Abstract
Objective:
To assess the risk factors for electrographic seizures among neonates treated with therapeutic hypothermia for hypoxic-ischemic encephalopathy (HIE).
Methods:
Three-center observational cohort study of 90 term neonates treated with hypothermia, monitored with continuous video-EEG (cEEG) within the first day of life (median age at onset of recording 9.5 hours, interquartile range 6.3–14.5), and continued for >24 hours (total recording 93.3 hours, interquartile range 80.1–112.8 among survivors). A pediatric electroencephalographer at each site reviewed cEEGs for electrographic seizures and initial EEG background category.
Results:
A total of 43 (48%) had electrographic seizures, including 9 (10%) with electrographic status epilepticus. Abnormal initial EEG background classification (excessively discontinuous, depressed and undifferentiated, burst suppression, or extremely low voltage), but not clinical variables (including pH <6.8, base excess ≤−20, or 10-minute Apgar ≤3), was strongly associated with seizures.
Conclusions:
Electrographic seizures are common among neonates with HIE undergoing hypothermia and are difficult to predict based on clinical features. These results justify the recommendation for cEEG monitoring in neonates treated with hypothermia.
Neonatal seizures are common,1–4 are often caused by hypoxic-ischemic encephalopathy (HIE),5 and may be harmful to the developing brain.6–10 Though therapeutic hypothermia may lower the risk of electrographic seizures among neonates with HIE,11,12 the incidence remains high, estimated at 30% to 95%.11,13,14
In 2011, the American Clinical Neurophysiology Society (ACNS) recommended that all neonates at high risk for brain injury, including those with neonatal encephalopathy, be monitored with continuous video-EEG (cEEG) to evaluate suspicious clinical events or to assess for the presence of subclinical seizures.15 In spite of these recommendations, access to cEEG remains limited at many centers, since application and interpretation require specialized equipment and training, and many centers lack access altogether or must limit use of cEEG to those patients considered at highest risk for seizures. One common paradigm is to monitor neonates who display clinical seizures, though evidence suggests that clinicians are poor at clinically distinguishing seizures from nonseizure events,16,17 and most seizures in newborns lack a clear clinical correlate.18–20
We aimed to identify risk factors for electrographic seizures among neonates who were treated with therapeutic hypothermia and monitored according to guidelines from the ACNS.
METHODS
This was a retrospective multicenter cohort study. Participating institutions were The Children's Hospital of Philadelphia, Children's National Medical Center, and the University of Michigan C.S. Mott Children's Hospital. Neonates were treated with whole-body therapeutic hypothermia according to National Institute of Child Health and Human Development eligibility criteria and protocol.21 All 3 sites monitor patients who undergo therapeutic hypothermia using cEEG from the time of admission and through rewarming. Consecutive cooled patients were included as study subjects if cEEG was initiated within the first day of life and continued for ≥24 hours. Enrollment periods were as follows: center 1, May 2006 to January 2008; center 2, March 2008 to January 2010; and center 3, January 2011 to December 2012. Data from a subset of subjects were previously reported.14 Clinical data were extracted from electronic medical records and bedside charts by a child neurologist at each center. A sentinel event was defined as a placental abruption, uterine rupture, cord rupture, cord knot, or tight nuchal cord. Study data were collected and managed using REDCap (Research Electronic Data Capture) tools hosted at the University of California, San Francisco.22
Subjects at each center were monitored using conventional video-EEG. A trained technologist applied surface electrodes according to the international 10 to 20 system, modified for neonates. Monitoring was initiated as part of routine care and according to institutional clinical guidelines as soon as possible after admission to the intensive care nursery (median age at onset of EEG 9.5 hours of life, interquartile range 6.3–14.5), and was maintained throughout the duration of therapeutic hypothermia and rewarming (median duration of EEG recording 93.3 hours among survivors, interquartile range 80.1–112.8).
A neurophysiologist with experience in neonatal EEG at each site reviewed the archived full-length video-EEG recordings to evaluate for the presence of electrographic seizures and electrographic status epilepticus. An electrographic seizure was defined as a repetitive, evolving, and stereotyped waveform, with a definite beginning and end, with a minimum duration of 10 seconds, and minimum amplitude of 2 μV.23,24 Electrographic status epilepticus was defined as continuous electrographic seizure lasting at least 30 minutes, or recurrent electrographic seizures for at least 50% of 1–3 hours of recording time.24,25 EEG background at the onset of recording was evaluated for at least 1 hour of recording and classified into 1 of 4 patterns: (1) normal for gestational age, including recordings with transient discontinuity for less than 50% of the recording, with presence of distinct state changes; (2) excessively discontinuous, bursts of activity separated by interburst intervals with amplitude ≥5 μV and <25 μV for at least 2 seconds, and with no state changes; (3) severely abnormal, depressed and undifferentiated, with persistently low-voltage background activity with amplitude between 5 μV and 15 μV and without normal features, burst suppression, invariant and unreactive pattern of bursts of paroxysmal activity with mixed features but no age-appropriate activity and alternating with periods of marked voltage attenuation with amplitude <5 μV, or extremely low voltage, invariant and unreactive pattern, with amplitude <5 μV or with no discernible cerebral activity; (4) electrographic status epilepticus at the onset of recording.
Clinical and electrographic seizures were treated with medications according to institutional guidelines and at the discretion of the treating physician. None of the study centers used prophylactic phenobarbital.
Standard protocol approvals, registrations, and patient consents.
The Committee on Human Research or Institutional Review Board at each institution approved the study, including waiver of consent.
Statistical analysis.
Statistical analyses were performed using Stata 11.2 software (Stata Corp., College Station, TX). Continuous data were dichotomized and χ2 and Fisher exact tests were used to compare categorical variables. All tests were 2-sided. Logistic regression analysis was used to examine the association between risk factors and presence of electrographic seizures. In cases where a clinical risk factor was not documented in the patient chart, it was considered as not present. We considered a p value <0.05 significant.
RESULTS
Patient population.
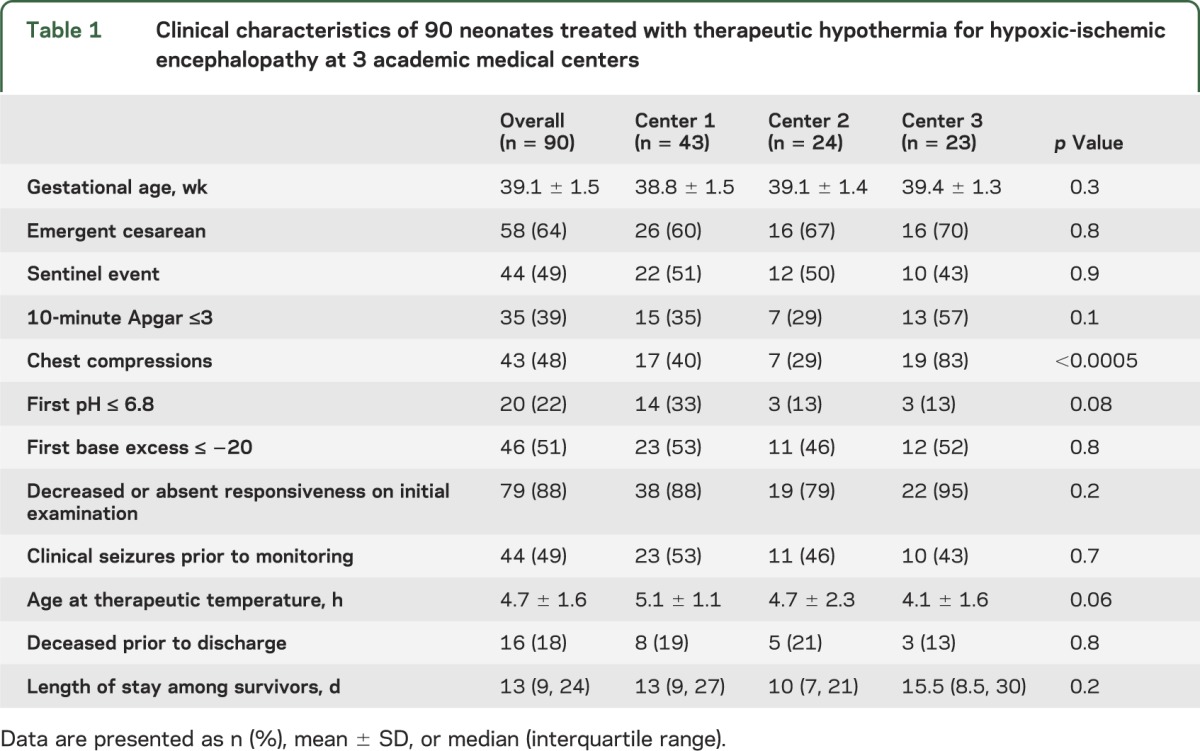
A total of 109 term neonates were treated with therapeutic hypothermia during the study period, and 90 met inclusion criteria. Subjects were excluded as follows: 2 were studied as part of a late-onset cooling trial and hypothermia was not initiated within the first 6 hours of life; in 14 subjects, cEEG monitoring was initiated only after 24 hours of life; in 2 subjects, initial cEEG recording could not be interpreted; and in 1 subject, cEEG was discontinued prior to 24 hours of life. The clinical characteristics of the subjects at each center are presented in table 1.
Table 1.
Clinical characteristics of 90 neonates treated with therapeutic hypothermia for hypoxic-ischemic encephalopathy at 3 academic medical centers
Electrographic and clinical seizures.
Overall, 43 of 90 subjects (48%) had electrographic seizures (range by institution 33%–71%, p = 0.01): 6% with only 1 or 2 seizures throughout the recording period, 32% with >2 seizures, and 10% with electrographic status epilepticus. The range of patients with a higher seizure burden (>2 seizures or status epilepticus) was 33% to 48% across institutions (p = 0.2). Electrographic seizure onset occurred at a median of 19.9 hours of life (interquartile range 16.7 hours–26.3 hours). Six subjects (7%) were in electrographic status epilepticus at the onset of recording. Only 4 of those with electrographic seizures (4% of the entire study population) had seizure onset de novo during rewarming.
Clinical seizures were suspected in 44 of 90 subjects (49%) prior to the onset of EEG monitoring. Of these, 35 (78%) received phenobarbital therapy. Half of subjects with clinically suspected seizures prior to monitoring had electrographic seizures (n = 22), while 46% (n = 21) of those without clinical seizures prior to onset of monitoring had electrographic seizures (relative risk 1.1, 95% confidence interval 0.7–1.7, p = 0.7). There was no difference in risk of electrographic seizures among those who received phenobarbital prior to cEEG monitoring.
Risk factors for electrographic seizures.
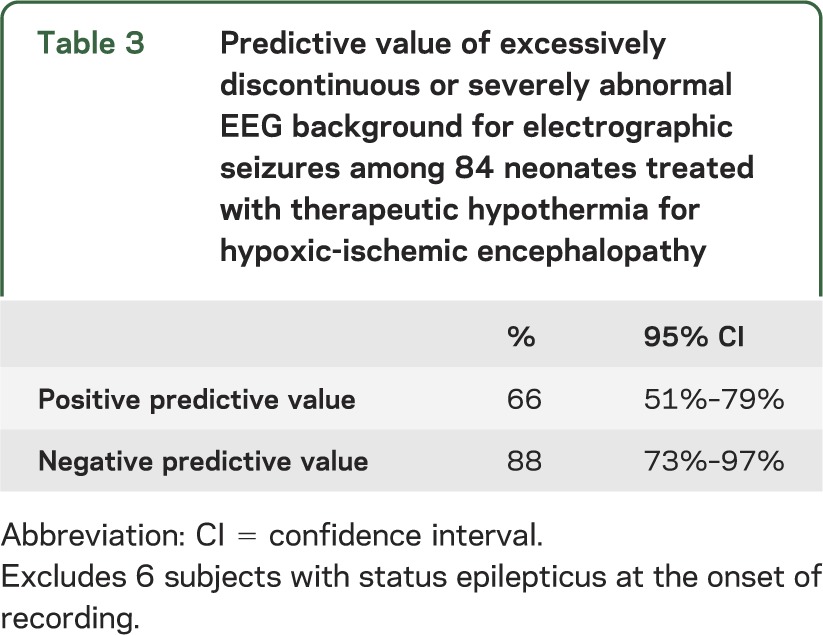
In the unadjusted analysis, the risk of electrographic seizures was higher among subjects with an abnormal EEG background. None of the perinatal clinical resuscitation variables predicted electrographic seizure occurrence (table 2). Presence of a perinatal sentinel event, degree of encephalopathy, and age at achieving therapeutic temperature for hypothermia were also not significantly associated with electrographic seizures. Initial EEG background pattern was electrographic status epilepticus in 6 subjects. Among the remaining subjects, initial EEG background was strongly associated with risk of seizures (and the risk persisted after adjusting for all clinical risk factors plus treatment center, p < 0.0001): the more severely abnormal the background pattern, the higher the risk for electrographic seizures. Furthermore, the higher risk of seizures among subjects with abnormal EEG background was independent of treatment with antiseizure medications prior to initiation of the EEG recording. Though subjects with excessively discontinuous and severely abnormal EEG background at the onset of recording were at highest risk (70% and 63%, respectively), 12% of subjects with a normal initial EEG background pattern had EEG seizures. Among subjects with EEG seizure onset during rewarming, the background was severely abnormal in 3 (75%), and normal in the remaining subject. The predictive value of an abnormal initial EEG background for electrographic seizures is presented in table 3.
Table 2.
Risk factors for electrographic seizures among 90 neonates treated with therapeutic hypothermia for hypoxic-ischemic encephalopathy
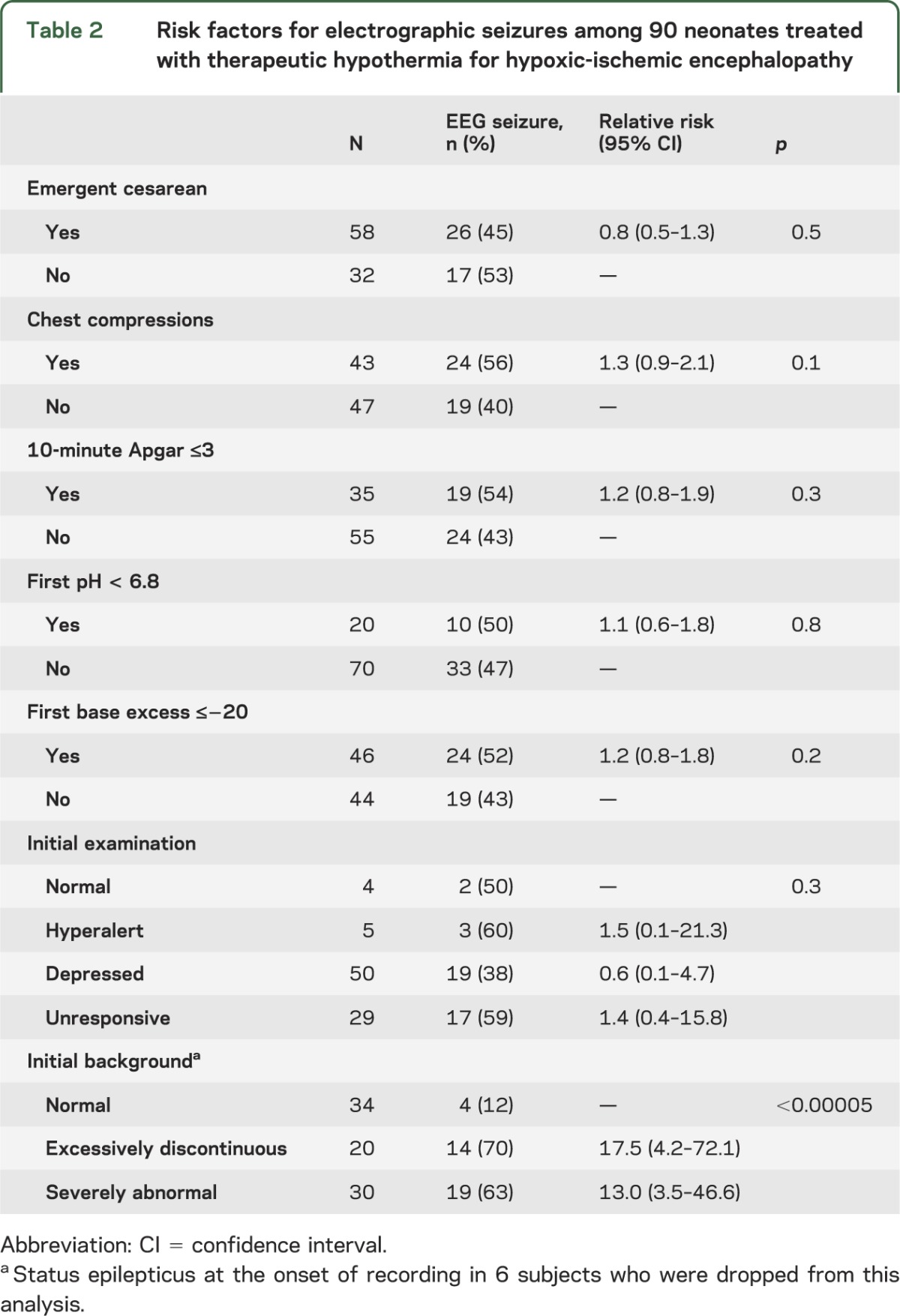
Table 3.
Predictive value of excessively discontinuous or severely abnormal EEG background for electrographic seizures among 84 neonates treated with therapeutic hypothermia for hypoxic-ischemic encephalopathy
DISCUSSION
Prior to the widespread use of therapeutic hypothermia, HIE was reported as the most common cause of neonatal seizures.3 Despite reports of reduced seizure burden among neonates treated with hypothermia,11,12 our multicenter data suggest that electrographic seizures remain common and are difficult to predict based on clinical features alone. EEG background at the onset of cEEG recording was most closely associated with risk of EEG seizures, with the highest risk among those with the most severely abnormal background pattern. Conversely, none of the perinatal clinical variables predicted electrographic seizures.
Our results are in keeping with single-center studies performed prior to the widespread use of therapeutic hypothermia. In a study of 49 term infants with perinatal asphyxia, as in our study, early cEEG monitoring was more accurate for predicting EEG seizures than either the clinical risk factors at birth or degree of acidosis.26 In a second study, a heterogeneous group of 51 neonates risk for seizures (including 30 with perinatal asphyxia) were examined with EEG recordings starting at a mean age of approximately 24 hours of life and found to have a very high association between initial EEG background and electrographic seizures.27 The less robust association between abnormal EEG and seizures in our cohort may be related to the difference in study population, effect of hypothermia or sedating medications on EEG background, or potential protective effect of hypothermia.
Our results support the recommendation that cEEG monitoring be implemented for all neonates who are treated with therapeutic hypothermia for HIE,15 rather than targeted subgroups, such as those with clinical seizures or depressed mental status. We found that the risk of electrographic seizures was similar among subjects with and without premonitoring clinical seizures, as well as those with normal or hyperalert mental status as compared to those with depressed or unresponsive mental status.
Increasingly, centers across North America and Europe are implementing cEEG monitoring for neonates with encephalopathy.28,29 Many centers are adopting modified montages, such as 2- or 3-channel amplitude-integrated EEG (aEEG), which has lower sensitivity and specificity for seizure detection than conventional video-EEG.30–33 Whether the increased accuracy of gold-standard full-montage video-EEG is clinically relevant is not known.
Though this study is strengthened by multicenter data, a large cohort size, gold-standard cEEG monitoring according to guidelines set out by the ACNS,15 and EEG review by pediatric neurophysiologists with experience in neonatal EEG, it is not without limitations. First, 3 separate pediatric neurophysiologists evaluated the EEG recordings without use of a consensus scoring system. Interrater differences are unlikely to have affected our primary result—that EEG background is associated with seizures among neonates treated with therapeutic hypothermia. However, misclassification of the outcome may have led to bias toward the null hypothesis and limited our ability to detect a difference among less robust predictors. Second, the lack of association with clinical risk factors may be related to limitations in clinical medical record documentation at each site or limited sample size. Nonetheless, we are able to determine that there is not a strong association between clinical risk factors and electrographic seizures. Third, subjects were enrolled over different study periods at the 3 centers, which may lead to heterogeneity within the study group, but again, should not affect the primary results. Fourth, we did not evaluate EEGs for elements such as normal named patterns, excessive abnormal sharp waves, degree of burst suppression, evolution of EEG background, or other more subtle EEG patterns, which may be useful for further refining risk of seizures. Finally, our data were insufficient to study additional perinatal risk factors such as maternal disease, placental abnormalities, and fetal causes (e.g., intrauterine growth retardation), findings that may further help to stratify the risk of seizures in this population.
Across 3 North American institutions, electrographic seizures were common among neonates treated with hypothermia and were difficult to predict based on clinical history alone. These findings justify the recommendation by the ACNS that EEG monitoring be used to detect electrographic seizures among neonates treated with hypothermia. Centers that have limited resources for prolonged conventional video-EEG could consider targeting neonates with moderate or severe initial EEG background abnormalities based on either early full-montage or an adapted montage such as single- or dual-channel aEEG, since these neonates are at highest risk. However, even neonates with normal initial EEG background may have electrographic seizures. Additional studies are warranted to further refine early EEG risk factors for seizures among neonates with HIE, as well as to determine the clinical impact of prolonged monitoring on medication use and long-term outcome.
Supplementary Material
ACKNOWLEDGMENT
The authors thank Jessica Kan, Ana Gutierrez-Colina, and Kaiwan Lin for their assistance with data collection and data management.
GLOSSARY
- ACNS
American Clinical Neurophysiology Society
- aEEG
amplitude-integrated EEG
- cEEG
continuous video-EEG
- HIE
hypoxic-ischemic encephalopathy
Footnotes
Editorial, page 1200
AUTHOR CONTRIBUTIONS
H.C. Glass drafted and revised the manuscript for content, participated in study concept and design, planned and performed analysis and interpretation of data, performed statistical analysis, and approved the submitted manuscript. C.J. Wusthoff revised the manuscript for content, participated in study concept and design, assisted with interpretation of data, acquired data, and approved the submitted manuscript. R.A. Shellhaas revised the manuscript for content, assisted with interpretation of data, acquired data, and approved the submitted manuscript. T.N. Tsuchida revised the manuscript for content, participated in study concept and design, assisted with interpretation of data, acquired data, and approved the submitted manuscript. S.L. Bonifacio revised the manuscript for content, assisted with interpretation of data, and approved the submitted manuscript. M. Cordeiro revised the manuscript for content, assisted with interpretation of data, acquired data, and approved the submitted manuscript. J. Sullivan revised the manuscript for content, participated in study concept and design, assisted with interpretation of data, and approved the submitted manuscript. N.S. Abend revised the manuscript for content, participated in study concept and design, assisted with interpretation of data, acquired data, and approved the submitted manuscript. T. Chang revised the manuscript for content, participated in study concept and design, assisted with interpretation of data, acquired data, and approved the submitted manuscript.
STUDY FUNDING
NIH/National Institute of Neurological Disorders and Stroke K23NS066137 and the Neonatal Brain Research Institute at UCSF support H.C.G. N.S.A. is supported by NIH/National Institute of Neurological Disorders and Stroke K23 NS076550. R.A.S. is supported by NIH/NICHD 5K23HD068402. S.L.B. is supported by KL2TR000143. The contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
DISCLOSURE
H. Glass receives research funding from NIH K23NS066137 and the Pediatric Epilepsy Research Foundation, has received travel expenses or honoraria for lectures not funded by industry, and has received payment for medical-legal review work. She serves on the editorial board of Pediatric Neurology. C. Wusthoff has received payment for medical-legal review work and has received travel expenses and honoraria for lectures and educational activities not funded by industry. She performs EEG studies as part of her clinical practice. R. Shellhaas receives research funding from NIH K23HD068402, the Child Neurology Foundation, and intramural grants from the University of Michigan's Department of Pediatrics and Communicable Diseases. She serves on the editorial boards of Pediatric Neurology and Journal of Child Neurology. T. Tsuchida reports no disclosures relevant to the manuscript. S. Bonifacio receives research funding from KL2TR000143. M. Cordeiro reports no disclosures relevant to the manuscript. J. Sullivan receives research funding from NIH (NIH 5 U01 NS053998-02), Pfizer, Inc., in support of his role as site PI for a study of pregabalin in pediatric epilepsy, and has also received payment for medical-legal work. N. Abend receives research funding from NIH (K23NS076550), receives royalties from Demos Medical Publishing, and has received payment for medical-legal review work. T. Chang reports no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Lanska MJ, Lanska DJ, Baumann RJ, Kryscio RJ. A population-based study of neonatal seizures in Fayette County, Kentucky. Neurology 1995;45:724–732 [DOI] [PubMed] [Google Scholar]
- 2.Saliba RM, Annegers JF, Waller DK, Tyson JE, Mizrahi EM. Incidence of neonatal seizures in Harris County, Texas, 1992-1994. Am J Epidemiol 1999;150:763–769 [DOI] [PubMed] [Google Scholar]
- 3.Ronen GM, Penney S, Andrews W. The epidemiology of clinical neonatal seizures in Newfoundland: a population-based study. J Pediatr 1999;134:71–75 [DOI] [PubMed] [Google Scholar]
- 4.Glass HC, Pham TN, Danielsen B, Towner D, Glidden D, Wu YW. Antenatal and intrapartum risk factors for seizures in term newborns: a population-based study, California 1998-2002. J Pediatr 2009;154:24–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Volpe JJ. Neonatal seizures. In: Volpe JJ, ed. Neurology of the Newborn, 5th ed Philadelphia: WB Saunders; 2008:203–244 [Google Scholar]
- 6.McCabe BK, Silveira DC, Cilio MR, et al. Reduced neurogenesis after neonatal seizures. J Neurosci 2001;21:2094–2103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lynch M, Sayin U, Bownds J, Janumpalli S, Sutula T. Long-term consequences of early postnatal seizures on hippocampal learning and plasticity. Eur J Neurosci 2000;12:2252–2264 [DOI] [PubMed] [Google Scholar]
- 8.Isaeva E, Isaev D, Holmes GL. Alteration of synaptic plasticity by neonatal seizures in rat somatosensory cortex. Epilepsy Res 2013;106:280–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miller SP, Weiss J, Barnwell A, et al. Seizure-associated brain injury in term newborns with perinatal asphyxia. Neurology 2002;58:542–548 [DOI] [PubMed] [Google Scholar]
- 10.Glass HC, Glidden D, Jeremy RJ, Barkovich AJ, Ferriero DM, Miller SP. Clinical neonatal seizures are Independently associated with outcome in infants at risk for hypoxic-ischemic brain injury. J Pediatr 2009;155:318–323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Low E, Boylan GB, Mathieson SR, et al. Cooling and seizure burden in term neonates: an observational study. Arch Dis Child Fetal Neonatal Ed 2012;97:F267–F272 [DOI] [PubMed] [Google Scholar]
- 12.Srinivasakumar P, Zempel J, Wallendorf M, Lawrence R, Inder T, Mathur A. Therapeutic hypothermia in neonatal hypoxic ischemic encephalopathy: electrographic seizures and magnetic resonance imaging evidence of injury. J Pediatr 2013;163:465–470 [DOI] [PubMed] [Google Scholar]
- 13.Glass HC, Nash KB, Bonifacio SL, et al. Seizures and magnetic resonance imaging-detected brain injury in newborns cooled for hypoxic-ischemic encephalopathy. J Pediatr 2011;159:731–735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wusthoff CJ, Dlugos DJ, Gutierrez-Colina A, et al. Electrographic seizures during therapeutic hypothermia for neonatal hypoxic-ischemic encephalopathy. J Child Neurol 2011;26:724–728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shellhaas RA, Chang T, Tsuchida T, et al. The American Clinical Neurophysiology Society's guideline on continuous electroencephalography monitoring in neonates. J Clin Neurophysiol 2011;28:611–617 [DOI] [PubMed] [Google Scholar]
- 16.Malone A, Ryan CA, Fitzgerald A, Burgoyne L, Connolly S, Boylan GB. Interobserver agreement in neonatal seizure identification. Epilepsia 2009;50:2097–2101 [DOI] [PubMed] [Google Scholar]
- 17.Murray DM, Boylan GB, Ali I, Ryan CA, Murphy BP, Connolly S. Defining the gap between electrographic seizure burden, clinical expression and staff recognition of neonatal seizures. Arch Dis Child Fetal Neonatal Ed 2008;93:F187–F191 [DOI] [PubMed] [Google Scholar]
- 18.Scher MS, Alvin J, Gaus L, Minnigh B, Painter MJ. Uncoupling of EEG-clinical neonatal seizures after antiepileptic drug use. Pediatr Neurol 2003;28:277–280 [DOI] [PubMed] [Google Scholar]
- 19.Biagioni E, Ferrari F, Boldrini A, Roversi MF, Cioni G. Electroclinical correlation in neonatal seizures. Eur J Paediatr Neurol 1998;2:117–125 [DOI] [PubMed] [Google Scholar]
- 20.Clancy RR, Legido A, Lewis D. Occult neonatal seizures. Epilepsia 1988;29:256–261 [DOI] [PubMed] [Google Scholar]
- 21.Shankaran S, Laptook A, Wright LL, et al. Whole-body hypothermia for neonatal encephalopathy: animal observations as a basis for a randomized, controlled pilot study in term infants. Pediatrics 2002;110:377–385 [DOI] [PubMed] [Google Scholar]
- 22.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap): a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clancy RR, Legido A. The exact ictal and interictal duration of electroencephalographic neonatal seizures. Epilepsia 1987;28:537–541 [DOI] [PubMed] [Google Scholar]
- 24.Scher MS, Hamid MY, Steppe DA, Beggarly ME, Painter MJ. Ictal and interictal electrographic seizure durations in preterm and term neonates. Epilepsia 1993;34:284–288 [DOI] [PubMed] [Google Scholar]
- 25.Pisani F, Cerminara C, Fusco C, Sisti L. Neonatal status epilepticus vs recurrent neonatal seizures: clinical findings and outcome. Neurology 2007;69:2177–2185 [DOI] [PubMed] [Google Scholar]
- 26.Murray DM, Boylan GB, Fitzgerald AP, Ryan CA, Murphy BP, Connolly S. Persistent lactic acidosis in neonatal hypoxic-ischaemic encephalopathy correlates with EEG grade and electrographic seizure burden. Arch Dis Child Fetal Neonatal Ed 2008;93:F183–F186 [DOI] [PubMed] [Google Scholar]
- 27.Laroia N, Guillet R, Burchfiel J, McBride MC. EEG background as predictor of electrographic seizures in high-risk neonates. Epilepsia 1998;39:545–551 [DOI] [PubMed] [Google Scholar]
- 28.Boylan G, Burgoyne L, Moore C, O'Flaherty B, Rennie J. An international survey of EEG use in the neonatal intensive care unit. Acta Paediatr 2010;99:1150–1155 [DOI] [PubMed] [Google Scholar]
- 29.Glass HC, Kan J, Bonifacio SL, Ferriero DM. Neonatal seizures: treatment practices among term and preterm infants. Pediatr Neurol 2012;46:111–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rennie JM, Chorley G, Boylan GB, Pressler R, Nguyen Y, Hooper R. Non-expert use of the cerebral function monitor for neonatal seizure detection. Arch Dis Child Fetal Neonatal Ed 2004;89:F37–F40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shellhaas RA, Soaita AI, Clancy RR. Sensitivity of amplitude-integrated electroencephalography for neonatal seizure detection. Pediatrics 2007;120:770–777 [DOI] [PubMed] [Google Scholar]
- 32.Frenkel N, Friger M, Meledin I, et al. Neonatal seizure recognition: comparative study of continuous-amplitude integrated EEG versus short conventional EEG recordings. Clin Neurophysiol 2011;122:1091–1097 [DOI] [PubMed] [Google Scholar]
- 33.Shah DK, Mackay MT, Lavery S, et al. Accuracy of bedside electroencephalographic monitoring in comparison with simultaneous continuous conventional electroencephalography for seizure detection in term infants. Pediatrics 2008;121:1146–1154 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


