Abstract
The association between neurocysticercosis (NCC) and epilepsy is well known and NCC is an important risk factor for epileptic seizures in many Taenia solium-endemic regions of the world. However, while the relationship between NCC and epilepsy is well known, the association between NCC and medically refractory (or surgically remediable epilepsy) has received little attention in the past. Our experience and review of the sparse literature available suggests that NCC is causally related to surgically remediable epilepsy albeit uncommonly so and that association derives its underpinnings from several different scenarios: (1) Medically refractory lesional epilepsy, in which seizures arise from the vicinity of the calcified neurocysticercus lesion (CNL), (2) Medically refractory epilepsy with dual pathology type of relationship between the hippocampal sclerosis (HS) and CNL in which both have been unequivocally demonstrated to give rise to independent seizures and (3) Mesial temporal lobe epilepsy due to HS with a distantly-located CNL, which is in itself not epileptogenic. A major point of controversy revolves around whether or not there exists a causal association between the CNL and HS. We believe that an association exists between NCC and HS and the most important factor influencing this association is the location of the CNL. Furthermore, NCC is a risk factor for medically-refractory epilepsy and that this might account for a considerable proportion of the intractable epilepsy population in endemic regions; the association has been largely ignored owing to the lack of availability of presurgical work-up facilities in these regions. Finally, from a clinical standpoint of presurgical evaluation, patients with CNL and HS should be evaluated on a case by case basis owing to disparate settings underlying the association.
Keywords: Association, medically-refractory epilepsy, mesial temporal sclerosis neurocysticercosis
Introduction
Infestation of the brain by the larval stage (cysticercus) of the flatworm parasite, Taenia solium, also known as neurocysticercosis (NCC) is a major risk factor for seizures and epilepsy in many endemic countries.[1,2,3] Recently, a metaanalysis of clinical presentations of NCC demonstrated that seizures might be the presenting manifestation in 80% of people with NCC.[4] Furthermore, although scarce, follow-up studies after a first seizure due to NCC have shown that seizures might recur in nearly 50%.[5] Other studies however, have shown that although seizures in people with NCC might recur, recurrence is mostly related to withdrawal of anti-epileptic drugs (AEDs).[6,7] Seizure control is excellent as long as adherence to AEDs is maintained.[8] Implicit with these data is the question whether NCC infestation of the brain is associated with poorly-controlled seizures or perhaps even medically refractory epilepsy requiring surgical treatment in the long term.
The treatment of NCC is largely medical and comprises of AEDs, antihelminthic drugs (albendazole and praziquantel) and corticosteroids. Indications for surgical treatment are few and are restricted to the amelioration of cysticercotic hydrocephalus, space-occupying lesions, intracranial hypertension and intraventricular cysticercosis.[9] Surgical treatment for control of epilepsy is rarely indicated.
Here, we review the association of NCC with medically-refractory epilepsy and the indications and approaches to presurgical evaluation and surgical treatment in people with NCC and medically-refractory epilepsy.
Calcific neurocysticercus lesion (CNL), seizures and epilepsy
Pathologic studies have shown that once a cysticercus larva lodges in the brain parenchyma, it passes through a series of evolutionary stages (vesicular, colloidal, granular-nodular, and fibrocalcified stages).[10] Thus the cysticercus appears to pass from a live stage (alive or active stage; corresponding to vesicular stage, i.e. without any breakdown of the blood-brain barrier and little host-inflammatory response in the surrounding brain parenchyma) eventually to a dead (or inactive; corresponding to the fibrocalcified pathologic stage) nodule. The intervening stage has been described as “degenerating,” “transitional,” “acute,” or “encephalitic” stage cysticercus (corresponding to the colloidal and granulonodular stages).[11,12] It is characterized by breakdown of the blood-brain barrier and consequent development of surrounding host-inflammatory response. Probably the inflammatory response and the cellular and molecular events are responsible for seizures during the degenerating stage of NCC.
It has been suggested that seizures occurring in relation to “degenerating” stage of NCC are provoked, whereas those occurring in relation to the “live” or the “inactive” phase are unprovoked.[13] This distinction however needs to be understood in context of recent attempts in revising the operational definitions of epileptic seizures and epilepsy. Whether a degenerating cysticercus presents an enduring tendency to manifest with seizures in which the seizure episodes are provoked in response to an inflammatory reaction to unpredictable and phasic release of antigenic material from the degenerating cysticercus remains a debatable issue. Traditionally the calcified lesions are thought to represent inactive cysticerci, but in a rather unpredictable manner from time to time, the CNLs might demonstrate contrast enhancement and perilesional edema on serial magnetic resonance imaging (MRI) studies suggesting breakdown of blood-brain barrier and inflammation.[14] Perilesional edema appears as bright signal on MRI fluid attenuated inversion recovery (FLAIR) or T2 imaging. This is often accompanied by enhancement around the calcified neurocysticercosis lesion (CNL). A Peruvian follow-up study of CNLs demonstrated that the episodes of perilesional edema might be associated with seizures in as many as 50% of the instances.[15] Thus, it appears that even in calcified lesions, the seizures might be provoked by the phasic release of antigen in an unpredictable manner.
Various population-based studies have shown that CNLs are the most common finding upon imaging studies in people with seizures.[1,2,3] In population-based case control studies, CNLs appear to be significantly common in people with seizures and epilepsy in comparison to healthy controls. However, selected population-based studies in high risk communities have also shown that CNLs might be detected in 9-18% of asymptomatic people in the community.[16,17] Thus, although it appears that the CNLs bear a causal association with epilepsy, which might be completely asymptomatic in a proportion of people among the community. An older, hospital-based study using computerized tomography (CT) scans in people with epilepsy and CNLs found that there was good correlation between electroclinical localization (including semiology based on historical descriptions) and the CT location of the CNLs in roughly one half of the cases.[18] In others correlation between could not be ascertained due to insufficient data, whereas in small proportion, the CNL was clearly co-incidental. Why CNLs present with seizures in some individuals and remain asymptomatic in others is unclear. Most commonly these calcified granulomas are located in the frontal cortex of the brain i.e. in About 50%, the parietal and occipital lobe having equal preponderance about 17.5%, with least common location in the temporal lobe i.e., 15%.[18,19] Therefore though CNLs are common finding in patients with or without epilepsy, the exact incidence and relation with epilepsy is not clear.
Association between NCC and medically-intractable epilepsy
In Sao Paulo, Brazil, a referral centre based cross-sectional study of 512 patients with medically-intractable epilepsy found evidence of CNL in 27%.[20] Similarly, other epilepsy centres from Brazil have observed a high incidence of CNLs in patients being subjected to presurgical work-up or epilepsy surgery for medically-refractory epilepsy.[21,22] These studies emphasize the high frequency of CNLs in patients with medically-intractable epilepsy in endemic regions. The observations as well as anecdotal reports of association between CNLs and mesial temporal lobe epilepsy have fuelled speculations about the association between CNLs and medically-intractable epilepsy. It remains conjectural whether or not these CNLs constitute a risk factor for medically refractory epilepsy. As mentioned earlier, CNLs are a fairly common finding on CT imaging of asymptomatic people in the community from endemic regions. Hence, these might represent a chance finding in people being worked up for epilepsy surgery.
We reviewed reports in published literature, which specifically alluded to the course including presurgical evaluation and outcome (including surgical treatment when undertaken) of medically-refractory epilepsy in the setting of calcified NCC and noted that several different types of situations existed [Figure 1a and b]:
Figure 1.
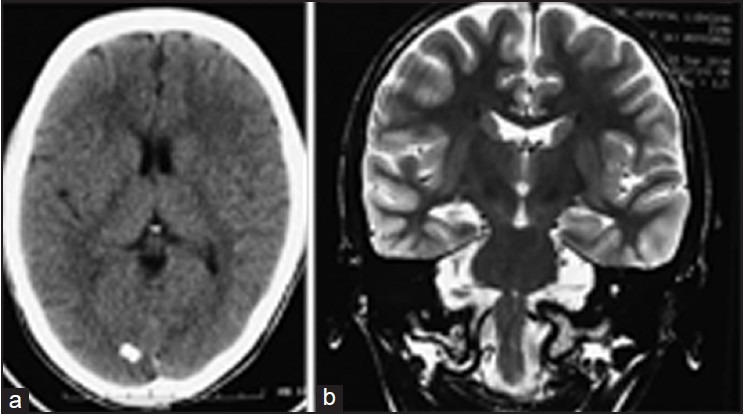
(a) Computed tomographic scan of a patient with medically-refractory mesial temporal lobe epilepsy showing an incidental calcific lesion in the occipital cortex. (b) Coronal MRI perpendicular to the long axis of the hippocampus revealed right sided MTS.
Medically refractory epilepsy with seizures arising from or in the vicinity of a CNL
This is apparently very rare. There were two cases among the cross-sectional study of 512 patients of medically-refractory epilepsy from a clinic in Brazil in whom seizures were demonstrated to arise from the calcified lesion itself based on data from invasive electroencephalogram (EEG) studies in one and otherwise in another.[20] Other cases have been reported anecdotally.[23,24] A recent report described surgical experience in five patients with seizures arising from a CNL, of whom four were rendered seizure free.[25] The CNL was most commonly located in the frontal lobe and was associated with a perilesional high intensity signal. In another report, it was observed that seizures occurring very frequently over short periods of time and the intervening periods were quiescent.[23] In such situation were calcified lesions are multiple, seizures (arising from other calcified lesions) might still recur after a lesionectomy (i.e., removal of a single CNL).[23]
Mesial temporal lobe epilepsy in relation to a calcified lesion within or close to the hippocampus
This appears to be a subtype of the first variety described above but appears to be more frequently reported in published literature. The CNL is located in or in close proximity to the hippocampus. The patient presents with typical mesial temporal lobe seizures. Imaging discloses hippocampal atrophy and sclerosis with a CNL located within or close to the hippocampus. The CNL might or might not enhance and be associated with surrounding oedema. It might be surmised that the hippocampus is implicated in the inflammatory reaction and ensuing gliosis and neuronal degneration surrounding a degenerating NCC lesion in its proximate neighborhood. Although highly selected, two surgical series of patients with mesial temporal lobe epilepsy plus CNL included several in whom the CNL was located within or in close proximity to the hippocampus, such that the resection of mesial temporal structures encompassed the CNL.[24,25,26] Even the cross-sectional study from Brazil comprised of an unspecified number of patients in whom the CNL was located very close to the hippocampus.[20] We surmise that the proximity of the CNL to the hippocampus, the vulnerability of the latter to insults lead to an overrepresentation of this location of the CNL in reported series.
Calcified neurocysticercosis lesions (CNLs) and mesial temporal sclerosis (MTS)
The association between NCC and MTS was initially suggested by anecdotal reports.[27,28,29] Subsequently, larger but mostly cross-sectional studies in cysticercosis endemic regions, found a high frequency of CNLs in populations of patients with medically refractory epilepsy drawn from tertiary care epilepsy facilities.[21,22,30,31,32] The frequency of CNLs was as high as 18-36% of patients undergoing presurgical evaluation for refractory mesial temporal lobe epilepsy and 28% of those undergoing surgical treatment for medically refractory mesial temporal lobe epilepsy. In comparison to the high frequency of CNLs in reports from Brazil, the proportion of CNLs among patients undergoing presurgical evaluation was much lesser (about one percent).[24,25,26] Cross-sectional studies also found a significant association between CNLs and MTS.[20,32]
A comparative evaluation of several clinical and histological parameters of hippocampal sclerosis (HS) in a sample of patients with HS with or without calcified brain cysticerci suggested that the occurrence of NCC lesions in association with HS or vice-versa may be merely coincidental.[21,33] These authors compared consecutive patients with HS with and without calcified brain cysticerci and found no difference in their clinical presentation, neuropsychological characteristics, pathological features as discerned upon histology of resected hippocampii and surgical outcome. They concluded that the calcified brain cysticerci were merely coincidental findings and that they in no way influenced the development of MTS. Other studies have however found that as compared to patients with HS alone, patients with MTS with CNL have a significantly lower incidence of typical febrile seizures, older age at initial precipitating injury (IPI), more frequent clustering of seizures and older age of onset of habitual complex partial seizures.[25,26,30] Patients with CNL with MTS had extratemporal and bitemporal interictal epileptiform discharges on electroencephalography as compared with patients with MTS alone. Patients with CNL and MTS had less frequent secondary generalized seizures and a lower age at the initial precipitating injury as compared with CNL alone. They concluded that probably association of CNL with MTS is not co-incidental as there were different clinical characteristics in this group compared with MTS alone and the location of CNL within the ipsilateral temporal lobe is likely to play a role in development of MTS in these patients.
One of the reasons for the inconsistency between studies described above might be the disparate mechanisms that underlie the association between NCC and MTS. A definitive evidence of a non-causal relationship is provided when patients with NCC and mesial temporal lobe epilepsy become seizure free after antero-mesial temporal lobectomy, without resection of the cysticercus.[21] We agree with the authors of this study that in a proportion of cases, NCC with HS may coexist purely by chance. HS is common substrate underlying epilepsy. Calcified NCC is a frequently noted upon imaging studies of both symptomatic and asymptomatic individuals in T. solium endemic regions.[2] Hence, it may be likely that the two conditions may occur co-incidentally in such regions. On the other hand, if there was a causal association between NCC and MTS it could be explained by a number of mechanisms. One hypothesis is the kindling model of epilepsy, wherein application of repeated subthreshold stimuli lead to the development of seizures, may also be relevant to the occurrence of temporal lobe epilepsy in association with NCC. Limbic structures are particularly susceptible to kindling and widespread connectivity of these structures with neocortical regions which is the site for NCC lesions may be responsible for temporal lobe seizures in patients with NCC. Another hypothesis for this association is that the occurrence of inflammation in the vicinity of the hippocampus results in clinical or electrographic seizures within that hippocampus leading ultimately to the development of an epileptic focus. Experimental studies have suggested that the injection of early, Taenia crassiceps-granuloma material in to the hippocampus of mice is highly epileptogenic.[34] These experiments provide support for a direct involvement of the hippocampus by brain inflammatory response to degenerating cysticerci.
From a clinical perspective, the association between CNLs and MTS can be approached in two different ways. An earlier study reported Engel's Class I outcome with standard antero-mesial temporal lobe resections alone in over 80% of 32 cases of MTS associated with one or more CNLs on imaging studies.[21] These rates are comparable with seizure freedom rates following surgery for isolated MTS. Contrary to this, in a series of 18 patients with MTS and CNLs of whom nine were operated, only one of the four patients who had a standard antero-mesial temporal lobe resection alone reported seizure freedom post-operatively, while all three patients who had an anteromesial temporal resection in addition to a lesionectomy (including two cases in which the CNL was located within the hippocampus) reported seizure freedom in the post-operative period.[25] In the same study, two patients with MTS and CNLs located in the parietal and occipital regions respectively had independent seizure onsets from both the hippocampus and the CNL, as a result of which both temporal lobe resection and lesionectomy were undertaken (cf dual pathology). Both patients were seizure free post-operatively. The latter situation is perhaps similar to previously reported dual pathology with other other conditions such as tumours and cortical developmental malformations, where surgical approaches targeting both the involved hippocampus and the lesion alone yield acceptable seizure freedom rates in the post-operative periods.[35]
The differences in the outcome reported in the two studies could relate to differences in case selection as well as presurgical evaluation and surgical strategies. However, these differences also bring forth the disparate mechanisms that underlie the association between NCC and MTS. Accordingly, the presence of the CNL might or might not influence surgical outcome and hence unified approach can be recommended to patients with medically-refractory mesial temporal lobe epilepsy with MTS who also present with CNLs on their imaging studies. Clearly, more studies with larger prospectively collected samples of patients are required in order to clarify the association between NCC and MTS.
Footnotes
Source of Support: Nil
Conflict of Interest: Nil
References
- 1.Montano SM, Villaran MV, Ylquimiche L, Figueroa JJ, Rodriguez S, Bautista CT, et al. Neurocysticercosis: Association between seizures, serology, and brain CT in rural Peru. Neurology. 2005;65:229–33. doi: 10.1212/01.wnl.0000168828.83461.09. [DOI] [PubMed] [Google Scholar]
- 2.Rajshekhar V, Raghava MV, Prabhakaran V, Oommen A, Muliyil J. Active epilepsy as an index of burden of neurocysticercosis in Vellore district, India. Neurology. 2006;67:2135–9. doi: 10.1212/01.wnl.0000249113.11824.64. [DOI] [PubMed] [Google Scholar]
- 3.Carabin H, Ndimubanzi PC, Budke CM, Nguyen H, Qian Y, Cowan LD, et al. Clinical manifestations associated with neurocysticercosis: A systematic review. PLoS Negl Trop Dis. 2011;5:e1152. doi: 10.1371/journal.pntd.0001152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carpio A, Hauser WA. Prognosis for seizure recurrence in patients with newly diagnosed neurocysticercosis. Neurology. 2002;59:1730–4. doi: 10.1212/01.wnl.0000036320.69823.ea. [DOI] [PubMed] [Google Scholar]
- 5.Del Brutto OH, Santibañez R, Noboa CA, Aguirre R, Díaz E, Alarcón TA. Epilepsy due to neurocysticercosis: Analysis of 203 patients. Neurology. 1992;42:389–92. doi: 10.1212/wnl.42.2.389. [DOI] [PubMed] [Google Scholar]
- 6.Singh G, Rajshekhar V, Murthy JM, Prabhakar S, Modi M, Khandelwal N, et al. A diagnostic and therapeutic scheme for a solitary cysticercus granuloma. Neurology. 2010;75:2236–45. doi: 10.1212/WNL.0b013e31820202dc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Singh G, Burneo JG, Sander JW. From seizures to epilepsy and its substrates: Neurocysticercosis. Epilepsia. 2013;54:783–92. doi: 10.1111/epi.12159. [DOI] [PubMed] [Google Scholar]
- 8.Scharf D. Neurocysticercosis. Two hundred thirty-eight cases from a California hospital. Arch Neurol. 1988;45:777–80. doi: 10.1001/archneur.1988.00520310087022. [DOI] [PubMed] [Google Scholar]
- 9.Escobar A. The pathology of neurocysticercosis, in Cysticercosis of the central nervous system, E. R.-C. In: Palacios J, Taveras JM, editors. Springfield, III: Charles C Thomas Publisher; 1983. pp. 27–54. [Google Scholar]
- 10.Carpio A, Placencia M, Santillán F, Escobar A. A proposal for classification of neurocysticercosis. Can J Neurol Sci. 1994;21:43–7. doi: 10.1017/s0317167100048757. [DOI] [PubMed] [Google Scholar]
- 11.Zee CS, Segall HD, Boswell W, Ahmadi J, Nelson M, Colletti P. MR imaging of neurocysticercosis. J Comput Assist Tomogr. 1988;12:927–34. doi: 10.1097/00004728-198811000-00004. [DOI] [PubMed] [Google Scholar]
- 12.Carpio A, Escobar A, Hauser WA. Cysticercosis and epilepsy: A critical review. Epilepsia. 1998;39:1025–40. doi: 10.1111/j.1528-1157.1998.tb01287.x. [DOI] [PubMed] [Google Scholar]
- 13.Nash TE, Del Brutto OH, Butman JA, Corona T, Delgado-Escueta A, Duron RM, et al. Calcific neurocysticercosis and epileptogenesis. Neurology. 2004;62:1934–8. doi: 10.1212/01.wnl.0000129481.12067.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nash TE, Pretell EJ, Lescano AG, Bustos JA, Gilman RH, Gonzalez AE, et al. Perilesional brain oedema and seizure activity in patients with calcified neurocysticercosis: A prospective cohort and nested case-control study. Lancet Neurol. 2008;7:1099–105. doi: 10.1016/S1474-4422(08)70243-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fleury A, Gomez T, Alvarez I, Meza D, Huerta M, Chavarria A, et al. High prevalence of calcified silent neurocysticercosis in a rural village of Mexico. Neuroepidemiology. 2003;22:139–45. doi: 10.1159/000068748. [DOI] [PubMed] [Google Scholar]
- 16.Prasad KN, Verma A, Srivastava S, Gupta RK, Pandey CM, Paliwal VK. An epidemiological study of asymptomatic neurocysticercosis in a pig farming community in northern India. Trans R Soc Trop Med Hyg. 2011;105:531–6. doi: 10.1016/j.trstmh.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 17.Singh G, Sachdev MS, Tirath A, Gupta AK, Avasthi G. Focal cortical-subcortical calcifications (FCSCs) and epilepsy in the Indian subcontinent. Epilepsia. 2000;41:718–26. doi: 10.1111/j.1528-1157.2000.tb00234.x. [DOI] [PubMed] [Google Scholar]
- 18.Murthy JM, Reddy VS. Clinical characteristics, seizure spread patterns and prognosis of seizures associated with a single small cerebral calcific CT lesion. Seizure. 1998;7:153–7. doi: 10.1016/s1059-1311(98)80072-1. [DOI] [PubMed] [Google Scholar]
- 19.Velasco TR, Zanello PA, Dalmagro CL, Araújo D, Jr, Santos AC, Bianchin MM, et al. Calcified cysticercotic lesions and intractable epilepsy: A cross sectional study of 512 patients. J Neurol Neurosurg Psychiatry. 2006;77:485–8. doi: 10.1136/jnnp.2005.078675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leite JP, Terra-Bustamante VC, Fernandes RM, Santos AC, Chimelli L, Sakamoto AC, et al. Calcified neurocysticercotic lesions and postsurgery seizure control in temporal lobe epilepsy. Neurology. 2000;55:1485–91. doi: 10.1212/wnl.55.10.1485. [DOI] [PubMed] [Google Scholar]
- 21.da Gama CN, Kobayashi E, Li LM, Cendes F. Hippocampal atrophy and neurocysticercosis calcifications. Seizure. 2005;14:85–8. doi: 10.1016/j.seizure.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 22.Ooi WW, Wijemanne S, Thomas CB, Quezado M, Brown CR, Nash TE. Short report: A calcified Taenia solium granuloma associated with recurrent perilesional edema causing refractory seizures: Histopathological features. Am J Trop Med Hyg. 2011;85:460–3. doi: 10.4269/ajtmh.2011.11-0221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chandra PS, Bal C, Garg A, Gaikwad S, Prasad K, Sharma BS, et al. Surgery for medically intractable epilepsy due to postinfectious etiologies. Epilepsia. 2010;51:1097–100. doi: 10.1111/j.1528-1167.2010.02538.x. [DOI] [PubMed] [Google Scholar]
- 24.Rathore C, Thomas B, Kesavadas C, Abraham M, Radhakrishnan K. Calcified neurocysticercosis lesions and antiepileptic drug-resistant epilepsy: A surgically remediable syndrome? Epilepsia. 2013;54:1815–22. doi: 10.1111/epi.12349. [DOI] [PubMed] [Google Scholar]
- 25.Rathore C, Thomas B, Kesavadas C, Radhakrishnan K. Calcified neurocysticercosis lesions and hippocampal sclerosis: Potential dual pathology? Epilepsia. 2012;53:e60–2. doi: 10.1111/j.1528-1167.2011.03386.x. [DOI] [PubMed] [Google Scholar]
- 26.Wichert-Ana L, Velasco TR, Terra-Bustamante VC, Alexandre V, Jr, Walz R, Bianchin MM, et al. Surgical treatment for mesial temporal lobe epilepsy in the presence of massive calcified neurocysticercosis. Arch Neurol. 2004;61:1117–9. doi: 10.1001/archneur.61.7.1117. [DOI] [PubMed] [Google Scholar]
- 27.Chung CK, Lee SK, Chi JG. Temporal lobe epilepsy caused by intrahippocampal calcified cysticercus: A case report. J Korean Med Sci. 1998;13:445–8. doi: 10.3346/jkms.1998.13.4.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Singla M, Singh P, Kaushal S, Bansal R, Singh G. Hippocampal sclerosis in association with neurocysticercosis. Epileptic Disord. 2007;9:292–9. doi: 10.1684/epd.2007.0122. [DOI] [PubMed] [Google Scholar]
- 29.Bianchin MM, et al. Clinical and electrophysiological differences between mesial temporal lobe epilepsy and mesial temporal lobe epilepsy plus neurocysticercosis. Epilepsia. 2006;47:244–5. [Google Scholar]
- 30.Bianchin MM, Velasco TR, Takayanagui OM, Sakamoto AC. Neurocysticercosis, mesial temporal lobe epilepsy, and hippocampal sclerosis: An association largely ignored. Lancet Neurol. 2006;5:20–1. doi: 10.1016/S1474-4422(05)70269-6. [DOI] [PubMed] [Google Scholar]
- 31.Bianchin MM, Velasco TR, Wichert-Ana L, Takayanagui OM, Leite JP, Sakamoto AC. How frequent is the association of neurocysticercosis and mesial temporal lobe epilepsy with hippocampal sclerosis? Epilepsia. 2010;51:2359–60. doi: 10.1111/j.1528-1167.2010.02735.x. [DOI] [PubMed] [Google Scholar]
- 32.Terra-Bustamante VC, Coimbra ER, Rezek KO, Escorsi-Rosset SR, Guarnieri R, Dalmagro CL, et al. Cognitive performance of patients with mesial temporal lobe epilepsy and incidental calcified neurocysticercosis. J Neurol Neurosurg Psychiatry. 2005;76:1080–3. doi: 10.1136/jnnp.2004.048934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stringer JL, Marks LM, White AC, Jr, Robinson P. Epileptogenic activity of granulomas associated with murine cysticercosis. Exp Neurol. 2003;183:532–6. doi: 10.1016/s0014-4886(03)00179-1. [DOI] [PubMed] [Google Scholar]
- 34.Li LM, Cendes F, Watson C, Andermann F, Fish DR, Dubeau F, et al. Surgical treatment of patients with single and dual pathology: Relevance of lesion and of hippocampal atrophy to seizure outcome. Neurology. 1997;48:437–44. doi: 10.1212/wnl.48.2.437. [DOI] [PubMed] [Google Scholar]
- 35.Cendes F, Cook MJ, Watson C, Andermann F, Fish DR, Shorvon SD, et al. Frequency and characteristics of dual pathology in patients with lesional epilepsy. Neurology. 1995;45:2058–64. doi: 10.1212/wnl.45.11.2058. [DOI] [PubMed] [Google Scholar]


