Abstract
Approximately 60% of all patients with epilepsy suffer from focal epilepsy syndromes. In about 15% of these patients, the seizures are not adequately controlled with antiepileptic drugs; such patients are potential candidates for surgical treatment and the major proportion is in the pediatric group (18 years old or less). Epilepsy surgery in children who have been carefully chosen can result in either seizure freedom or a marked (>90%) reduction in seizures in approximately two-thirds of children with intractable seizures. Advances in structural and functional neuroimaging, neurosurgery, and neuroanaesthesia have improved the outcomes of surgery for children with intractable epilepsy. Early surgery improves the quality of life and cognitive and developmental outcome and allows the child to lead a normal life. Surgically remediable epilepsies should be identified early and include temporal lobe epilepsy with hippocampal sclerosis, lesional temporal and extratemporal epilepsy, hemispherical epilepsy, and gelastic epilepsy with hypothalamic hamartoma. These syndromes have both acquired and congenital etiologies and can be treated by resective or disconnective surgery. Palliative procedures are performed in children with diffuse and multifocal epilepsies who are not candidates for resective surgery. The palliative procedures include corpus callosotomy and vagal nerve stimulation while deep brain stimulation in epilepsy is still under evaluation. For children with “surgically remediable epilepsy,” surgery should be offered as a procedure of choice rather than as a treatment of last resort.
Keywords: Children, epilepsy surgery, temporal lobe epilepsy, extratemporal epilepsy, hemispherotomy
Introduction
Approximately 60% of all patients with epilepsy suffer from focal epilepsy syndromes. In about 15% of these patients, the seizures are not adequately controlled with anticonvulsive drugs, and such patients are potential candidates for surgical treatment. Around 65% of newly diagnosed epilepsy patients have a good response to antiepileptic drugs (AEDs). However, about 35% of patients have incompletely controlled epilepsy.[1] A major proportion of epileptic patients falls in the pediatric group (18 years or less), and approximately 25% of them have medically intractable epilepsy.
Advances in neuroimaging and neurosurgical techniques have helped in selecting an increasing number of children with refractory epilepsies into the pediatric epilepsy surgery programs. Surgery has become an accepted treatment modality for carefully selected adults with intractable focal epilepsy. Epilepsy surgery in children who have been carefully chosen can result in either seizure freedom or a marked (>90%) reduction in seizures in approximately two-thirds of the children with intractable seizures.[2,3] Infants and children benefit from epilepsy surgery, with encouraging results published in recent series.[4,5,6,7,8]
Consequences of ongoing seizures in childhood
Poor developmental outcome in children is associated with earlier seizure onset.[9] Ongoing seizures in severe epilepsy has been shown to be associated with developmental delay and cognitive decline, and improvement in developmental outcome may be noted with early cessation of seizures.[10] Chronic AED therapy may be associated with adverse effects. Recurrent seizures are associated with behavioral and psychiatric problems, psychosocial consequences, poor quality of life of the child and family, and increased risk of injury and sudden death.[11]
Unique features of pediatric epilepsy surgery
Children are not small adults. Epilepsy surgery in children requires a careful approach during presurgical evaluation and surgical approach, as it is different from adults.[4,12] 1. Seizure frequency is high in children when compared to adults. 2. Frequent seizures in infants and children is associated with developmental arrest or regression, especially in children younger than 2 years. 3. Focal epilepsy in childhood is often associated with age-specific etiologies. Dysplasia is the commonest substrate in children, whereas hippocampal sclerosis is common in adults. 4. The presentation of intractable localization-related epilepsy is often heterogeneous in childhood. Pediatric patients with hemispheric or unilateral focal etiologies can have generalized seizures, generalized and multifocal electroencephalography (EEG) patterns, and rapid evolution of electroclinical features. 5. The child's brain is capable of significant reorganization of neurologic function after insult and surgery, a unique and complex phenomenon that is critical for surgical planning. For example, interhemispheric language transfer occurs in children operated below the age of 6 years.
Selection of the ideal candidate
Epilepsy surgery is considered in children when 1. epilepsy is refractory, which is defined as inadequate control of seizures despite proper drug therapy with AED or the adequate control of epileptic seizures but with unacceptable side effects.[12] In adults, medical intractability may be considered as failure to respond to at least two anticonvulsant drugs over at least 2 years. These rules may not be appropriate in children and infants with catastrophic-onset epilepsies as seizure frequency may be such that a greater number of drugs are tried over a shorter time. In such cases, there is a need for early surgery for seizure freedom and prevention of developmental delay. 2. To be suitable for temporal or extratemporal epilepsy surgery, it should be proven that the seizures arise exclusively from one area of the brain that is functionally silent. Such an area of the brain may be relatively small or large, dependent partly on the underlying pathology and partly on the area of brain involved. The goals of epilepsy surgery should be clear [Table 1], and one should also understand when not to consider epilepsy surgery [Table 2].
Table 1.
Goals of epilepsy surgery

Table 2.
When not to consider epilepsy surgery

Types of epilepsy surgery
Currently, four types of surgical procedures are in practice[13] [Table 3]. They include the following: 1. Resection of the epileptogenic region responsible for the patient's habitual seizures. 2. Interruption of pathways propagating seizures. 3. Decreasing brain excitability by stimulating structures exerting a restraining influence. 4. Prevention of neuronal synchronization.
Table 3.
Surgical techniques

Presurgical evaluation
The goals of presurgical evaluation are 1. To establish the diagnosis of epileptic seizure. 2. Define the electroclinical syndrome. 3. Delineate the lesion(s) responsible for the seizures. 4. Evaluate the past AED treatments and ensure that an adequate medical treatment had been provided. 5. Select ideal surgical candidates with optimal electro-clinico-radiologic correlation. 6. Ensure that the surgery will not result in disabling neuropsychological deficits.[14] Different diagnostic tools area being used by epileptologists to identify different cortical zones — symptomatogenic zone, irritative and ictal-onset zones, epileptogenic zone, and functional deficit zone, each one of which is more or less a precise index of the epileptogenic zone.[15] The current diagnostic techniques used in the definition of these cortical zones are video EEG monitoring, magnetic resonance imaging (MRI), ictal single-photon emission computerized tomography (SPECT), and positron emission tomography (PET). A detailed developmental, neuropsychological assessment and evaluation for behavioral problems should be performed. Intracarotid amobarbital (WADA) test and functional MRI (fMRI) are indicated in selected cases but can be performed in older co-operative children and adolescents. Ictal SPECT and interictal PET may be used as adjunctive tests to add further information. Magnetoencephalography (MEG) can complement the scalp EEG data in defining the extent and location of the epileptogenic zone. In children, a noninvasive approach is preferred, more recently made possible by developments in neuroimaging.
Role of imaging in presurgical evaluation of epilepsy
The goals of neuroimaging in patients with medically refractory epilepsy are 1. delineation of structural and functional abnormalities in the suspected epileptogenic region, 2. prediction of the nature of structural pathology, 3. detection of abnormalities distant from the epileptogenic region (dual pathology), and 4. identification of the eloquent brain regions such as language, memory, and sensorimotor areas and the relation of these regions to the epileptogenic region.[16] The images should be reviewed by radiologists specially interested and experienced in the evaluation of patients with epilepsy.
Structural neuroimaging: MRI brain
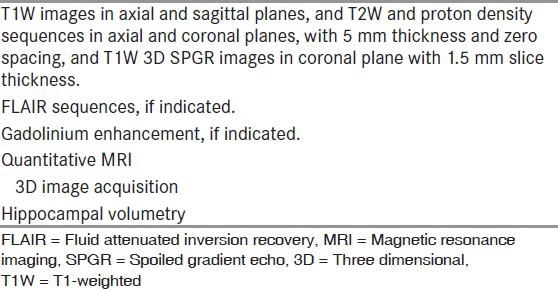
Positive pathology is a favorable predictor of outcome after surgery, and MRI has enabled the detection of pathology preoperatively.[17] The common abnormalities identified by MRI in children with refractory epilepsy are mesial temporal atrophy and sclerosis (MTS), malformations of cortical development, primary brain tumors, vascular malformations, and focal atrophic lesions [Figures 1a–g]. The structural MRI protocol for patients with chronic epilepsy is summarized in Table 4.[18] In MTS, the hippocampus is best visualized by acquiring thin slices (1-3 mm) orthogonal to its long axis. The important MRI features of MTS are 1. abnormal increased signal of hippocampus and amygdala relative to other gray matter on T2-weighted (T2W) images, 2. atrophic changes in the hippocampus/amygdala or temporal lobe in T1W images, 3. abnormal increased signal in gray/white matter of the temporal lobe relative to the gray matter elsewhere, 4. atrophy of the ipsilateral fornix, 5. dilatation of the temporal horn, and 6. blurring of gray and white-matter margin in the temporal neocortex. In addition, there may be lesions associated with ipsilateral MTS such as migrational disorders, porencephalic cysts, and neoplasms (dual pathology). MRI has about 90% sensitivity and specificity in detecting MTS and other abnormalities in the rest of the temporal lobe. Detection of temporal lobe abnormalities may be enhanced by using quantitative and semiquantitative techniques such as T2 relaxometry, hippocampal volumetry, and proton magnetic resonance spectroscopy. Such techniques are mostly available only in centers specializing in epilepsy surgery, and therefore a normal routine MRI in the presence of clinical or EEG suspicion of focal epilepsy or both does not preclude referral.
Figure 1.
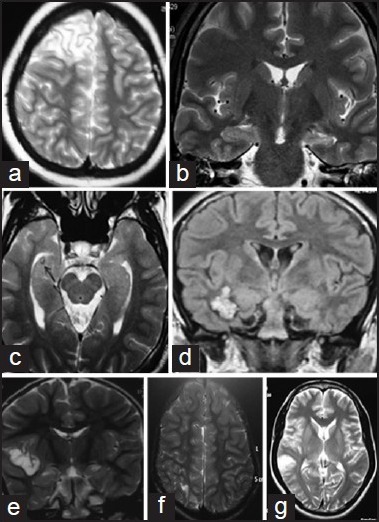
Common imaging abnormalities in children with refractory epilepsy. (a) Focal cortical dysplasia (T2 weighted images showing right frontal Taylor's type focal cortical dysplasia.) (b) Left mesial temporal sclerosis (FLAIR image showing hyperintesity of the hippocampus) in a 14 year child with refractory TLE. (c). Right hippocampal calcification. (d) Right temporal cavernoma.(e) Right perisylvian dysembryoblastic neuroepthelail tumor (DNET). (f) Right parietal gliosis in a 9 year child with refractory epilepsy. (g) Right temporal low grade glioma. FLAIR = Fluid attenuated inversion recovery
Table 4.
MRI protocol for patients with chronic epilepsy[18]
Malformations of cortical development are being increasingly recognized in patients with refractory epilepsy. They may be focal cortical dysplasia (FCD), lissencephaly, heterotopia, polymicrogyria, and schizencephaly.[19] Patients with low-grade primary brain tumors frequently present with seizures. The underlying histopathologies include dysembryoplastic neuroepithelial tumor, ganglioglioma, gangliocytoma, and pilocytic and fibrillary astrocytoma. These lesions have low signal on T1 and high signal on T2W images. Cyst formation and enhancement with gadolinium may occur. Calcification is present in some cases. Cavernous angiomas are circumscribed and have the characteristic appearance of a range of blood products on MRI. Newly developed MRI techniques, diffusion weighted imaging (DWI), diffusion tensor imaging (DTI), and tractography improve the sensitivity of MRI. In children undergoing evaluation for hemispherotomy, MRI plays a major role, not only in giving information regarding the extent and nature of the hemispheric abnormality but also in excluding the presence of any abnormality on the contralateral side, which may be a predictor of an unfavorable outcome. One should carefully look for a hypothalamic hamartoma (HH) in children with normal MRI brain and refractory epilepsy especially with gelastic seizures.
Functional imaging: Role of SPECT and PET
Ictal SPECT and interictal fluorodeoxyglucose (FDG)-PET remain important imaging tools in the presurgical evaluation of children with refractory partial epilepsy. The two commonly used tracers for SPECT are 99-TC-hexamethylene propylaxamine (99Tc-HMPAO) and 99m Tc ethyl cysteinate dimer (99m Tc-ECD). SPECT measures blood flow and by comparing interictal and ictal SPECT studies, the increase in blood flow of certain brain regions during the ictal phase with respect to the interictal period can be evaluated. During ictal SPECT, due to epileptic activation, the neurons located in these areas are hyperactive, and there is an increase in blood flow as an autoregulatory response. The limitations of ictal SPECT are the following: 1. The dye reaches the brain at least 1 minute after the seizure onset, a time at which significant seizure spread has already occurred. 2. The spatial resolution of the images is low. An ictal SPECT displays both the ictal-onset zone and seizure propagation pathways. In common practice, the region with the largest and most intense hyperperfusion is considered as the ictal-onset zone [Figures 2a and b]. An ictal injection delay of less than 20 seconds after seizure onset significantly correlates with correct localization.[20]
Figure 2.
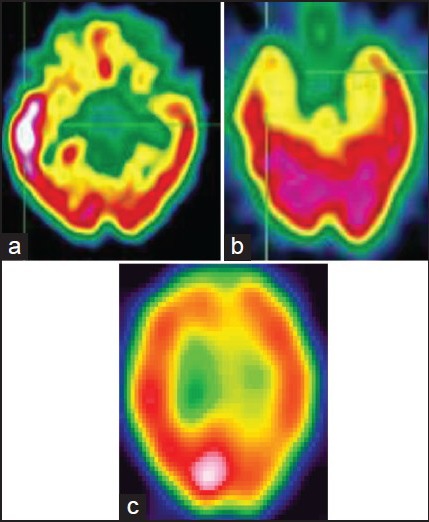
Hyperperfusion on ictal SPECT (a) and hypoperfusion on interictal SPECT (b) in a child with TLE. (c) Posterior parietal ictal hyperperfusion in a child with refractory extratemporal epilepsy. SPECT = Single-photon emission computerized tomography; TLE = Temporal lobe epilepsy
FDG-PET is more useful for lateralizing than localizing the epileptic focus.[18] Patients with MTS have low glucose metabolism in the whole temporal lobe [Figure 3], whereas patients with mesiobasal temporal tumors show only a slight decrease in metabolism. There is no correlation found between the degree of hypometabolism and the location of the epileptic focus. Unilateral focal temporal hypometabolism in 18FDG-PET predicts a good outcome after surgery for temporal lobe epilepsy (TLE).[19] The diagnostic sensitivity of FDG-PET as analyzed by statistical parametric mapping (SPM) was 44% in patients with refractory partial epilepsy and normal MRI. Video EEG semiology and ictal onset may be misleading in infants and young children.[21,22] In such cases, noninvasive functional imaging in the form of ictal SPECT or interictal PET may provide useful information. The value of ictal SPECT has been enhanced by the use of subtraction ictal SPECT coregistered with MRI (SISCOM).[23]
Figure 3.
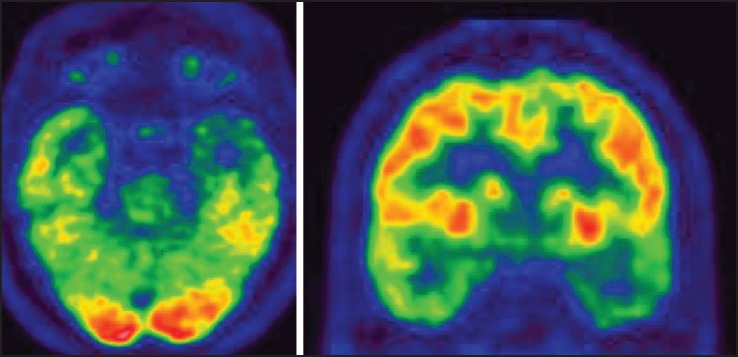
Interictal FDG-PET (axial and coronal images) showing left temporal hypometabolism in a 12-year-old child with TLE and left HS. FDG-PET = Fluorodeoxyglucose positron emission tomography; HS = Hippocampal sclerosis; TLE = Temporal lobe epilepsy
Neuropsychology
Neuropsychological assessment plays an important role in the determination of verbal and nonverbal function in older children and in the determination of cerebral dominance.[12] Wada (sodium amytal) testing may be required in older children to lateralize language function, particularly where there has been relatively late onset of the epilepsy. However, in the presence of early-onset localization-related epilepsy, particularly associated with a structural abnormality, relocalization of function (i.e., language) is likely. Functional imaging techniques (in the form of MRI or PET) are being increasingly used to localize language function, although they are likely to be reliable only in the older child who is able to understand the required task and is able to lie within the magnet unsedated.[24] Such investigative techniques may preclude the need for Wada testing.
Role of noninvasive EEG in presurgical evaluation
In TLE, adolescents and older children have semiology similar to adults. Infants and toddlers are more likely to display seizure semiologies reminiscent of extratemporal and generalized epilepsies. An inverse relationship between the occurrences of ictal motor manifestations and age was shown in previous studies.[25,26,27,28,29] Such motor manifestations include tonic, clonic, myoclonic, and hypermotor seizures and epileptic spasms and may be mistaken for extratemporal seizures. The occurrence of epileptic spasms in young children with temporal lobe pathology is likely secondary to rapid secondary generalization of focal-onset seizures via dysfunctional cortical–subcortical interactions (particularly involving the thalamus, basal ganglia, and other brainstem structures). This can result in the generalized semiologic and EEG appearances, making identification of the ictal-onset zone difficult. It is also difficult to assess for the presence of auras in the very young. Beyond the age of 6 years, most children with TLE display much of the same seizure semiology as their adult counterparts.
The interictal and ictal EEG
The interictal EEG in TLE is typically characterized by temporal spike or sharp-wave discharges and temporal intermittent rhythmic delta activity (TIRDA). Temporal spike or sharp-wave discharges are highly epileptogenic discharges that are maximal over the anterior temporal region. There is often increased activation of spike and sharp-wave discharges during drowsiness and sleep, with nearly 90% of the patients with temporal lobe seizures showing spikes during sleep. The spike-wave discharges may occur independently or synchronously over the bilateral temporal regions. However, most patients with bitemporal interictal EEG patterns are found to have unilateral temporal lobe seizures. However, the interictal EEG frequently shows generalized and multifocal EEG abnormalities. The scalp ictal and interictal EEG recordings in children may be poorly localizing, even in TLE, due to incomplete or abnormal brain maturation. A focal lesion can present with generalized or multifocal epileptiform discharges in infants and young children. Similarly, the ictal EEG in a focal seizure of anterior temporal lobe origin may initially demonstrate lateralized or generalized scalp EEG changes.[30] Typically, the scalp EEG during a temporal lobe seizure demonstrates moderate- to high-amplitude rhythmic paroxysmal activity that is maximal over a unilateral temporal region. This may progress to generalized rhythmic slowing that is maximal on the side of seizure onset.
Invasive monitoring
Intracranial EEG recording is indicated for precise localization of the epileptogenic zone, when located close to the functional cortex for planning a safe resection. Stereotactically inserted depth electrode area indicates when EEG recording is needed from buried gray matter that is not accessible with other electrodes. According to Mayo clinic experience, invasive recordings in TLE were deemed necessary for (a) inability to accurately localize the site of seizure onset by surface EEG, (b) suspected multifocal onset, and (c) discrepancies between MRI findings and video EEG monitoring [Figure 4a].[31] Stereotactic depth recordings, when combined with scalp EEG recording, the so-called stereo EEG (SEEG), helps in clear delineation of the epileptogenic zone in complex cases. The complications of depth electrodes are intracerebral hemorrhage, in 1-4% cases, with rare fatalities and infection. The current use of subdural electrode grids by experienced centers has been shown to be well tolerated, even in young children, and allows not only accurate localization of seizure onset, but also localization of function.[32] This may be particularly necessary in children with extratemporal epilepsy, either in those with normal structural imaging (with highly concordant functional data) or with a structural abnormality thought to be close in proximity to the cortex involved in useful function [Figure 4b].
Figure 4.
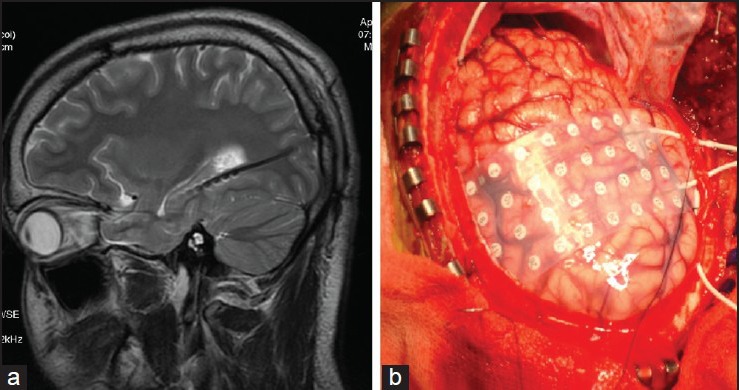
Invasive EEG: Temporal depth electrodes (a) in a child patient with refractory TLE and discordant presurgical data and subdural grid (b) in a child. EEG = Electroencephalography
Magnetoencephalography
Whole-head MEG facilitates simultaneous recording from the entire brain surface. Localizations of interictal spike zone with MEG showed excellent agreement with invasive EEG recordings. MEG is useful for the study of patients with nonlesional neocortical epilepsy and in patients with large lesions; it provides unique information on the epileptogenic zone. MEG can be used to map the sensorimotor cortex and language cortex. Both EEG and MEG yield complementary and confirmatory information.[33]
Successful epilepsy surgery requires a multidisciplinary team approach with discussion of individual patient presurgical evaluation data in detail in a patient management conference. It will improve patient care and communication among members of the team. None of the currently available preoperative workups can exactly delineate the epileptogenic zone. However, with the multimodality presurgical evaluation approach, sufficient concordance could be established among various independent investigation, thus identifying location and extent of the epileptogenic zone with a high degree of confidence. This will result in a good surgical outcome.
Surgically remediable epilepsy syndromes of childhood
Surgery can be considered at any age in the pediatric population from infancy through early childhood and adolescence. The surgically remediable epilepsies in children are TLE with hippocampal sclerosis (HS), lesional temporal and extratemporal epilepsy, hemispherical epilepsy syndrome, and HH. Table 5 summarizes the criteria for selection of children with refractory epilepsy and the various surgical procedures.
Table 5.
Selection criteria for childhood epilepsy surgery

Temporal lobe epilepsy
Surgery for TLE is the commonest surgery performed in adults, and most of them have their seizure onset in childhood or adolescence. Many children with TLE continue to have seizures in spite of adequate AED therapy. Children with medically refractory TLE should be evaluated appropriately for surgical management. Hippocampal sclerosis and dual pathology (hippocampal sclerosis and other lesion) were associated with only 11% and 3% seizure freedom at last follow-up in medically treated cases, respectively.[34] Early surgical intervention avoids the long-term risks associated with AED therapy. Children undergoing temporal lobectomy for refractory epilepsy show improvement in quality of life, visual memory, and attentional functions after surgery.[34] The complication rates are less than 5%.[7,35]
Presurgical evaluation
Ictal scalp EEG monitoring is essential for determining the region of seizure onset, especially for differentiating mesial versus neocortical onset. Patients with mesial TLE may have higher seizure-free outcomes with surgery compared to patients with neocortical temporal and extratemporal lobe epilepsies, particularly if a single MRI lesion is present.[36] MRI-positive TLE is associated with a favorable outcome. Patients with lesional TLE, such as hippocampal sclerosis or foreign tissue lesions (tumor, vascular anomaly), have a higher probability of seizure freedom after resection than those with normal MRI.[37] Postsurgical seizure-free outcome is seen in 70% to 90% patients with mesial TLE with hippocampal sclerosis compared to lowered seizure-free outcome of 60% in patients with nonlesional TLE.[36] Multimodality noninvasive imaging modalities are increasingly used to determine the presumed epileptogenic focus when the scalp EEG and MRI are nonlocalizing or normal. These include ictal SPECT, SISCOM, PET, MEG, and MR spectroscopy. The ability of SISCOM to detect epileptogenic lesions is 88% as compared to 39% by ictal SPECT alone. In patients with TLE and normal MRI, unilateral PET hypometabolism has a positive predictive value between 70% and 80%.[38]
Neuropsychological assessment prior to pediatric epilepsy surgery includes age-appropriate, standardized tests to evaluate multiple domains: intelligence, language, memory, attention, problem solving/executive function, visuospatial and perceptual analysis and reasoning, academic skills, motor and sensory function, behavior, personality, emotional status, and adaptive functioning. It identifies areas of existing dysfunction, assists in determining language lateralization, and provides guidance in weighing the risks and benefits of surgery. It has been shown that verbal memory dysfunction was worst among children with left temporal lesions compared to those with right-sided lesions and controls, whereas nonverbal dysfunction was most prominent among children with right TLE. Children with TLE were found to have a long-lasting impact on verbal learning and memory. When compared to controls, children and adolescents with epilepsy failed to build up an adequate learning and memory performance.[39]
Intracarotid sodium amobarbital testing (Wada test) is used to determine language lateralization and to screen for verbal memory dominance. Among children who underwent temporal lobectomy, better verbal memory performance after injection ipsilateral to the side of surgery than after contralateral injection (Wada memory asymmetries) predicted preserved postoperative verbal memory capacity.[40] The difficulty is co-operation of the child. Those at greater risk of poor co-operation include children with full-scale intelligence quotient (IQ) <80, age <10 years, and seizures arising from the dominant left hemisphere. fMRI is a noninvasive option to determine language lateralization as well as identify patients at a higher risk of verbal memory decline following surgery. If both Wada and fMRI are not feasible, language lateralization and verbal memory capacity may be determined by neuropsychologic testing.
Standard temporal lobectomy with amygdalohippocampectomy
The standard resection includes 4 to 5 cm of the temporal lobe from the temporal pole and 2 to 3 cm of the mesial temporal structures. Better outcomes are associated with larger temporal lobe resection; however, some authors attribute it to larger mesial temporal resection. Also for neocortical resection, it is restricted to 4 cm in the dominant hemisphere versus 5.5 to 6 cm in the nondominant hemisphere.[41]
Role of electrocorticography in TLE surgery
Intraoperative electrocorticography (ECoG) has been used to localize the irritative zone and guide the extent of surgical resection. ECoG is unlikely to influence surgical resection in standard anterior temporal lobectomy in patients with mesial TLE with HS but helps in the presence of dual pathologies, tumoral lesions, and dysplasias. In tailored temporal lobectomies, intraoperative hippocampal ECoG may guide the posterior extent of hippocampal resection. In 140 consecutive patients undergoing this procedure, McKhann et al. showed that removal of all ECoG confirmed epileptogenic hippocampal tissue, but not the size of resection, correlated with seizure-free outcome.[42] ECoG is important in children with dual pathology and ensures complete resection of regions of cortical dysplasia.
Complications
Complications of dominant temporal lobectomy include language and memory impairments, with naming and fluency deficits seen in 50%.[43] Verbal memory impairment depends on whether resection was done in the dominant hemisphere as well as preoperative level of function; those with higher preoperative function are more likely to show decline. Transient diplopia due to trochlear nerve palsy occurred in 1.5% of cases. Significant visual field deficits have been described in about 35% of patients undergoing temporal lobectomy but approximately 38% of these patients experience improvement within the first year after surgery.[44] The other rare complications are hemiparesis, dysphasia, and hemianopia.
Nonresective epilepsy surgery
Ablative surgery, radiofrequency, and thermal ablation are currently under investigation. Seizure remission is delayed up to one year after stereotactic radiosurgery for mesial TLE, occurring around the time of vasogenic edema on MRI.[45]
Pathology
The most common etiologies among pediatric surgical candidates are low-grade tumors and focal malformations of cortical development, which together accounted for the etiologies in 57% of adolescents, 70% of preadolescent children, and 90% of infants.[4,7] Duchowny and colleagues reported low-grade tumors or malformations of cortical development in 90% of the infants in the series from Miami Children's Hospital.[46] In children, if hippocampal sclerosis is present, it is frequently associated with extrahippocampal pathology such as cortical dysplasia or low-grade tumors. Such dual pathology has been reported in 31% to 79% of all cases of MTS in children.[47,48]
Extratemporal surgery
Surgery for extratemporal epilepsy in adults and children results in a lower frequency of seizure freedom (45%) compared with that of temporal lobectomy (68%); however, patients who undergo extratemporal resections experience substantial improvement in seizure control (35%) compared with temporal lobectomies (24%).[7] In a study by Adler et al.,[49] in 35 patients whose seizures initially occurred before the age of 16 years, 40% were seizure-free, and another 23% had a 75% or higher reduction in seizure frequency. In another long-term follow-up study of 45 children (<16 years of age) who underwent frontal lobe surgery, after a median follow-up of 15 years, it was noted that 20% had a seizure-free response (with or without auras), and another 9% had fewer than three seizures per year.[17] Gilliam et al. described 15 children who underwent extratemporal surgery; the seizure freedom was 60% with frontal lobe epilepsy, and another 20% had more than a 95% seizure reduction. Pathology in the form of cortical dysplasia, gliosis, or tumor was found in all extratemporal specimens and did not appear to predict outcome.[50] Wyllie et al. reported 48 extratemporal and multilobar resections in children and adolescents monitored for longer than 1 year.[4] Of these individuals, 54% were seizure-free, and another 20% had only rare seizures. Focal abnormalities were present on MRI in 85% of the patients. The use of modern techniques has effected a considerable improvement in seizure relief for both children and adults with seizures of extratemporal onset. However, for patients who have no lesions present on neuroimaging, studies remain a challenge. If no structural abnormality is identified, additional noninvasive testing (SPECT, PET, MEG) should be considered to facilitate intracranial electrode implantation.
Surgical techniques
The major factors that potentially complicate surgical intervention in extratemporal epilepsy include multifocal epileptogenic foci, high incidence of nonlesional or MRI-negative epilepsy, and frequent proximity of the epileptogenic zone to the eloquent cortex. Hence, the surgical options should be tailored to the individual risk–benefit profile.
During extratemporal resective surgery, the standard method to know the extent of resectability on the table would be by the use of intraoperative ECoG.[51] The resection can be tailored based on ECoG. ECoG helps to decide the extent of resection, which is determined by the extent of interictal and ictal activity. It is used for mapping the irritative and epileptogenic zone during epilepsy surgery and determining the prognosis of surgical outcome based on residual epileptiform activity. For focal lesions, focal resection or lesionectomy or both are performed. If the lesion involves the entire lobe, lobectomy has to be performed. Either of the above involving the eloquent cortex would limit the extent of resection sparing the eloquent cortex. In lesions involving the eloquent cortex, the choices would be dependent on the location. For motor cortex lesions, the resection needs to be done by performing mapping of the motor cortex with either a) central sulcus mapping — somatosensory evoked potentials (SSEP), b) cortical stimulation, or c) awake craniotomy. For language area lesions, awake craniotomy with language mapping guides the resection. The above options may be used alone or in combination.
For eloquent cortex resections, mapping can be carried out with subdural grid electrode placement. This can be supplemented with preoperative functional MRI fusion with neuronavigation systems, making functional and anatomical landmarks overlap with or without real-time intraoperative MRI integration. If one finds that there is an overlap of the seizure foci with the eloquent cortex, the choice would be to do a multiple subpial transection (MST). Preresection spike distribution and postresection spike reduction may have prognostic significance.[52]
Pathology
Developmental brain abnormalities (e.g., cortical dysplasia, tuberous sclerosis complex, Sturge–Weber syndrome) and low-grade cortical tumors (gangliogliomas, desmoplastic infantile gangliogliomas, oligodendrogliomas, astrocytomas) are the commonest pathologies in children with epilepsies of extratemporal origin.[4,5,7]
Posterior quadrantic resection and disconnection
This technique is used in posterior hemispherical extratemporal epilepsies and accounts for less than 5% of epilepsy surgeries. This is done when the epileptogenic zone encompasses large areas of the temporal, parietal, and occipital lobes and spares the central and frontal areas. The commonest etiologies include cortical dysplasia, Sturge — Weber syndrome, and ischemic perinatal lesions.[53] Intraoperative functional mapping using cortical stimulation under light general anesthesia is done to identify and preserve the motor areas, and ECoG is used to delineate the extent of the epileptogenic foci. Stage I involves mesial temporal resection through infrainsular window. In stage II, parieto-occipital disconnection of the posterior temporal, parietal, and occipital lobes is performed.
Hemispherotomy
Candidates for hemispherotomy
Children with intractable partial seizures of all types who have a structural abnormality that involves most of one hemisphere and who are already hemiparetic with a homonymous hemianopia should be considered for hemispherotomy. Early surgery should be contemplated for several underlying pathologies that have a favorable outcome (e.g., Rasmussen syndrome, Sturge — Weber syndrome, and hemimegalencephaly due to malformations of cortical development [Figure 5a–c]).[54] Hemispherotomy should not be delayed in patients with Rasmussen syndrome and cortical dysplasia, even when full hemiparesis and hemianopia have not developed.[55] Furthermore, language function improved in most patients in one of the series, independent of the resected side, suggesting that the constant storm of epileptiform activity was inhibiting the normally functioning hemisphere.[56]
Figure 5.
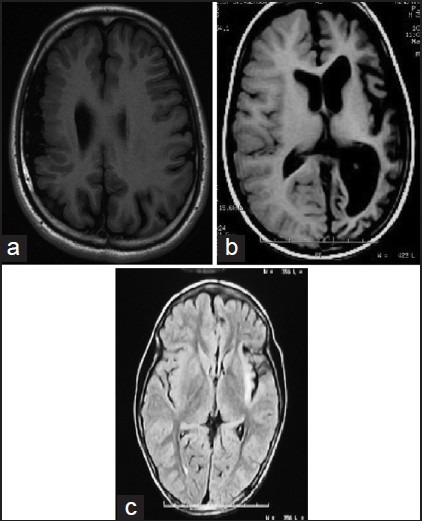
Hemispherical epilepsy syndromes: (a) Right hemispherical malformation, (b) left hemispherical atrophy, (c) Rassmussen's encephalitis involving the left hemisphere
The two very novel and recent techniques of functional hemispherotomies are parasagittal insular hemispherotomy (PIH) and vertical parasagittal hemispherotomy (VPH) as popularized by Villemure and Delalande, respectively. Functional hemispherectomy as described by Rasmussen involves anterior temporal lobectomy with amygdalohippocampectomy, followed by frontoparietal central zone parasagittal excision, including the parasagittal tissue, corpus callosotomy from genu to splenium via the ventricle, and finally resection of the insular cortex.[57,58]
Corpus callosotomy
Corpus callosotomy is a palliative surgical procedure for children with intractable seizures who are not candidates for focal resective surgery. It is a particularly useful technique for atonic, tonic — clonic, and tonic seizures leading to injuries. Corpus callosotomy was first used in children in the 1940s. Surgeries include complete section of the corpus callosum, anterior two-thirds section with or without the hippocampal commissure, and two-stage procedures. Generalized tonic — clonic and atonic seizures were reduced by 80% to 100%. In another study, 83% of the children had more than an 80% reduction in tonic, tonic — clonic, or atonic seizures after a partial or two-stage callosotomy with 2 or more years of follow-up.[59] The second procedure completing the callosotomy was performed months after the anterior section if adequate seizure relief was not achieved. No mortality and only transient hemiparesis was reported in this series. Other complications reported include transient dysphasia, more intense focal seizures, memory problems, and split-brain syndromes (e.g, inability to name an object placed in the right hand due to disconnection of the involved sensory cortex from the left hemisphere language centers). However, individuals with severe developmental deficits and little or no language skills may undergo a single-stage complete callosotomy without apparent harm.
Vagus nerve stimulation therapy
Vagus nerve stimulation (VNS) was approved by the Food and Drug Administration in 1997 as an adjunctive therapy for refractory partial-onset seizures in patients aged 12 years and older. The precise mechanism of action of the VNS is not clear. It is believed to work through both immediate and long-term changes. Immediate mechanisms include changes in the nucleus of the tractus solitarius and its connections. This probably causes synchronization and desynchronization of brain electrical activity. Long-term mechanisms are thought to include changes in neurotransmitter concentrations and regional cerebral blood flow. Some researchers hypothesize that the VNS works through increased activity of the noradrenergic and serotoninergic pathways, thus increasing the seizure threshold.
Efficacy with VNS therapy
There are no randomized, double-blind, controlled trials performed exclusively with children. In a study of 60 children with partial seizures or generalized seizures, 29% had more than a 50% reduction in seizure frequency.[60] The median reductions in seizure frequency were 23%, 31%, 34%, and 42% at 3, 6, 12, and 18 months, respectively. The response rate was not different among the various seizure types. VNS was also found to be useful in the management of refractory generalized epilepsy both in adults and children. A study of 13 patients with Lennox–Gastaut syndrome treated with VNS reported a median reduction in seizure frequency of 52% in the first 6 months.[61] VNS was found to improve independence, learning, and mood, at times independent of its antiepileptic effect.
Open-label and small blinded trials have provided promising evidence for the use of deep brain stimulation (DBS) in refractory seizures. The targets used were amygdalohippocampus, anterior nucleus of thalamus, head of caudate nucleus, cerebellum, subthalamic nuclei, and centromedian nucleus of thalamus.[62,63]
Multiple Subpial Transection
The results of an international meta-analysis have suggested that MST alone has efficacy and causes only minimal neurologic compromise in patients with intractable seizures who cannot be treated with resective surgery.[64] It is a novel technique for the treatment of intractable epilepsy originating from the eloquent cortex like the language cortex or the sensorimotor cortex. The aim of MST is to impair the capacity of the cortical tissue to generate sufficient neuronal synchrony to produce epileptiform discharges, without interfering with its capacity to mediate physiologic functions. MST induces minimal or no functional deficits after surgery due to preservation of the cortical vertical columns.[65] The rationale is that 5 mm wide cortical area with horizontal connection is needed to be able to support paroxysmal discharges. The vertical column is the master organizational principle of the cerebral cortex. Hence, the aim is to transect the horizontal fibers of the cerebral cortex while preserving the vertical column. In the initial phases of seizure spread, this disconnection interferes with cell synchronization and ictal propagation.
Hypothalamic Hamartoma
Hypothalamic hamartomas are ectopic masses of neuronal and glial tissue, which may be small and pedunculated or sessile and relatively large. The association of gelastic or dacrystic seizures or both with HH is now well recognized.[66] Associated symptoms include other seizure types, precocious puberty, behavioral disturbances, and progressive cognitive deterioration. In the majority of cases, epilepsy begins during the neonatal or early childhood period, usually in the form of gelastic seizures. In the majority of patients, epilepsy proves to be drug resistant, manifesting with multiple seizure types and progressive cognitive and developmental deterioration. Surgical treatment can result in seizure freedom in up to 50% of the patients and can be accompanied by significant improvements in behavior, cognition, and quality of life. Partial treatment of HHs may be sufficient to reduce seizure frequency and improve behavior and quality of life with less risk.
High-resolution MR imaging remains the procedure of choice for identifying HHs, which may be as small as a few millimeters to a few centimeters in size. Hamartomas are usually isointense to gray matter on T1-weighted imaging and hyperintense or isointense on T2-weighted imaging and do not enhance after gadolinium administration. They vary considerably in size and location, and these influence the surgical approach. In particular, the relationship of the lesion to the hypothalamus, the interpeduncular cistern, and the wall of the third ventricle are all important and need to be demonstrated by the imaging.
Surgery
A variety of surgical approaches to remove HHs have been described, including subfrontal, subtemporal, pterional, and frontotemporal. Total or subtotal resection or partial treatment of an HH is needed to achieve seizure freedom [Figure 6a, b]. Surgical disconnection has been proposed as an alternative to removal in cases in which the tumor is large and complete and safe resection is not possible.[67,68] The transcallosal interforniceal approach is the most successful with 69% seizure freedom. There are complications in about 24% of patients, the same as with other approaches, but the complications are milder and include fewer neurological deficits than the other routes. Radiosurgery, interstitial radiotherapy, and stereotactic radiofrequency thermocoagulation can result in seizure freedom in up to 50% of the patients.
Figure 6.
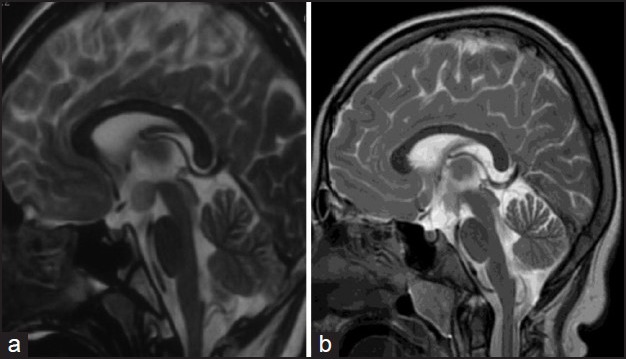
Magnetic resonance imaging (MRI) of the brain in a child with hypothalamic hamartoma (a: Pre resection, b: Post resection)
Outcome of epilepsy surgery in children
Based on results from several recent pediatric surgical series, the chance for favorable seizure outcome after surgery is not adversely affected by young age, with seizure-free postoperative outcome reported for 60-65% of infants, 59-67% of children, and 69% of adolescents, compared to 64% reported in a large, predominantly adult series.[69] Results are better, and more patients attain seizure freedom after temporal resection (78%) than after extratemporal or multilobar resection (54%), with intermediate results after hemispherotomy (69%).[4] Seizure-free outcome was significantly more frequent when the etiology was tumor (82%) than when it was cortical dysplasia (52%), and this difference persisted whether the resection was temporal or extratemporal/multilobar. In children with intractable TLE due to hippocampal sclerosis, 78% seizure freedom was attained, and these results are similar to those in adults.[69] The presence of a focal lesion on MRI appears to be a favorable prognostic feature.
Potential risks of epilepsy surgery
The most serious risk of epilepsy surgery is perioperative mortality. Isolated deaths were reported in several pediatric series, with a frequency of 1.3%.[4] The risk may be higher in infants, because of their tendency to require extensive surgery in the face of a small blood volume. The mortality of epilepsy surgery must be balanced against the mortality of medically treated uncontrolled seizures. Other risks of epilepsy surgery include new postoperative neurologic deficits. However, young age confers some advantages because of developmental plasticity.
Summary
Advances in structural and functional neuroimaging, neurosurgery, and neuroanesthesia have improved the outcomes of surgery for children with intractable epilepsy. Children with intractable epilepsies should be evaluated early at specialized epilepsy surgery centers to avoid the long-term consequences and morbidity associated with epilepsy. Surgically remediable epilepsies should be identified early and include TLE with hippocampal sclerosis, lesional temporal and extratemporal epilepsy, hemispherical epilepsy, and gelastic epilepsy with HH. Palliative procedures include corpus callosotomy and VNS while deep brain stimulation in epilepsy is still under evaluation.
Footnotes
Source of Support: Nil
Conflict of Interest: Nil
References
- 1.Kwan P, Brodie MJ. Early identification of refractory epilepsy. N Engl J Med. 2000;342:314–9. doi: 10.1056/NEJM200002033420503. [DOI] [PubMed] [Google Scholar]
- 2.Pellock J.M. Managing pediatric epilepsy syndromes with new antiepileptic drugs. Pediatrics. 1999;104:1106–16. doi: 10.1542/peds.104.5.1106. [DOI] [PubMed] [Google Scholar]
- 3.Camfield P.R, Camfield C.S. Antiepileptic drug therapy: When is epilepsy truly intractable? Epilepsia. 1996;37(Suppl 1):S60–5. doi: 10.1111/j.1528-1157.1996.tb06023.x. [DOI] [PubMed] [Google Scholar]
- 4.Wyllie E, Comair YG, Kotagal P, Bulacio J, Bingaman W, Ruggieri P. Seizure outcome after epilepsy surgery in children and adolescents. Ann Neurol. 1998;44:740–8. doi: 10.1002/ana.410440507. [DOI] [PubMed] [Google Scholar]
- 5.Paolicchi JM, Jayakar P, Dean P, Yaylali I, Morrison G, Prats A, et al. Predictors of outcome in pediatric epilepsy surgery. Neurology, 2000;54:642–7. doi: 10.1212/wnl.54.3.642. [DOI] [PubMed] [Google Scholar]
- 6.Vining EP, Freeman JM, Pillas DJ, Uematsu S, Carson BS, Brandt J, et al. Why would you remove half a brain? The outcome of 58 children after hemispherectomy-the Johns Hopkins experience: 1968 to 1996. Pediatrics. 1997;100:163–71. doi: 10.1542/peds.100.2.163. [DOI] [PubMed] [Google Scholar]
- 7.Jayalakshmi S, Panigrahi M, Kulkarni DK, Uppin M, Somayajula S, Challa S. Outcome of epilepsy surgery in children after evaluation with non-invasive protocol. Neurol India. 2011;59:30–6. doi: 10.4103/0028-3886.76854. [DOI] [PubMed] [Google Scholar]
- 8.Dagar A, Chandra PS, Chaudhary K, Avnish C, Bal CS, Gaikwad S, et al. Epilepsy surgery in a pediatric population: a retrospective study of 129 children from a tertiary care hospital in a developing country along with assessment of quality of life. Pediatr Neurosurg. 2011;47:186–93. doi: 10.1159/000334257. [DOI] [PubMed] [Google Scholar]
- 9.Vasconcellos E, Wyllie E, Sullivan S, Stanford L, Bulacio J, Kotagal P, et al. Mental retardation in pediatric candidates for epilepsy surgery: The role of early seizure onset. Epilepsia. 2001;42:268–74. doi: 10.1046/j.1528-1157.2001.12200.x. [DOI] [PubMed] [Google Scholar]
- 10.Czochañska J, Langner-Tyszka B, Losiowski Z, Schmidt-Sidor B. Children who develop epilepsy in the first year of life: A prospective study. Dev Med Child Neurol. 1994;36:345–50. [PubMed] [Google Scholar]
- 11.Nashef L, Fish DR, Garner S, Sander JW, Shorvon SD. Sudden death in epilepsy: A study of incidence in a young cohort with epilepsy and learning difficulty. Epilepsia. 1995;36:1187–94. doi: 10.1111/j.1528-1157.1995.tb01061.x. [DOI] [PubMed] [Google Scholar]
- 12.Cross J.H. Epilepsy surgery in childhood. Epilepsia. 2002;43(Suppl 3):65–70. doi: 10.1046/j.1528-1157.43.s.3.6.x. [DOI] [PubMed] [Google Scholar]
- 13.Lee B.I. Overview of epilepsy surgery. J Korean Med Sci. 1992;7:91–109. doi: 10.3346/jkms.1992.7.2.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Radhakrishnan K. Radhakrishnan K, editor. Medically refractory epilepsy. Medically refractory epilepsy Trivandrum: Sree Chitra Tirunal Institute for Medical Sciences and Technology. 1999:1–39. [Google Scholar]
- 15.Chugani HT, Shewmon DA, Shields WD, Sankar R, Comair Y, Vinters HV, et al. Surgery for intractable infantile spasms: Neuroimaging perspectives. Epilepsia. 1993;34:764–71. doi: 10.1111/j.1528-1157.1993.tb00459.x. [DOI] [PubMed] [Google Scholar]
- 16.Guidelines for neuroimaging evaluation of patients with uncontrolled epilepsy considered for surgery. Commission on Neuroimaging of the International League Against Epilepsy. Epilepsia. 1998;39:375–6. doi: 10.1111/j.1528-1157.1998.tb01341.x. [DOI] [PubMed] [Google Scholar]
- 17.Fish DR, Smith SJ, Quesney LF, Andermann F, Rasmussen T. Surgical treatment of children with medically intractable frontal or temporal lobe epilepsy: Results and highlights of 40 years’ experience. Epilepsia. 1993;34:244–7. doi: 10.1111/j.1528-1157.1993.tb02405.x. [DOI] [PubMed] [Google Scholar]
- 18.Gupta A.K. Radhakrishnan K, editor. The role of imaging in presurgical evaluation of epilepsy. Medically refractory epilepsy Trivandrum: Sree Chitra Tirunal Institute for Medical Sciences and Technology. 1999:1–39. [Google Scholar]
- 19.Salmenpera T.M, Duncan J.S. Imaging in epilepsy. J Neurol Neurosurg Psychiatry. 2005;76(Suppl 3):iii2–10. doi: 10.1136/jnnp.2005.075135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dupont P, Van Paesschen W, Palmini A, Ambayi R, Van Loon J, Goffin J, et al. Ictal perfusion patterns associated with single MRI-visible focal dysplastic lesions: Implications for the noninvasive delineation of the epileptogenic zone. Epilepsia. 2006;47:1550–7. doi: 10.1111/j.1528-1167.2006.00628.x. [DOI] [PubMed] [Google Scholar]
- 21.Holmes G. L. Partial complex seizures in children: An analysis of 69 seizures in 24 patients using EEG FM radiotelemetry and videotape recording. Electroencephalogr Clin Neurophysiol. 1984;57:13–20. doi: 10.1016/0013-4694(84)90003-8. [DOI] [PubMed] [Google Scholar]
- 22.Wyllie E, Chee M, Granström ML, DelGiudice E, Estes M, Comair Y, et al. Temporal lobe epilepsy in early childhood. Epilepsia. 1993;34:859–68. doi: 10.1111/j.1528-1157.1993.tb02103.x. [DOI] [PubMed] [Google Scholar]
- 23.O’Brien TJ, So EL, Mullan BP, Hauser MF, Brinkmann BH, Bohnen NI, et al. Subtraction ictal SPECT co-registered to MRI improves clinical usefulness of SPECT in localizing the surgical seizure focus. Neurology. 1998;50:445–54. doi: 10.1212/wnl.50.2.445. [DOI] [PubMed] [Google Scholar]
- 24.Hertz-Pannier L, Gaillard WD, Mott SH, Cuenod CA, Bookheimer SY, Weinstein S, et al. Noninvasive assessment of language dominance in children and adolescents with functional MRI: A preliminary study. Neurology. 1997:797–800. doi: 10.1212/wnl.48.4.1003. [DOI] [PubMed] [Google Scholar]
- 25.Ray A, Kotagal P. Temporal lobe epilepsy in children: Overview of clinical semiology. Epileptic Disord. 2005;7:299–307. [PubMed] [Google Scholar]
- 26.Fogarasi A, Tuxhorn I, Janszky J, Janszky I, Rásonyi G, Kelemen A, et al. Age-dependent seizure semiology in temporal lobe epilepsy. Epilepsia. 2007;48:1697–702. doi: 10.1111/j.1528-1167.2007.01129.x. [DOI] [PubMed] [Google Scholar]
- 27.Bourgeois B.F. Temporal lobe epilepsy in infants and children. Brain Dev. 1998;20:135–41. doi: 10.1016/s0387-7604(98)00010-2. [DOI] [PubMed] [Google Scholar]
- 28.Brockhaus A, Elger CE. Complex partial seizures of temporal lobe origin in children of different age groups. Epilepsia. 1995;36:1173–81. doi: 10.1111/j.1528-1157.1995.tb01059.x. [DOI] [PubMed] [Google Scholar]
- 29.Terra-Bustamante VC, Inuzuca LM, Fernandes RM, Funayama S, Escorsi-Rosset S, Wichert-Ana L, et al. Temporal lobe epilepsy surgery in children and adolescents: Clinical characteristics and post-surgical outcome. Seizure. 2005;14:274–81. doi: 10.1016/j.seizure.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 30.Wyllie E, Lachhwani DK, Gupta A, Chirla A, Cosmo G, Worley S, et al. Successful surgery for epilepsy due to early brain lesions despite generalized EEG findings. Neurology. 2007;69:389–97. doi: 10.1212/01.wnl.0000266386.55715.3f. [DOI] [PubMed] [Google Scholar]
- 31.Schiller Y, Cascino GD, Sharbrough FW. Chronic intracranial EEG monitoring for localizing the epileptogenic zone: an electroclinical correlation. Epilepsia. 1998;39:1302–8. doi: 10.1111/j.1528-1157.1998.tb01328.x. [DOI] [PubMed] [Google Scholar]
- 32.Duchowny M. Epilepsy surgery in children. Curr Opin Neurol. 1995;8:112–6. doi: 10.1097/00019052-199504000-00004. [DOI] [PubMed] [Google Scholar]
- 33.Pataraia E, Baumgartner C, Lindinger G, Deecke L. Magnetoencephalography in presurgical epilepsy evaluation. Neurosurg Rev. 2002;25:141–59. doi: 10.1007/s10143-001-0197-2. [DOI] [PubMed] [Google Scholar]
- 34.Gleissner U, Sassen R, Schramm J, Elger CE, Helmstaedter C. Greater functional recovery after temporal lobe epilepsy surgery in children. Brain. 2005;128:2822–9. doi: 10.1093/brain/awh597. [DOI] [PubMed] [Google Scholar]
- 35.Sinclair DB, Aronyk KE, Snyder TJ, Wheatley BM, McKean JD, Bhargava R, et al. Pediatric epilepsy surgery at the University of Alberta: 1988-2000. Pediatr Neurol. 2003;29:302–11. doi: 10.1016/s0887-8994(03)00307-2. [DOI] [PubMed] [Google Scholar]
- 36.Bell M.L, Rao S, So EL, Trenerry M, Kazemi N, Stead SM, et al. Epilepsy surgery outcomes in temporal lobe epilepsy with a normal MRI. Epilepsia. 2009;50:2053–60. doi: 10.1111/j.1528-1167.2009.02079.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McIntosh AM, Kalnins RM, Mitchell LA, Fabinyi GC, Briellmann RS, Berkovic SF, et al. Temporal lobectomy: long-term seizure outcome, late recurrence and risks for seizure recurrence. Brain. 2004;127:2018–30. doi: 10.1093/brain/awh221. [DOI] [PubMed] [Google Scholar]
- 38.Willmann O, Wennberg R, May T, Woermann FG, Pohlmann-Eden B. The contribution of 18F-FDG PET in preoperative epilepsy surgery evaluation for patients with temporal lobe epilepsy A meta-analysis. Seizure. 2007;16:509–20. doi: 10.1016/j.seizure.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 39.Helmstaedter C, Elger CE. Chronic temporal lobe epilepsy: A neurodevelopmental or progressively dementing disease? Brain. 2009;132:2822–30. doi: 10.1093/brain/awp182. [DOI] [PubMed] [Google Scholar]
- 40.Szabo CA, Wyllie E. Intracarotid amobarbital testing for language and memory dominance in children. Epilepsy Res. 1993;15:239–46. doi: 10.1016/0920-1211(93)90061-b. [DOI] [PubMed] [Google Scholar]
- 41.Cataltepe O, Weaver J. Anteromesial temporal lobectomy. Pediatric Epilepsy Surgery: Preoperative assessment and surgical treatment. In: Cataltepe O, Jallo GI, editors. Pediatric Epilepsy Surgery Preoperatiev asessment and Surgical treatment. New York, NY: Thieme Medical Publishers Inc; 2010. pp. 136–46. [Google Scholar]
- 42.McKhann GM, 2nd, Schoenfeld-McNeill J, Born DE, Haglund MM, Ojemann GA. Intraoperative hippocampal electrocorticography to predict the extent of hippocampal resection in temporal lobe epilepsy surgery. J Neurosurg. 2000;93:44–52. doi: 10.3171/jns.2000.93.1.0044. [DOI] [PubMed] [Google Scholar]
- 43.Lassonde M, Sauerwein HC, Jambaqué I, Smith ML, Helmstaedter C. Neuropsychology of childhood epilepsy: Pre- and postsurgical assessment. Epileptic Disord. 2000;2:3–13. [PubMed] [Google Scholar]
- 44.Yam D, Nicolle D, Steven DA, Lee D, Hess T, Burneo JG. Visual field deficits following anterior temporal lobectomy: Long-term follow-up and prognostic implications. Epilepsia. 2010;51:1018–23. doi: 10.1111/j.1528-1167.2009.02427.x. [DOI] [PubMed] [Google Scholar]
- 45.Chang EF, Quigg M, Oh MC, Dillon WP, Ward MM, Laxer KD, et al. Predictors of efficacy after stereotactic radiosurgery for medial temporal lobe epilepsy. Neurology. 2010;74:165–72. doi: 10.1212/WNL.0b013e3181c9185d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Duchowny M, Jayakar P, Resnick T, Harvey AS, Alvarez L, Dean P, et al. Epilepsy surgery in the first three years of life. Epilepsia. 1998;39:737–43. doi: 10.1111/j.1528-1157.1998.tb01159.x. [DOI] [PubMed] [Google Scholar]
- 47.Lee YJ, Kang HC, Bae SJ, Kim HD, Kim JT, Lee BI, et al. Comparison of temporal lobectomies of children and adults with intractable temporal lobe epilepsy. Childs Nerv Syst. 2010;26:177–83. doi: 10.1007/s00381-009-1015-3. [DOI] [PubMed] [Google Scholar]
- 48.Mohamed A, Wyllie E, Ruggieri P, Kotagal P, Babb T, Hilbig A, et al. Temporal lobe epilepsy due to hippocampal sclerosis in pediatric candidates for epilepsy surgery. Neurology. 2001;56:1643–9. doi: 10.1212/wnl.56.12.1643. [DOI] [PubMed] [Google Scholar]
- 49.Adler J, Erba G, Winston KR, Welch K, Lombroso CT. Results of surgery for extratemporal partial epilepsy that began in childhood. Arch Neurol. 1991;48:133–40. doi: 10.1001/archneur.1991.00530140025013. [DOI] [PubMed] [Google Scholar]
- 50.Gilliam F, Wyllie E, Kashden J, Faught E, Kotagal P, Bebin M, et al. Epilepsy surgery outcome: Comprehensive assessment in children. Neurology. 1997;48:1368–74. doi: 10.1212/wnl.48.5.1368. [DOI] [PubMed] [Google Scholar]
- 51.Davies KG, Weeks RD. Results of cortical resection for intractable epilepsy using intra-operative corticography without chronic intracranial recording. Br J Neurosurg. 1995;9:7–12. doi: 10.1080/02688699550041683. [DOI] [PubMed] [Google Scholar]
- 52.Hamiwka LD, Grondin RT, Madsen JR. Surgical approaches in cortical dysplasias. In: Cataltepe O, Jallo GI, editors. Pediatric Epilepsy Surgery. Preoperatiev asessment and Surgical treatment. New York, NY: Thieme Medical Publishers Inc; 2010. pp. 185–95. [Google Scholar]
- 53.Daniel RT, Babu KS, Jacob R. Posterior Quadrantic resection and disconnection. In: Cataltepe O, Jallo GI, editors. Pediatric Epilepsy Surgery. Preoperatiev asessment and Surgical treatment. New York, NY: Thieme Medical Publishers Inc; 2010. pp. 197–204. [Google Scholar]
- 54.Buchhalter JR, Jarrar RG. Therapeutics in pediatric epilepsy, Part 2: Epilepsy surgery and vagus nerve stimulation. Mayo Clin Proc. 2003;78:371–8. doi: 10.4065/78.3.371. [DOI] [PubMed] [Google Scholar]
- 55.Rasmussen T. Hemispherectomy for seizures revisited. Can J Neurol Sci. 1983;10:71–8. doi: 10.1017/s0317167100044668. [DOI] [PubMed] [Google Scholar]
- 56.Curtiss S, De Bode S, Mathern GW. Spoken language outcomes after hemispherectomy: Factoring in etiology. Brain Lang. 2001;79:379–96. doi: 10.1006/brln.2001.2487. [DOI] [PubMed] [Google Scholar]
- 57.Delalande O, Bulteau C, Dellatolas G, Fohlen M, Jalin C, Buret V, et al. Vertical parasagittal hemispherotomy: Surgical procedures and clinical long-term outcomes in a population of 83 children. Neurosurgery. 2007;60:19–32. doi: 10.1227/01.NEU.0000249246.48299.12. [DOI] [PubMed] [Google Scholar]
- 58.Villemure JG, Mascott CR. Peri-insular hemispherotomy: Surgical principles and anatomy. Neurosurgery. 1995;37:975–81. doi: 10.1227/00006123-199511000-00018. [DOI] [PubMed] [Google Scholar]
- 59.Nordgren RE, Reeves AG, Viguera AC, Roberts DW. Corpus callosotomy for intractable seizures in the pediatric age group. Arch Neurol. 1991;48:364–72. doi: 10.1001/archneur.1991.00530160028010. [DOI] [PubMed] [Google Scholar]
- 60.Murphy JV. Left vagal nerve stimulation in children with medically refractory epilepsy. The Pediatric VNS Study Group. J Pediatr. 1999;134:563–6. doi: 10.1016/s0022-3476(99)70241-6. [DOI] [PubMed] [Google Scholar]
- 61.Hosain S, Nikalov B, Harden C, Li M, Fraser R, Labar D. Vagus nerve stimulation treatment for Lennox-Gastaut syndrome. J Child Neurol. 2000;15:509–12. doi: 10.1177/088307380001500803. [DOI] [PubMed] [Google Scholar]
- 62.Halpern CH, Samadani U, Litt B, Jaggi JL, Baltuch GH. Deep brain stimulation for epilepsy. Neurotherapeutics. 2008;5:59–67. doi: 10.1016/j.nurt.2007.10.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fisher R, Salanova V, Witt T, Worth R, Henry T, Gross R, et al. Electrical stimulation of the anterior nucleus of thalamus for treatment of refractory epilepsy. Epilepsia. 2010;51:899–908. doi: 10.1111/j.1528-1167.2010.02536.x. [DOI] [PubMed] [Google Scholar]
- 64.Spencer SS, Schramm J, Wyler A, O’Connor M, Orbach D, Krauss G, et al. Multiple subpial transection for intractable partial epilepsy: An international meta-analysis. Epilepsia. 2002;43:141–5. doi: 10.1046/j.1528-1157.2002.28101.x. [DOI] [PubMed] [Google Scholar]
- 65.Schramm J, Aliashkevich AF, Grunwald T. Multiple subpial transections: Outcome and complications in 20 patients who did not undergo resection. J Neurosurg. 2002;97:39–47. doi: 10.3171/jns.2002.97.1.0039. [DOI] [PubMed] [Google Scholar]
- 66.Berkovic SF, Arzimanoglou A, Kuzniecky R, Harvey AS, Palmini A, Andermann F. Hypothalamic hamartoma and seizures: A treatable epileptic encephalopathy. Epilepsia. 2003;44:969–73. doi: 10.1046/j.1528-1157.2003.59102.x. [DOI] [PubMed] [Google Scholar]
- 67.Polkey CE. Resective surgery for hypothalamic hamartoma. Epileptic Disord. 2003;5:281–6. [PubMed] [Google Scholar]
- 68.Addas B, Sherman EM, Hader WJ. Surgical management of hypothalamic hamartomas in patients with gelastic epilepsy. Neurosurg Focus. 2008;25:E8. doi: 10.3171/FOC/2008/25/9/E8. [DOI] [PubMed] [Google Scholar]
- 69.Wyllie E. Surgical treatment of epilepsy in pediatric patients. Can J Neurol Sci. 2000;27:106–10. [PubMed] [Google Scholar]


