Abstract
A 31-year-old woman presented with severe dystonia-parkinsonism. She had nonprogressive psychomotor retardation and cognitive dysfunction from childhood without evidence of dystonia or parkinsonism. At age 30, she then developed severe dystonia and gait disturbance. There was neither dystonia nor parkinsonism before age 30. MRI revealed cerebral atrophy and iron accumulation in the globus pallidus and substantia nigra (figure 1, A–D). The characteristic MRI findings were hyperintensity of the substantia nigra with a central band of hypointensity in T1-weighted axial slices (figure 1, B). Beta-propeller protein-associated neurodegeneration (BPAN) was diagnosed based on MRI findings and identification of a novel heterozygous mutation in the WDR45 gene (NM_007075.3: c.519+1_519+3del) (figure 2). This is a neurodegeneration involving brain iron accumulation (NBIA) characterized by psychomotor retardation from childhood and dystonia-parkinsonism in midadulthood.1,2 Although we could not analyze the father's gene since he had died, the mother had no mutation in the WDR45 gene (figure 2). Thus, it might be a de novo mutation in the WDR45 gene, as reported previously.1,2
A 31-year-old woman presented with severe dystonia-parkinsonism. She had nonprogressive psychomotor retardation and cognitive dysfunction from childhood without evidence of dystonia or parkinsonism. At age 30, she then developed severe dystonia and gait disturbance. There was neither dystonia nor parkinsonism before age 30. MRI revealed cerebral atrophy and iron accumulation in the globus pallidus and substantia nigra (figure 1, A–D). The characteristic MRI findings were hyperintensity of the substantia nigra with a central band of hypointensity in T1-weighted axial slices (figure 1, B). Beta-propeller protein-associated neurodegeneration (BPAN) was diagnosed based on MRI findings and identification of a novel heterozygous mutation in the WDR45 gene (NM_007075.3: c.519+1_519+3del) (figure 2). This is a neurodegeneration involving brain iron accumulation (NBIA) characterized by psychomotor retardation from childhood and dystonia-parkinsonism in midadulthood.1,2 Although we could not analyze the father's gene since he had died, the mother had no mutation in the WDR45 gene (figure 2). Thus, it might be a de novo mutation in the WDR45 gene, as reported previously.1,2
Figure 1. Brain MRI findings in the patient.
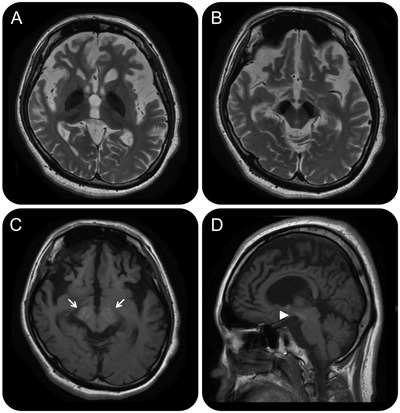
(A, B) T2-weighted MRI shows marked hypointensity in the globus pallidus and substantia nigra in axial slices. (C) T1-weighted MRI shows hyperintensity of the substantia nigra with a central band of hypointensity in an axial slice (arrows). (D) T1-weighted MRI shows cerebral atrophy and hyperintensity of the substantia nigra (arrowhead) in a sagittal slice.
Figure 2. Identification of a novel heterozygous mutation in the WDR45 gene.
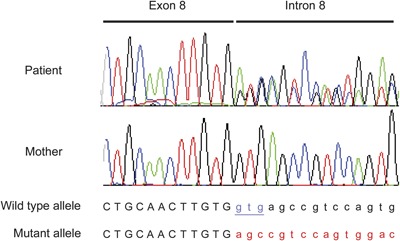
Electropherograms of the patient and her mother using a forward primer are shown. A novel heterozygous mutation (NM_007075.3: c.519+1_519+3del, underlined), which may result in abnormal splicing, was identified in intron 8 (splice donor site) of the WDR45 gene in the patient, as shown by the double signals due to the 3 bp deletion. Meanwhile, the mother had no mutation in the WDR45 gene. The colored peaks denote nucleotide bases as follows: black, guanine; red, thymine; blue, cytosine; and green, adenine.
l-Dopa/decarboxylase inhibitor treatment (200 mg/day orally) led to improvement of the rigidity and bradykinesia in our patient. She became able to move by herself by crawling on her hands and knees and no longer needed meal assistance. In addition, she became able to utter a few words like “thanks” and “bye,” although the dystonia of the lower limbs remained unchanged. Since T1-weighted hyperintensity of the substantia nigra with a central band of hypointensity has not been found in other NBIA disorders, including neuroferritinopathy, aceruloplasminemia, and pantothenate kinase-associated neurodegeneration, this finding should facilitate the diagnosis of BPAN.
STUDY FUNDING
No targeted funding reported.
DISCLOSURES
Y. Ichinose, M. Miwa, A. Onohara, K. Obi, and K. Shindo report no disclosures. H. Saitsu receives funding from a Grant-in-Aid for Scientific Research (B) from the Japan Society for the Promotion of Science. N. Matsumoto serves on editorial advisory boards for Clinical Genetics, Journal of Human Genetics, and American Journal of Medical Genetics Part A; receives funding from the Ministry of Health, Labour and Welfare, the Japan Science and Technology Agency, a Grant-in-Aid for Scientific Research on Innovative Areas (Foundation of Synapse and Neurocircuit Pathology) from the Ministry of Education, Culture, Sports, Science and Technology of Japan, a Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science, and a grant from the Takeda Science Foundation. Y. Takiyama serves on the editorial advisory board for Rinsho Shinkeigaku. Full disclosure form information provided by the authors is available with the full text of this article at Neurology.org/cp.
REFERENCES
- 1.Haack TB, Hogarth P, Kruer MC, et al. Exome sequencing reveals de novo WDR45 mutations causing a phenotypically distinct, X-linked dominant form of NBIA. Am J Hum Genet 2012;91:1144–1149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saitsu H, Nishimura T, Muramatsu K, et al. De novo mutations in the autophagy gene WDR45 cause static encephalopathy of childhood with neurodegeneration in adulthood. Nat Genet 2013;45:445–449 [DOI] [PubMed] [Google Scholar]


