Abstract
Objectives:
The aim of this study was to determine the prevalence of idiopathic normal-pressure hydrocephalus (iNPH) in elderly persons in a large population-based sample using radiologic and clinical examinations.
Methods:
We examined representative elderly populations aged 70 years and older that had undergone neuropsychiatric evaluations and CT of the brain between 1986 and 2000 (n = 1,238). Gait was evaluated by clinical examination and history of walking difficulty. Cognitive function was evaluated with the Mini-Mental State Examination and urinary incontinence by self-report. iNPH was diagnosed in concordance with the American-European iNPH guidelines. Exclusion criteria were history of meningitis, severe head trauma, and subarachnoid hemorrhage.
Results:
The prevalence of probable iNPH was 0.2% in those aged 70–79 years (n = 2) and 5.9% (n = 24) in those aged 80 years and older, with no difference between men and women. Only 2 of these persons had been treated for iNPH. Hydrocephalic ventricular enlargement, i.e., a CT image consistent with NPH, was found in 56 persons (4.5%). An Evans Index >0.3 was found in 256 (20.7%) and occluded sulci at the high convexity in 67 persons (5.4%). All of these findings were more common in the older age groups.
Conclusions:
Many elderly possess clinical and imaging features of iNPH, especially those older than 80 years. The number of persons with iNPH is probably much higher than the number of persons currently treated.
The number of elderly and people with dementia is increasing in most parts of the world.1 It is therefore important to learn more about the prevalence of treatable causes of dementia. Idiopathic normal-pressure hydrocephalus (iNPH) is a treatable neurologic disorder first described by Salomón Hakim in 1965.2 iNPH is characterized by gait and balance impairment, cognitive deterioration, and urinary incontinence, and radiologically by a communicating ventricular enlargement.3 Treatment by diversion of CSF to the peritoneal cavity or heart is successful in reversing symptoms in more than 80% of the patients.4 iNPH is thus one of the few causes of reversible dementia, but it is still underdiagnosed.5,6 The prevalence of iNPH in selected populations and in community-based studies on young elderly varies between 0.1% and 2.9%.5,7–10 However, few large population-based studies have investigated the prevalence of iNPH, and most included few persons older than 80 years. Thus, the prevalence still has to be examined in population-based studies including many persons older than 80 years.
The aim of this study was to determine the prevalence of iNPH by examining CT images of the brain and clinical signs of iNPH in a large representative elderly population.
METHODS
Study population.
Between 1986 and 2000, studies on representative elderly populations in Gothenburg, Sweden, were conducted using identical examinations (including neuropsychiatric examinations and key informant interviews) at each occasion.11 All participants were systematically obtained from the Swedish population register based on birth dates and included people living in private households and in residential care. Subsamples were examined with CT of the brain. CT scans made between 1986 and 2000 were available and used in this study. The studies included the H85 studies, the NORA (Nordic Research on Ageing) Studies, the Prospective Population Study of Women, and the Gerontological and Geriatric Population Studies (H70) in Gothenburg, Sweden. These samples have been described in detail previously12–17 and were examined with identical methods by the same research team.
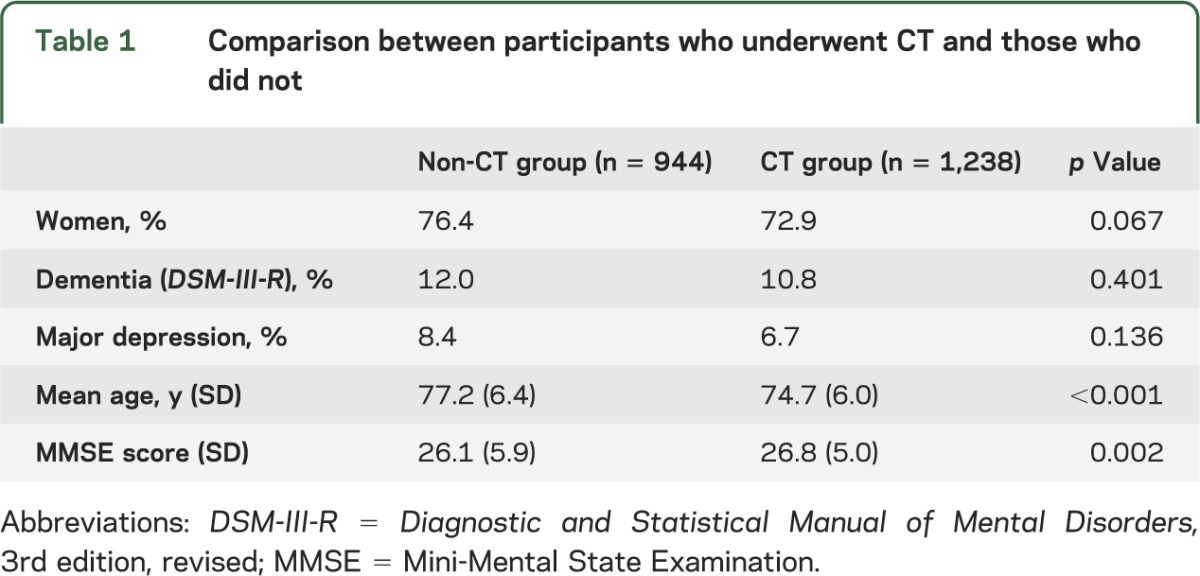
In total, 3,246 individuals were invited and 2,182 accepted to take part in a clinical examination (response rate 67%), with no significant difference in response rate between men and women (65% vs 68%; p = 0.107). Of those who took part in the clinical examination, 1,238 accepted a CT scan of the head (response rate 58.1%; 60% for men and 56% for women; p = 0.067). These are described in figure 1 and table 1. The age and sex distribution among those that were included in the study is shown in table e-1 on the Neurology® Web site at Neurology.org. Participants in the CT study were on average slightly younger and performed better on the Mini-Mental State Examination (MMSE). There was no significant difference in the prevalence of dementia and major depression between the CT group and non-CT group (table 1). Because of oversampling of persons older than 80 years, we stratified prevalences into the age groups 70–79 years and 80 years and older.
Figure 1. Selection of study participants.
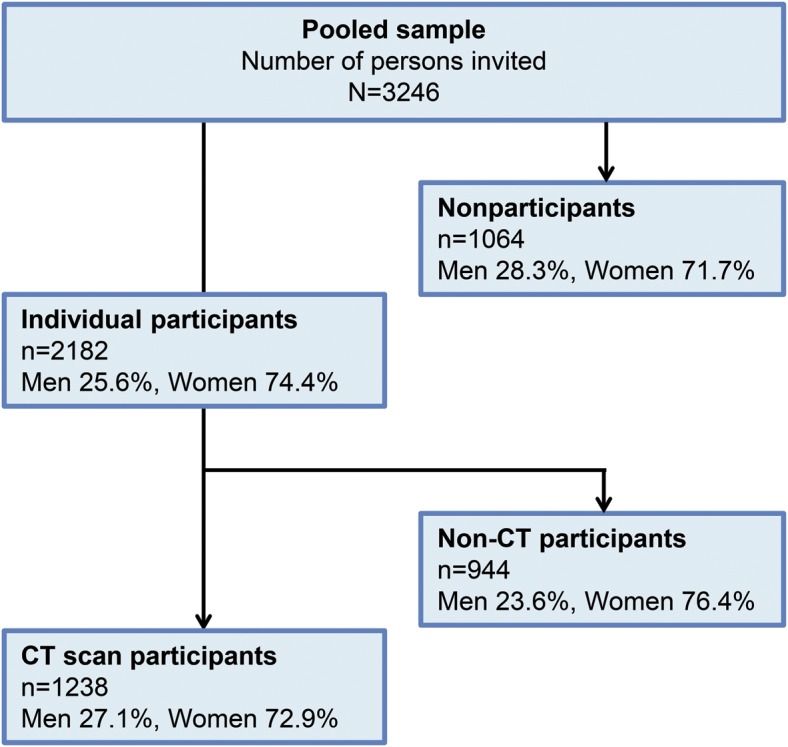
Table 1.
Comparison between participants who underwent CT and those who did not
Radiologic assessment.
Ten-millimeter continuous slices were obtained on CT scans from 1986 to 1992 (n = 383) and 8-mm continuous slices from 1995 to 2000 (n = 855). All images were in the transverse plane, and no contrast was used. Three observers, a medical student (D.J.), a resident in neurosurgery (K.R.), and a consultant in neurology (C.W.), collectively made an initial screening assessment of all CT images. The observers were blinded to all clinical data. Each case was evaluated for hydrocephalic ventricular enlargement (HcVe). This was based on previous descriptions of the radiologic signs of iNPH3,18–20 and was defined as enlargement of all 4 ventricles without equivalent widening of cortical sulci or obstruction of CSF flow or structural lesions. No extracerebral mass lesions or other pathologies influencing ventricular morphology were allowed. Cases in which ventricular dilation was caused by conditions other than NPH, such as brain atrophy, obstructive hydrocephalus, or loss of brain parenchyma, were not classified as HcVe. Evans Index (EI) was defined as the ratio between the largest width of the frontal horns and the largest inner diameter of the skull in the same slice. A value higher than 0.3 is considered abnormal.21 Occluded sulci at the high convexity (OccSul) was defined as not having any sulcus extending to the midline at the falx cerebri on the 2 uppermost CT slices.
A consultant neuroradiologist (C.J.) reevaluated all images that in the initial assessment screened positive for HcVe or had uncertain findings and made the final decisions. In one individual, we could not accurately determine sulcal occlusion and therefore excluded this person from further analyses. We created a reference group to compare the frequency and risk of clinical features between persons with radiologic features of iNPH and persons without. This reference group consisted of all persons without iNPH-associated CT abnormality (n = 955).
Clinical assessment.
The neuropsychiatric examinations were semistructured and included both psychiatric symptoms and examinations to detect possible diseases of the nervous system. Clinical problems of cognition and/or behavior caused by brain injury or brain disease were addressed. Common signs and symptoms of dementia were rated, and several test batteries of cognition were performed. They were made by psychiatrists until 1999 and in 2000 by experienced psychiatric nurses. Interrater reliability between psychiatrists and psychiatric nurses was studied in 50 individuals. Interrater agreement for signs and symptoms of cognitive dysfunction was between 89.4% and 100% (κ values between 0.74 and 1.00). We assessed gait disturbance by evaluating data from clinical examinations and interviews. Specialists in geriatrics performed physical examinations in which a general assessment of gait was made. An examiner graded walking difficulties as nonexistent, slight, or extensive. Participants had also been asked questions regarding gait and walking difficulties on interviews. We defined gait disturbance in the following way: presence of slight or extensive walking difficulties and/or presence of self-reported walking difficulty. Cognitive function was evaluated with the MMSE; a score ≤25 was defined as cognitive impairment. Urinary incontinence was assessed by self-report and defined as involuntary leakage of urine occurring more than once per week. Data on gait were available in 941 persons, cognitive function in 942, and urinary incontinence in 745. Probable iNPH was diagnosed in concordance with the American-European iNPH guidelines,3 i.e., HcVe together with gait disturbance and either MMSE ≤25 or urinary incontinence. Exclusion criteria were history of severe head trauma, meningitis, and subarachnoid hemorrhage, which were assessed by self-report and on information from the Swedish Hospital Discharge Register.
Statistical analysis.
The prevalence of iNPH was calculated by dividing the number of persons with probable iNPH with the number of study participants. Differences between proportions for men and women, and between age 70–79 years vs 80 years and older, were tested using Fisher exact test. Multiple binary logistic regressions models including age and sex were run to analyze the influence of CT features on gait disturbance, urinary incontinence, and cognitive impairment. All statistical tests were 2-sided, and statistical significance was assumed for p values <0.05. Tests were performed using SPSS 17.0 (SPSS Inc., Chicago, IL).
Standard protocol approvals, registrations, and patient consents.
The Ethics Committee for Medical Research at Gothenburg University approved all studies. Informed consent was obtained from the participants, their nearest relatives, or both.
RESULTS
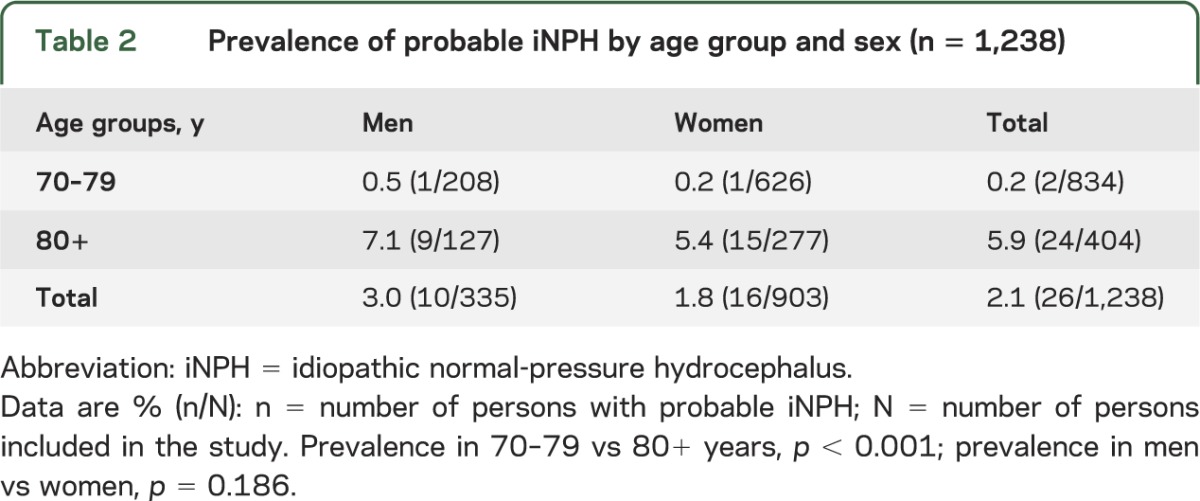
The prevalence of probable iNPH was 0.2% in those aged 70–79 years and 5.9% in those aged 80 years and older (table 2). The mean age of those who fulfilled the criteria for iNPH was 85.3 years (SD 4.0). iNPH was associated with being older than 80 years (odds ratio 35.5; 95% confidence interval 4.8–262.7) but not with sex.
Table 2.
Prevalence of probable iNPH by age group and sex (n = 1,238)
Radiologic findings.
Among all images, 235 (19%) screened positive for HcVe or had uncertain findings and were therefore reevaluated by a consultant neuroradiologist (C.J.).
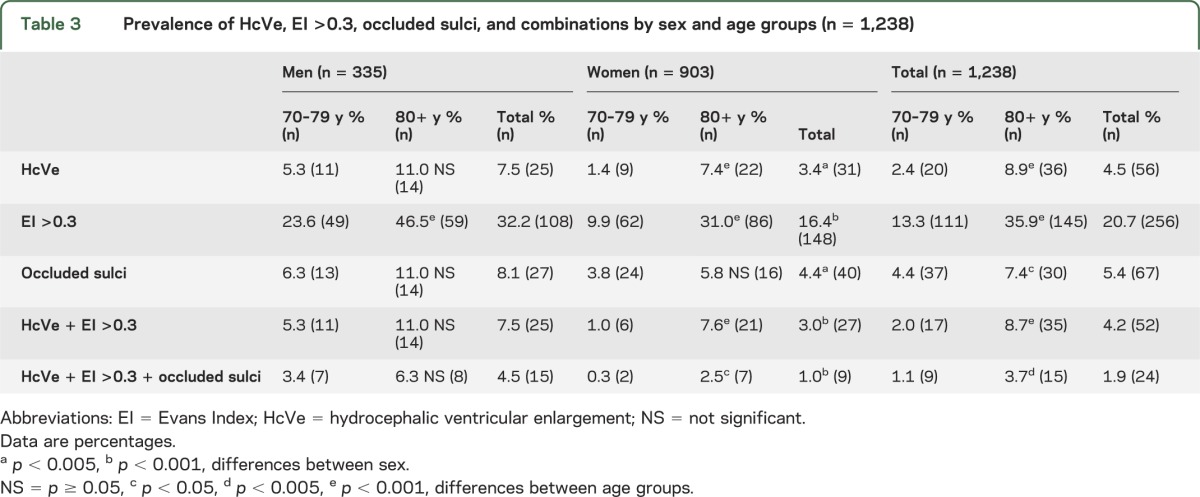
The final prevalence of HcVe was 4.5%. EI >0.3 was found in 20.7% and OccSul in 5.4% (table 3). All of these findings and their combinations were more common in men than in women and in the older age groups. Twenty-four (1.9%) had all 3 radiologic signs. Two hundred eighty-three (22.9%) had either HcVe or EI >0.3 or OccSul (figure e-1). All except 4 persons with HcVe had EI >0.3 (none of these 4 met the criteria of probable iNPH). The mean value of EI in the population was 0.28 (SD 0.04). Men had higher values than women (0.29 vs 0.27, p < 0.001). Two persons with iNPH had ventricular shunts on CT.
Table 3.
Prevalence of HcVe, EI >0.3, occluded sulci, and combinations by sex and age groups (n = 1,238)
Clinical findings.
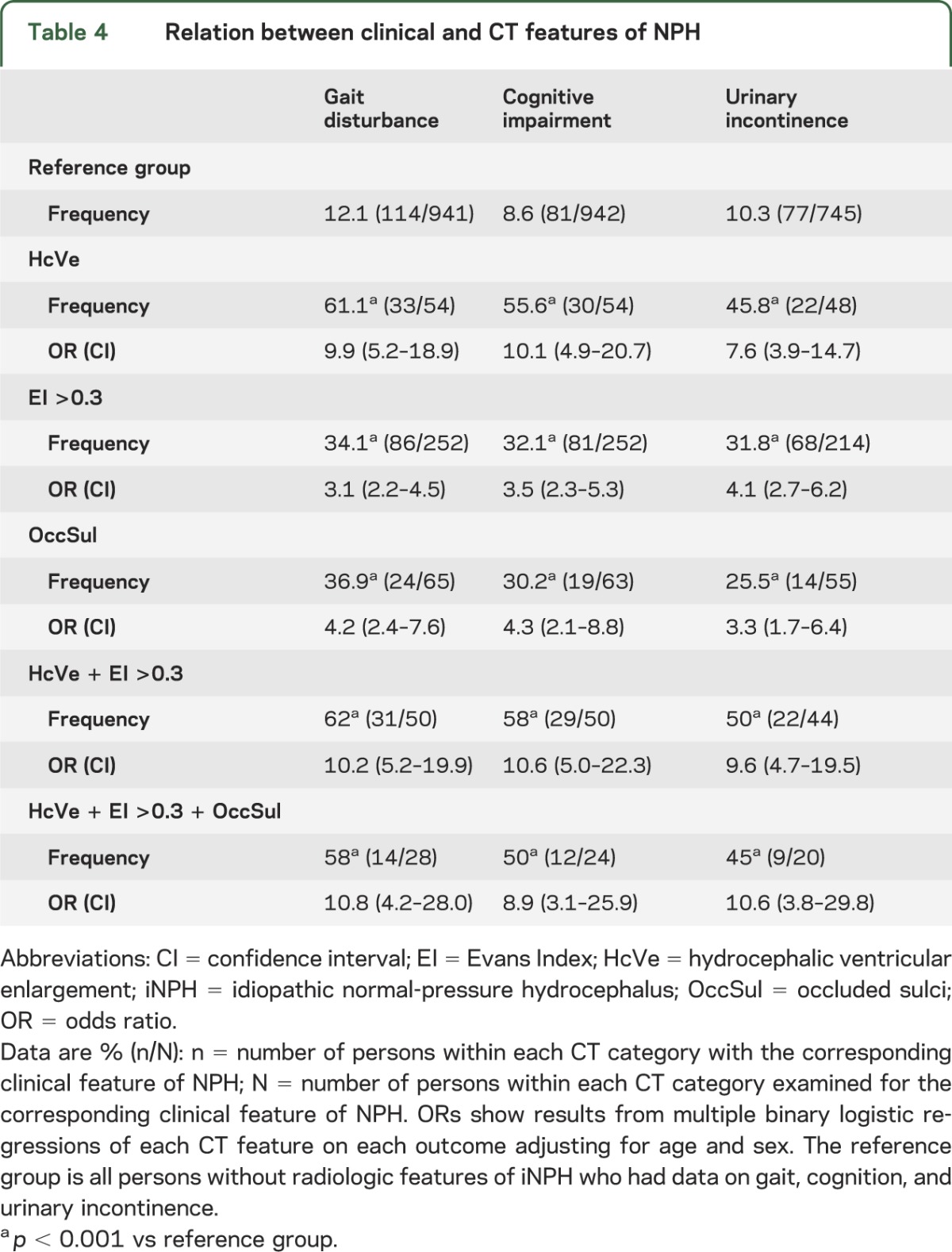
The frequency and risk of gait disturbance, cognitive impairment, and urinary incontinence was higher in those with HcVe, EI >0.3, OccSul, and combinations of these (table 4). The strongest associations were with HcVe. Of 56 probands with HcVe, 26 (46%) fulfilled the criteria for probable iNPH. Among those, 18 had all 3 clinical signs of NPH, i.e., gait disturbance, cognitive impairment, and urinary incontinence. Of 24 probands with all 3 radiologic NPH features, 11 (45.8%) fulfilled the criteria of iNPH. Each individual case of HcVe is described in table e-2.
Table 4.
Relation between clinical and CT features of NPH
DISCUSSION
To our knowledge, this is the largest population-based study on the prevalence of iNPH. We found that 0.2% among those aged 70–79 years and 5.9% of those 80 years and older matched guideline criteria for probable iNPH. Furthermore, 4.5% had HcVe, i.e., a CT image consistent with NPH. All but 2 with iNPH were older than 80 years. This finding is important because persons older than 80 years constitute the age group that is increasing the most worldwide.22 Based on these results, we estimate that approximately 2 million persons in Europe (114,394 aged 70–79 years and 1,842,983 aged 80 years or older) and700,000 in the United States (33,808 aged 70–79 years and 669,178 aged 80 years or older) may have iNPH. Furthermore, several authors have argued that many with iNPH are not properly evaluated for their symptoms and do not receive the correct diagnosis.6,23 This is supported by our finding that only 2 persons in the study population had previously undergone shunt surgery. Our study shows that many elderly persons possess the clinical and imaging features of iNPH. There is a pressing need to identify the most appropriate surgical candidates, and a critical need to conduct a well-designed, well-powered, randomized controlled trial of CSF diversion.
Few previous studies have investigated the prevalence of iNPH, and most included few people older than 80 years. The prevalence of iNPH in persons older than 65 years was 0.4% in a door-to-door survey in 2 German villages.7 However, iNPH was examined only in those who screened positive for parkinsonism, thus underestimating the prevalence. A Norwegian study reported a prevalence of probable iNPH of 0.12% in persons older than 65 years.5 The prevalence is similar to that in our study in the age group 70–79 years. The Norwegian study found a prevalence of 0.1% in those older than 80 years. However, the study was not population-based. Participants were recruited from an advertisement campaign directed to the general population and primary care physicians. The low prevalence could thus be attributable to recruitment bias. A community-based study in Tajiri, Japan, found a prevalence of 2.9% among 170 community-dwelling persons aged 65 years or older. The mean age of the study participants was 72.4 years.8 Another community-based study from the same area reported a prevalence of possible iNPH of 1.4% when examining 497 persons who were 65 years or older.9 A third Japanese study conducted in 790 community-dwelling elderly persons found that 1.5% had features of NPH on MRI, and 0.5% met the criteria of possible iNPH.10 The study population comprised only 2 age groups: 61 years and 70–72 years. The 3 Japanese studies did not use the same criteria to diagnose iNPH as in our study. The diagnostic criteria were hydrocephalus on MRI together with one of the clinical features of NPH. If we had used the same methodology, our prevalence estimate would have been even higher. The main reason for the lower prevalence in the studies mentioned compared with our study is that these studies were mainly conducted among younger elderly.
We found that the frequency of hydrocephalic symptoms was surprisingly lower among those with all 3 radiologic features than in those with only HcVe. The reason was mainly because OccSul and EI >0.3 were less associated with symptoms than HcVe. Considering that assessment of sulcal narrowing was part of the evaluation by the neuroradiologist, all persons classified with HcVe had some degree of sulcal narrowing but only a small proportion of these fulfilled our criteria for OccSul. The criteria for OccSul in this study were therefore probably too strict and we thus believe that the most accurate estimate of the prevalence of iNPH is the one that used the radiologic feature of HcVe. It might also be that OccSul has no or only a weak association with NPH. The risk of having symptoms was lower among those with EI >0.3, indicating that EI by itself has limited relevance in the elderly. Although radiologic features of NPH were more common among men and in the older age groups, there was no significant difference in the prevalence of probable iNPH between men and women and it might be that radiologic features of NPH are also related to concomitant pathophysiologic processes.
Among the strengths of the study are the large representative population, the comprehensive examinations, and that all CT scans with suspected hydrocephalus were evaluated by an experienced neuroradiologist.
The main limitation is that the diagnosis was based on retrospective analysis. Gait disturbance, cognitive impairment, and urinary incontinence are common and nonspecific symptoms among the elderly and there were many confounders that we were unable to adjust for. The diagnosis was based on the American-European iNPH guidelines.3 However, we were not able to include all variables and exclusion criteria listed in the guidelines. Onset, duration, and progression of symptoms over time, step height and length, walking speed, base of standing, and retropulsion are examples of variables that we were unable to assess. This diagnostic bluntness might have led to an overestimation of the prevalence of NPH. However, some participants lacked information regarding certain clinical variables. In these cases, we were unable to establish a diagnosis even in the presence of HcVe. This might have led to an underestimation of the prevalence.
Gait and urinary incontinence were evaluated by rather crude ratings and other causes were not ruled out. This could have resulted in an overestimation of the results. However, it should also have decreased our possibilities to find associations with radiologic findings. Nonetheless, the associations found were surprisingly strong. The risk of having gait disturbance and cognitive impairment was approximately 10 times higher in those with HcVe. This could indicate that we made a correct diagnosis in most cases. Furthermore, we are confident that the CT findings in those with HcVe were so convincing and indicative of NPH that the CT results alone would justify at least further evaluations. Evaluation of gait was partially based on participants’ own, subjective reporting of walking difficulties. This might have overestimated hydrocephalic gait disturbance, but in some cases may have resulted in underestimation.
The CT images included only transverse sections with thicker slices and somewhat lower resolution than most modern images. The reason for this is that it was the technical standard for imaging in Gothenburg, Sweden, during the time the images were made. The majority of the scans were made after 1992 and had better quality. Although MRI would have been better, CT is sufficient for detecting the majority of iNPH cases. We are thus convinced that the imaging method did not have a large influence on the results.
Possible selection bias and low participation rate might limit the results of this study. Persons who underwent CT in this study were younger and it might be that the very oldest and those with dementia were less likely to participate. However, there was no significant difference in the prevalence of dementia between CT and non-CT participants. Furthermore, cognitive impairment is one of the main features of iNPH, and the prevalence of iNPH was particularly high in those older than 80 years. Considering this, selection bias and low participation rate could have led to underestimation of the prevalence of iNPH.
There was also oversampling of women and persons older than 80 years. For this reason, we stratified prevalences into age groups and calculated prevalence according to sex. The reason for oversampling of women was that the Prospective Population Study of Women was included. This was a representative population-based cohort consisting only of women. However, because there was no difference in the prevalence between men and women, we consider the total sample representative. The calculated prevalence of iNPH in this study should thus not be affected.
The time span in which the study population was examined is also important. CT images were done over several decades. Thus, if the prevalence of NPH has changed over time, it may have affected the results.
Many elderly possess the clinical and imaging features of iNPH and are in need of further evaluation. The results of this study suggest that the number of persons with iNPH is much higher than the number of patients treated. Considering the detrimental consequences of untreated iNPH, it is essential to increase awareness and improve the possibilities for diagnosis and treatment of iNPH. It is conceivable that the number of individuals with iNPH will increase as the life expectancy rises in the general population.
Supplementary Material
ACKNOWLEDGMENT
The authors thank Dr. Mats Tullberg at the University of Gothenburg, Department of Neuroscience and Physiology, for help with planning the study and the initial work with the CT assessments. The authors also thank Tina Jacobsson and Cecilia Mellqvist at the University of Gothenburg, Department of Neuroscience and Physiology, for help with collecting and organizing the data, and the staff at the Hydrocephalus Research Unit for guidance and encouragement.
Glossary
- EI
Evans Index
- HcVe
hydrocephalic ventricular enlargement
- iNPH
idiopathic normal-pressure hydrocephalus
- MMSE
Mini-Mental State Examination
- OccSul
occluded sulci
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
Daniel Jaraj made the screening assessment of the CT images, participated in the design of the study, helped coordinate the study, and drafted the manuscript. Katrin Rabiei made the screening assessment of the CT images, participated in the design of the study, and helped revise the manuscript. Thomas Marlow participated in the design of the study, performed all statistical analyses and interpreted the data, and helped revise the manuscript. Christer Jensen made the final radiologic assessment of the CT images, participated in the design of the study, and helped revise the manuscript. Ingmar Skoog was principal investigator of the population studies, interpreted the data, participated in the design of the study, and participated in drafting the manuscript. Carsten Wikkelsø conceived the study, participated in the design of the study, participated in the screening assessment of the CT images, helped coordinate the study, and participated in drafting the manuscript. All authors have read and approved the final manuscript.
STUDY FUNDING
The Swedish Research Council (11267, 2005-8460, 825-2007-7462, 825-2012-5041, 2013-8717), Swedish Research Council for Health, Working Life and Welfare (2001-2646, 2003-0234, 2004-0150, 2006-0020, 2008-1229, 2004-0145, 2006-0596, 2008-1111, 2010-0870, AGECAP 2013-2300, 2013-2496, EpiLife 2006-1506, Forte 2013-2496, 2013-2300), Swedish Brain Power, the Alzheimer's Association Zenith Award (ZEN-01-3151), the Alzheimer's Association Stephanie B. Overstreet Scholars (IIRG-00-2159), the Bank of Sweden Tercentenary Foundation, Eivind och Elsa K: son Sylvans stiftelse, Stiftelsen Söderström-Königska Sjukhemmet, Stiftelsen för Gamla Tjänarinnor, Handlanden Hjalmar Svenssons Forskningsfond, Swedish Alzheimerfonden, Alma och Anna Yhlen's Foundation.
DISCLOSURE
D. Jaraj has received funding from the University of Gothenburg. K. Rabie, T. Marlow, and C. Jensen report no disclosures relevant to the manuscript. I. Skoog has served on scientific advisory boards for Pfizer Inc. and AstraZeneca; serves as Triage Editor for the European Journal of Psychiatry; has served on an editorial advisory board for International Psychogeriatrics; receives publishing royalties for Alzheimers sjukdom och andra kognitiva sjukdomar (English title: Alzheimer's Disease and Other Cognitive Disorders [Liber, 2003]); serves on speakers bureaus for Shire plc, Janssen-Cilag, Pfizer Inc., Novartis, GE Healthcare, and Eisai Inc.; and has received research support from the Swedish Research Council, the Alzheimer's Association, and the Bank of Sweden Tercentenary Foundation. C. Wikkelsø receives honorarium for lecturing and consulting by Codman and Johnson & Johnson. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Prince M, Bryce R, Albanese E, Wimo A, Ribeiro W, Ferri CP. The global prevalence of dementia: a systematic review and metaanalysis. Alzheimers Dement 2013;9:63–75 [DOI] [PubMed] [Google Scholar]
- 2.Hakim S, Adams RD. The special clinical problem of symptomatic hydrocephalus with normal cerebrospinal fluid pressure: observations on cerebrospinal fluid hydrodynamics. J Neurol Sci 1965;2:307–327 [DOI] [PubMed] [Google Scholar]
- 3.Relkin N, Marmarou A, Klinge P, Bergsneider M, Black PM. Diagnosing idiopathic normal-pressure hydrocephalus. Neurosurgery 2005;57:S4–S16 [DOI] [PubMed] [Google Scholar]
- 4.Klinge P, Hellstrom P, Tans J, Wikkelso C. One-year outcome in the European multicentre study on iNPH. Acta Neurol Scand 2012;126:145–153 [DOI] [PubMed] [Google Scholar]
- 5.Brean A, Eide PK. Prevalence of probable idiopathic normal pressure hydrocephalus in a Norwegian population. Acta Neurol Scand 2008;118:48–53 [DOI] [PubMed] [Google Scholar]
- 6.Brean A, Fredo HL, Sollid S, Muller T, Sundstrom T, Eide PK. Five-year incidence of surgery for idiopathic normal pressure hydrocephalus in Norway. Acta Neurol Scand 2009;120:314–316 [DOI] [PubMed] [Google Scholar]
- 7.Trenkwalder C, Schwarz J, Gebhard J, et al. Starnberg trial on epidemiology of Parkinsonism and hypertension in the elderly: prevalence of Parkinson's disease and related disorders assessed by a door-to-door survey of inhabitants older than 65 years. Arch Neurol 1995;52:1017–1022 [DOI] [PubMed] [Google Scholar]
- 8.Hiraoka K, Meguro K, Mori E. Prevalence of idiopathic normal-pressure hydrocephalus in the elderly population of a Japanese rural community. Neurol Med Chir 2008;48:197–199 [DOI] [PubMed] [Google Scholar]
- 9.Tanaka N, Yamaguchi S, Ishikawa H, Ishii H, Meguro K. Prevalence of possible idiopathic normal-pressure hydrocephalus in Japan: the Osaki-Tajiri project. Neuroepidemiology 2009;32:171–175 [DOI] [PubMed] [Google Scholar]
- 10.Iseki C, Kawanami T, Nagasawa H, et al. Asymptomatic ventriculomegaly with features of idiopathic normal pressure hydrocephalus on MRI (AVIM) in the elderly: a prospective study in a Japanese population. J Neurol Sci 2009;277:54–57 [DOI] [PubMed] [Google Scholar]
- 11.Skoog I. Psychiatric epidemiology of old age: the H70 study—the NAPE lecture 2003. Acta Psychiatr Scand 2004;109:4–18 [DOI] [PubMed] [Google Scholar]
- 12.Skoog I, Nilsson L, Palmertz B, Andreasson LA, Svanborg A. A population-based study of dementia in 85-year-olds. N Engl J Med 1993;328:153–158 [DOI] [PubMed] [Google Scholar]
- 13.Skoog I, Olesen PJ, Blennow K, Palmertz B, Johnson SC, Bigler ED. Head size may modify the impact of white matter lesions on dementia. Neurobiol Aging 2012;33:1186–1193 [DOI] [PubMed] [Google Scholar]
- 14.Palsson S, Aevarsson O, Skoog I. Depression, cerebral atrophy, cognitive performance and incidence of dementia: population study of 85-year-olds. Br J Psychiatry 1999;174:249–253 [DOI] [PubMed] [Google Scholar]
- 15.Bengtsson C, Blohme G, Hallberg L, et al. The study of women in Gothenburg 1968–1969: a population study—general design, purpose and sampling results. Acta Med Scand 1973;193:311–318 [DOI] [PubMed] [Google Scholar]
- 16.Simoni M, Pantoni L, Pracucci G, et al. Prevalence of CT-detected cerebral abnormalities in an elderly Swedish population sample. Acta Neurol Scand 2008;118:260–267 [DOI] [PubMed] [Google Scholar]
- 17.Olesen PJ, Gustafson DR, Simoni M, et al. Temporal lobe atrophy and white matter lesions are related to major depression over 5 years in the elderly. Neuropsychopharmacology 2010;35:2638–2645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Evans WA., Jr An encephalographic ratio for estimating the size of the cerebral ventricles: further experience with serial observations. Am J Dis Child 1942;64:820–830 [Google Scholar]
- 19.Tullberg M, Hultin L, Ekholm S, Mansson JE, Fredman P, Wikkelso C. White matter changes in normal pressure hydrocephalus and Binswanger disease: specificity, predictive value and correlations to axonal degeneration and demyelination. Acta Neurol Scand 2002;105:417–426 [DOI] [PubMed] [Google Scholar]
- 20.Hashimoto M, Ishikawa M, Mori E, Kuwana N. Diagnosis of idiopathic normal pressure hydrocephalus is supported by MRI-based scheme: a prospective cohort study. Cerebrospinal Fluid Res 2010;7:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Evans WA., Jr An encephalographic ratio for estimating ventricular enlargement and cerebral atrophy. Arch Neurol Psychiatry 1942;47:931–937 [Google Scholar]
- 22.Kinsella K, He W. An Aging World: 2008. Washington, DC: US Government Printing Office; 2009. Report P95/09-1 [Google Scholar]
- 23.Stein SC, Burnett MG, Sonnad SS. Shunts in normal-pressure hydrocephalus: do we place too many or too few? J Neurosurg 2006;105:815–822 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


