagulant treatment of £120 000, making initiatives to increase anticoagulation by 10% highly cost-effective.
The cost of these basic tools for improvement is modest in comparison with the cost of not taking action.42 The entire cost of the programme — producing and circulating guidelines, the educational sessions, the development and installation of the APEL tool in all practices, the data extraction, reports and their circulation — was approximately £15 000 per CCG (£300 per practice), although this utilised a pre-existing infrastructure of academic support and web-connected record systems. This does not take account of the time that many clinicians gave for guideline development, APEL tool testing, and staff training, and the costs incurred by GPs from cleaning registers, reviewing patients, and altering treatment, which often required haematology referrals. To maintain this programme beyond 2 years, there is a recurrent annual cost of approximately £100 per practice, £5000 per CCG per annum to update guidelines and computer software, and produce and distribute evaluative reports.
This intervention was effective in increasing the number of appropriately treated patients with AF; however, the untreated proportion remained high, with considerable variability between practices. Additional investment by CCGs in supportive programmes is required to increase the slope of improvement and attain 80% levels of anticoagulation without inappropriate delay.
Addendum
This programme has since received support from University College London Partners, Academic Health Science Network to support improvement in anticoagulation across 21 CCGs with a population of 6 million.
Acknowledgments
The CEG is managed by Keith Prescott and the AF programme was facilitated by Arun Chinnaraj and other CEG staff. We are grateful to over 500 east London GPs, practice nurses, practice managers, and PCT/CCG staff including prescribing advisers and GP clinical leads for their help and cooperation with this programme.
Funding
This research received no specific funding, but the principle investigator and Clinical Effectiveness Group (CEG) staff who supported this programme were funded by Newham, City and Hackney, and Tower Hamlets primary care trusts/clinical commissioning groups.
Ethical approval
All data were anonymised and managed according to the UK NHS information governance requirements. Ethical approval was not required for the use of anonymised data in this observational study.
Provenance
Freely submitted; externally peer reviewed.
Competing interests
The authors have declared no competing interests.
Discuss this article
Contribute and read comments about this article: www.bjgp.org/letters
REFERENCES
- 1.Heeringa J, van der Kuip DA, Hofman A, et al. Prevalence, incidence and lifetime risk of atrial fibrillation: the Rotterdam study. Eur Heart J. 2006;27(8):949–953. doi: 10.1093/eurheartj/ehi825. [DOI] [PubMed] [Google Scholar]
- 2.Mathur R, Pollara E, Hull S, et al. Ethnicity and stroke risk in patients with atrial fibrillation. Heart. 2013;99:1087–1092. doi: 10.1136/heartjnl-2013-303767. [DOI] [PubMed] [Google Scholar]
- 3.QStroke 2013. Risk calculator: http://qstroke.org (accessed 6 Mar 2014).
- 4.QRisk2 2013. Risk calculator: http://qrisk.org (accessed 6 Mar 2014).
- 5.Camm AJ, Lip YHG, De Caterina R, et al. 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation. Developed with with the special contribution of the European Heart Rhythm Association. Eur Heart J. 2012;33(21):2719–2747. doi: 10.1093/eurheartj/ehs253. [DOI] [PubMed] [Google Scholar]
- 6.Ogilvie IM, Newton N, Welner SA, et al. Underuse of oral anticoagulants in atrial fibrillation: a systematic review. Am J Med. 2010;123(7):638–645.e4. doi: 10.1016/j.amjmed.2009.11.025. [DOI] [PubMed] [Google Scholar]
- 7.National Institute for Health and Care Excellence . Atrial fibrillation. The management of atrial fibrillation. London: NICE; 2006. CG036. [PubMed] [Google Scholar]
- 8.Lip GYH, Halperin JL. Improving stroke risk stratification in atrial fibrillation. Am J Med. 2010;123(6):484–488. doi: 10.1016/j.amjmed.2009.12.013. [DOI] [PubMed] [Google Scholar]
- 9.Sato H, Ishikawa K, Kitabatake A, et al. Low-dose aspirin for prevention of stroke in low-risk patients with atrial fibrillation: Japan Atrial Fibrillation Stroke Trial. Stroke. 2006;37(2):447–451. doi: 10.1161/01.STR.0000198839.61112.ee. [DOI] [PubMed] [Google Scholar]
- 10.Aguilar MI, Hart R, Pearce LA. Oral anticoagulants versus antiplatelet therapy for preventing stroke in patients with non-valvular atrial fibrillation and no history of stroke or transient ischemic attacks. Cochrane Database Syst Rev. 2007;3:CD006186. doi: 10.1002/14651858.CD006186.pub2. [DOI] [PubMed] [Google Scholar]
- 11.Mant J, Hobbs FD, Fletcher K, et al. Warfarin versus aspirin for stroke prevention in an elderly community population with atrial fibrillation (the Birmingham Atrial Fibrillation Treatment of the Aged Study, BAFTA): a randomised controlled trial. Lancet. 2007;370(9586):493–503. doi: 10.1016/S0140-6736(07)61233-1. [DOI] [PubMed] [Google Scholar]
- 12.National Institute for Health and Care Excellence CMG49: Support for commissioning: anticoagulation therapy. http://www.nice.org.uk/usingguidance/commissioningguides/AnticoagulationTherapy.jsp (accessed 6 Mar 2014).
- 13.Fang MC, Stafford RS, Ruskin JN, Singer DE. National trends in antiarrhythmic and antithrombotic medication use in atrial fibrillation. Arch Intern Med. 2004;164(1):55–60. doi: 10.1001/archinte.164.1.55. [DOI] [PubMed] [Google Scholar]
- 14.Holt TA, Hunter TD, Gunnarsson C, et al. Risk of stroke and oral anticoagulant use in atrial fibrillation: a cross-sectional survey. Br J Gen Pract. 2012 doi: 10.3399/bjgp12X656856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murphy NF, Simpson CR, Jhund PS, et al. A national survey of the prevalence, incidence, primary care burden and treatment of atrial fibrillation in Scotland. Heart. 2007;93(5):606–612. doi: 10.1136/hrt.2006.107573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marshall IJ, Wang Y, McKevitt C, et al. Trends in risk factor prevalence and management before first stroke: data from the South London Stroke Register 1995–2011. Stroke. 2013;44(7):1809–1816. doi: 10.1161/STROKEAHA.111.000655. [DOI] [PubMed] [Google Scholar]
- 17.Majeed A, Moser K, Carroll K. Trends in the prevalence and management of atrial fibrillation in general practice in England and Wales, 1994–1998: analysis of data from the general practice research database. Heart. 2001;86(3):284–288. doi: 10.1136/heart.86.3.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee S, Shafe ACE, Cowie MR. UK stroke incidence, mortality and cardiovascular risk management 1999–2008: time-trend analysis from the General Practice Research Database. BMJ Open. 2011;1(2) doi: 10.1136/bmjopen-2011-000269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Scowcroft ACE, Lee S, Mant J. Thromboprophylaxis of elderly patients with AF in the UK: an analysis using the General Practice Research Database (GPRD) 2000–2009. Heart. 2013;99(2):127–132. doi: 10.1136/heartjnl-2012-302843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sandhu RK, Bakal JA, Ezekowitz JA, McAlister FA. Risk stratification schemes, anticoagulation use and outcomes: the risk–treatment paradox in patients with newly diagnosed non-valvular atrial fibrillation. Heart. 2011;97(24):2046–2050. doi: 10.1136/heartjnl-2011-300901. [DOI] [PubMed] [Google Scholar]
- 21.Bungard TJ, Ghali WA, Teo KK, et al. Why do patients with atrial fibrillation not receive warfarin? Arch Intern Med. 2000;160(1):41–46. doi: 10.1001/archinte.160.1.41. [DOI] [PubMed] [Google Scholar]
- 22.Craig P, Dieppe P, Macintyre S, et al. Developing and evaluating complex interventions: new guidance. London: MRC; 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Michie S, Johnston M, Abraham C, et al. Making psychological theory useful for implementing evidence based practice: a consensus approach. Qual Saf Health Care. 2005;14(1):26–33. doi: 10.1136/qshc.2004.011155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grimshaw JM, Thomas RE, MacLennan G, et al. Effectiveness and efficiency of guideline dissemination and implementation strategies. Health Technol Assess. 2004;8(6):iii–iv. 1–72. doi: 10.3310/hta8060. [DOI] [PubMed] [Google Scholar]
- 25.Grol R, Grimshaw J. From best evidence to best practice: effective implementation of change in patients’ care. Lancet. 2003;362(9391):1225–1230. doi: 10.1016/S0140-6736(03)14546-1. [DOI] [PubMed] [Google Scholar]
- 26.Oakeshott P, Kerry SM, Williams JE. Randomized controlled trial of the effect of the Royal College of Radiologists’ guidelines on general practitioners’ referrals for radiographic examination. Br J Gen Pract. 1994;44(382):197–200. [PMC free article] [PubMed] [Google Scholar]
- 27.Thomas RE, Croal BL, Ramsay C, et al. Effect of enhanced feedback and brief educational reminder messages on laboratory test requesting in primary care: a cluster randomised trial. Lancet. 2006;367(9527):1990–1996. doi: 10.1016/S0140-6736(06)68888-0. [DOI] [PubMed] [Google Scholar]
- 28.Holt TA, Thorogood M, Griffiths F. Changing clinical practice through patient specific reminders avialable at the time of the clinical encounter: systematic review and meta-analysis. J Gen Intern Med. 2012;27(8):974–984. doi: 10.1007/s11606-012-2025-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baskerville NB, Liddy C, Hogg W. Systematic review and meta-analysis of practice facilitation within primary care settings. Ann Fam Med. 2012;10(1):63–74. doi: 10.1370/afm.1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ivers N, Jamtvedt G, Flottorp S, et al. Audit and feedback: effects on professional practice and healthcare outcomes. Cochrane Database Syst Rev. 2012;6:CD000259. doi: 10.1002/14651858.CD000259.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Campbell S, Reeves D, Kontopantelis E, et al. Quality of primary care in England with the introduction of pay for performance. N Engl J Med. 2007;357(2):181–190. doi: 10.1056/NEJMsr065990. [DOI] [PubMed] [Google Scholar]
- 32.Team H-QBR New GMS Contract QOF Implementation Dataset and Business Rules Atrial fibrillation indicator sets. http://www.pcc-cic.org.uk/sites/default/files/articles/attachments/atrial_fibrillation_ruleset_v25.0.pdf (accessed 6 Mar 2014).
- 33.CEG. Clinical Effectiveness Group Clinical Guidelines. Improving Anticoagulation 2012. http://blizard.qmul.ac.uk/ceg-resource-library/clinical-guidance/cat_view/1-clinical-guidance/2-clinical-guidelines.html (accessed 20 Mar 2014).
- 34.STROBE STROBE Statement. Strengthening the reporting of observational studies in epidemiology. http://www.strobe-statement.org/ (accessed 6 Mar 2014).
- 35.England PH. Technical briefing 2: Statistical process control methods in public health intelligence. http://www.apho.org.uk/default.aspx?RID=39306 (accessed 6 Mar 2014).
- 36.Atrial fibrillation association . Grasp the initiative Prevent AF-related Stroke, Anticoagulate! Supporting the use of GRASP-AF in primary care to help reduce the risk of AF-related Stroke. Shipston-on-Stour: AF Association; 2012. [Google Scholar]
- 37.Cowan C, Healicon R, Robson I, et al. The use of anticoagulants in the management of atrial fibrillation among general practices in England. Heart. 2013;99(16):1166–1172. doi: 10.1136/heartjnl-2012-303472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.NHS QOF Database UK AF3. http://www.gpcontract.co.uk/timeline/UK/AF%203 (accessed 6 Mar 2014).
- 39.HSCIC Quality and Outcome Framework Results 2012/13. http://qof.hscic.gov.uk/ (accessed 6 Mar 2014).
- 40.Jacobs LG, Billett HH, Freeman K, et al. Anticoagulation for stroke prevention in elderly patients with atrial fibrillation, including those with falls and/or early-stage dementia: a single-center, retrospective, observational study. Am J Geriatr Pharmacother. 2009;7(3):159–166. doi: 10.1016/j.amjopharm.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 41.Lip GY, Lim HS. Atrial fibrillation and stroke prevention. Lancet Neurol. 2007;6(11):981–993. doi: 10.1016/S1474-4422(07)70264-8. [DOI] [PubMed] [Google Scholar]
- 42.Saka O, McGuire A, Wolfe C. Cost of stroke in the United Kingdom. Age Ageing. 2009;38(1):27–32. doi: 10.1093/ageing/afn281. [DOI] [PubMed] [Google Scholar]