Abstract
Objective:
To elucidate the neuropathology in cerebellar ataxia with neuropathy and bilateral vestibular areflexia syndrome (CANVAS), a novel cerebellar ataxia comprised of the triad of cerebellar impairment, bilateral vestibular hypofunction, and a peripheral sensory deficit.
Method:
Brain and spinal neuropathology in 2 patients with CANVAS, together with brain and otopathology in another patient with CANVAS, were examined postmortem.
Results:
Spinal cord pathology demonstrated a marked dorsal root ganglionopathy with secondary tract degeneration. Cerebellar pathology showed loss of Purkinje cells, predominantly in the vermis.
Conclusion:
The likely underlying sensory pathology in CANVAS is loss of neurons from the dorsal root and V, VII, and VIII cranial nerve ganglia—in other words, it is a “neuronopathy” rather than a “neuropathy.” Clinically, CANVAS is a differential diagnosis for both spinocerebellar ataxia type 3 (or Machado-Joseph disease) and Friedreich ataxia. In addition, there are 6 sets of sibling pairs, implying that CANVAS is likely to be a late-onset recessive or autosomal dominant with reduced penetrance disorder, and identification of the culprit gene is currently a target of investigation.
An abnormal visually enhanced vestibulo-ocular reflex (VVOR) represents a compound impairment of the 3 key corrective oculomotor reflexes, namely, smooth pursuit, the vestibulo-ocular reflex (VOR), and the opticokinetic reflex. We refer the reader to our earlier work for details of the VVOR.1 While the initial description of a syndrome of cerebellar ataxia and bilateral vestibulopathy noted the presence of a peripheral neuropathy in 3 of the 4 index cases, we later showed that in 18 patients a peripheral nerve disease was an integral component of the syndrome we renamed cerebellar ataxia with neuropathy and bilateral vestibular areflexia syndrome (CANVAS).2 At this time, we noted that a neuronopathy (ganglionopathy) could not be definitively excluded. Subsequently, temporal bone histopathology1 revealed a vestibular, facial, and trigeminal sensory neuronopathy. In an effort to unify the underlying pathology in CANVAS, we speculated that the peripheral sensory deficit, invariably seen in this syndrome, was more likely to be a neuronopathy than a neuropathy. Our efforts at testing this hypothesis were initially limited by the difficulty in differentiating these 2 entities with conventional neurophysiologic protocols. On obtaining the first spinal cord postmortem samples in cases of diagnosed CANVAS, it appears that the peripheral sensory deficit in CANVAS may be due to a dorsal root ganglionopathy. We hope to develop neurophysiologic protocols that may be used to identify this pathology in the living patient.
METHODS
Standard protocol approvals, registrations, and patient consents.
Approval to conduct this study was obtained from the Royal Victorian Eye and Ear Hospital Ethics Committee. Written informed consent for research was obtained from all patients (or guardians of patients) participating in the study.
We have gathered neuropathologic data on 3 cases of CANVAS: with brain pathology on all 3, together with spinal pathology in 2 of these and temporal bone histopathology on the other.
RESULTS
The brains of 3 patients with CANVAS were examined postmortem. Patient 1 was a 71-year-old woman with a 10-year history of progressive imbalance, dysarthria, and dysphagia. Examination revealed an ataxic gait, cerebellar dysarthria, downbeat nystagmus, severe bidirectional impairment of horizontal and vertical VOR on impulsive testing, and saccadic eye movements on testing the VVOR. While normal in the upper limbs, proprioception was impaired in the lower limbs and deep tendon reflexes were absent. Pinprick sensation was preserved throughout. Temporal bone pathology was obtained from this patient (see figure 1).3 Patient 2 was an 81-year-old woman with a more than 40-year history of lower limb dysesthesia, gait unsteadiness complicated by falls, and dysphagia. On examination, there was cerebellar dysarthria, a bilaterally positive horizontal head impulse test, saccadic dysmetria, horizontal gaze-evoked downbeat nystagmus, and an abnormal VVOR. Vibration perception in the lower limbs was impaired with total body loss of pinprick perception. Patient 3 was a 90-year-old man with a 30-year history of progressive imbalance and peripheral sensory impairment. On examination, he displayed a bilaterally abnormal horizontal head impulse test, saccadic smooth pursuit and VVOR, hypermetric saccades to target, and bilateral horizontal gaze-evoked nystagmus. Deep tendon reflexes were globally absent with distal loss of proprioception in the upper limbs; however, pinprick perception remained intact.
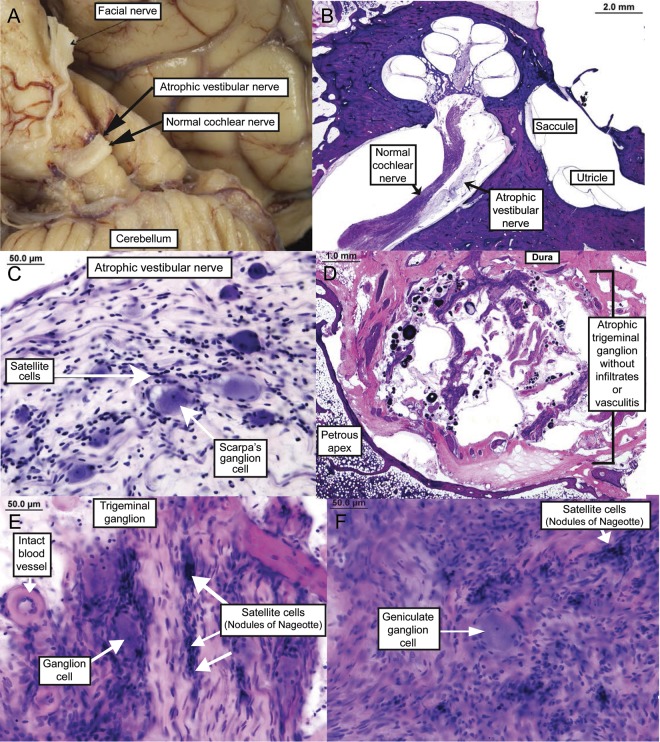
Figure 1. Temporal bone histopathology (some of this pathology has been previously reported3).
(A) Marked atrophy of the vestibular component of the eighth cranial nerve in the cerebellopontine angle (compared with the adjacent cochlear component of the eighth cranial nerve). (B) Severely atrophic vestibular nerve; low-power hematoxylin & eosin (H&E). (C) Atrophic vestibular nerve with marked diminution in Scarpa ganglion cells; high-power H&E. (D) Severely atrophic trigeminal ganglion where the bulk of the ganglion has been replaced by arachnoid tissue, psammoma bodies, and CSF; low-power H&E. (E) Severely atrophic trigeminal ganglion with a marked decrease in ganglion cells, atrophy of nerve fibers, and presence of nodules of Nageotte; high-power H&E. (F) Atrophic facial nerve with near-total loss of ganglion cells; high-power H&E.
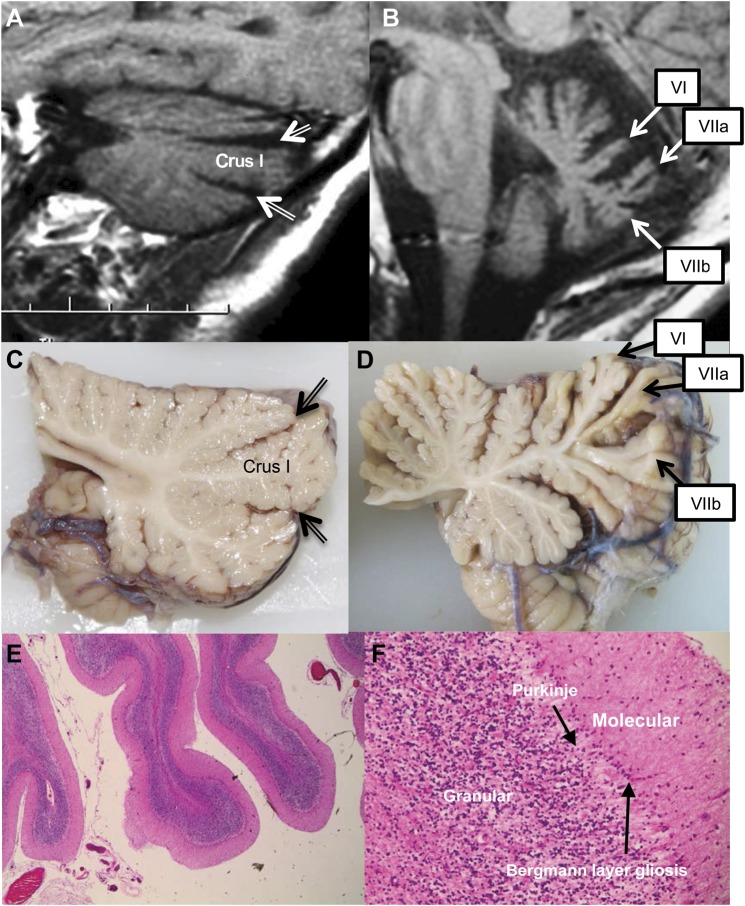
On neuropathologic examination the main changes seen were in the cerebellum and spinal cord. The cerebrum showed minor generalized atrophy and on coronal section showed minor blunting of the angles of the lateral ventricle. Basal ganglia and diencephalon appeared normal. Microscopic examination of the cortex showed age-related changes only. Basal ganglia, thalamus, subthalamic nuclei, and hypothalamus showed no microscopic pathology. On external examination of the brainstem and cerebellum, atrophy of cerebellar folia (figure 2) and atrophy of the vestibular component of the eighth cranial nerve in the cerebellopontine angles (figure 1A) were noted. On parasagittal examination, the cerebellum of patients 1 and 2 displayed prominent atrophy in the superior and inferior aspects of the vermis that was more marked than the atrophy of the hemispheres (figure 2). In the third patient, there was minor atrophy of the folia of the cerebellar superior vermis. Cross-section examination of the brainstem in all 3 cases was unremarkable. In all subjects, microscopic sections of cerebellum showed vermal atrophy with neuronal loss in the Purkinje cell layer in association with Bergmann layer gliosis and occasional torpedo body formation. These changes were less prominent laterally. There was relatively minor atrophy of the granular cell layer and secondary loss of white matter tracts (see figure 2). The cerebellar dentate nuclei were unremarkable. Sections of the brainstem showed very minor pigment incontinence from the neurons of the substantia nigra with no evident Lewy bodies. Pontine and cranial nerve (including the eighth cranial nerve) nuclei showed a normal neuronal content with no gliosis. The medial lemniscus was normally myelinated. The inferior olivary nuclei showed lateral aspect loss of neurons and gliosis with secondary tract degeneration in the cerebellomedullary tracts. There were no neuronal or astrocytic inclusions, tangles, or neuritic threads in the cerebellum, brainstem, or spinal cord on immunoreaction with tau (Dako, Glostrup, Denmark) or α-synuclein (Dako).
Figure 2. Cerebellum.
(A) MRI of patient 2 showing a parasagittal pattern of atrophy predominantly affecting hemispheric crus I. Double-lined arrows indicate widening of the superior posterior and horizontal fissures. (B) MRI of patient 2 showing midsagittal pattern of atrophy predominantly affecting vermal lobules VI, VIIa, and VIIb. (C) Parasagittal cerebellum showing minor atrophy of the folia. Double-lined arrows indicate the superior posterior and horizontal fissures. (D) Midline cerebellar vermis showing marked atrophy of folia, particularly in the superior and dorsal aspect. (E) Cerebellar atrophy ×100; hematoxylin & eosin (H&E). (F) Purkinje cell loss and Bergmann layer gliosis; ×400 H&E.
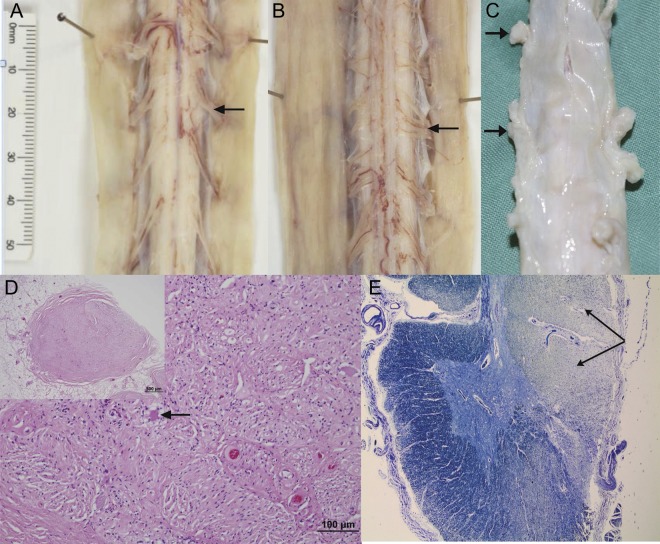
Spinal cord examination was undertaken in patients 2 and 3 and revealed markedly atrophic dorsal root ganglia (DRG) with atrophy of the dorsal roots. On cross-section, atrophic posterior columns were seen (see figure 3). Subtotal neuronal loss in the DRG with no satellite cell proliferation was noted on sections. The posterior columns showed severe loss of myelinated axons (see figure 3). Slight pallor of the corticospinal tracts was also evident. Apart from a mild loss in the lumbar anterior horns in case 3, there was no neuronal loss from the anterior or lateral horns, and lateral thoracic columns showed a normal neuronal population.
Figure 3. Spinal cord dorsal root ganglia.
(A) Normal anterior spinal cord nerve rootlets (arrow). (B) Atrophic posterior spinal cord nerve rootlets (arrow). (C) Spinal cord and coverings showing atrophic dorsal root ganglia (arrows). (D) Atrophic dorsal root ganglion showing marked neuronal loss with scant residual neurons (arrow in higher magnification main image; low-power whole dorsal root ganglion shown in inset). No evidence of satellite cell hypertrophy. (E) Cross-section of cervical spinal cord showing secondary demyelination of the posterior columns (arrows); ×20, Luxol fast blue.
Microscopic examination of the temporal bone resection in case 13 revealed severely atrophic vestibular ganglia with marked diminution in Scarpa ganglion cells. The associated vestibular nerve was atrophic. Severely atrophic trigeminal ganglion with loss of ganglion cells and replacement psammoma bodies were seen. The trigeminal ganglion was also atrophic with a marked decrease in ganglion cells, atrophy of nerve fibers, and presence of nodules of Nageotte. The facial nerve ganglion showed near-total loss of cells.
DISCUSSION
In total, we have gathered clinical data on 51 patients with CANVAS. These patients have been diagnosed by a small number of neurologists in the past 5 to 10 years, so CANVAS is very unlikely to be a rare syndrome.
Pathophysiologically, DRG neuronal loss leads to axonal degeneration with secondary demyelination in the posterior columns. The DRG neuronal loss described here supports our clinical suspicion that the peripheral sensory deficit in CANVAS is a sensory neuronopathy in at least a subgroup of patients with CANVAS. This finding also reinforces our previous elucidation of a vestibular (Scarpa), trigeminal, and facial neuronopathy.3 However, it should be noted that there remains the possibility of phenotypic heterogeneity in CANVAS or even bilateral vestibulopathy comorbid with cerebellar impairment (so-called cerebellar ataxia with bilateral vestibulopathy4) such that it may manifest with or without a neuropathy or neuronopathy. It has been noted that in the DRG of patients with Friedreich ataxia, there is a thickening of the layer of ferritin-reactive satellite cells that normally surround DRG neurons.5 Satellitosis was not seen in the DRG of the patient with CANVAS. Consistent with our previous MRI findings,1 the cerebellar pathology is not diffuse and specifically involves the anterior and dorsal vermis, and the hemispheric crus I. As with CANVAS, Friedreich ataxia often manifests as the combination of cerebellar ataxia, a bilateral vestibulopathy, and DRG atrophy6,7; however, in Friedreich ataxia, the deep cerebellar nuclei are involved.8
While the cerebellar impairment in multiple system atrophy of the cerebellar type (MSAc) has been reported to be accompanied by a bilateral vestibulopathy,9,10 this is somewhat controversial11 and clinico-radiologically MSAc bears a limited resemblance to CANVAS. No α-synuclein immunoreactive inclusions typical for MSA were seen in sections. The association of cerebellar ataxia, bilateral vestibulopathy, and loss of small-fiber sensation is seen in spinocerebellar ataxia type 3 (SCA3); however, because it is an autosomal dominantly inherited ataxia, the family pedigree is likely to be far more populated with patients than that of autosomal recessive inheritance—the presumed mode of inheritance in CANVAS. Other SCAs may manifest a sensory neuropathy, more frequently SCAs 4 and 2512,13 and occasionally SCAs 1, 8, and 27.14–16
Elucidation of the underlying spinal cord pathology supports the development of a targeted neurophysiologic testing protocol to aid in the clinical diagnosis of patients with ataxia and a comorbid peripheral sensory deficit. Our cohort of patients with CANVAS includes 6 sibling pairs, which implies either an autosomal recessive or autosomal dominant with reduced penetrance pattern of inheritance.
ACKNOWLEDGMENT
This work is dedicated to the exceptionally fine work and mentorship of the late Dr. Saumil Merchant.
GLOSSARY
- CANVAS
cerebellar ataxia with neuropathy and bilateral vestibular areflexia syndrome
- DRG
dorsal root ganglia
- MSAc
multiple system atrophy with predominant cerebellar ataxia
- SCA
spinocerebellar ataxia
- VOR
vestibulo-ocular reflex
- VVOR
visually enhanced vestibulo-ocular reflex
AUTHOR CONTRIBUTIONS
Dr. Szmulewicz: analysis and interpretation, critical revision of the manuscript for important intellectual content, study supervision, study concept and design. Dr. McLean and Dr. Rodriguez: acquisition of data, analysis and interpretation, critical revision of the manuscript for important intellectual content. Dr. Chancellor and Dr. Mossman: critical revision of the manuscript for important intellectual content. Dr. Lamont: acquisition of data. Dr. Roberts: acquisition of data, analysis and interpretation, critical revision of the manuscript for important intellectual content. Dr. Storey: critical revision of the manuscript for important intellectual content. Dr. Halmagyi: critical revision of the manuscript for important intellectual content, study supervision, study concept and design.
STUDY FUNDING
The authors thank the Garnet Passe and Rodney Williams Memorial Foundation, and the National Health and Medical Research Council for financial support.
DISCLOSURE
D. Szmulewicz, C. McLean, and M. Rodriguez report no disclosures relevant to the manuscript. A. Chancellor has received funding for travel from Biogen Idec and Sanofi-Aventis and serves on the editorial board for Practical Neurology. S. Mossman, D. Lamont, and L. Roberts report no disclosures relevant to the manuscript. E. Storey has received funding for travel (and speaker honoraria payable to his institution) from Pfizer Inc.; serves as Neurology coeditor for Journal of Clinical Neuroscience; and receives research support from the National Health and Medical Research Council (Australia), the NIH, Alfred Hospital Research Foundation, Wicking Foundation, and Bethlehem-Griffiths Foundation. G. Halmagyi serves on the scientific board for the Brain Foundation of Australia; serves on the editorial boards of Acta Otolaryngologica, Otology, Neurotology, Audiology, Neuro-otology, and the Italian Journal of Otolaryngology; has served as a consultant for GN Otometrics; and receives research support from the National Health and Medical Research Council. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Szmulewicz DJ, Waterston JA, MacDougall HG, et al. Cerebellar ataxia, neuropathy, vestibular areflexia syndrome (CANVAS): a review of the clinical features and video-oculographic diagnosis. Ann NY Acad Sci 2011;1233:139–147 [DOI] [PubMed] [Google Scholar]
- 2.Szmulewicz DJ, Waterston JA, Halmagyi GM, et al. Sensory neuropathy as part of the cerebellar ataxia neuropathy vestibular areflexia syndrome. Neurology 2011;76:1903–1910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Szmulewicz DJ, Merchant SN, Halmagyi GM. Cerebellar ataxia with neuropathy and bilateral vestibular areflexia syndrome: a histopathologic case report. Otol Neurotol 2011;32:e63–e65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Migliaccio A, Halmagyi G, Mcgarvie L, Cremer P. Cerebellar ataxia with bilateral vestibulopathy: description of a syndrome and its characteristic clinical sign. Brain 2004;127:280–293 [DOI] [PubMed] [Google Scholar]
- 5.Koeppen AH, Morral JA, Davis AN, et al. The dorsal root ganglion in Friedreich's ataxia. Acta Neuropathol 2009;118:763–776 [DOI] [PubMed] [Google Scholar]
- 6.Koeppen AH. Friedreich's ataxia: pathology, pathogenesis, and molecular genetics. J Neurol Sci 2011;303:1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fahey MC, Cremer PD, Aw ST, et al. Vestibular, saccadic and fixation abnormalities in genetically confirmed Friedreich ataxia. Brain 2008;131(pt 4):1035–1045 [DOI] [PubMed] [Google Scholar]
- 8.Koeppen AH, Davis AN, Morral JA. The cerebellar component of Friedreich's ataxia. Acta Neuropathol 2011;122:323–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Suarez H, Rosales B, Claussen CF. Plastic properties of the vestibulo-ocular reflex in olivo-ponto-cerebellar atrophy. Acta Otolaryngol 1992;112:589–594 [DOI] [PubMed] [Google Scholar]
- 10.Anderson T, Luxon L, Quinn N, Daniel S, Marsden CD, Bronstein A. Oculomotor function in multiple system atrophy: clinical and laboratory features in 30 patients. Mov Disord 2008;23:977–984 [DOI] [PubMed] [Google Scholar]
- 11.Rascol OJ, Clanet M, Senard JM, Montastruc JL, Rascol A. Vestibulo-ocular reflex in Parkinson's disease and multiple system atrophy. Adv Neurol 1993;60:395–397 [PubMed] [Google Scholar]
- 12.Flanigan K, Gardner K, Alderson K, et al. Autosomal dominant spinocerebellar ataxia with sensory axonal neuropathy (SCA4): clinical description and genetic localization to chromosome 16q22.1. Am J Hum Genet 1996;59:392–399 [PMC free article] [PubMed] [Google Scholar]
- 13.Stevanin G, Bouslam N, Thobois S, et al. Spinocerebellar ataxia with sensory neuropathy (SCA25) maps to chromosome 2p. Ann Neurol 2004;55:97–104 [DOI] [PubMed] [Google Scholar]
- 14.Subramony SH, Vig PJS. Clinical aspects of spinocerebellar ataxia 1. In: Wells RD, Warren ST, editors. Genetic Instabilities and Hereditary Neurological Diseases. San Diego: Academic Press; 1998:231–239 [Google Scholar]
- 15.Koob MD, Moseley ML, Schut LJ, et al. An untranslated CTG expansion causes a novel form of spinocerebellar ataxia (SCA8). Nat Genet 1999;21:379–384 [DOI] [PubMed] [Google Scholar]
- 16.Dalski A, Atici J, Kreuz FR, et al. Mutation analysis in the fibroblast growth factor 14 gene: frameshift mutation and polymorphisms in patients with inherited ataxias. Eur J Hum Genet 2005;13:118–120 [DOI] [PubMed] [Google Scholar]