Abstract
Objective:
To study the impact of negative expectation related to receiving a placebo (the “lessebo effect”) on efficacy outcome measures of symptomatic treatments in Parkinson disease (PD).
Methods:
We conducted meta-analyses of double-blind randomized controlled trials (RCTs) of dopamine agonists in PD and compared the pooled mean score change of the motor section of the Unified Parkinson's Disease Rating Scale (mUPDRS) across active treatment arms according to the presence of a placebo arm or the probability of placebo assignment (0%, <50%, and 50%) of the original RCT. A mixed-effects model was used. Heterogeneity was assessed by subgroup analyses and meta-regression modeling.
Results:
A total of 28 study arms were extracted from active-controlled trials (3,277 patients) and 42 from placebo-controlled trials (4,554 patients). The overall difference between groups in the pooled mean score change in the mUPDRS was 1.6 units (95% confidence interval [CI] 0.2, 3.0; p = 0.023), in favor of the active-controlled group. In subgroup analyses, this difference was of higher magnitude in the early PD group without motor fluctuations (3.3 mUPDRS units, 95% CI 1.1, 5.4; p = 0.003) and for study duration ≤12 weeks (4.1 mUPDRS units, 95% CI 1.0, 7.2; p = 0.009). There was no between-group difference using probability of placebo assignment as criterion.
Conclusions:
This study shows that the use of a placebo can be associated with a clinically significant reduction in the magnitude of change of the mUPDRS after an active treatment in RCTs for PD. These new findings have potential implications in the development of new treatments and appraisal of current treatment options for PD and possibly for other neurologic disorders.
The use of a placebo is the norm in randomized controlled trials (RCTs) of interventions for conditions for which there is no established standard of care. The rationale to support the use of placebo derives from the need to distinguish the benefit observed in a patient related to the expectation of receiving a potentially beneficial treatment from the effect of the study intervention that relates to its pharmacologic properties. In Parkinson disease (PD), a placebo response has been well-demonstrated.1 A meta-analysis of RCTs of symptomatic medical therapies conducted in different stages of PD severity found that 18% of patients demonstrated a placebo response as defined by the authors.1
A possible effect of the use of a placebo in RCTs that has been little studied is the negative expectation it can generate in a study participant, related to the possibility of not receiving the active treatment. This negative expectation, engendered by the possibility of placebo assignment and coined the “lessebo effect,” may have an important impact on the measured response to a treatment.2 With the exception of a small number of studies in psychiatry,2–6 this novel concept, alternatively considered a “negative placebo effect,” has not been reported in other branches of medicine, including neurology. Our aim was to assess the presence and magnitude of the lessebo effect in PD, and to determine the influence of factors such as demographics, disease characteristics, and study design.
METHODS
Study selection.
Randomized double-blind placebo- or active-controlled clinical trials of at least 4 weeks' duration for symptomatic treatment of PD were included. Crossover trials were excluded. All pharmacologic interventions investigated for an antiparkinsonian effect in patients with PD were included, regardless of the route of administration. Surgical or physical therapy interventions were excluded. Studies including patients with a concomitant diagnosis of dementia were excluded.
Outcome measure.
The mean score change in the motor section of the Unified Parkinson's Disease Rating Scale (mUPDRS) from baseline to the end of treatment was the outcome measure.
Search strategy.
A literature search was conducted using the electronic medical databases Medline (1965–November 2012), EMBASE (1974–November 2012), The Cochrane Controlled Trials Register (issue 11, November 2012), Clinical Trials Database of the US NIH (last accessed in November 2012), and published guidelines on the management of PD.7–14 We used a broad search strategy using the terms Parkinson* disease, treatment, limited to human studies, clinical trials, guidelines, or reviews. We contacted the authors or sponsors to obtain data not available in the original publications (see Acknowledgment). We also searched assessment reports of the US Food and Drug Administration and European Medicines Agency (November 2012).
Data collection.
The unit of observation was a study arm. Year of publication, study design, allocation ratio to placebo and active treatment, intervention, duration of treatment, and outcome measures of the included studies were collected. Data from the original publication as reported were collected for analyses, and when the SD of the mean change between baseline and end of treatment was not available (indicated by # in additional list of references on the Neurology® Web site at Neurology.org), we estimated this using the baseline and end of treatment SD, allowing for a correlation factor of 0.5.15
Data analysis.
All included studies were assessed for the risk of bias using the Cochrane risk of bias tool.16 The pooled mean score change in the mUPDRS from baseline to end of treatment across all active treatment study arms, regardless of being used as control group (i.e., an active comparator), was calculated using a random-effects model with inverse-variance weighting (Der-Simonian and Laird method),17 as heterogeneity was expected to be high. The index I2 was used for assessment of heterogeneity and values greater than 50% were considered high.15
As primary analysis, we stratified the active treatment study arms into 2 groups:
Study arms extracted from RCTs using another pharmacologic intervention for PD as control, i.e., active comparator (active-controlled group).
Study arms extracted from placebo-controlled RCTs (placebo-controlled group).
As secondary analyses, we further stratified the placebo-controlled group into 2 categories according to the probability of placebo assignment being 50% or less than 50%. We used 2 definitions for this stratification: criterion A—probability of placebo assignment as a function of the number of study arms (i.e., 1 divided by the total number of study arms); criterion B—probability of placebo assignment according to the number of subjects assigned to placebo. For example, a 2-arm study with an allocation ratio of 2:1 (active: placebo) had a probability of placebo allocation of 0.5 using criterion A (2 treatment arms) and 0.33 using criterion B (2:1 allocation). The pooled mean mUPDRS score change in the different strata was compared using a meta-regression mixed-effects model. The same modelling strategy was used to compare patient-level characteristics between active-controlled and placebo-controlled groups. Study-level characteristics were compared between the 2 groups using the Wilcoxon rank sum test for continuous variables, the χ2 test for binary variables, and the Kruskal-Wallis test for multicategorical (>2 levels) variables. Subgroup analyses were conducted to assess sources of heterogeneity from study-associated factors such as duration of the trial, levodopa equivalent daily dose (LEDD), data analysis according to intention-to-treat principle, and patient or disease-associated factors (mean age, median duration of disease, stage of disease [early PD vs PD with motor fluctuations], and baseline mUPDRS score). For stage of disease, early PD was defined as patients in need of symptomatic treatment who were untreated or taking only monoamine oxidase type B inhibitors or amantadine. Continuous variables were dichotomized by the median value except for study duration, which was dichotomized using threshold (12 weeks) determined as clinically meaningful according to the opinion of the investigators (T.A.M., C.M., A.E.L.). Subgroup analyses were conducted with meta-regression modelling using a mixed-effects model and a test for interaction between the subgroup variable and type of control group (active vs placebo). To calculate the LEDD, we used the following conversion factors: bromocriptine dose (×10), ropinirole dose (×20), pergolide dose (×100), pramipexole dose (×100), cabergoline dose (×66.6), piribedil dose (×1), dihidro-ergotamine dose (×20), rotigotine dose (×30).18 A p value ≤ 0.05 was deemed statistically significant. The statistical analyses were performed using Stata v12.0 (StataCorp, College Station, TX: StataCorp LP).
RESULTS
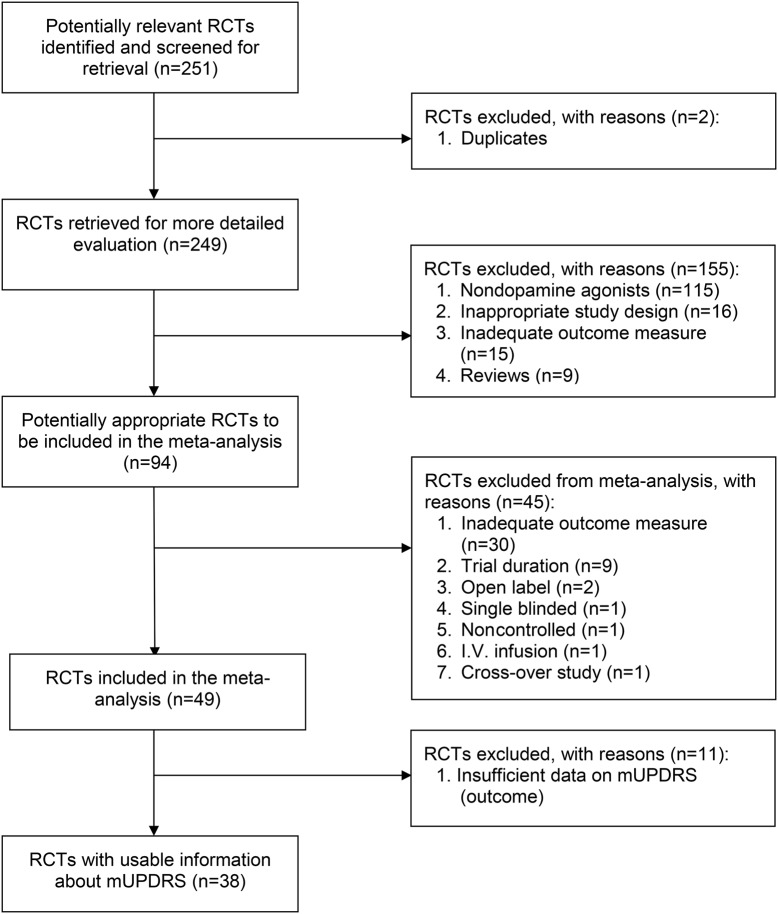
The initial search identified 249 studies, of which 155 were excluded after assessment of eligibility criteria based on the study title and abstract. By consensus, we decided to exclusively include trials of dopamine agonists (DAs) to ensure a greater degree of homogeneity across studies. Of the 94 studies with DAs, 56 were excluded after reviewing the full-text article (figure 1). Thirty-eight studies (see e-references) met inclusion criteria with a total of 70 study arms of active treatment and 7,831 randomized participants (table e-1). Assessment of risk of bias revealed that the majority of included studies had a low risk of bias (figure e-1). The 70 active treatment study arms were divided into those from studies using an active comparator (active-controlled group, n = 28, 3,277 patients) and those using a placebo control (placebo-controlled group, n = 42, 4,554 patients).
Figure 1. Flowchart for selection of included studies (according to PRISMA guidelines)27.
mUPDRS = motor section of the Unified Parkinson's Disease Rating Scale; RCT = randomized controlled trial.
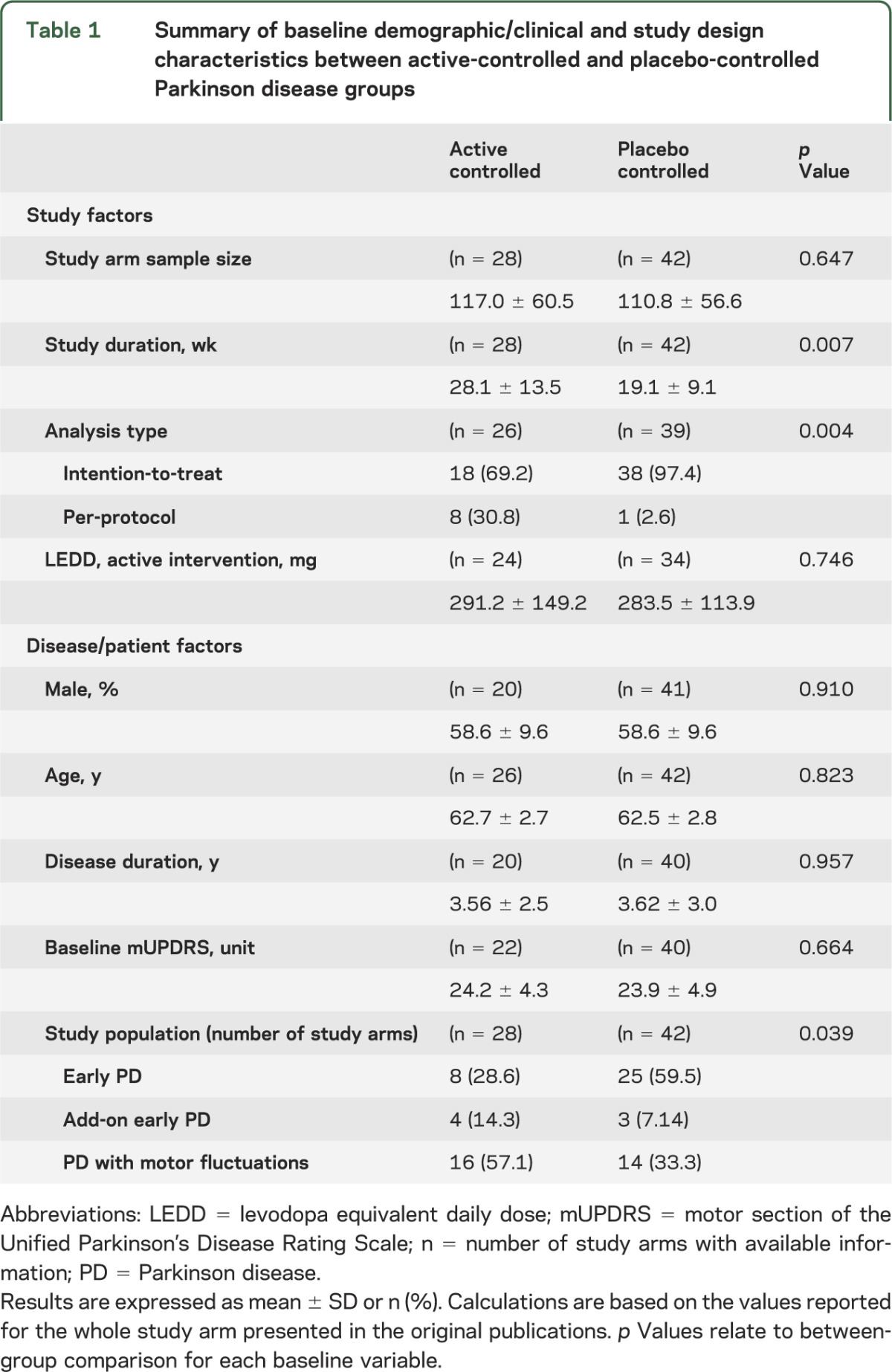
The active-controlled group had longer study duration compared with the placebo-controlled group (28.1 ± 13.5 vs 19.1 ± 9.1 week, p = 0.007) and were less frequently extracted from RCTs in early PD (8, 28.6% vs 25, 59.5%, overall p = 0.039) or with an intention-to-treat type of data analysis (18, 69.2% vs 38, 97.4%, p = 0.004). The remaining baseline demographic (age, sex), clinical (duration of disease, baseline mUPDRS), and study (LEDD) variables were not statistically different between the 2 groups (table 1).
Table 1.
Summary of baseline demographic/clinical and study design characteristics between active-controlled and placebo-controlled Parkinson disease groups
Mean score change in the mUPDRS.
Primary analysis.
The pooled mean mUPDRS score change from baseline to end of treatment was 6.0 units (95% confidence interval [CI] 5.2, 6.8) in the placebo-controlled group (figure 2A) and 7.6 units (95% CI 6.5, 8.7) in the active-controlled group (figure 2B). The between-group difference was 1.6 units (95% CI 0.2, 3.0; p = 0.023). There was significant heterogeneity in both meta-analyses with an I2 value of 91% in each. For the subgroup of arms with DA only, the difference between the placebo-controlled group and active-controlled group was 1.4 units (95% CI 0.3; 2.7; p = 0.044).
Figure 2. Forest plots.
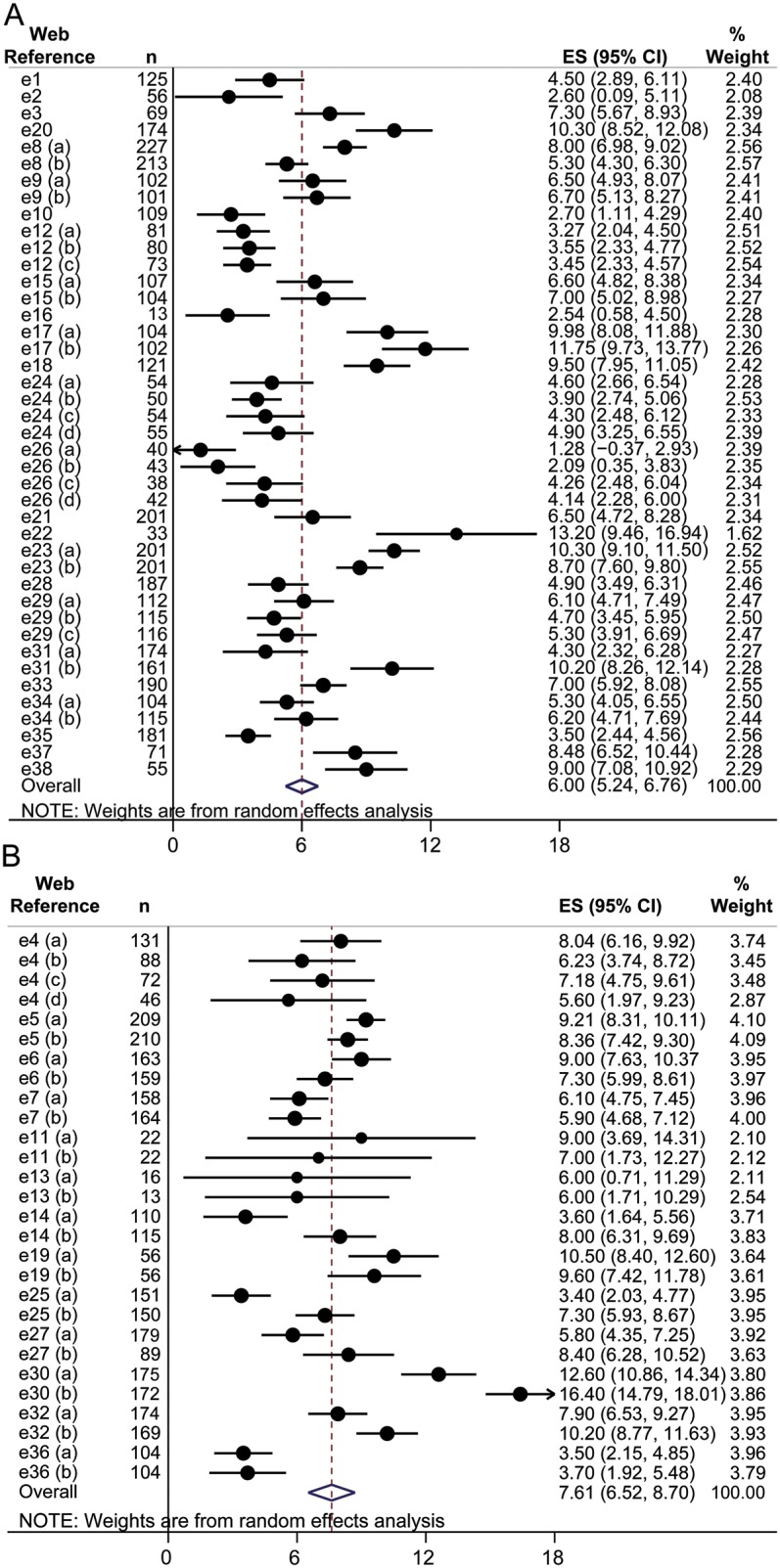
(A) Forest plot of the meta-analysis for the change in the motor section of the Unified Parkinson's Disease Rating Scale (mUPDRS) after an active treatment in placebo-controlled studies in Parkinson disease (PD). (B) Forest plot of the meta-analysis for the change in the mUPDRS after an active treatment in active-controlled studies in PD. Results from individual studies and pooled results are presented. Reference numbers refer to e-references. CI = confidence interval; ES = effect estimate/mean difference; n = sample size of study arm; weight = inverse variance weighing.
Secondary analyses.
A difference in the pooled mean mUPDRS score change was found among the different probabilities of placebo assignment (0, <50%, or 50%) regardless of using criterion A (number of study arms) or criterion B (allocation ratio). Only the difference between the active-controlled group and the group with a probability of <50% of placebo assignment was significant (1.9 units, 95% CI 0.3, 3.4, p = 0.017 for criterion A, and 2.18 units, 95% CI 0.51, 3.75, p= 0.023 for criterion B). There was no significant difference between the groups with a probability of placebo assignment of 50% and <50% (see table e-2).
Patient- and disease-associated factors.
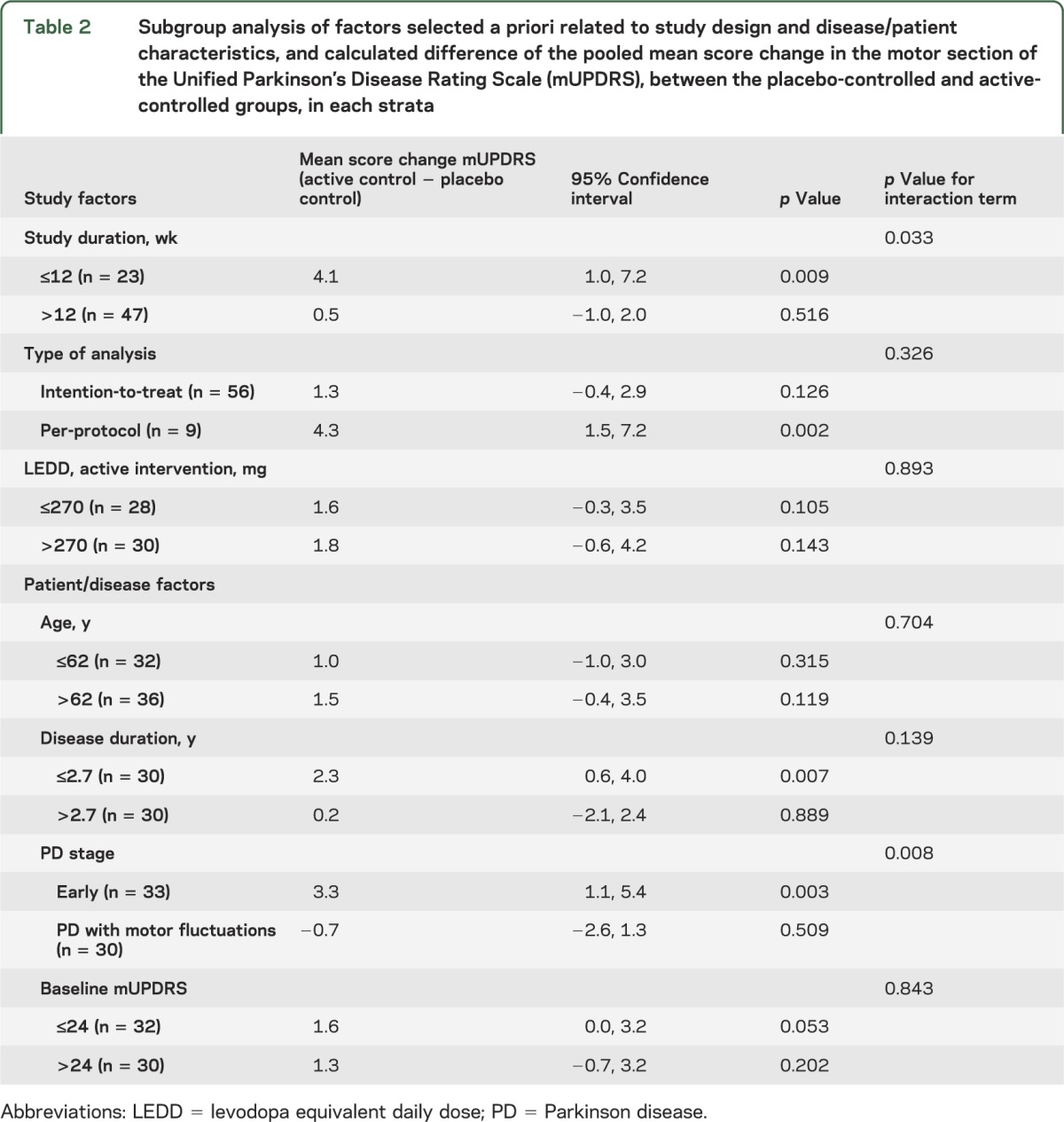
The difference in the pooled mean mUPDRS score change between the placebo- and active-controlled groups was significantly greater in studies enrolling participants with early PD than in studies where patients had PD with motor fluctuations (table 2). There were no significant subgroup effects for age, disease duration, or baseline mUPDRS.
Table 2.
Subgroup analysis of factors selected a priori related to study design and disease/patient characteristics, and calculated difference of the pooled mean score change in the motor section of the Unified Parkinson's Disease Rating Scale (mUPDRS), between the placebo-controlled and active-controlled groups, in each strata
Study-associated factors.
The difference in the pooled mean mUPDRS score change between active-controlled and placebo-controlled groups was significantly larger for studies of ≤12 weeks in duration than for longer studies (table 2). There were no significant subgroup effects for type of data analysis or LEDD.
DISCUSSION
In these meta-analyses, we have demonstrated that the presence of a placebo arm in RCTs of symptomatic therapy for PD is associated with a reduction in the magnitude of change in the investigator-derived mUPDRS, compared to studies involving only active comparators. Thus, for antiparkinsonian drugs within the same drug class, a study with an active comparator can be associated with a change in the mUPDRS of larger magnitude compared with a placebo-controlled study, other aspects of study design (e.g., blinding, randomization, allocation concealment) and study population being equal. The estimated magnitude of the lessebo effect was 1.60 mUPDRS units across all studies, and as high as 3.3 units for the early PD group, and 4.1 units for the group of studies with duration less than or equal to 12 weeks. A “minimal clinically important difference,” i.e., the smallest change perceived and valued by a patient, is reported to be in the range of 1.519 to 5.120 mUPDRS units in PD. Thus, the lessebo effect is within this clinically relevant range. Within a given clinical trial, it is not known if the lessebo effect has an equal impact on the different arms of the trial. If not, then it would alter the effect size for that study, with resulting implications for trial design (sample size, choice of comparator). Such a change in effect size would also have implications when making judgments of comparative effectiveness of agents by examining differences in magnitude of effect between studies.
Expectation of benefit in clinical trials may have several contributors. It is necessary to consider the effect of the expectation of benefit at the baseline of the study (e.g., related to the likelihood of placebo allocation) and the perceived allocation by the patient at the end of the study, the latter reflecting the cumulative patient experience during the trial. Given that the mUPDRS is a physician-rated scale, the attitudes of the physician executing the rating scale could also contribute to the observed difference, if the physician were conditioned by the perceived beliefs of treatment allocation. Understanding the contributions of these factors to the measured outcome will require prospective measurement of expectation of benefit on the part of both patients and physicians at the beginning and end of a trial. Compared with prior studies on the lessebo effect conducted in psychiatry,2–6 the results are much less dependent on the patient's subjective assessment, since the mUPDRS is a physician-rated assessment of motor function in PD.
Our results support the hypothesis that a physiologic change (e.g., increase in brain dopamine levels leading to a reduction in parkinsonism) can be triggered by the patient's expectation of benefit per se or by a greater motivation in following physician's instructions. As mentioned, the beliefs of the rating physician could also contribute to the observed difference. Along these lines, it would be interesting to assess the presence of the lessebo effect in more subjective or health-related quality of life outcomes measures in PD, such as the experience of daily living sections of the new Movement Disorders Society–UPDRS.21
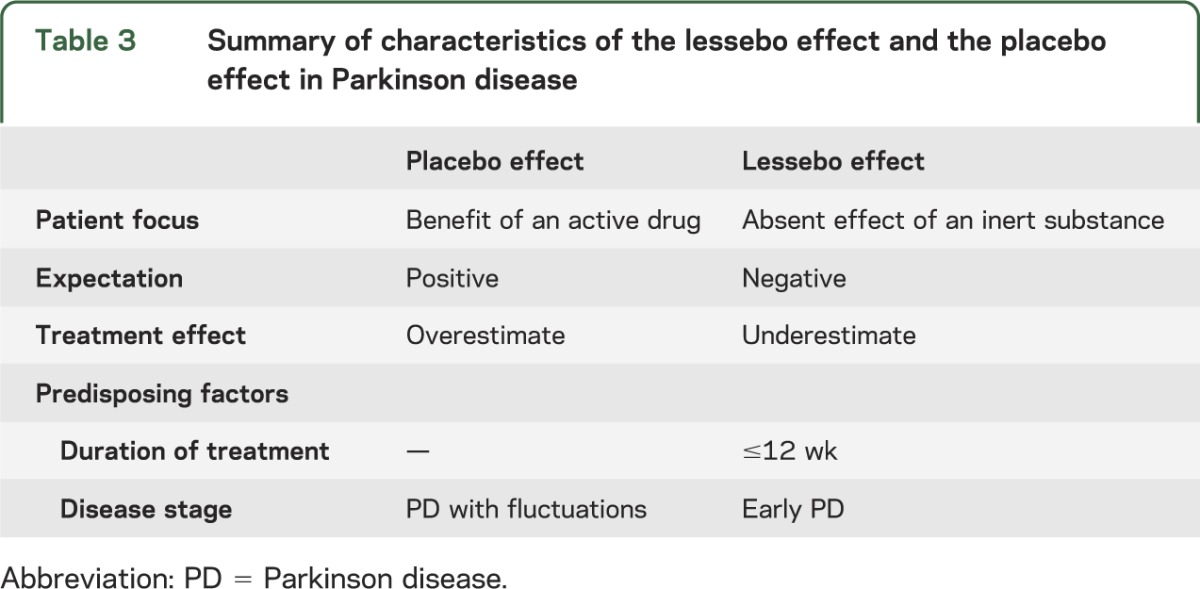
Neuroimaging offers potential insights into the brain mechanisms underlying the effects of patient's expectation of benefit in PD and the use of placebo, which relate to the lessebo effect. It has been proposed that the placebo effect is mediated by reward systems.22 [11C] raclopride PET studies in PD have shown that a placebo response and the expectation of being given an active treatment is associated with the release of endogenous dopamine in the striatum accompanied by an observable improvement in parkinsonian motor function.23 In light of these findings, we hypothesize that a lessebo effect could be explained by a relative reduction in endogenous dopamine release, when patients with PD participating in a placebo-controlled trial have a dominant expectation of being given an inert substance. We hypothesize that placebo and lessebo effects can even coexist in a patient or in a group of patients participating in a placebo-controlled trial and the prevailing negative or positive expectation of benefit would determine a decrease or increase of cerebral dopamine release and, consequently, the relative influences of each. The results of the subgroup analyses suggest that the lessebo effect is greater in the population of patients with early PD. We found no evidence of a lessebo effect in clinical studies of patients with PD with motor fluctuations. These findings are in striking contrast with the reported increased likelihood of a positive placebo response in patients with motor fluctuations and higher baseline mUPDRS scores.1 This suggests that the magnitude of the placebo and lessebo effects could change in an inverse manner (table 3).
Table 3.
Summary of characteristics of the lessebo effect and the placebo effect in Parkinson disease
We did not identify a difference in the pooled mean mUPDRS score change between the groups with 50% vs <50% probability of placebo assignment. Different scenarios can explain these results. (1) Only the existence of a placebo arm per se could be the relevant factor for a study participant but not the higher or lower likelihood of being assigned to an active treatment. A study of the impact of placebo assignment in clinical trials in PD showed that patients with PD generally do not retain information on the different likelihoods of placebo allocation: 71% to 100% of patients enrolled in placebo-controlled studies with more than one active arm did not understand their likelihood of placebo allocation,24 while 90% of patients enrolled in a 2-arm placebo-controlled study understood the 50% likelihood of placebo allocation. This finding is consistent with the lack of association between allocation ratio and the lessebo effect in our study. (2) The lessebo effect could be maximal for intermediate probabilities of placebo allocation. Support for this hypothesis is mixed: [11C] raclopride PET studies in PD report that a placebo response is also maximal for intermediate probabilities of uncertainty of placebo allocation.25 However, clinical trial data have shown an increased likelihood of a positive placebo response with a higher likelihood of a placebo assignment (50% vs <50%).1 (3) We cannot exclude a lack of power as the reason for finding no difference in the magnitude of the lessebo effect for different probabilities of placebo assignment.
It is possible that the lessebo effect is also present in other neurologic diseases and medical conditions. To our knowledge, the lessebo effect has only been evaluated in psychiatry2–6 through meta-analyses and shown to be present. In these studies, the outcomes are intrinsically and strongly biased by patients' subjective impression of benefit. It would be informative to ascertain whether the lessebo effect is present in conditions where, for example, dopaminergic function is not obviously impacted or the placebo response is less frequent or pronounced.
The results of these meta-analyses provide the seeds for future research into the existence and nature of the lessebo effect in PD and other disorders. A meta-analysis of individual patient data would magnify the sample size substantially and overcome the aggregation bias created by pooling mean results at a study level.26 Other possible avenues of research to prospectively test the validity of our hypotheses could include imaging studies to determine the differential activation of brain areas in the “pessimistic” and “optimistic” study participant. Finally, the attitudes and influences of the evaluating investigator also need to be considered in understanding both the placebo and lessebo effects. Randomly assigning patients with PD to one of 2 RCTs of a dopamine agonist, one with an active control (a comparable dopamine agonist) and the other with a placebo control, using raters blinded to the study design, could further help determine the influence of the evaluating investigator. In addition, the implication of the lessebo effect in disease-modifying treatment studies has to be assessed, as these studies routinely use placebo as control.
Overall, our results emphasize the need to consider the existence of a lessebo effect in the design and interpretation of interventional studies in patients with PD and how patient characteristics and study factors can condition its magnitude.
Supplementary Material
ACKNOWLEDGMENT
The authors thank Professor Christopher Goetz, Professor Claudia Trenkwalder, Professor Cristina Sampaio, Dr. Giménez Roldán, Professor Donald Grosset, Professor Michael McDermott, Dr. Karl Kieburtz, Dr. Peter Le Witt, Professor Lucilla Parnetti, Dr. Karen Blindauer, Professor Joaquim Ferreira, Professor Olivier Rascol, Dr. Yoshi Mizuno, and the Parkinson Study Group for facilitating the acquisition of original unpublished data; and Polichem SA, UCB Pharma, and GlaxoSmithKline for their assistance in obtaining further trial data.
GLOSSARY
- CI
confidence interval
- DA
dopamine agonist
- LEDD
levodopa equivalent daily dose
- mUPDRS
motor section of the Unified Parkinson's Disease Rating Scale
- PD
Parkinson disease
- RCT
randomized controlled trial
Footnotes
Editorial, page 1390
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
T.A.M.: literature search, study design, methodology, data collection, data analysis, data interpretation, writing. P.S.: methodology, data analysis, data interpretation, critical review of manuscript. C.M.: data interpretation, critical review of manuscript. G.T.: methodology, data analysis, critical review of manuscript. A.E.L.: study design, data analysis, data interpretation, and critical review of manuscript.
STUDY FUNDING
Supported by the Edmond J. Safra Fellowship in Neurodegenerative Diseases and the Mohammad Al Zaibak Clinical Research Fellowship in Movement Disorders (to T.A.M.) and the New Investigator Award from the Canadian Institutes of Health Research (to C.M.).
DISCLOSURE
T. Mestre, P. Shah, C. Marras, and G. Tomlinson report no disclosures relevant to the manuscript. A. Lang has been a consultant for Biovail, Ceregene, Novartis, Merck Serono, Solvay, Teva, Abbott, Allon Therapeutics, Astra Zeneca, Eisai, and GlaxoSmithKline; served as an expert testimony for welding industry; is funded by the Ontario Problem Gambling Research Centre, Canadian Institutes of Health Research, Michael J. Fox Foundation, and National Parkinson Foundation; and has received royalties from Saunders, Wiley-Blackwell, Johns Hopkins Press, and Cambridge University Press. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Goetz CG, Wuu J, McDermott MP, et al. Placebo response in Parkinson's disease: comparisons among 11 trials covering medical and surgical interventions. Mov Disord 2008;23:690–699 [DOI] [PubMed] [Google Scholar]
- 2.Sinyor M, Levitt AJ, Cheung AH, et al. Does inclusion of a placebo arm influence response to active antidepressant? J Clin Psychiatry 2010;71:270–279 [DOI] [PubMed] [Google Scholar]
- 3.Moncrieff J. A comparison of antidepressant trials using active and inert placebos. Int J Methods Psychiatr Res 2003;12:117–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Woods SW, Gueorguieva RV, Baker CB, Makuch RW. Control group bias in randomized atypical antipsychotic medication trials for schizophrenia. Arch Gen Psychiatry 2005;62:961–970 [DOI] [PubMed] [Google Scholar]
- 5.Sneed JR, Rutherford BR, Rindskopf D, Lane DT, Sackeim HA, Roose SP. Design makes a difference: a meta-analysis of antidepressant response rates in placebo-controlled versus comparator trials in late-life depression. Am J Geriatr Psychiatry 2008;16:65–73 [DOI] [PubMed] [Google Scholar]
- 6.Henkel V, Casaulta F, Seemuller F, et al. Study design features affecting outcome in antidepressant trials. J Affect Disord 2012;141:160–167 [DOI] [PubMed] [Google Scholar]
- 7.Seppi K, Weintraub D, Coelho M, et al. The Movement Disorder Society evidence-based medicine review update: treatments for the non-motor symptoms of Parkinson's disease. Mov Disord 2011;26(suppl 3):S42–S80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miyasaki JM, Martin W, Suchowersky O, Weiner WJ, Lang AE. Practice parameter: initiation of treatment for Parkinson's disease: an evidence-based review: report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2002;58:11–17 [DOI] [PubMed] [Google Scholar]
- 9.Pahwa R, Factor SA, Lyons KE, et al. Practice parameter: treatment of Parkinson disease with motor fluctuations and dyskinesia (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2006;66:983–995 [DOI] [PubMed] [Google Scholar]
- 10.Horstink M, Tolosa E, Bonuccelli U, et al. Review of the therapeutic management of Parkinson's disease: report of a joint task force of the European Federation of Neurological Societies (EFNS) and the Movement Disorder Society-European Section (MDS-ES): part II: late (complicated) Parkinson's disease. Eur J Neurol 2006;13:1186–1202 [DOI] [PubMed] [Google Scholar]
- 11.Goetz CG, Poewe W, Rascol O, Sampaio C. Evidence-based medical review update: pharmacological and surgical treatments of Parkinson's disease: 2001 to 2004. Mov Disord 2005;20:523–539 [DOI] [PubMed] [Google Scholar]
- 12.Horstink M, Tolosa E, Bonuccelli U, et al. Review of the therapeutic management of Parkinson's disease: report of a joint task force of the European Federation of Neurological Societies (EFNS) and the Movement Disorder Society-European Section (MDS-ES): part I: late (complicated) Parkinson's disease. Eur J Neurol 2006;13:1170–1185 [DOI] [PubMed] [Google Scholar]
- 13.Nieuwboer A, Odin P, Poewe W, et al. Late (complicated) Parkinson’s disease. In: Gilhus NE, Barnes MP, Brainin M, eds. European Handbook of Neurological Management, 2nd ed Hoboken, NJ: Wiley-Blackwell; 2011 [Google Scholar]
- 14.Oertel WH, Berardelli A, Bloem BR, et al. Early (uncomplicated) Parkinson's disease. In: Gilhus NE, Barnes MP, Brainin M, eds. European Handbook of Neurological Management, 2nd ed Hoboken, NJ: Wiley-Blackwell; 2011 [Google Scholar]
- 15.Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. Available at: http://handbook.cochrane.org/. Accessed March 12, 2014. [Google Scholar]
- 16.The Nordic Cochrane Centre TCC. Review Manager (RevMan) [Computer program], Version 5.1. Oxford: The Nordic Cochrane Centre TCC; 2011 [Google Scholar]
- 17.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–188 [DOI] [PubMed] [Google Scholar]
- 18.Tomlinson CL, Stowe R, Patel S, Rick C, Gray R, Clarke CE. Systematic review of levodopa dose equivalency reporting in Parkinson's disease. Mov Disord 2010;25:2649–2653 [DOI] [PubMed] [Google Scholar]
- 19.Hauser RA, Auinger P, Group PS. Determination of minimal clinically important change in early and advanced Parkinson's disease. Mov Disord 2011;26:813–818 [DOI] [PubMed] [Google Scholar]
- 20.Schrag A, Sampaio C, Counsell N, Poewe W. Minimal clinically important change on the Unified Parkinson's Disease Rating Scale. Mov Disord 2006;21:1200–1207 [DOI] [PubMed] [Google Scholar]
- 21.Goetz CG, Tilley BC, Shaftman SR, et al. Movement Disorder Society–sponsored revision of the Unified Parkinson's Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov Disord 2008;23:2129–2170 [DOI] [PubMed] [Google Scholar]
- 22.De La Fuente-Fernández R, Stoessl AJ. The biochemical bases for reward: implications for the placebo effect. Eval Health Prof 2002;25:387–398 [DOI] [PubMed] [Google Scholar]
- 23.de la Fuente-Fernández R, Ruth TJ, Sossi V, Schulzer M, Calne DB, Stoessl AJ. Expectation and dopamine release: mechanism of the placebo effect in Parkinson's disease. Science 2001;293:1164–1166 [DOI] [PubMed] [Google Scholar]
- 24.Goetz CG, Janko K, Blasucci L, Jaglin JA. Impact of placebo assignment in clinical trials of Parkinson's disease. Mov Disord 2003;18:1146–1149 [DOI] [PubMed] [Google Scholar]
- 25.Lidstone SC, Schulzer M, Dinelle K, et al. Effects of expectation on placebo-induced dopamine release in Parkinson disease. Arch Gen Psychiatry 2010;67:857–865 [DOI] [PubMed] [Google Scholar]
- 26.Thompson SG, Higgins JP. How should meta-regression analyses be undertaken and interpreted? Stat Med 2002;21:1559–1573 [DOI] [PubMed] [Google Scholar]
- 27.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009;339:b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.