Abstract
Objective:
To test whether level of perceived stress and reductions in levels of perceived stress (i.e., “let-down”) are associated with the onset of migraine attacks in persons with migraine.
Methods:
Patients with migraine from a tertiary headache center were invited to participate in a 3-month electronic diary study. Participants entered data daily regarding migraine attack experience, subjective stress ratings, and other data. Stress was assessed using 2 measures: the Perceived Stress Scale and the Self-Reported Stress Scale. Logit-normal, random-effects models were used to estimate the odds ratio for migraine occurrence as a function of level of stress over several time frames.
Results:
Of 22 enrolled participants, 17 (median age 43.8 years) completed >30 days of diaries, yielding 2,011 diary entries including 110 eligible migraine attacks (median 5 attacks per person). Level of stress was not generally associated with migraine occurrence. However, decline in stress from one evening diary to the next was associated with increased migraine onset over the subsequent 6, 12, and 18 hours, with odds ratios ranging from 1.5 to 1.9 (all p values < 0.05) for the Perceived Stress Scale. Decline in stress was associated with migraine onset after controlling for level of stress for all time points. Findings were similar using the Self-Reported Stress Scale.
Conclusions:
Reduction in stress from one day to the next is associated with migraine onset the next day. Decline in stress may be a marker for an impending migraine attack and may create opportunities for preemptive pharmacologic or behavioral interventions.
Migraine is a common headache disorder with substantial personal and societal burden.1,2 This burden may be amplified by the unpredictable nature of attacks,3 although attacks are sometimes predictable based on trigger factors and premonitory features.4–22 Premonitory features include changes in mood or behavior that precede the onset of an attack. Trigger factors, measurable precipitants associated with an increased probability of an attack, can include stressful events, hormonal changes, weather changes, and certain foods, although some studies demonstrate reduced odds for migraine attacks in relation to certain variables.5–22 In patient surveys, perceived stress was associated with migraine onset in up to 80% of respondents.13–16 Diary studies have shown that “daily hassles” significantly increase in the 2- to 3-day period before a migraine day.15,16 In an electronic diary study, emotional factors including irritability and intolerance (40.0% vs 33.7%) were more common on migraine days than control days.4 Some studies have examined the effects of reduction in stress or “let-down” as a migraine trigger factor, often focused on “weekend” headache attacks.14–22 The roles of level of stress and reduction in stress as migraine triggers remain to be disentangled. Our objective was to examine the association between stress and reduction in stress on migraine attack onset in persons with migraine using electronic diaries.4,23 We assessed the influence of self-reported subjective stress ratings daily and change from one day to the next on the occurrence of migraine among persons with migraine over an observation window of 6 to 24 hours. Understanding the time course of stress effects may provide clues to biological mechanisms and inform the choice of interventions.
METHODS
Subject recruitment and inclusion and exclusion criteria.
This study was conducted at Montefiore Headache Center, a tertiary headache center in Bronx, NY. Twenty-two patients meeting International Classification of Headache Disorders–224 criteria for migraine with or without aura were recruited. Eligible participants were aged 18 years or older, had 3 to 10 migraine attacks and fewer than 15 headache days/month (episodic migraine), and reported an awareness of impending migraines. Subjects with severe depression based on a Patient Health Questionnaire–9 item (PHQ-9)25 score of ≥15 and/or severe anxiety based on a Generalized Anxiety Disorder–7 item (GAD-7)26 score of ≥20 were excluded. Subjects were allowed to use migraine prophylaxis and antidepressant medications.
Standard protocol approvals, registrations, and patient consents.
The study was approved by the Institutional Review Board of the Montefiore Medical Center, and participants completed a written informed consent.
Data collection.
Data collection included baseline and exit questionnaires and an electronic diary. The electronic diary was a custom-programmed, palm-based, electronic Patient Reported Outcome device provided by Symfo (Boston, MA). Subjects were trained to enter and transmit data via a landline telephone to a secure central server. Diary completion was monitored by the study coordinator. Noncompliant subjects were contacted.
Baseline and exit questionnaire.
At baseline, information on sociodemographic and headache features was obtained. Additional instruments included the 12-item Allodynia Symptom Checklist,27 Migraine Disability Assessment Scale,28 PHQ-9, and GAD-7. At study completion, participants reported their experience in the study, knowledge of their triggers and premonitory features, perception about their ability to predict a migraine attack, the PHQ-9, and the GAD-7.
Electronic diary data collection.
Diaries alarmed every morning and evening to trigger data collection. At initiation of each entry, subjects were prompted with a stem question (“How are you feeling now?”), which had 4 response options including: “I do not anticipate a migraine”; “I think I might get a migraine”; “I am currently experiencing a migraine”; and “I had a migraine since the last entry but I am now free of pain.” The reply to the stem question initiated a branched logic to the appropriate questionnaire. Participants who reported that they were currently experiencing a migraine were directed to enter data at the conclusion of the migraine attack.
Morning data collection included hours of sleep the previous night, number of alcoholic drinks since the last data entry, menstruation status and premenstrual syndrome symptoms (females), belief about the chance of experiencing a migraine that day, and 6 items from the mood circumplex,29 which asked for ratings on a visual analog scale ranging from 0 (not at all) to 100 (extremely) for each of the following mood states: happy, sad, relaxed, nervous, lively, and bored. Evening data collection included medication consumption (for headache and other reasons), menstruation status and premenstrual syndrome symptoms (females), the 12-item Allodynia Symptom Checklist, alcohol and caffeine consumption, 6 moods from the mood circumplex, and 2 ratings of stress: the Self-Reported Stress Scale (SRSS) and the Perceived Stress Scale (PSS).30 The SRSS, created for this study, was a single-item question: “Overall, how stressful was today?” Response options ranged from 0 (“not at all stressful”) to 10 (“extremely stressful”). We adapted the 4-item version of the PSS to assess the experience of stress over the past day. Items included, “Today, how often did you feel…
that you were unable to control the important things in your life?
confident about your ability to handle your personal problems?
that things were going your way?
that difficulties were piling up so high that you could not overcome them?”
Response options were: never (0), almost never (0.25), sometimes (0.50), fairly often (0.75), and very often (1.0) for items 1 and 4. Responses to items 2 and 3 were reverse-coded. Responses to the 4 items were summed to create a total score ranging from 0 to 4. Higher scores indicate a higher degree of perceived stress.
Analyses.
Stata versions 11 and 12 (StataCorp, College Station, TX) were used for data analyses. Migraine occurrence was modeled as a binary outcome. Because each subject contributed many days of observations, random-effects models were used to take within-subject correlation into account. Specifically, logit-normal models with a random subject-specific intercept were used to estimate a subject-specific odds ratio (OR) for the association of the stress variables from the evening diaries with migraine occurrence within specific time frames (6, 12, 18, and 24 hours after diary entry). Understanding the time course may clarify mechanisms and contribute to the development of therapies. Random-effects models assess the risk of headache onset within the individual obviating the need for additional adjustment for demographic factors. The models weight individuals who contribute more data more heavily. The ORs represent the relative odds of migraine over a specified time frame, after an exposure to stress or change in stress, in comparison to periods without the exposure. Additional analyses used a dichotomous variable as to whether there was a decline in stress as measured by the PSS or SRSS in the logit-normal models. Mantel-Haenszel ORs were estimated for the association of the categorical variables (decline in stress as measured with PSS or with SRSS) with migraine incidence. The Mantel-Haenszel analysis uses different weights than the logit-normal models in combining information across the subjects, and thus serves as a sensitivity analysis. In addition, we were able to report exact confidence intervals and p values for the Mantel-Haenszel analysis.31 All reported results are unadjusted. However, the cohort in this study is small and homogeneous with only one male; therefore, demographic factors are not likely to confound analyses.
RESULTS
Subject sociodemographics.
Of 22 subjects, 5 were excluded for insufficient or unreliable diary data, leaving 17 subjects with more than 30 days of reliable diary data and providing 1,015 eligible diary days for analysis. Sixteen participants were women (94.1%). The median age was 43.8 years (range 23.4–55.4 years). The sample reported race/ethnicity as follows: white, non-Hispanic 47.1%; Hispanic 23.5%; African American 23.5%; and other 5.9%. All participants completed high school, 65% completed a bachelor's degree, and 29% had postgraduate education. Fifty-three percent used prophylactic migraine medication during the study. Of these, 6 participants were using nortriptyline (an antidepressant) for migraine prophylaxis. Two participants used other psychotropic medications including quetiapine for bipolar disorder and citalopram for depression. Subjects completed diaries over a 12-week period, contributing a total of 2,011 diaries including 1,015 eligible evening diaries (average 59.7 days per subject). Participants reported a total of 110 migraine attacks that were eligible for analysis.
Association of level of stress (status score) and migraine attack.
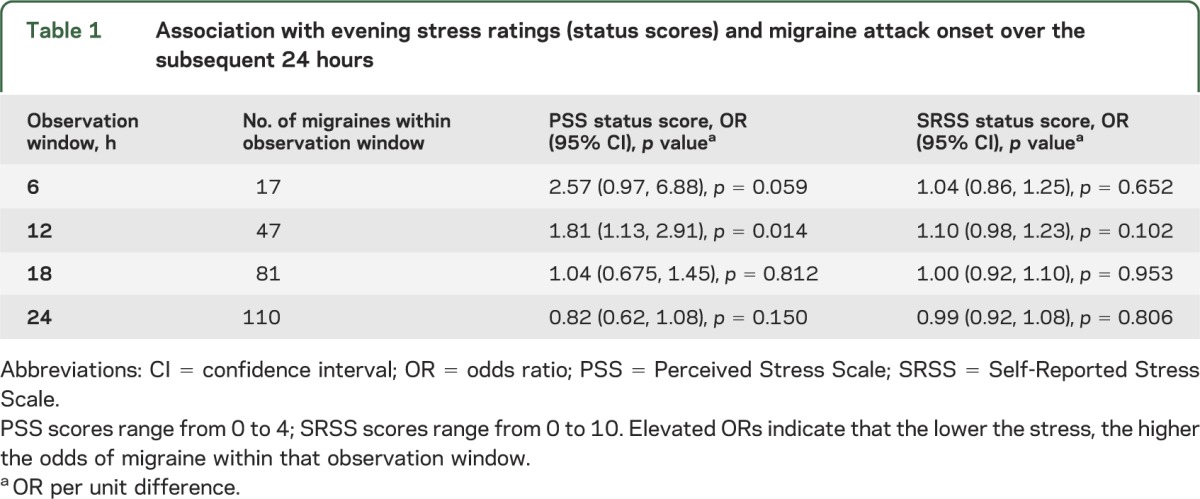
The average weighted mean PSS score was 0.99 (standard error 0.12). The average weighted mean SRSS score was 4.06 (standard error 0.40). Table 1 shows the association of level of stress as measured by PSS and SRSS scores in the evening and migraine attacks over the subsequent 6, 12, 18, and 24 hours. Among 8 models, results were significant only for the PSS score predicting migraine over the subsequent 12 hours.
Table 1.
Association with evening stress ratings (status scores) and migraine attack onset over the subsequent 24 hours
Association of change in stress and migraine attack.
We analyzed the association of change scores from one night to the next on migraine attack onset over the subsequent 24 hours in 771 eligible sets of observations. For each unit decline in stress for the PSS, there was a statistically significant increase in the relative odds of migraine over 6, 12, and 18, but not 24 hours (table e-1 on the Neurology® Web site at Neurology.org). For the SRSS, results were significant at all 4 time intervals. For the PSS change score, the OR at 6 hours is 1.92 (1.04, 3.54), indicating that a 1-unit decline on the PSS was associated with a 1.92-fold increase in the relative odds of migraine over the next 6 hours. ORs for a migraine attack were similarly elevated at 12 and 18 hours but lost significance at 24 hours.
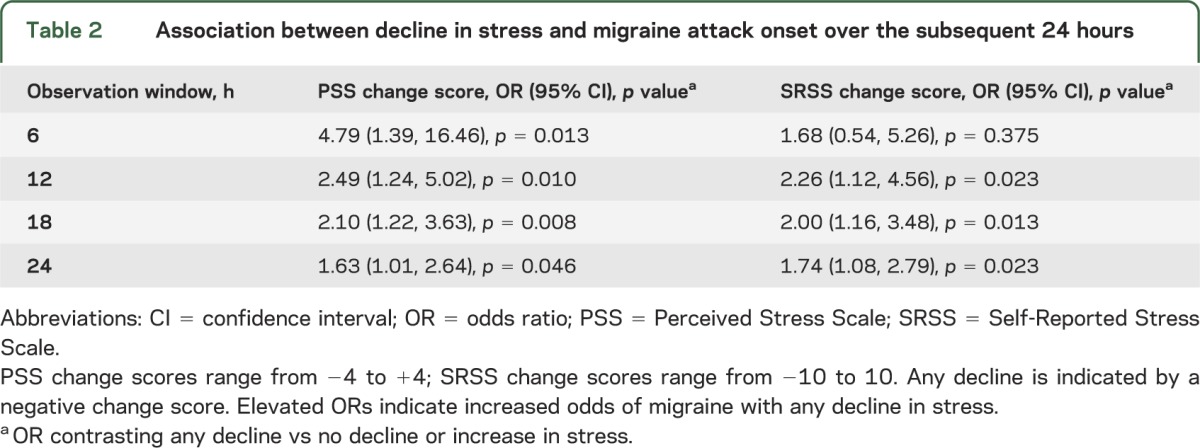
To further assess the influence of decline in stress, we dichotomized the change in stress variables to any decline (i.e., change score <0) vs no decline (including increase; i.e., change score ≥0). Any decline in perceived stress was associated with a greater occurrence of migraine across all time periods on the PSS and SRSS, with the exception of the 6-hour observation window for the SRSS (table 2). As a function of any decline in the PSS, the odds of migraine increased more than 4-fold at 6 hours and more than doubled at 12 hours. For the SRSS, declining stress was associated with headache onset at 12, 18, and 24 hours.
Table 2.
Association between decline in stress and migraine attack onset over the subsequent 24 hours
Association between level and change in stress and migraine attack.
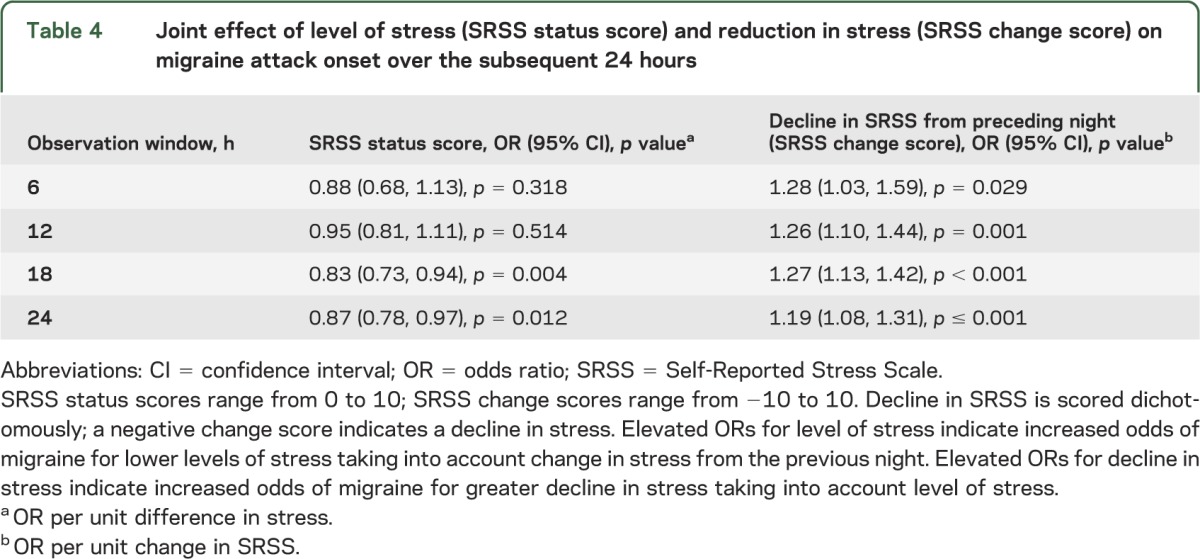
Although change in stress was more strongly associated with migraine attack onset and level of stress reached significance at a single time point, we assessed their joint influence. We modeled headache onset with level of stress (status score) and change in stress (dichotomized as decline or no decline) for the PSS. Results showed that decline in stress was significantly associated with migraine attack onset even after controlling for level of stress (table 3). Level of stress was not significantly associated with migraine onset at any time point. Adjusting for level of stress minimally attenuated the magnitude of this association (comparing results in table 3 with table 2). These results suggest that change in perceived stress rather than level of stress is associated with migraine attack onset. In table 4, we depict change in the SRSS, controlling for level of SRSS. We found that change in SRSS was significantly associated with migraine attack onset at all points in time whereas level of stress was independently associated only at 18 and 24 hours. We then fit models with any decline (dichotomized) for the PSS and the measured decline in the SRSS both included. The effect of the SRSS was reduced compared with a model without PSS for all time horizons, and the PSS was no longer significantly associated with migraine attack for any time window (table e-2).
Table 3.
Joint effect of level of stress (PSS status score) and decline in stress (any decline vs no decline on PSS) on migraine attack onset over the subsequent 24 hours
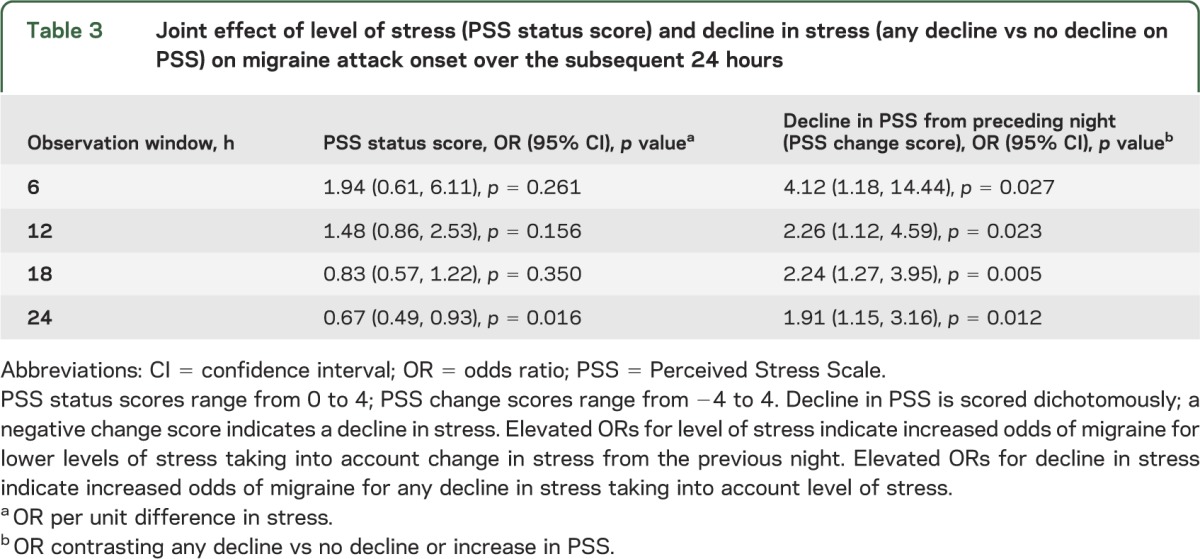
Table 4.
Joint effect of level of stress (SRSS status score) and reduction in stress (SRSS change score) on migraine attack onset over the subsequent 24 hours
DISCUSSION
In this study, we assessed stress using 2 different stress measures and examined the influence of both status and change in stress scores. Stress status scores in the evening were not significantly associated with migraine attack onset for most time windows (table 1). After taking change in stress into account, status scores were never significantly associated with migraine occurrence. However, decline in stress from one evening to the next was consistently associated with migraine attack onset on the third day. Decline in both PSS and SRSS was associated with migraine attack onset at 6, 12, and 18 hours, and the SRSS was significantly associated with onset at 24 hours. Similarly, any decline in stress tested as a dichotomous variable was robustly associated with migraine onset.
We also assessed the separate and joint contribution of status and change scores on the PSS by including them in a single model. In these models, PSS status score was not significantly associated with migraine attack onset at any time point whereas decline in PSS score was strongly related to migraine attack onset at each time point over 24 hours. Similarly, the SRSS status score was associated with headache onset at 18 and 24 hours whereas the change score was associated with an increased risk of migraine attack at all time windows. These results are consistent with and extend prior research.11–20
In aggregate, these findings and the prior literature support the let-down hypothesis, which predicts that decline in perceived stress is associated with an increased probability of migraine onset. There are at least 2 alternative mechanisms to explain this phenomenon. Stress may be associated with another factor that leads to an increased probability of migraine. These “unmeasured mediators” of stress may include missed medications, skipped meals, or disturbed sleep, any of which could arise as a consequence of stress. This explanation does not negate our findings, but suggests a direction for future testing of mediation models. Alternatively, we may have reversed cause and effect. That is, during the premonitory phase of migraine, there may be a period of increased vulnerability to stress followed by a phase of decreased vulnerability to stress. Under this hypothesis, stress followed by reduction in stress is not a trigger factor for migraine but a manifestation of the impending attack. This could represent a form of “neural normalization” if migraine increases brain activity resulting in a reduction in stress perception.32 This seems unlikely because stress is often tied to identifiable external events and the let-down effect persists for up to 24 hours.
There are several plausible biological mechanisms for let-down migraine, although the mechanism remains uncertain. Stressors are known to activate both the autonomic nervous system and neuroendocrine mechanisms in a manner that may be adaptive or maladaptive.33,34 Activation of the hypothalamic-pituitary axis leads to short-term elevation of glucocorticoids, which have anti-inflammatory and antinociceptive effects.35 When acute stress ends, hypothalamic-pituitary axis activation declines and glucocorticoid levels fall. Steroids and their withdrawal produce a complex array of sometimes contradictory effects including both pain relief and hyperalgesia.35 Glucocorticoid withdrawal may contribute to let-down migraine. In addition to the role of stress in attack initiation, migraine attacks are stressful events in their own right. Migraine attacks with recurrent episodes of pain, central sensitization, and concomitant hormonal and inflammatory changes may alter brain structure and function.31,34
This study has several limitations. Generalizability of findings to primary care settings is uncertain. The SRSS has not been validated; it is face valid, correlated with the validated PSS, and was consistently associated with an increased risk of migraine attack onset. There were missing diary entries and data for most subjects. Finally, the sample was small; however, each individual serves as their own control (analyses are within-person), which adjusts for a broad array of characteristics. Despite the modest number of participants, the study included 2,011 daily diary entries and 110 migraine attacks, supporting robust and consistent results. Larger studies are needed to assess both generalizability and individual differences in the relationship of stress and attack occurrence. Appendix e-1 contains tables e-3 through e-7, which provide information on variance components and intraclass correlations to facilitate powering future studies. We specifically examined the effects of stress on day 1 and day 0. Level of stress on day 2 was protective (data not shown). We hope to examine a broader range of temporal relations in a larger sample in future work.
Despite these limitations, this study demonstrates a striking association between reduction in perceived stress and migraine occurrence utilizing time-stamped electronic diaries. Results demonstrate that electronic diaries coupled with appropriate analytic strategies provide a powerful tool for assessing headache triggers.11–22 Random-effects logistic regression modeling provides more accurate summaries of effects both within and among participants. Methodologic issues in studying stress and relaxation after stress exemplify the problems that arise for studying provocative factors for migraine in general. Self-reported beliefs about migraine triggers are best regarded as hypothesis-generating. Reports of triggers can be followed up either with observational diary studies, or for some triggers, with randomized trials. Experimental interventions (randomized blinded exposure) are, in some respects, the most rigorous design but they have limitations. For some exposures, experimental administration is not possible. For others, response to placebo or sham triggers may be high. Most trials involve exposure on a single occasion. If migraine thresholds vary over time and trigger factors interact, a laboratory-based, single-blinded exposure study may miss important associations. This weakness is offset either in multiattack observational studies or with longer-term blinded exposure in a randomized clinical trial. In this regard, studies of aspartame are informative.36–39 If variation in perceived stress triggers migraine attacks, for at least some individuals, strategies that reduce stress may decrease attack frequency. Awareness of perceived stress may improve migraine prediction and provides targets for nonpharmacologic and pharmacologic interventions. Because the let-down effect occurs over 6 to 24 hours, proposed interventions should work quickly and maintain effects over 24 hours. Empirically supported therapies include cognitive behavioral therapy, biofeedback training, mindfulness-based stress reduction, and healthy lifestyle practices (e.g., exercise, proper sleep hygiene), which may help moderate increases in perceived stress.40 Teaching individuals to monitor stress levels at multiple time points during the day and engage in brief interventions may help protect against additional increases and possibly reduce levels of stress. Interventions that stabilize stress, including relaxation practice, diaphragmatic breathing, and guided visual imagery, may be helpful in this context.40 Future work should supplement patient-reported measures with physiologic measures of sympathetic and neuroendocrine effectors.
Supplementary Material
ACKNOWLEDGMENT
The authors thank C. Mark Sollars, MS, and Emily L. Polak, PhD, for editorial support.
GLOSSARY
- GAD-7
Generalized Anxiety Disorder–7 item
- OR
odds ratio
- PHQ-9
Patient Health Questionnaire–9 item
- PSS
Perceived Stress Scale
- SRSS
Self-Reported Stress Scale
Editorial, page 1388
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
Drs. Lipton, Buse, Haut, Hall, Borkowski, and Tennen participated in study design and conceptualization of the study. Drs. Lipton, Buse, Haut, Hall, Grosberg, and Borkowski, and Ms. DeFreitas participated in data collection. Drs. Lipton, Buse, Haut, Hall, and Tennen participated in analysis or interpretation of the data. All authors participated in drafting or revising the manuscript for intellectual content.
STUDY FUNDING
Supported by an investigator-initiated grant from Endo Pharmaceuticals, Chadds Ford, PA.
DISCLOSURE
R. Lipton receives research support from the NIH (PO1 AG03949) (Program Director, Project and Core Leader), RO1AG025119 (investigator), RO1AG022374-06A2 (investigator), RO1AG034119 (investigator), RO1AG12101 (investigator), K23AG030857 (mentor), K23NS05140901A1 (mentor), and K23NS47256 (mentor), the National Headache Foundation, and the Migraine Research Fund; serves on the editorial board of Neurology®, has reviewed for the National Institute on Aging and National Institute of Neurological Disorders and Stroke, holds stock options in eNeura Therapeutics; serves as consultant, advisory board member, or has received honoraria from: Allergan, American Headache Society, Autonomic Technologies, Boehringer-Ingelheim Pharmaceuticals, Boston Scientific, Bristol-Myers Squibb, CogniMed, CoLucid, Eli Lilly, Endo, eNeura Therapeutics, GlaxoSmithKline, Merck, Novartis, NuPathe, Pfizer, and Vedanta. D. Buse has received grant support from the National Headache Foundation and research support or honoraria from Allergan Pharmaceuticals, MAP Pharmaceuticals, Merck & Co., Inc., NuPathe, and Novartis. C. Hall receives or has received research support from the National Institute on Aging (P01 AG03949, P01 AG027734, R01 AG022092, R01 AG034087), the National Center for Research Resources (1-UL1-RR025750-01), the National Cancer Institute (P30 CA13330-35), the National Institute of Occupational Safety and Health (5-U1O-OH008242 and contract 200-2011-39489), and Endo Pharmaceuticals, has consulted for research projects at the University of Connecticut Health Center, is and has been a member of Data and Safety Monitoring Committees at Columbia University, received honoraria from Washington University St. Louis and from Oregon Health and Science University, has received travel funding from Washington University St. Louis, Yale University, Oregon Health and Science University, and the University of Victoria, is a member of the American Statistical Association Media Experts Panel, and is a member of the editorial board of The Open Neurology Journal. H. Tennen receives research support from the NIH (5R21AA017584-04, co-principal investigator [PI]; 5P60AA003510-33, Center Component PI and Center Component investigator; 3R01AGO026006, subcontractor; 5R01AA016599-03, subcontractor; 5R01AA12827-07, investigator); he serves on the editorial boards of Journal of Social and Clinical Psychology, Journal of Personality Assessment, Journal of Personality and Social Psychology, Social Psychology Compass, and The Journal of Positive Psychology, and he is the editor of Journal of Personality. Dr. Tennen serves as a consultant or has received honoraria from John Wiley & Sons, Pfizer, and Best Practice Project Management. T. DeFreitas and T. Borkowski report no disclosures relevant to the manuscript. B. Grosberg served on a scientific advisory board for Kowa Pharmaceuticals America, Inc., and Tribute Pharmaceuticals; has received speaker honoraria from Zogenix; and receives research support from Allergan, Inc., Boston Scientific, and ElectroCore. S. Haut receives grant support from NIH (RO1 NS053998) and the Shor Foundation for Epilepsy Research. She has consulted for Acorda, Impax, and Upsher-Smith. She is on the editorial board of Epilepsy & Behavior and Epilepsy Currents. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Lipton RB, Bigal ME, Diamond M, Freitag F, Reed ML, Stewart WF. Migraine prevalence, disease burden, and the need for preventive therapy. Neurology 2007;68:343–349 [DOI] [PubMed] [Google Scholar]
- 2.Stovner LJ, Hagen K, Jensen R, et al. The global burden of headache: a documentation of headache prevalence and disability worldwide. Cephalalgia 2007;27:193–210 [DOI] [PubMed] [Google Scholar]
- 3.Haut SR, Bigal ME, Lipton RB. Chronic disorders with episodic manifestations: focus on epilepsy and migraine. Lancet Neurol 2006;5:148–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Giffin NJ, Ruggiero L, Lipton RB, et al. Premonitory symptoms in migraine: an electronic diary study. Neurology 2003;60:935–940 [DOI] [PubMed] [Google Scholar]
- 5.Andress-Rothrock D, King W, Rothrock J. An analysis of migraine triggers in a clinic-based population. Headache 2010;50:1366–1370 [DOI] [PubMed] [Google Scholar]
- 6.Haque DB, Rahman DK, Hoque DA, et al. Precipitating and relieving factors of migraine versus tension-type headache. BMC Neurol 2012;12:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hauge AW, Kirchman M, Olesen J. Characterization of consistent triggers of migraine with aura. Cephalalgia 2011;31:416–438 [DOI] [PubMed] [Google Scholar]
- 8.Hougaard A, Amin F, Hauge AW, Ashina M, Olesen J. Provocation of migraine with aura using natural trigger factors. Neurology 2013;80:428–431 [DOI] [PubMed] [Google Scholar]
- 9.Schürks M, Buring JE, Kurth T. Migraine features, associated symptoms and triggers: a principal component analysis in the Women's Health Study. Cephalalgia 2011;31:861–869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cooke LJ, Rose MS, Becker WJ. Chinook winds and migraine headache. Neurology 2000;54:302–307 [DOI] [PubMed] [Google Scholar]
- 11.Zebenholzer K, Rudel E, Frantal S, et al. Migraine and weather: a prospective diary-based analysis. Cephalalgia 2011;31:391–400 [DOI] [PubMed] [Google Scholar]
- 12.Kelman L. The triggers or precipitants of the acute migraine attack. Cephalalgia 2007;27:394–402 [DOI] [PubMed] [Google Scholar]
- 13.Sauro KM, Becker WJ. The stress and migraine interaction. Headache 2009;49:1378–1386 [DOI] [PubMed] [Google Scholar]
- 14.Wöber C, Holzhammer J, Zeitlhofer J, Wessely P, Wöber-Bingöl C. Trigger factors of migraine and tension-type headache: experience and knowledge of the patients. J Headache Pain 2006;7:188–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spierings EL, Sorbi M, Haimowitz BR, Tellegen B. Changes in daily hassles, mood, and sleep in the 2 days before a migraine headache. Clin J Pain 1996;12:38–42 [DOI] [PubMed] [Google Scholar]
- 16.Hashizume M, Yamada U, Sato A, et al. Stress and psychological factors before a migraine attack: a time-based analysis. Biopsychosoc Med 2008;2:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Couturier EG, Hering R, Steiner TJ. Weekend attacks in migraine patients: caused by caffeine withdrawal? Cephalalgia 1992;12:99–100 [DOI] [PubMed] [Google Scholar]
- 18.Hering R, Couturier EG, Steiner TJ. Weekend migraine in men. Lancet 1992;339:67. [DOI] [PubMed] [Google Scholar]
- 19.Morrison DP. Occupational stress in migraine: is weekend headache a myth or reality? Cephalalgia 1990;10:189–193 [DOI] [PubMed] [Google Scholar]
- 20.Wöber C, Brannath W, Schmidt K, et al. ; PAMINA Study Group. Prospective analysis of factors related to migraine attacks: the PAMINA Study. Cephalalgia 2007;27:304–314 [DOI] [PubMed] [Google Scholar]
- 21.Torelli P, Cologno D, Manzoni GC. Weekend headache: a retrospective study in migraine without aura and episodic tension-type headache. Headache 1999;39:11–20 [DOI] [PubMed] [Google Scholar]
- 22.Nattero G, De Lorenzo C, Biale L, Allais G, Torre E, Ancona M. Psychological aspects of weekend headache sufferers in comparison with migraine patients. Headache 1989;29:93–99 [DOI] [PubMed] [Google Scholar]
- 23.Hufford MR, Stone AA, Shiffman S, Schwartz JE, Broderick JE. Patient non-compliance with paper diaries. BMJ 2002;324:1193–1194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Headache Classification Subcommittee of the International Headache Society. International Classification of Headache Disorders, 2nd ed. Cephalalgia 2004;24(suppl 1):9–160 [DOI] [PubMed] [Google Scholar]
- 25.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med 2001;16:606–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spitzer RL, Kroenke K, Williams JBW, Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med 2006;166:1092–1097 [DOI] [PubMed] [Google Scholar]
- 27.Lipton RB, Bigal ME, Ashina S, et al. Cutaneous allodynia in the migraine population. Ann Neurol 2008;63:148–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stewart WF, Lipton RB, Kolodner KB, Sawyer J, Lee C, Liberman JN. Validity of the Migraine Disability Assessment (MIDAS) score in comparison to a diary-based measure in a population sample of migraine sufferers. Pain 2000;88:41–52 [DOI] [PubMed] [Google Scholar]
- 29.Tugade MM, Conner TS, Barrett LF. Assessment of mood. In: Ayers S, Baum A, McManus C, et al., editors. The Cambridge Handbook of Psychology, Health, and Medicine, 2nd ed Cambridge, UK: Cambridge University Press; 2007:278–286 [Google Scholar]
- 30.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav 1983;24:385–396 [PubMed] [Google Scholar]
- 31.Liao J, Hall CB. Fast and accurate computation of the exact confidence limits for the common odds ratio in several 2 × 2 tables. J Comput Graph Stat 1995;4:173–179 [Google Scholar]
- 32.Carandini M, Heeger DJ. Normalization as a canonical neural computation. Nat Rev Neurosci 2012;13:51–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McEwen BS, Stellar E. Stress and the individual: mechanisms leading to disease. Arch Intern Med 1993;153:2093–2101 [PubMed] [Google Scholar]
- 34.Borsook D, Maleki N, Becerra L, McEwen B. Understanding migraine through the lens of maladaptive stress responses: a model disease of allostatic load. Neuron 2012;73:219–234 [DOI] [PubMed] [Google Scholar]
- 35.McEwen BS, Kalia M. The role of corticosteroids and stress in chronic pain conditions. Metabolism 2010;59(suppl 1):S9–S15 [DOI] [PubMed] [Google Scholar]
- 36.Schiffman SS, Buckley CE, Sampson HA, et al. Aspartame and susceptibility to headache. N Engl J Med 1987;317:1181–1185 [DOI] [PubMed] [Google Scholar]
- 37.Koehler SM, Glaros A. The effect of aspartame on migraine headache. Headache 1988;28:10–14 [DOI] [PubMed] [Google Scholar]
- 38.Lipton RB, Newman LC, Solomon S. Aspartame as a dietary trigger in migraine. Headache 1989;29:90–92 [DOI] [PubMed] [Google Scholar]
- 39.Lipton RB, Newman LC, Solomon S. Aspartame and headache. N Engl J Med 1988;318:1200–1202 [DOI] [PubMed] [Google Scholar]
- 40.Buse DC, Andrasik F. Behavioral medicine for migraine. Neurol Clin 2009;27:445–465 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


