Abstract
Functional neuro-imaging techniques are helpful in the pre-surgical evaluation of epilepsy for localization of the epileptogenic zone as ancillary tools to electroencephalography (EEG) and magnetic resonance imaging (MRI) or when other localization techniques are normal, non-concordant or discordant. Positron emission tomography (PET) and ictal single photon emission computed tomography (ictal SPECT) imaging are traditional tests that have been reported to have good sensitivity and specificity although the results are better with more expertise as is true for any technique. More recently magnetoencephalogram/magnetic source imaging (MEG/MSI), diffusion tensor imaging and functional magnetic resonance imaging (fMRI) have been used in localization and functional mapping during the pre-surgical work-up of epilepsy. Newer techniques such as fMRI-EEG, functional connectivity magnetic resonance imaging and near infra-red spectroscopy, magnetic resonance spectroscopy and magneto nanoparticles hold promise for further development that could then be applied in the work-up of epilepsy surgery. In this manuscript, we review these techniques and their current position in the pre-surgical evaluation of epilepsy.
Keywords: Diffusion tensor imaging, functional connectivity magnetic resonance imaging, functional magnetic resonance imaging, functional magnetic resonance imaging-electroencephalography, magnetoencephalogram, positron emission tomography, single photon emission computed tomography, temporal lobe epilepsy
Introduction
Epilepsy surgery is the most effective treatment option for pharmacoresistant epilepsy, but is often hindered by difficulty in accurately delineating the epileptogenic zone. Although there have been great advances in surgical localization from the use of magnetic resonance imaging (MRI) during the previous decades, MRI is sometimes insufficient to identify the epileptogenic zone or could be non-concordant/discordant to other localization techniques such as clinical semiology, electroencephalography (EEG), video EEG monitoring, and neuropsychology testing. At these times, testing using functional techniques can help provide additional information in identifying the epileptogenic zone.
Some such techniques include positron emission tomography (PET), ictal single photon emission computed tomography (ictal SPECT) and magnetoencephalogram/magnetic source imaging (MEG/MSI) which have already found their place in routine clinical practice with varying degrees of use at different centers. In addition, newer techniques such as diffusion tensor imaging (DTI) and functional magnetic resonance imaging (fMRI-EEG) have been extensively used at a fewer number of surgical epilepsy centers to aid localization or for functional mapping to assess surgical safety. Functional connectivity magnetic resonance imaging (fcMRI), magnetic resonance spectroscopy (MRS), arterial spin labeling (ASL), near infra-red spectroscopy (NIRS) and magento nanoparticle (MNP) imaging are newer techniques that hold promise, but are yet to find their place in the pantheon of functional neuroimaging techniques available to today's epileptologist for identification of the epileptogenic zone or in helping the placement of intra-cranial electrodes. In addition, improvement of these methods may help avert the need for intracranial EEG, which can be an expensive and occasionally risky investigation on account of its invasive nature.[1]
In this manuscript, we review some of the functional neuro-imaging techniques including the history of development, principles and their current/potential role in the pre-surgical evaluation of epilepsy. Relevant articles were located on PubMed by searching with the keyword related to the functional neuroimaging technique and the keyword “epilepsy” and were selected for relevance to epilepsy, in particular pre-surgical work-up.
Positron Emission Tomography
PET conveys functional representations of various aspects of brain activity. Depending upon the radionuclide tracer utilized, PET has been applied to study local glucose utilization (fluorine-18 fluorodeoxy-glucose [FDG]) and quantification of specific neurotransmitter-receptor relationships e.g. [11C] flumazenil with gamma-Aminobutyric acid (GABAA) receptors, [18F] 2’-methoxyphenyl-(N-2’-pyridinyl)-p-fluoro-benzamidoethyipiperazine with serotonin receptions, [18F] Fallypride with dopamine receptors, opioid-based ligands on opioid receptors. The focus of this review will be on glucose metabolism imaging with FDG-PET.
Brain glucose metabolism is a representation of neural activity. As a glucose analog, FDG is transported into tissues and phosphorylated by hexokinase in the same manner as glucose. However, unlike glucose, FDG accumulates in the cell as it cannot undergo further metabolism. As proton-rich isotopes such as FDG decay, positrons are emitted. Subsequently, positrons annihilate on contact with nearby electrons. Each annihilation produces two 511 keV photons travelling in opposite directions and these photons are then captured by multiple pairs of oppositely situated detectors surrounding the subject.[2] Utilizing MR images from the same patient, FDG-PET and MR images can be co-registered. Fused images are color-graded, such that each color change reflects a difference in FDG uptake.[3] In cases for which visual asymmetry of FDG uptake across homologous structures cannot be confidently discerned, the use of standard uptake value ratios can be supplemented to discern areas of abnormality. A difference of more than 10% is considered to be significant.[4]
Hypometabolism on FDG-PET has been pathophysiologically attributed to factors such as neuronal loss or reduction in synaptic density.[5] Correspondingly, brain regions containing the epileptogenic zone have been shown to have hypometabolism on inter-ictal FDG-PET, with an in-plane resolution of 2-3 mm. Temporal lobe hypometabolism ipsilateral to the seizure focus has been observed in 60-90% of patients with temporal lobe epilepsy (TLE).[6,7,8] For patients with extratemporal lobe epilepsy, hypometabolism on FDG-PET correctly localizes the seizure focus in 67%.[8] In presurgical evaluation for medically refractory epilepsy, FDG-PET is particularly advantageous for patients with apparently normal brain MRI findings. In these cases, FDG-PET has an overall diagnostic sensitivity of 44% in detecting “non-lesional” epileptogenic substrates.[9]
PET co-registered to MRI (PCOM) has been shown to offer the additional advantage in some clinical scenarios [Figure 1]. For focal cortical dysplasias, FDG-PET frequently demonstrates wider boundaries of abnormality than MRI. PCOM has the advantage of superimposing metabolic abnormalities upon anatomic landmarks (such as particular gyri or sulci) and thus may enhance surgical planning toward more complete lesional resection.[4] Epileptogenic tumor's, particularly gangliogliomas and dysembryoplastic neuroepithelial tumor's, are frequently associated with cortical dysplasias that extend beyond the tumor's. PCOM can help identify and define the borders of cortical dysplasias surrounding these tumor's such that a more complete resection of epileptogenic substrate can be pursued.[10] Moreover, in patients with tuberous sclerosis complex, multiple tubers are frequently present, but often only one of the tubers may be epileptogenic. PET co-registered to DTI sequences of MRI protocols has shown promise for distinguishing epileptogenic tubers.[11]
Figure 1.
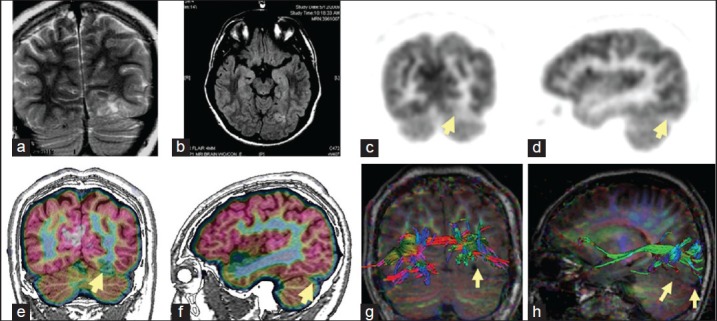
Multi-modality imaging in a patient with left occipital lobe seizures. Magnetic resonance imaging (MRI) shows left sub-occipital cortical dysplasia in T2 and fluid attenuated inversion recovery sequences (a, b). Positron emission tomography (PET) imaging shows hypometabolism in corresponding areas visible as a lighter shade of gray in grayscale images (c, d), but is much easier appreciated as a break in the pink cortical ribbon in color coded PET imaging co-registered to MRI (e, f). Prior to surgery the patient also had diffusion tensor imaging tractography of the visual pathway fibers which are seen to be displaced superiorly by the lesion (yellow arrows in g, h)
FDG-PET, when done during or shortly following an epileptic seizure, often reveals complex patterns of both hypometabolism and hypermetabolism. Therefore, caution should be exercised to rule out the occurrence of seizures near the time of FDG injection. Antiepileptic medications can decrease glucose metabolic rates and additional steps to analyze metabolic rates normalized to the whole brain metabolism may be necessary in some cases.
Single Photon Emission Computed Tomography
SPECT is a functional neuroimaging modality that is able to measure or compare differences in blood flow through tissues and organs. More specifically, brain perfusion SPECT allows for a quantitative estimation of regional cerebral blood flow. When utilized in the pre-surgical evaluation of patients with refractory epilepsy, SPECT scanning is performed during both the ictal as well as the inter-ictal periods. Local neuronal hyperactivity during an epileptic seizure triggers an autoregulatory response that contributes to preferential hyperperfusion of the seizure onset zone. Taking advantage of this phenomenon, ictal SPECT is performed with injection of the radionuclide tracer during the electro-clinical manifestation of a seizure — preferably within 20 s of seizure onset to optimize seizure focus localization.[12] Ictal SPECT requires video-EEG monitoring as well as appropriate logistical support to insure prompt injection of the radionuclide tracer. Ictal SPECT is the only routine clinical imaging modality capable of detecting ictal brain functional changes, allowing for visualization of the seizure onset zone during an actual seizure. Similar to PET imaging, ictal SPECT is particularly advantageous for patients with apparently normal brain MRI findings.
The radionuclide tracers frequently used in SPECT, 99mTc-hexa-methyl-propyleneamine-oxime or 99mTc-ethyl cysteinate dimer, are characterized by the ability to cross the blood brain barrier rapidly due to their small molecular size and lipophilicity and are distributed proportional to the blood flow in cerebral tissues. Upon cerebral uptake and intracellular conversion to polar metabolites, they are retained with minimal regional redistribution or washout — allowing sufficient time for image acquisition up to 4 hours after injection. Once trapped in cerebral tissues, the gamma radiation emitted from the radionuclide tracer can be directly detected by a gamma camera. Symmetry in terms of perfusion across homologous structures is analyzed, with asymmetry of more than 10% considered as abnormal.
Across several investigations of patients with unilateral TLE proven by either EEG, brain MRI, or post-resection seizure freedom, ictal SPECT has been claimed to correctly identify the seizure focus in over 90% of the cases.[13,14,15] For patients with mesial temporal ictal onset zones, the area of hyperperfusion primarily involves the anterior temporal pole and mesial temporal structures, while the lateral temporal cortex is more variably involved. For patients with lateral temporal epileptogenic substrates, studies have shown bilateral temporal lobe hyperperfusion (greater on the side of the lesion) or posterolateral temporal hyperperfusion ipsilateral to the lesion.[16,17] Because it may take 15-20 s for the tracer to reach the brain, the ictal SPECT image may display not only the seizure onset zone, but also the areas of seizure propagation. Similarly, longer injection delay from the time of seizure onset may also result in more likelihood of including seizure propagation areas. Another caveat associated with longer injection delays is the phenomenon of “postictal switch” in which the seizure onset zone switches to hypoperfusion, while seizure propagation regions become hyperperfused — resulting in false localization of the seizure focus. In patients with TLE, common areas of seizure propagation can include ipsilateral insula, basal ganglia, frontal lobe, as well as contralateral temporal lobe.[5]
Ictal SPECT of patients with extratemporal epilepsy has been shown to have a lower sensitivity (66%) for detecting relevant abnormality, when compared to patients with TLE.[18] This lower sensitivity is possibly related to rapid seizure propagation and secondary generalization that are most frequently associated with extratemporal epilepsy. For example, in patients with occipital lobe epilepsy, very early ictal injection is recommended in order to avoid incorrect demonstration of hyperperfusion in the temporal lobe — representing propagated seizure activity.[19] Moreover, for seizures arising from the supplementary motor area (SMA), seizure propagation can be very rapid, while the seizure duration is often very short. Such presentation lowers the usefulness of ictal SPECT, which correctly localizes the seizure focus in only 40% of such cases.[20]
The accuracy of ictal SPECT can be enhanced by subtraction ictal SPECT co-registered to MRI (SISCOM) analysis [Figure 2]. Using a computer-aided voxel-based co-registration technique, the inter-ictal SPECT is subtracted from the ictal SPECT and a mean image is generated using the mean and standard deviation of the differences in all brain voxels. This mean image is then co-registered to the patient's brain MRI. This technique has been reported to significantly improve seizure focus localization when compared with side-by-side visual inspection of ictal and inter-ictal SPECT images.[21,22] For patients with extratemporal epilepsy where rapid seizure propagation occurs and where perfusion changes can be subtle, SISCOM analysis may be particularly useful.[23]
Figure 2.
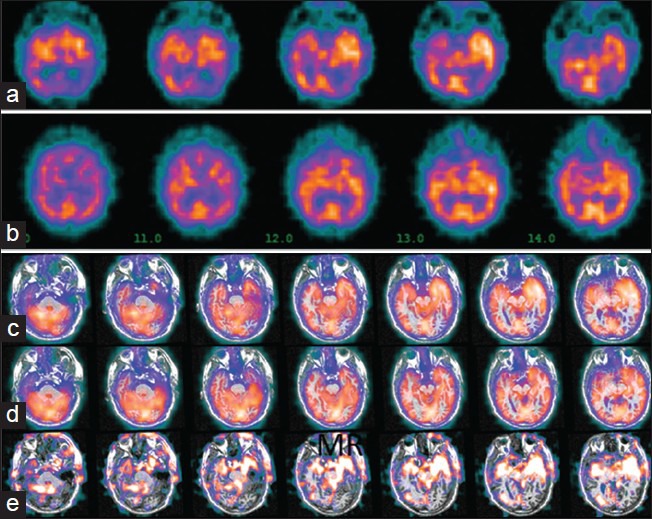
Single photon emission computed tomography (SPECT) imaging and subtraction ictal SPECT co-registered to MRI (SISCOM): Patient with non-lesional left temporal lobe seizures. SPECT images show left temporal hypermetabolism in ictal images (a), compared to inter-ictal (b). Co-registration of these images to MRI makes the asymmetry more obvious in ictal (c) and inter-ictal (d) reconstructions (note that the slice angle and thickness in the reconstructions c and d are different from the raw SPECT images in a and b. Subtraction of the inter-ictal from the ictal SPECT shows the regions of maximal difference in intensity (e). Image orientation is radiological (patient left is on image right)
Inter-ictal SPECT has a sensitivity of only about 50% in identifying the seizure onset zone.[13,14,15] Therefore, inter-ictal SPECT on its own has been considered insufficient for confident localization of the seizure focus.
Diffusion Tensor Imaging
DTI is a relatively novel imaging method that provides improved summary of substrate cellular and molecular properties. This modality may allow for increased sensitivity for microstructural abnormalities, particularly in tissues with highly organized microstructure such as the white matter — allowing visualization of the location, orientation, and anisotropy of the axon tracts.
DTI derives from novel algorithms built on a classic MRI protocol-diffusion weighted imaging (DWI). This DWI modality uses the motion of water molecules to probe cellular and molecular structures, and is presently used in clinical practice to identify ischemia. Whereas DWI measurements are made in three directions and the results are summarized by three DWI images, DTI derives from computational methods that integrate diffusion data acquired in more than three directions (usually 7 or more). These measurements are summarized using a Gaussian model of diffusion, specifically by a tensor (ellipsoid) that approximates how far the water molecules diffuse in all directions from a point. This computational integration of diffusion data, the diffusion tensor, yields a sensitive detector of tissue microstructural pathology that influences freedom of water molecular diffusion.[24] Common ways to summarize the freedom of molecular movement in DTI include fractional anisotropy (FA-an index describing the degree of anisotropy or directional constraints) and mean diffusivity (MD-a measurement of the amplitude of diffusion of motion). Another approach to DTI analysis is the method of fiber tractography (FT), which allows for a measure of white matter tract integrity. FT is determined by a computational algorithm that determines the likely fiber patterns that pass through one selected region or between multiple selected regions [Figure 1]. Compared with the region of interest (ROI) analyses utilized to determine FA and MD measure, FT has been shown to be less operator dependent and can generally include a more homogeneous sampling of data to yield more robust results.[25]
Although the proximity of the sphenoid bone and air cells of the sphenoid sinus are known to produce susceptibility artifacts during temporal lobe imaging with DTI, successful investigation of the hippocampus has been well-documented. Pathophysiologically, it is hypothesized that the hippocampal cell loss and architectural disruption may correlate with expansion of extracellular space, allowing increased freedom of molecular diffusivity.[26] The gliosis component of hippocampal sclerosis (if present) may add directional constraints and is reflected by a decreased FA. Among patients with known mesial temporal sclerosis (MTS), two investigators have independently shown significantly increased MD indices in the hippocampus ipsilateral to the known MTS, when compared with values in the contralateral hippocampus and with control subjects. Additional evidence suggests that the DTI may reveal temporal lobe abnormalities that are not detected on conventional MRI. Among two patients reported by Assaf et al. and one patient reported by Salmenpera et al. with mesial temporal epilepsy, but normal MRI findings, elevated MD indices were significantly evident.[26,27] Furthermore, among another nine MRI negative patients with EEG documented seizures localizing to the left temporal region, ROI analysis within the left temporal white matter demonstrated both a significant increase in MD and a significant decrease in FA.[28]
Conventional MRI and fMRI methods provide limited information regarding anatomic relationships between the epileptogenic substrate and white matter fiber tracts associated with eloquent cortical areas. DTI tractography may be able to address this limitation through its mapping of white matter fiber anatomy in relation to planned resection zones. In a study of 49 patients undergoing surgical planning for extratemporal resective epilepsy surgery, DTI tractography assisted in the surgical decision making in two-thirds of the cases. More specifically, DTI tractography helped modify the surgical procedure in one-third of patients with frontal lobe resections, one-fourth of patients with occipital resections and two-thirds of patients with parietal or multilobar resections.[29]
Magnetoenceophalogram
MEG [Figure 3] refers to the detection of minute magnetic flux induced by the electrical activity of cortical neurons. MSI is the co-registration of MEG data on the patient's structural MRI to help in the accurate localization of detected brain activity. As the cortical activity induced magnetic flux is only in the order of 10-100 fT (1 femtoTesla is 10−15 Tesla), extremely sensitive magnetometers are needed for its detection. The initial measurement of the MEG signal in 1968 by David Cohen at the University of Illinois required a magnetometer consisting of a ferrite core surrounded by 2 million turns of a copper wire.[30] MEG became technically easier with the development of superconducting quantum interference devices (SQUIDs), which dramatically increased the sensitivity of magnetometers. By the 1980s commercial manufacturers had made arrays of multiple sensors (150-300) that enabled the simultaneous measurement of MEG signals from different areas of the brain surface akin to the measurement of EEG signals from different surface regions using scalp electrodes. However, as of 2011, only 160 laboratories around the world have MEG capability.[31]
Figure 3.
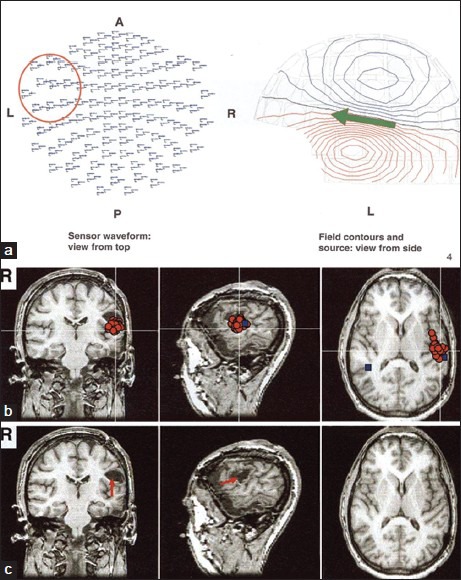
MEG acquired with a 306-channel whole-head MEG system showing epileptiform spike activity mainly from the posterior-lateral aspect of the left inferior-frontal/superior-temporal lobes (red circle and green arrow in a). MSI shows that the MEG spikes (red circles in b) are localized to the inferior margin of a small cavity in the posterior part of the left inferior frontal gyrus (red arrow in c). The primary auditory cortex in the left hemisphere was located in the vicinity of the spike cluster (blue box in b)
The principle of MEG is based on the induction of magnetic flux perpendicular to the electric current generated by post-synaptic (intracellular) currents in pyramidal neurons.[32] Since cortical neurons on the gyral crests are oriented in a radial direction from the scalp surface, they would be expected to create the maximal magnetic field radial to the cortical surface. Similarly, neurons in the sulci are oriented parallel to the scalp surface and induce magnetic fields radial to the scalp surface, which are best detected by MEG.[31] Since the cortical neurons create very minute magnetic fluctuations (in the order of fT), ambient magnetism in the atmosphere (in the order of microteslas) can significantly interfere with their recording. Hence a magnetically shielded room is necessary to perform testing, which represents a significant cost in installing and operating these machines. The method of analyzing MEG signals uses several techniques, the simplest and most powerful of which is the equivalent current dipole model (ECD). ECD analysis requires expertise, especially with multi-dipole analysis. Other approaches include minimum-norm estimates or minimum-current estimates and beamforming.[31] Source analysis can be used to localize the MEG activity and to determine artifacts, (e.g., a source close to eyeballs or tongue is likely to be artifact).[31] MEG has advantages in that the skull and scalp are “magnetically transparent” and do not attenuate the cortical signals as happens with EEG. The drawback of using MEG/MSI, as with EEG, for source localization is the so-called “inverse problem” where an infinite number of potential sources can explain a detected signal.
MEG/MSI has been used since the early 1980s in identifying epileptic foci.[33,34] MEG is used in detecting epileptic spikes. As tangential spikes are better detected, MEG can often detect spikes, which are completely missed by surface EEG. Inter-ictal spikes with MEG have been reported to correctly localize epilepsy in 52-89% of patients.[35,36] Unique information was provided by MEG/MSI in 24-58% and altered or informed surgical decision making in 9-11%.[36,37,38] It has been reported that the direction of the MEG dipole can help differentiate types of temporal lobe epilepsy into mesial, lateral and diffuse onsets.[39] MEG/MSI also helps in accurate placement of intra-cranial electrodes.[37,40] A high coverage of the MEG signal area by the surgical resection and a homogeneous distribution of MEG localizations correlate to a favorable outcome after surgery.[34]
Ictal MEG has been claimed to be a useful tool and potentially provides better localization than inter-ictal MEG.[41,42] Other uses of MEG include real-time tracking of brain activity to study sensory, motor, language and cognitive functions and the study of brain rhythms.[31]
Functional MRI
FMRI depends upon the property of differential magnetic susceptibilities of deoxygenated and oxygenated hemoglobin. In 1990, Ogawa used this concept to develop a contrast between oxygenated and deoxygenated hemoglobin called blood oxygenation level dependent (BOLD) contrast to demonstrate activity in the visual cortex.[43] Deoxygenated hemoglobin is paramagnetic leading to distortion of magnetic fields and a shorter T2 relaxation time. At areas of increased brain activity, reactive vasodilatation occurs that overcompensates for the metabolic demand leading to an influx of oxygenated hemoglobin, thus prolonging the T2 relaxation time. Subtracting the BOLD image of rest from a specific task (such as a language paradigm) thus leads to a prolonged T2 signal in areas of the brain activated by the task.
Language fMRI is the most frequently used fMRI paradigm in epilepsy and has been claimed to be a substitute for the Wada test for purposes of memory lateralization. Memory fMRI tests do not yield a robust activation although several groups are continuing research in making a useful paradigm. Newer modifications of fMRI such as fMRI-EEG and functional connectivity MRI are also reviewed below.
Language fMRI
Language fMRI uses a language paradigm to visualize the language areas of the cortex. Several studies have compared Wada test findings to language fMRI which is thought to be a viable and non-invasive alternative.[44,45] Several epilepsy centers have gravitated toward using language fMRI as a surrogate marker for lateralization by the Wada test. Several language paradigms exist. In general, verb generation tasks (i.e., think of action associated with an object. e.g., think of “drive” when shown a “car”) activate the Broca's area, while semantic tasks and reading activate the posterior language areas.[46] Using a panel of language tests improves reliability of lateralization compared to using a single test.[47] Studies on patients with epilepsy have shown that activation is reduced in typical language regions and is shifted to other areas,[48,49] although this shifting of language functions may be dysfunctional.[50] However, such shifting of language portends better post-operative cognitive outcomes.
Memory fMRI
Memory fMRI is technically more challenging than language fMRI and has found less robust results.[46] Better memory has been correlated with greater mesial temporal lobe activation.[51] In agreement with the “functional adequacy model,” it has been shown that the degree of activation in the dominant (left) hemisphere correlates with verbal memory in left TLE and non-verbal memory in right TLE.[52] Several studies of lateralized epilepsy have found that activation during memory tasks is reduced on the ipsi-lesional side[53,54] and was shifted to the contra-lateral undamaged hemisphere, although this reached significance only for left TLE.[54] An older study showed that memory fMRI may correlate to Wada test results,[53] and subsequent studies have shown that pre-operative memory fMRI results may be helpful in predicting post-operative memory outcomes.[55,56,57] Future advancements in memory fMRI testing may eventually produce a viable non-invasive alternative to the Wada test.
EEG-Functional MRI
EEG-fMRI is a special application of fMRI incorporating information from EEG and was developed in 1992 by John Ives et al. EEG-fMRI strives to merge the structural resolution of fMRI (in the order of 3 mm3) and the temporal resolution of EEG (in the order of milliseconds).[58]
The technique generally uses epileptiform discharges in the EEG as triggers to evaluate changes in the BOLD signal in a fashion analogous to using a verb generation task as a trigger in the traditional task-based fMRI described earlier. The BOLD activations of several such discharges are grouped together by the image analysis software to show the location of the fMRI activation that corresponds to the EEG signal of interest [Figure 4]. There are several technical challenges that need to be overcome to record the EEG-fMRI signal.[58]
Figure 4.
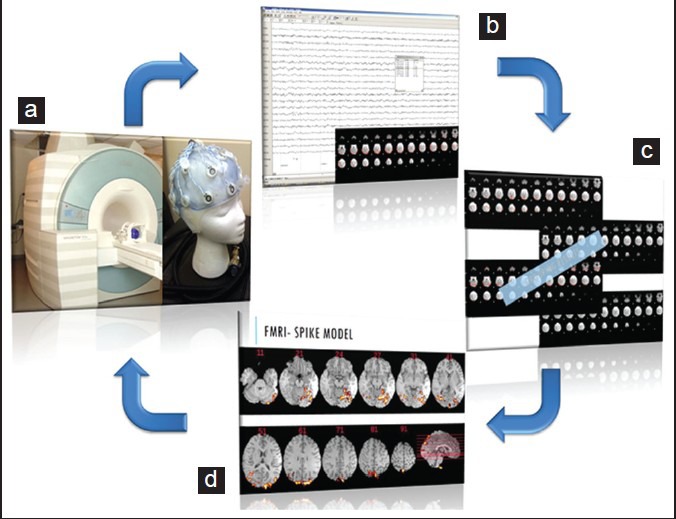
Schematic showing analysis methodology in functional magnetic resonance imaging-electroencephalography (fMRI-EEG). EEG is acquired using a specialized system within the magnetic resonance imaging (MRI) machine while acquiring blood oxygenation level dependent (BOLD) sequences (a). Subsequently the EEG is analyzed for spikes and the corresponding BOLD fMRI change is detected (b). Multiple spike related BOLD signal changes are summated to improve the signal to noise ratio (c). The resultant summated signal is co-registered to the structural brain MRI to show the location of the summated BOLD signal change (d). This is the same patient in Figure 1. See how the summated signal in d corresponds to the lesion visualized with other structural and functional imaging modalities
EEG-fMRI detected BOLD activity in patients with epilepsy has been reported to have concordance with the scalp EEG in 88% and was found to have contributed information in addition to the scalp findings about the epileptic focus in 64%.[59] EEG-fMRI has been suggested as a very useful test to guide implantation of intra-cranial electrodes.[60] More recent developments in EEG-fMRI include the advances in performing simultaneous intracranial EEG recordings as part of the technique.[61] EEG-fMRI activation during incidentally recorded seizures has also been reported to be of value in the localization of epileptogenic cortex.
Functional Connectivity MRI
FcMRI utilizes the principles of fMRI to demarcate brain networks and was first described in 1995.[62] The basic underlying principle involves the correlation of signal changes over time (time series) in different voxels (the smallest individual unit of the acquired MRI volume) of the brain to detect correlated voxels. Such voxels are put together on a brain map to reveal the underlying network. Different techniques of fcMRI have been described including ROI/seed-based techniques, independent component analysis (ICA), graph theory based analysis, amplitude of low frequency fluctuations, regional homogeneity analysis and Granger causality analysis.
Since epilepsy is thought to be a network disorder[63] with structural changes demonstrated in different regions of the brain distant from the primary epileptic focus,[64] techniques evaluating brain networks such as DTI and fcMRI assume significance in elucidating the pathophysiology of the disease and have the potential to guide surgical treatment. Using fcMRI, it has been shown that the language network is disrupted in left TLE compared with the controls.[65] It has also been shown that the default mode network (DMN) shows disconnection between its anterior and posterior aspects as well as between the posterior DMN and the hippocampus.[66,67] In right TLE, greater connectivity between the left MTL and the medial frontal cortex improves non-verbal memory, suggesting potential adaptive changes. However, in left TLE, greater connectivity between the left MTL and posterior cingulum worsens verbal memory suggesting maladaptive changes.[68] Attempts have been made to lateralize TLE based on functional connectivity, although independent confirmation of results by other groups is lacking at this time.[69,70]
Near Infra-Red Spectroscopy
NIRS is a low resolution functional imaging technique which has advantages of being portable and in being easy to use with existing techniques. First described in 1977,[71] the NIRS setup consists of a transmitter probe, which transmits near infra-red spectrum wavelength rays that pass through the cranium to a depth of approximately 2 cm during which phase it is absorbed by hemoglobin in the tissue. Reflected rays from here is detected by a sensor probe and the strength of reflected rays is inversely related to the concentration of hemoglobin in the brain tissue.[72] The low resolution images obtained can then be co-registered to the structural brain images to lateralize and localize the signal changes. Such optical properties of the brain can be used to detect the several parameters including oxygenated hemoglobin concentration, deoxygenated hemoglobin concentration, total hemoglobin concentration, cytochrome-oxidase redox state, regional oxygen saturation, tissue oxygenation index, blood flow index and others.[73]
NIRS has been shown to correctly identify the affected hemisphere during seizures, as corroborated by simultaneous ictal SPECT and intracranial EEG.[72] Studies have also shown the use of NIRS in detecting extra-temporal and temporal lobe seizures, including sub-clinical seizures.[74,75] Although the literature in this field is still evolving, NIRS has the potential to be a useful addition to the pre-surgical work-up of epilepsy, particularly given its ease of use and ability to combine with other tests such as video EEG monitoring and SPECT scans. In addition to seizure detection and localization, NIRS has also been shown to be useful in language lateralization and could be an additional or alternative procedure to Wada and fMRI testing.[76] One disadvantage of NIRS imaging is the inability to measure functional changes in buried cortex such as deep sulci, insula, parasagittal mesial cortex, mesial and basal temporal lobes, infra-tentorial cortex and the basal ganglia.[73]
ASL
ASL is a technique of functional imaging of the brain using MRI. Similar to other functional imaging techniques, ASL uses blood labeling to image functional areas of the brain. In ASL, arterial blood is magnetically labeled using a 180° RF inversion pulse prior to imaging the ROI (imaging slice in MRI). This tracer then flows into the ROI and reduces the MR signal and image intensity at this area. Subtracting this image from the baseline MRI creates the perfusion image, which reflects the amount of blood delivered to each voxel. ASL is non-invasive and repeatable without known harmful effects. Licensing and post-processing issues have prevented ASL from gaining wider clinical use.[77] In epilepsy, ASL has been used to show mesial temporal hypometabolism on the side of seizures, which correlated with PET hypometabolism[78] as well as hippocampal volume loss.[79]
Magnetic Resonance Spectroscopy
MRS can be used to non-invasively measure creatine (Cr), N-acetyl aspartate (NAA), choline (Cho), lactate, myo-inositol and GABA in the brain tissue. Localization of the epileptogenic zone has been attempted using MRS and has been shown to be helpful when conventional MRI does not reveal the lesion.[80] Reduced NAA/Cho and NAA/Cr was found in the lesional temporal lobe in TLE[81] and in the epileptogenic/irritative zone in FLE.[82] Such MRS changes are more likely to be due to cell dysfunction than cell loss as corresponding histological changes are not found in resected specimens.[80,83] A meta-analysis of MRS studies concluded that MRS remains a research tool with clinical potential and found an association of ipsilateral MRS abnormality with a better outcome following epilepsy surgery.[84] MRS changes have also been noted in IGE and are not reviewed here.
Magneto Nano-Particle Imaging
In animal studies using rat epilepsy models, MNP imaging has been evaluated in “proof of principle” studies showing success in crossing the blood brain barrier and in demonstrating uptake by epileptogenic tissue using alpha-methyl-tryptophan and 2-deoxy glucose labeled MNPs. 2-deox glucose MNP imaging has been approved for human use. Future studies may investigate the usefulness of MNP imaging in human epilepsy.[85]
Acknowledgments
Epilepsy Foundation of America.
Footnotes
Source of Support: Epilepsy Foundation of America
Conflict of Interest: Nil
References
- 1.Knowlton RC. The role of FDG-PET, ictal SPECT, and MEG in the epilepsy surgery evaluation. Epilepsy Behav. 2006;8:91–101. doi: 10.1016/j.yebeh.2005.10.015. [DOI] [PubMed] [Google Scholar]
- 2.Juhász CC, Chugani DC, Muzik O, Chugani HT. Positron-emisson tomography in epilepsy. In: Kuzniecky RI, Jackson GD, editors. Magnetic Resonance in Epilepsy: Neuroimaging Techniques. 2nd ed. Oxford: Elsevier; 2005. pp. 395–411. [Google Scholar]
- 3.Lee KK, Salamon N. [18F] fluorodeoxyglucose-positron-emission tomography and MR imaging coregistration for presurgical evaluation of medically refractory epilepsy. AJNR Am J Neuroradiol. 2009;30:1811–6. doi: 10.3174/ajnr.A1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Salamon N, Kung J, Shaw SJ, Koo J, Koh S, Wu JY, et al. FDG-PET/MRI coregistration improves detection of cortical dysplasia in patients with epilepsy. Neurology. 2008;71:1594–601. doi: 10.1212/01.wnl.0000334752.41807.2f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goffin K, Dedeurwaerdere S, Van Laere K, Van Paesschen W. Neuronuclear assessment of patients with epilepsy. Semin Nucl Med. 2008;38:227–39. doi: 10.1053/j.semnuclmed.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 6.Drzezga A, Arnold S, Minoshima S, Noachtar S, Szecsi J, Winkler P, et al. 18F-FDG PET studies in patients with extratemporal and temporal epilepsy: Evaluation of an observer-independent analysis. J Nucl Med. 1999;40:737–46. [PubMed] [Google Scholar]
- 7.Breier JI, Mullani NA, Thomas AB, Wheless JW, Plenger PM, Gould KL, et al. Effects of duration of epilepsy on the uncoupling of metabolism and blood flow in complex partial seizures. Neurology. 1997;48:1047–53. doi: 10.1212/wnl.48.4.1047. [DOI] [PubMed] [Google Scholar]
- 8.Casse R, Rowe CC, Newton M, Berlangieri SU, Scott AM. Positron emission tomography and epilepsy. Mol Imaging Biol. 2002;4:338–51. doi: 10.1016/s1536-1632(02)00071-9. [DOI] [PubMed] [Google Scholar]
- 9.Lee SK, Lee SY, Kim KK, Hong KS, Lee DS, Chung CK. Surgical outcome and prognostic factors of cryptogenic neocortical epilepsy. Ann Neurol. 2005;58:525–32. doi: 10.1002/ana.20569. [DOI] [PubMed] [Google Scholar]
- 10.Takahashi A, Hong SC, Seo DW, Hong SB, Lee M, Suh YL. Frequent association of cortical dysplasia in dysembryoplastic neuroepithelial tumor treated by epilepsy surgery. Surg Neurol. 2005;64:419–27. doi: 10.1016/j.surneu.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 11.Chandra PS, Salamon N, Huang J, Wu JY, Koh S, Vinters HV, et al. FDG-PET/MRI coregistration and diffusion-tensor imaging distinguish epileptogenic tubers and cortex in patients with tuberous sclerosis complex: A preliminary report. Epilepsia. 2006;47:1543–9. doi: 10.1111/j.1528-1167.2006.00627.x. [DOI] [PubMed] [Google Scholar]
- 12.Lee SK, Lee SY, Yun CH, Lee HY, Lee JS, Lee DS. Ictal SPECT in neocortical epilepsies: Clinical usefulness and factors affecting the pattern of hyperperfusion. Neuroradiology. 2006;48:678–84. doi: 10.1007/s00234-006-0106-z. [DOI] [PubMed] [Google Scholar]
- 13.Spanaki MV, Spencer SS, Corsi M, MacMullan J, Seibyl J, Zubal IG. Sensitivity and specificity of quantitative difference SPECT analysis in seizure localization. J Nucl Med. 1999;40:730–6. [PubMed] [Google Scholar]
- 14.Devous MD, Sr, Thisted RA, Morgan GF, Leroy RF, Rowe CC. SPECT brain imaging in epilepsy: A meta-analysis. J Nucl Med. 1998;39:285–93. [PubMed] [Google Scholar]
- 15.Zaknun JJ, Bal C, Maes A, Tepmongkol S, Vazquez S, Dupont P, et al. Comparative analysis of MR imaging, ictal SPECT and EEG in temporal lobe epilepsy: A prospective IAEA multi-center study. Eur J Nucl Med Mol Imaging. 2008;35:107–15. doi: 10.1007/s00259-007-0526-y. [DOI] [PubMed] [Google Scholar]
- 16.Ho SS, Berkovic SF, McKay WJ, Kalnins RM, Bladin PF. Temporal lobe epilepsy subtypes: Differential patterns of cerebral perfusion on ictal SPECT. Epilepsia. 1996;37:788–95. doi: 10.1111/j.1528-1157.1996.tb00653.x. [DOI] [PubMed] [Google Scholar]
- 17.Duncan R, Rahi S, Bernard AM, Biraben A, Devillers A, Lecloirec J, et al. Ictal cerebral blood flow in seizures originating in the posterolateral cortex. J Nucl Med. 1996;37:1946–51. [PubMed] [Google Scholar]
- 18.Weil S, Noachtar S, Arnold S, Yousry TA, Winkler PA, Tatsch K. Ictal ECD-SPECT differentiates between temporal and extratemporal epilepsy: Confirmation by excellent postoperative seizure control. Nucl Med Commun. 2001;22:233–7. doi: 10.1097/00006231-200102000-00016. [DOI] [PubMed] [Google Scholar]
- 19.Kim SK, Lee DS, Lee SK, Kim YK, Kang KW, Chung CK, et al. Diagnostic performance of [18F]FDG-PET and ictal [99mTc]-HMPAO SPECT in occipital lobe epilepsy. Epilepsia. 2001;42:1531–40. doi: 10.1046/j.1528-1157.2001.21901.x. [DOI] [PubMed] [Google Scholar]
- 20.Ebner A, Buschsieweke U, Tuxhorn I, Witte OW, Seitz RJ. Supplementary sensorimotor area seizure and ictal single-photon emission tomography. Adv Neurol. 1996;70:363–8. [PubMed] [Google Scholar]
- 21.O’Brien TJ, So EL, Mullan BP, Hauser MF, Brinkmann BH, Bohnen NI, et al. Subtraction ictal SPECT co-registered to MRI improves clinical usefulness of SPECT in localizing the surgical seizure focus. Neurology. 1998;50:445–54. doi: 10.1212/wnl.50.2.445. [DOI] [PubMed] [Google Scholar]
- 22.Kaiboriboon K, Lowe VJ, Chantarujikapong SI, Hogan RE. The usefulness of subtraction ictal SPECT coregistered to MRI in single- and dual-headed SPECT cameras in partial epilepsy. Epilepsia. 2002;43:408–14. doi: 10.1046/j.1528-1157.2002.21201.x. [DOI] [PubMed] [Google Scholar]
- 23.Rowe CC. Single-photon-emission computed tomography in epilepsy. In: Kuzniecky RI, Jackson GD, editors. Magnetic Resonance in Epilepsy: Neuroimaging Techniques. 2nd ed. Oxford: Elsevier; 2005. pp. 385–94. [Google Scholar]
- 24.Connelly A. MR diffusion and perfusion imaging in epilepsy. In: Kuzniecky RI, Jackson GD, editors. Magnetic Resonance in Epilepsy: Neuroimaging Techniques. 2nd ed. Oxford: Elsevier; 2005. pp. 315–32. [Google Scholar]
- 25.Kanaan RA, Shergill SS, Barker GJ, Catani M, Ng VW, Howard R, et al. Tract-specific anisotropy measurements in diffusion tensor imaging. Psychiatry Res. 2006;146:73–82. doi: 10.1016/j.pscychresns.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 26.Assaf BA, Mohamed FB, Abou-Khaled KJ, Williams JM, Yazeji MS, Haselgrove J, et al. Diffusion tensor imaging of the hippocampal formation in temporal lobe epilepsy. AJNR Am J Neuroradiol. 2003;24:1857–62. [PMC free article] [PubMed] [Google Scholar]
- 27.Salmenpera TM, Symms MR, Boulby PA, Barker GJ, Duncan JS. Postictal diffusion weighted imaging. Epilepsy Res. 2006;70:133–43. doi: 10.1016/j.eplepsyres.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 28.Rugg-Gunn FJ, Eriksson SH, Symms MR, Barker GJ, Duncan JS. Diffusion tensor imaging of cryptogenic and acquired partial epilepsies. Brain. 2001;124:627–36. doi: 10.1093/brain/124.3.627. [DOI] [PubMed] [Google Scholar]
- 29.Radhakrishnan A, James JS, Kesavadas C, Thomas B, Bahuleyan B, Abraham M, et al. Utility of diffusion tensor imaging tractography in decision making for extratemporal resective epilepsy surgery. Epilepsy Res. 2011;97:52–63. doi: 10.1016/j.eplepsyres.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 30.Cohen D. Magnetoencephalography: Evidence of magnetic fields produced by alpha-rhythm currents. Science. 1968;161:784–6. doi: 10.1126/science.161.3843.784. [DOI] [PubMed] [Google Scholar]
- 31.Hari R, Salmelin R. Magnetoencephalography: From SQUIDs to neuroscience. Neuroimage 20th anniversary special edition. Neuroimage. 2012;61:386–96. doi: 10.1016/j.neuroimage.2011.11.074. [DOI] [PubMed] [Google Scholar]
- 32.Hari R. The neuromagnetic method in the study of the human auditory cortex. In: Grandori F, Hoke M, Romani G, editors. Auditory Evoked Magnetic Fields and Potentials Advances in Audiology. Basel: Karger; 1990. pp. 222–82. [Google Scholar]
- 33.Barth DS, Sutherling W, Engel J, Jr, Beatty J. Neuromagnetic localization of epileptiform spike activity in the human brain. Science. 1982;218:891–4. doi: 10.1126/science.6813968. [DOI] [PubMed] [Google Scholar]
- 34.Fischer MJ, Scheler G, Stefan H. Utilization of magnetoencephalography results to obtain favourable outcomes in epilepsy surgery. Brain. 2005;128:153–7. doi: 10.1093/brain/awh333. [DOI] [PubMed] [Google Scholar]
- 35.Wheless JW, Willmore LJ, Breier JI, Kataki M, Smith JR, King DW, et al. A comparison of magnetoencephalography, MRI, and V-EEG in patients evaluated for epilepsy surgery. Epilepsia. 1999;40:931–41. doi: 10.1111/j.1528-1157.1999.tb00800.x. [DOI] [PubMed] [Google Scholar]
- 36.Stefan H, Hummel C, Scheler G, Genow A, Druschky K, Tilz C, et al. Magnetic brain source imaging of focal epileptic activity: A synopsis of 455 cases. Brain. 2003;126:2396–405. doi: 10.1093/brain/awg239. [DOI] [PubMed] [Google Scholar]
- 37.Sutherling WW, Mamelak AN, Thyerlei D, Maleeva T, Minazad Y, Philpott L, et al. Influence of magnetic source imaging for planning intracranial EEG in epilepsy. Neurology. 2008;71:990–6. doi: 10.1212/01.wnl.0000326591.29858.1a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pataraia E, Simos PG, Castillo EM, Billingsley RL, Sarkari S, Wheless JW, et al. Does magnetoencephalography add to scalp video-EEG as a diagnostic tool in epilepsy surgery? Neurology. 2004;62:943–8. doi: 10.1212/01.wnl.0000115122.81621.fe. [DOI] [PubMed] [Google Scholar]
- 39.Pataraia E, Lindinger G, Deecke L, Mayer D, Baumgartner C. Combined MEG/EEG analysis of the interictal spike complex in mesial temporal lobe epilepsy. Neuroimage. 2005;24:607–14. doi: 10.1016/j.neuroimage.2004.09.031. [DOI] [PubMed] [Google Scholar]
- 40.Knowlton RC, Razdan SN, Limdi N, Elgavish RA, Killen J, Blount J, et al. Effect of epilepsy magnetic source imaging on intracranial electrode placement. Ann Neurol. 2009;65:716–23. doi: 10.1002/ana.21660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Eliashiv DS, Elsas SM, Squires K, Fried I, Engel J., Jr Ictal magnetic source imaging as a localizing tool in partial epilepsy. Neurology. 2002;59:1600–10. doi: 10.1212/01.wnl.0000032493.83875.0b. [DOI] [PubMed] [Google Scholar]
- 42.Assaf BA, Karkar KM, Laxer KD, Garcia PA, Austin EJ, Barbaro NM, et al. Ictal magnetoencephalography in temporal and extratemporal lobe epilepsy. Epilepsia. 2003;44:1320–7. doi: 10.1046/j.1528-1157.2003.14303.x. [DOI] [PubMed] [Google Scholar]
- 43.Ogawa S, Lee TM, Kay AR, Tank DW. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc Natl Acad Sci U S A. 1990;87:9868–72. doi: 10.1073/pnas.87.24.9868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Abou-Khalil B. An update on determination of language dominance in screening for epilepsy surgery: The Wada test and newer noninvasive alternatives. Epilepsia. 2007;48:442–55. doi: 10.1111/j.1528-1167.2007.01012.x. [DOI] [PubMed] [Google Scholar]
- 45.Binder JR. Preoperative prediction of verbal episodic memory outcome using FMRI. Neurosurg Clin N Am. 2011;22:219–32. doi: 10.1016/j.nec.2010.12.002. ix. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Richardson M. Current themes in neuroimaging of epilepsy: Brain networks, dynamic phenomena, and clinical relevance. Clin Neurophysiol. 2010;121:1153–75. doi: 10.1016/j.clinph.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 47.Gaillard WD, Balsamo L, Xu B, McKinney C, Papero PH, Weinstein S, et al. fMRI language task panel improves determination of language dominance. Neurology. 2004;63:1403–8. doi: 10.1212/01.wnl.0000141852.65175.a7. [DOI] [PubMed] [Google Scholar]
- 48.Billingsley RL, McAndrews MP, Crawley AP, Mikulis DJ. Functional MRI of phonological and semantic processing in temporal lobe epilepsy. Brain. 2001;124:1218–27. doi: 10.1093/brain/124.6.1218. [DOI] [PubMed] [Google Scholar]
- 49.Berl MM, Balsamo LM, Xu B, Moore EN, Weinstein SL, Conry JA, et al. Seizure focus affects regional language networks assessed by fMRI. Neurology. 2005;65:1604–11. doi: 10.1212/01.wnl.0000184502.06647.28. [DOI] [PubMed] [Google Scholar]
- 50.Weber B, Wellmer J, Schür S, Dinkelacker V, Ruhlmann J, Mormann F, et al. Presurgical language fMRI in patients with drug-resistant epilepsy: Effects of task performance. Epilepsia. 2006;47:880–6. doi: 10.1111/j.1528-1167.2006.00515.x. [DOI] [PubMed] [Google Scholar]
- 51.Dupont S, Samson Y, Van de Moortele PF, Samson S, Poline JB, Hasboun D, et al. Bilateral hemispheric alteration of memory processes in right medial temporal lobe epilepsy. J Neurol Neurosurg Psychiatry. 2002;73:478–85. doi: 10.1136/jnnp.73.5.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Powell HW, Richardson MP, Symms MR, Boulby PA, Thompson PJ, Duncan JS, et al. Reorganization of verbal and nonverbal memory in temporal lobe epilepsy due to unilateral hippocampal sclerosis. Epilepsia. 2007;48:1512–25. doi: 10.1111/j.1528-1167.2007.01053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Detre JA, Maccotta L, King D, Alsop DC, Glosser G, D’Esposito M, et al. Functional MRI lateralization of memory in temporal lobe epilepsy. Neurology. 1998;50:926–32. doi: 10.1212/wnl.50.4.926. [DOI] [PubMed] [Google Scholar]
- 54.Vannest J, Szaflarski JP, Privitera MD, Schefft BK, Holland SK. Medial temporal fMRI activation reflects memory lateralization and memory performance in patients with epilepsy. Epilepsy Behav. 2008;12:410–8. doi: 10.1016/j.yebeh.2007.11.012. [DOI] [PubMed] [Google Scholar]
- 55.Rabin ML, Narayan VM, Kimberg DY, Casasanto DJ, Glosser G, Tracy JI, et al. Functional MRI predicts post-surgical memory following temporal lobectomy. Brain. 2004;127:2286–98. doi: 10.1093/brain/awh281. [DOI] [PubMed] [Google Scholar]
- 56.Richardson MP, Strange BA, Duncan JS, Dolan RJ. Memory fMRI in left hippocampal sclerosis: Optimizing the approach to predicting postsurgical memory. Neurology. 2006;66:699–705. doi: 10.1212/01.wnl.0000201186.07716.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Janszky J, Jokeit H, Kontopoulou K, Mertens M, Ebner A, Pohlmann-Eden B, et al. Functional MRI predicts memory performance after right mesiotemporal epilepsy surgery. Epilepsia. 2005;46:244–50. doi: 10.1111/j.0013-9580.2005.10804.x. [DOI] [PubMed] [Google Scholar]
- 58.Stern JM. Simultaneous electroencephalography and functional magnetic resonance imaging applied to epilepsy. Epilepsy Behav. 2006;8:683–92. doi: 10.1016/j.yebeh.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 59.Pittau F, Dubeau F, Gotman J. Contribution of EEG/fMRI to the definition of the epileptic focus. Neurology. 2012;78:1479–87. doi: 10.1212/WNL.0b013e3182553bf7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.van Houdt PJ, Ossenblok PP, Colon AJ, Boon PA, de Munck JC. A framework to integrate EEG-correlated fMRI and intracerebral recordings. Neuroimage. 2012;60:2042–53. doi: 10.1016/j.neuroimage.2012.02.023. [DOI] [PubMed] [Google Scholar]
- 61.Carmichael DW, Vulliemoz S, Rodionov R, Thornton JS, McEvoy AW, Lemieux L. Simultaneous intracranial EEG-fMRI in humans: Protocol considerations and data quality. Neuroimage. 2012;63:301–9. doi: 10.1016/j.neuroimage.2012.05.056. [DOI] [PubMed] [Google Scholar]
- 62.Biswal B, Yetkin FZ, Haughton VM, Hyde JS. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med. 1995;34:537–41. doi: 10.1002/mrm.1910340409. [DOI] [PubMed] [Google Scholar]
- 63.Spencer SS. Neural networks in human epilepsy: Evidence of and implications for treatment. Epilepsia. 2002;43:219–27. doi: 10.1046/j.1528-1157.2002.26901.x. [DOI] [PubMed] [Google Scholar]
- 64.Lin JJ, Salamon N, Lee AD, Dutton RA, Geaga JA, Hayashi KM, et al. Reduced neocortical thickness and complexity mapped in mesial temporal lobe epilepsy with hippocampal sclerosis. Cereb Cortex. 2007;17:2007–18. doi: 10.1093/cercor/bhl109. [DOI] [PubMed] [Google Scholar]
- 65.Waites AB, Briellmann RS, Saling MM, Abbott DF, Jackson GD. Functional connectivity networks are disrupted in left temporal lobe epilepsy. Ann Neurol. 2006;59:335–43. doi: 10.1002/ana.20733. [DOI] [PubMed] [Google Scholar]
- 66.Haneef Z, Lenartowicz A, Yeh HJ, Engel J, Jr, Stern JM. Effect of lateralized temporal lobe epilepsy on the default mode network. Epilepsy Behav. 2012;25:350–7. doi: 10.1016/j.yebeh.2012.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liao W, Zhang Z, Pan Z, Mantini D, Ding J, Duan X, et al. Default mode network abnormalities in mesial temporal lobe epilepsy: A study combining fMRI and DTI. Hum Brain Mapp. 2011;32:883–95. doi: 10.1002/hbm.21076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Doucet G, Osipowicz K, Sharan A, Sperling MR, Tracy JI. Hippocampal functional connectivity patterns during spatial working memory differ in right versus left temporal lobe epilepsy. Brain Connect. 2013;3:398–406. doi: 10.1089/brain.2013.0158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bettus G, Bartolomei F, Confort-Gouny S, Guedj E, Chauvel P, Cozzone PJ, et al. Role of resting state functional connectivity MRI in presurgical investigation of mesial temporal lobe epilepsy. J Neurol Neurosurg Psychiatry. 2010;81:1147–54. doi: 10.1136/jnnp.2009.191460. [DOI] [PubMed] [Google Scholar]
- 70.Morgan VL, Sonmezturk HH, Gore JC, Abou-Khalil B. Lateralization of temporal lobe epilepsy using resting functional magnetic resonance imaging connectivity of hippocampal networks. Epilepsia. 2012;53:1628–35. doi: 10.1111/j.1528-1167.2012.03590.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jöbsis FF. Noninvasive, infrared monitoring of cerebral and myocardial oxygen sufficiency and circulatory parameters. Science. 1977;198:1264–7. doi: 10.1126/science.929199. [DOI] [PubMed] [Google Scholar]
- 72.Watanabe E, Nagahori Y, Mayanagi Y. Focus diagnosis of epilepsy using near-infrared spectroscopy. Epilepsia. 2002;43(Suppl 9):50–5. doi: 10.1046/j.1528-1157.43.s.9.12.x. [DOI] [PubMed] [Google Scholar]
- 73.Obrig H. NIRS in clinical neurology - A ‘promising’ tool? Neuroimage. 2013 Apr 2; doi: 10.1016/j.neuroimage.2013.03.045. doi:pii: S1053-8119(13)00295-4. [DOI] [PubMed] [Google Scholar]
- 74.Nguyen DK, Tremblay J, Pouliot P, Vannasing P, Florea O, Carmant L, et al. Non-invasive continuous EEG-fNIRS recording of temporal lobe seizures. Epilepsy Res. 2012;99:112–26. doi: 10.1016/j.eplepsyres.2011.10.035. [DOI] [PubMed] [Google Scholar]
- 75.Nguyen DK, Tremblay J, Pouliot P, Vannasing P, Florea O, Carmant L, et al. Noninvasive continuous functional near-infrared spectroscopy combined with electroencephalography recording of frontal lobe seizures. Epilepsia. 2013;54:331–40. doi: 10.1111/epi.12011. [DOI] [PubMed] [Google Scholar]
- 76.Gallagher A, Thériault M, Maclin E, Low K, Gratton G, Fabiani M, et al. Near-infrared spectroscopy as an alternative to the Wada test for language mapping in children, adults and special populations. Epileptic Disord. 2007;9:241–55. doi: 10.1684/epd.2007.0118. [DOI] [PubMed] [Google Scholar]
- 77.Deibler AR, Pollock JM, Kraft RA, Tan H, Burdette JH, Maldjian JA. Arterial spin-labeling in routine clinical practice, part 1: Technique and artifacts. AJNR Am J Neuroradiol. 2008;29:1228–34. doi: 10.3174/ajnr.A1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lim YM, Cho YW, Shamim S, Solomon J, Birn R, Luh WM, et al. Usefulness of pulsed arterial spin labeling MR imaging in mesial temporal lobe epilepsy. Epilepsy Res. 2008;82:183–9. doi: 10.1016/j.eplepsyres.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wolf RL, Alsop DC, Levy-Reis I, Meyer PT, Maldjian JA, Gonzalez-Atavales J, et al. Detection of mesial temporal lobe hypoperfusion in patients with temporal lobe epilepsy by use of arterial spin labeled perfusion MR imaging. AJNR Am J Neuroradiol. 2001;22:1334–41. [PMC free article] [PubMed] [Google Scholar]
- 80.Hajek M, Krsek P, Dezortova M, Marusic P, Zamecnik J, Kyncl M, et al. 1H MR spectroscopy in histopathological subgroups of mesial temporal lobe epilepsy. Eur Radiol. 2009;19:400–8. doi: 10.1007/s00330-008-1156-x. [DOI] [PubMed] [Google Scholar]
- 81.Hammen T, Kerling F, Schwarz M, Stadlbauer A, Ganslandt O, Keck B, et al. Identifying the affected hemisphere by (1)H-MR spectroscopy in patients with temporal lobe epilepsy and no pathological findings in high resolution MRI. Eur J Neurol. 2006;13:482–90. doi: 10.1111/j.1468-1331.2006.01293.x. [DOI] [PubMed] [Google Scholar]
- 82.Guye M, Ranjeva JP, Le Fur Y, Bartolomei F, Confort-Gouny S, Regis J, et al. 1H-MRS imaging in intractable frontal lobe epilepsies characterized by depth electrode recording. Neuroimage. 2005;26:1174–83. doi: 10.1016/j.neuroimage.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 83.Kuzniecky R, Palmer C, Hugg J, Martin R, Sawrie S, Morawetz R, et al. Magnetic resonance spectroscopic imaging in temporal lobe epilepsy: Neuronal dysfunction or cell loss? Arch Neurol. 2001;58:2048–53. doi: 10.1001/archneur.58.12.2048. [DOI] [PubMed] [Google Scholar]
- 84.Willmann O, Wennberg R, May T, Woermann FG, Pohlmann-Eden B. The role of 1H magnetic resonance spectroscopy in pre-operative evaluation for epilepsy surgery. A meta-analysis. Epilepsy Res. 2006;71:149–58. doi: 10.1016/j.eplepsyres.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 85.Akhtari M, Bragin A, Moats R, Frew A, Mandelkern M. Imaging brain neuronal activity using functionalized magnetonanoparticles and MRI. Brain Topogr. 2012;25:374–88. doi: 10.1007/s10548-012-0231-4. [DOI] [PubMed] [Google Scholar]


