Abstract
Magnetoencephalography (MEG) is the measurement of the magnetic field generated by the electrical activity of neurons. It is usually combined with a magnetic resonance imaging to get what is called magnetic source imaging. The technology that has helped record these minute magnetic fields is super-conducting quantum interference detector which is like a highly sensitive magnetic field meter. To attenuate the external magnetic noise the MEG is housed inside a magnetically shielded room. The actual sensors recording magnetic fields are magnetometers and/or gradiometers. MEG fields pass through the head without any distortion. This is a significant advantage of MEG over electroencephalography. MEG provides a high spatial and temporal resolution. The recording and identification information should be according to the American Clinical Magnetoencephalography Society guidelines published in 2011. MEG currently has two approved indications in the United States, one is for pre-operative brain mapping and the other is for use in epilepsy surgery. MEG studies have shown functional brain tissue inside brain tumors.
Keywords: Cortical mapping, epilepsy, magnetic source imaging, magnetoencephalography
Introduction
Magnetoencephalography (MEG) measures the magnetic fields generated by electric currents in the brain. The magnetic field measurements are in the range of femto-tesla to pico-tesla. MEG provides a very accurate resolution of the timing of neuronal activity.[1] This is a non-invasive test.
MEG is a direct measure of brain function and it has a very high temporal resolution. It also has a good spatial resolution. MEG is usually combined with a magnetic resonance imaging (MRI) Brain to get a good structural perspective. This combination of MEG and MRI is called magnetic source imaging (MSI). Currently, its approved clinical uses in the United States of America are in Epilepsy Surgery and for pre-operative brain mapping. Most clinical MEG's in the United States are associated with Epilepsy Centers.[1]
Basic Physiology
MEG records magnetic fields generated by electric currents in the brain. An electric current is always associated with a magnetic field perpendicular to its direction as per the right-hand rule. The magnetic permeability of biological tissues is almost the same as that of empty space and so the magnetic field is not distorted by scalp or skull. However, the magnetic fields diminish as 1/r3 with the distance of ‘r’.
When neurons are activated synchronously they generate electric currents and thus magnetic fields, which are then recorded by MEG outside the head. Like electroencephalography (EEG), the source of the magnetic fields is dendritic current of pyramidal neurons that fire synchronously and in parallel. Axonal and synaptic currents and their magnetic fields cancel out.[2]
The usual amplitude of magnetic fields created by the brain are extremely small, they do not exceed a few hundred femto tesla (10−15 T). Compared with this the Earth's magnetic field is between 10−4 and 10−5 T and an MRI is usually 1.5-3 T.
Recording
To record these minute magnetic fields presents a unique engineering dilemma. There are two basic issues, one is to record these small magnetic fields and the other is to shield out the earth's relatively stronger magnetic fields. The technology that has helped record these minute magnetic fields is super-conducting quantum interference detector which is like a highly sensitive magnetic field meter. To maintain super conductors one needs to provide an extremely cold environment, which is achieved by using liquid helium which is only 3° about absolute zero (−452°F or −270°C). The Figure 2 gives a schematic representation of MEG.
Figure 2.
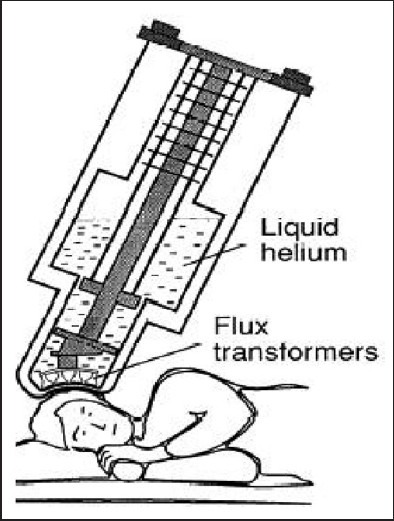
Magnetoencephalography equipment (Hamalainen et al. Rev Mod Phys 1993)
To attenuate the external magnetic noise the MEG is housed inside a magnetically shielded room.
The actual sensors recording magnetic fields are magnetometers and/or gradiometers. There are 2 types of gradiometers — axial and planar. Magnetometers provide the best signal and are most sensitive to deep brain sources but are also more sensitive to competing magnetic noise. Gradiometers are better at noise reduction.
MEG Basics
MEG does not detect radial dipoles but tangential dipoles are seen on MEG, this is because the corresponding magnetic field of the radial dipole remains within the cranial cavity and thus cannot be detected by sensors on the outside.[3]
The MEG has a basic source identification problem. Determining the active site in the brain from magnetic fields recorded outside the head presents the “inverse problem”. This problem has no unique solution. Thus, source localization represents approximations based on certain assumptions that have to be made. A commonly used assumption is presuming simplified models of neuronal generators like equivalent current dipole (ECD) [Figures 1–3].[4]
Figure 1.
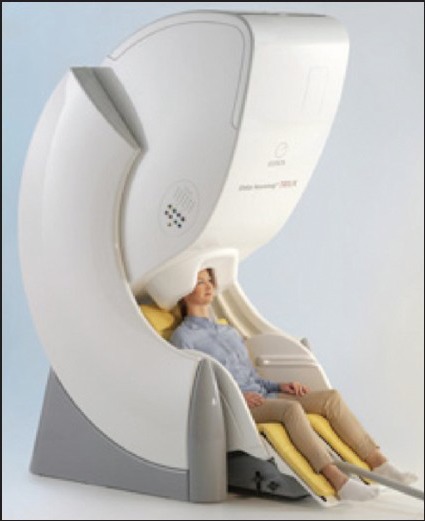
Magnetoencephalography
Figure 3.
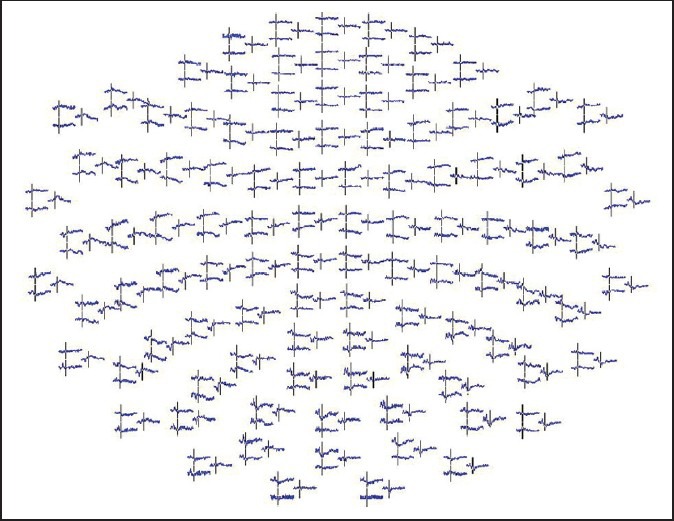
Magnetoencephalography recording
MEG fields pass through the head without any distortion. This is a significant advantage of MEG over EEG. MEG provides a high spatial and temporal resolution [Figure 4].
Figure 4a and b.
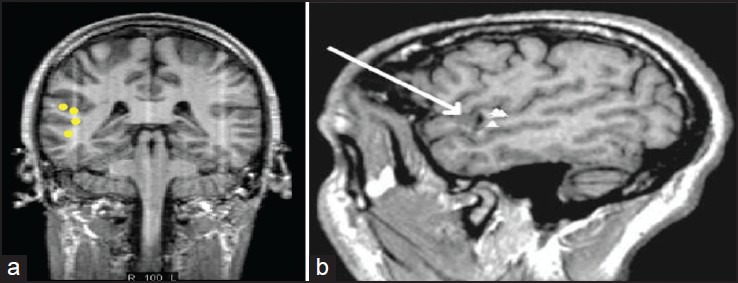
Magnetic source imaging – Combining magnetoencephalography with magnetic resonance imaging
The decay of magnetic fields as a function of distance is more pronounced than for electric fields. MEG is therefore more sensitive to superficial cortical activity, which should be useful for the study of neocortical epilepsy.
MEG data is always co-registered with MRI Brain to provide MSI. This provides a unique combination of good anatomical detail with neurophysiologic data.
MEG versus EEG
It is important to understand the differences between neurophysiological tools we know from a novel neurophysiological tool we are trying to learn about. The first obvious difference is that EEG records the electrical activity and MEG records magnetic activity of the brain.
In EEG the electrodes are placed on the scalp. MEG is performed using a dewar that contains multiple sensor coils, which do not touch the patient's head. MEG primarily detects the magnetic fields induced by intracellular currents, whereas scalp EEG is sensitive to electrical fields generated by extracellular currents.
Although MEG is more sensitive in detecting currents that are tangential to the surface of the scalp, EEG is sensitive to tangential and radial neuronal activities. MEG requires 3-4 cm2 of synchronized cortical epileptic activity to detect an epileptic spike, whereas at least 6-20 cm2 of synchronized cortical area is needed for scalp EEG spike detection. Magnetic fields are not distorted by the tissue conductivity of the scalp, skull, cerebrospinal fluid (CSF) and brain; in contrast, electrical fields may be distorted by the skull and CSF. MEG provides better spatial resolution of source localization (2-3 mm) than EEG (7-10 mm).
A recent study comparing the signal-to-noise ratios (SNRs) of hypothetical sources in different areas of the brain indicated an advantage for MEG over EEG in the study of neocortical epilepsies, although the estimated SNRs were comparable in the temporal lobe. MEG data can be analyzed by source modeling techniques, which allow for localization in three dimensions. Although source modeling techniques are available for scalp EEG, MEG source modeling is generally simpler and more accurate for technical reasons. MEG is certainly more expensive than an EEG. Here in the United States MEG costs a few thousand dollars.
Clinical Applications
MEG currently has two approved indications in the United States, one is for pre-operative Brain Mapping and the other is for use in epilepsy surgery.
MEG in epilepsy
In the surgical treatment of epilepsy, the most critical piece of information is the location of the seizure focus and MEG can substantially contribute to this localization. MEG also helps to identify the eloquent cortex and this helps determine the area that can be safely resected during epilepsy surgery.
MEG is primarily used as a noninvasive device that detects interictal epileptic discharges like spikes to determine the epileptic focus by using appropriate source localization techniques.
EEG is almost always simultaneously recorded with MEG. And so both EEG and MEG data are available for analysis in all spontaneous MEG recordings done for Epilepsy.
Recording and analysis of MEG for epilepsy (American Clinical Magnetoencephalography Society [ACMEGS] Clinical Practice Guidelines - 2011)
The following are considered “minimum standards” for the routine clinical recording and analysis of spontaneous MEG and EEG in all age-groups.
Equipment and recording
Magnetically shielded room that conforms to the current operational and safety standards should be used. The entire magnetically shielded room, including adjustable lighting system and audio-visual communication system, has to be inspected regularly to ensure proper operation. Patient bed and/or chair must be nonmagnetic and appropriate for use with the MEG system. Food and Drug Administration — approved whole head system is necessary to record simultaneously from the entire cerebrum. All components of the MEG system, both hardware and software, must be Food and Drug Administration approved. Simultaneous recording of MEG and EEG is most beneficial for a clinical epilepsy study.
Because exact information about the relative position of the head with respect to the sensor array is necessary for source localization, a reliable digitization system must be used to locate the head position. Most often, this is accomplished by determining the position of several “head position indicator” coils while the patient is in the array. Transient electrical signals within the head position indicator coils on the head create magnetic sources that can be localized by MEG, thus providing the position of the head in sensor space. Before recording, the positions of at least three external fiducial points (usually nasion, left preauricular point and right preauricular point), head position indicator coils and/or other anatomic landmarks for creating the Cartesian coordinate that allows co-registration of MEG data with MRI for source localization.
Head position measurement is recommended before and after each recording segment (block), or continuously where available, to quantify any head movement and to determine the quality of the data recorded in the segment.
In preparation for an MEG-EEG study, a standardized digitization procedure commensurate with the MEG system must be followed to ensure accurate head localization in the sensor space, continuous head position tracking where available and accurate co registration of MEG data with subject's MRI for source localization.
Sampling frequency of the MEG system must be set appropriately to ensure adequate acquisition of the signals of interest. The frequency of a low-pass filter of one half or less of the sampling frequency should be applied to the data before digital conversion to avoid aliasing. A high-pass filter is usually required to minimize effects of large low-frequency signals, but unlike EEG, spontaneous MEG recordings can be performed without a high-pass filter (“direct current coupled”).
The ongoing waveforms of a sampling of MEG and EEG channels should be displayed in real time to monitor the quality of the recording. Displays of electrooculogram, electrocardiography (ECG) and electromyogram may also be useful. All recorded data must include the same synchronized time signal irrespective of the applied method of synchronization. Appropriate sensor tuning and overall quality control procedures must be performed regularly according to operational instructions of the particular MEG and EEG systems. Confirmation of accurate system performance using a phantom should be performed as often as feasible, preferably weekly.
Minimum duration of spontaneous MEG-EEG recording sessions should be 30 min and preferably, this will include both wakefulness and sleep. A longer recording is recommended if interictal epileptiform discharges (IIED's) are insufficiently frequent to permit a reasonable clinical interpretation. A repeated study with longer recording times, additional sleep deprivation and antiepileptic drug manipulation coordinated with the patient's epileptologist, sedation, or other clinically acceptable means for increasing diagnostic yield may be necessary.
Recording during sleep should be a standard part of a spontaneous MEG-EEG study for epilepsy because of the activating effect of sleep on IIEDs. Hyperventilation is a standard activating procedure for clinical EEG for epilepsy studies and it may be implemented during MEG-EEG study. However, the MEG can be contaminated by large artifacts caused by associated head movements. Thus, if hyperventilation is used, the MEG data immediately after hyperventilation may be most useful.
Analysis of data
Waveforms of MEG and EEG (“raw data,” original data as collected) for the entire recording should be visually examined, following the principles established for clinical EEG. Visual inspection of time series is an obligatory initial step in the analysis of spontaneous MEG-EEG data that is aimed at the (1) identification of artifacts, (2) evaluation of overall data quality and integrity and (3) identification of background rhythms, asymmetries and other background characteristics and IIEDs, including morphologic and temporal characteristics, in both MEG and EEG. These findings should be evaluated and reported systematically for each study.
Use of filters is usually necessary to eliminate irrelevant biologic signals and the inherent noise of MEG system and environment the particular selection of a high-pass, low-pass, band-pass and/or notch filters depends on the analysis to be performed and the characteristics of the MEG system used. This selection requires an appropriate conceptual understanding of the filtering method and practical experience in their use. Most current analytical routines used for the analysis of spontaneous MEG-EEG data for localization of epileptic foci benefit from using the high-pass filter of 1-4 Hz and low-pass filter of 40-70 Hz.
Source analysis is used for estimating the location of the cortical generators of neuromagnetic activity of interest. For epilepsy studies, identified IIEDs are most often used for this purpose. However, source analysis of slow-wave activity or fast activity is currently under investigation and may become standard practice in the future, if proven useful. If a seizure is recorded during a study, the onset of the seizure may be localized using methods for spike analysis if the potential differences between interictal and ictal discharge generation are taken into consideration during source localization.
Source localization by the ECD modeling should be performed on all well-defined IIEDs; this includes spikes (20-70 ms) and sharp waves (70-200 ms). The clinical significance of both types of IIEDs in epileptic focus localization is equivalent the morphology, localization and temporal characteristics of visually identified IIEDs should be reported in a standard fashion. Although not routinely used by most clinical magnetoencephalographers, principal component analysis and independent component analysis can be useful to estimate the reasonable number of sources in the signal above background noise. If the background noise level has also been estimated, independent component analysis may be useful to identify and remove certain artifacts, such as ECG or eye movement artifact.
Several time points in the IIED waveform can be selected for source analysis. These include the spike peak or a point on the rising phase of the spike. Selecting the peak of a large-amplitude spike will guarantee a high SNR that minimizes the calculation errors; however, the field at this latency may not represent the spike origin. If an assessment of sequential field maps over a single spike phase shows no rotation, one can assume a stable source and model only at the spike peak for greatest SNR. If field rotation is evident, it is useful to model time points before the peak to seek an earlier source and throughout the spike time course to identify possible propagation. Note that modeling time points off the peak will mean lesser SNR and a larger confidence volume. This requires a more careful interpretation of the results. Cortical generators after the spike peak, such as the after coming wave, are typically complex and not well modeled by an ECD.
The Single ECD model is currently the most common and accepted method for modeling sources of IIEDs for the purpose of the epileptic focus localization.
MRI brain and co-registration
A volumetric scan with recognized high neuroanatomic fidelity (such as T1-weighted gradient echo or multi-echo flash with two different flip angles) is required. Voxel dimensions should be isotropic (1 mm is optimal) with scan and reconstruction matrix of at least 256 × 256 (higher resolution is not necessary) to allow good overlays. The field of view should be skin to skin, that is, include the face, ears and entire scalp (sagittal orientation of slice acquisition is best) such that an accurate identification of superficial fiducial points is possible for MEG to MRI co-registration.
Referring physicians should receive MEG results in the form of EEG and MEG tracings of representative spikes or sharp waves used for source analysis in addition to MSI that contain one dipole source localization and its moment per spike co registered with the patient's brain MRI. Methods of co-registration depend on MEG system and additional software used for source localization. Any approved, reliable, accurate and established method of co registration may be implemented.
Reporting
For a spontaneous MEG study done as a pre-surgical evaluation, the use of the phrase, “clinical correlation necessary” is considered insufficient. Additional, clinically relevant information must be provided because source localizations may guide intracranial electrode placement. Interictal discharges and when available ictal rhythms, should be described as focal, multifocal, or generalized at a minimum. Source lateralization and localization, in terms of lobar or sub lobar area, should be summarized. Any propagation of interictal or ictal activity should also be described. In addition, this part of the report should state whether the MEG-EEG source localization is consistent with the presumed focus based on the previous EEG findings and the patient's seizure semiology. If disparate, plausible reason(s) for the difference should be provided. Furthermore, the anatomic relationship of MEG-EEG source estimates to any MRI lesion should be described.
To determine the usefulness of MEG in epilepsy surgery we need to also review a few landmark studies done to explore this subject.
Knowlton et al.[5] (Ann Neurol 2008;64:35-41) studied the value of MSI to predict the seizure-free outcome following epilepsy surgery in patients who require intracranial electroencephalography (ICEEG). This was a NIH funded prospective observation study of epilepsy surgery candidates not sufficiently localized with scalp EEG and MRI. Of 160 patients enrolled 62 completed ICEEG and subsequent surgical resection. Nearly 61% resulted in an Engel I seizure-free outcome at a minimum of 1-year follow-up (mean-3.4 years). The results showed MSI sensitivity for a conclusively localized study was 55% with a positive predictive value of 78%. Eliminating non-diagnostic MSI cases (no spikes captured during recording) yielded a corrected negative predictive value of 64%. MSI has a clinical value in predicting seizure-free surgical outcome in epilepsy surgery candidates who typically require ICEEG.
A study by Knowlton et al.[6] explored the predictive and prognostic value of MSI as compared with ICEEG localization in epilepsy surgery. This work was part of a cohort study of epilepsy surgery candidates not sufficiently localized with non-invasive studies. Of 160 patients enrolled over 4 years, 77 completed ICEEG seizure monitoring. Seizures were not captured in five patients. Of the 72 diagnostic ICEEG studies, seizure localization results were 74% localized, 10% multifocal and 17% non-localized respectively. Nearly 61% were localized to neocortical regions. Depending on patient subgroup pairs, sensitivity ranged from 58% to 64% with MSI and specificity ranges were 79% to 88% with MSI respectively. Conclusively positive MSI has a high predictive value for seizures localized with ICEEG.
In a study by Stefan et al.[7] showed that the average sensitivity of MEG for specific electrical activity is 70% in a series of 455 patients undergoing a pre-surgical epilepsy evaluation. Information crucial for final decision making was obtained in 10% of patients.
Sutherling et al.[8] studied where MSI changed surgical decisions. This was a prospective, blinded, crossover-controlled, single-treatment, observational case series. 69 sequential patients diagnosed with partial epilepsy of suspected neocortical origin had video-EEG and imaging. All met criteria for ICEEG. At a surgical conference, a decision was made before and after presentation of MSI. Cases where MSI altered the decision were noted. Results showed that MSI gave non-redundant information in 23 patients (33%). MSI added ICEEG electrodes in 9 (13%) and changed the surgical decision in another 14 (20%). Based on the MSI, 16 patients (23%) were scheduled for different ICEEG coverage. 28 have gone to ICEEG, 29 to resection and 14 to vagal nerve stimulation, including 17 where MSI changed the decision. Additional electrodes in 4 patients covered the correct : Hemisphere in 3, lobe in 3 and sublobar ictal onset zone in 1. MSI avoided contralateral electrodes in 2, who both localized on ICEEG. MSI added information to ICEEG in 1. MSI provided non-redundant information in 33% of patients.
One limitation of MEG is demonstrated when it fails to detect an IIED during the recording. We looked at the yield of MEG in mesial temporal lobe epilepsy; this data was presented at the American Epilepsy Society meeting in 2010. This was a retrospective study[9] of intractable mesial temporal lobe epilepsy patients who underwent MEG/MSI for seizure focus localization. MEG can detect mesial temporal spikes in about 50% of cases. In these cases, it localizes the seizure focus accurately.
MEG in Pre-operative Mapping of Eloquent Cortex
MEG provides an accurate means of functional mapping of eloquent cortex; this includes identification of motor, sensory, visual and auditory areas. Language cortex mapping can also be attempted. MEG studies have shown functional brain tissue inside brain tumors.[10]
The recording and identification information should be according to the ACMEGS guidelines published in 2011.[11,12]
The accuracy of MEG functional localization has been confirmed by intra-operative recordings. An interesting and critical finding has been the identification of functional brain tissue within tumors. About 8-18% of patients were found to have functional activity within a glioma.
Future
MEG is also showing great potential in early identification of Autistic children by studying auditory processing by MEG. It is also showing potential use in identifying mild cognitive deficit patients in Alzheimer's. MEG is showing promise in the study of Psychiatric disorders and also in Head Injury.
Footnotes
Source of Support: Nil
Conflict of Interest: Nil
References
- 1.Singh S. Magnetoencephalography. Prog Clin Neurosciences. 2012;26:164–74. [Google Scholar]
- 2.Hämäläinen MS. Basic principles of magnetoencephalography. Acta Radiol Suppl. 1991;377:58–62. [PubMed] [Google Scholar]
- 3.Hämäläinen MS. Magnetoencephalography: A tool for functional brain imaging. Brain Topogr. 1992;5:95–102. doi: 10.1007/BF01129036. [DOI] [PubMed] [Google Scholar]
- 4.Hamalainen M, Hari R, Ilmoniemi RJ, Knuutila J, Lounasmaa OV. Magnetoencephalography — Theory, instrumentation, and application to noninvasive studies of the working human brain. Rev Mod Phys. 1993;65:413–97. [Google Scholar]
- 5.Knowlton RC, Elgavish RA, Bartolucci A, Ojha B, Limdi N, Blount J, et al. Functional imaging: II. Prediction of epilepsy surgery outcome. Ann Neurol. 2008;64:35–41. doi: 10.1002/ana.21419. [DOI] [PubMed] [Google Scholar]
- 6.Knowlton RC, Elgavish RA, Limdi N, Bartolucci A, Ojha B, Blount J, et al. Functional imaging: I. Relative predictive value of intracranial electroencephalography. Ann Neurol. 2008;64:25–34. doi: 10.1002/ana.21389. [DOI] [PubMed] [Google Scholar]
- 7.Stefan H, Hummel C, Scheler G, Genow A, Druschky K, Tilz C, et al. Magnetic brain source imaging of focal epileptic activity: A synopsis of 455 cases. Brain. 2003;126:2396–405. doi: 10.1093/brain/awg239. [DOI] [PubMed] [Google Scholar]
- 8.Sutherling WW, Mamelak AN, Thyerlei D, Maleeva T, Minazad Y, Philpott L, et al. Influence of magnetic source imaging for planning intracranial EEG in epilepsy. Neurology. 2008;71:990–6. doi: 10.1212/01.wnl.0000326591.29858.1a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singh S, Antony A, Thakur N. Yield of magnetoencephalography in mesial temporal lobe epilepsy. Epilepsia AES. 2010 Abst 2081. [Google Scholar]
- 10.Schiffbauer H, Ferrari P, Rowley HA, Berger MS, Roberts TP. Functional activity within brain tumors: A magnetic source imaging study. Neurosurgery. 2001;49:1313–20. doi: 10.1097/00006123-200112000-00005. [DOI] [PubMed] [Google Scholar]
- 11.Bagiæ AI, Knowlton RC, Rose DF, Ebersole JS ACMEGS Clinical Practice Guideline (CPG) Committee. American Clinical Magnetoencephalography Society Clinical Practice Guideline 1: Recording and analysis of spontaneous cerebral activity. J Clin Neurophysiol. 2011;28:348–54. doi: 10.1097/WNP.0b013e3182272fed. [DOI] [PubMed] [Google Scholar]
- 12.Burgess RC, Funke ME, Bowyer SM, Lewine JD, Kirsch HE, Bagiæ AI, et al. American Clinical Magnetoencephalography Society Clinical Practice Guideline 2: Presurgical functional brain mapping using magnetic evoked fields. J Clin Neurophysiol. 2011;28:355–61. doi: 10.1097/WNP.0b013e3182272ffe. [DOI] [PMC free article] [PubMed] [Google Scholar]


