Abstract
There are 50 million people living with epilepsy worldwide, and most of them reside in developing countries. About 10 million persons with epilepsy are there in India. Many people with active epilepsy do not receive appropriate treatment for their condition, leading to large treatment gap. The lack of knowledge of antiepileptic drugs, poverty, cultural beliefs, stigma, poor health infrastructure, and shortage of trained professionals contribute for the treatment gap. Infectious diseases play an important role in seizures and long-term burden causing both new-onset epilepsy and status epilepticus. Proper education and appropriate health care services can make tremendous change in a country like India. There have been many original researches in various aspects of epilepsy across India. Some of the geographically specific epilepsies occur only in certain regions of our country which have been highlighted by authors. Even the pre-surgical evaluation and epilepsy surgery in patients with drug-resistant epilepsy is available in many centers in our country. This article attempts to provide a complete preview of epilepsy in India.
Keywords: Epilepsy in India, epilepsy surgery, hot water epilepsy, progressive myoclonic epilepsy, status epilepticus, treatment gap, women with epilepsy
Introduction
Epilepsy is a disorder of the brain which is characterized by an enduring predisposition to generate seizures and by its neurobiological, cognitive, psychological, and social consequences. Epilepsy is defined by International League Against Epilepsy (ILAE; 1993) as a condition characterized by recurrent (two or more) epileptic seizures, unprovoked by any immediate identified cause.[1] According to the World Health Organization (WHO), of the 50 million people with epilepsy worldwide, 80% reside in developing countries.[2] Epilepsy was estimated to account for 0.5% of the global burden of disease, accounting for 7,307,975 disability adjusted life years (DALYs) in 2005.[2]
Descriptive Epidemiology and Treatment Gap
It is estimated that there are more than 10 million persons with epilepsy (PWE) in India. Its prevalence is about 1% in our population.[3] The prevalence is higher in the rural (1.9%) compared to urban population (0.6%)[4,5] In the Bangalore Urban Rural Neuro-epidemiological Survey (BURNS), a prevalence rate of 8.8/1000 population was observed, with the rate in rural communities (11.9) being twice that of urban areas (5.7).[6] The estimated burden of epilepsy using the DALYs accounts for 1% of the total burden of disease in the world, excluding that due to social stigma and isolation, which further add to the disease burden.[7] Here, we provide an overview of epilepsy in India, and the recent developments and their applications in the field of epilepsy. This review also deals with the epidemiological estimates, causes, and risk factors of epilepsy in India, and the role of epilepsy surgery in India and its need. In India, with less than 2000 neurologists and an estimated 5-6 million patients with active epilepsy, there is a huge need to strengthen epilepsy services, particularly in the rural and underserved areas, as the prevalence in rural areas is much higher [Tables 1 and 2].
Table 1.
Bangalore Urban Rural Neuro-epidemiological Survey (BURNS) prevalence data of epilepsy: Age specific
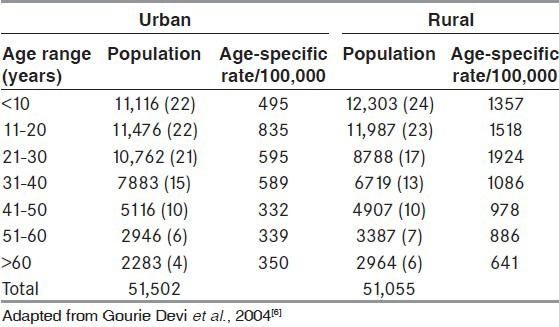
Table 2.
Epidemiology of epilepsy in India
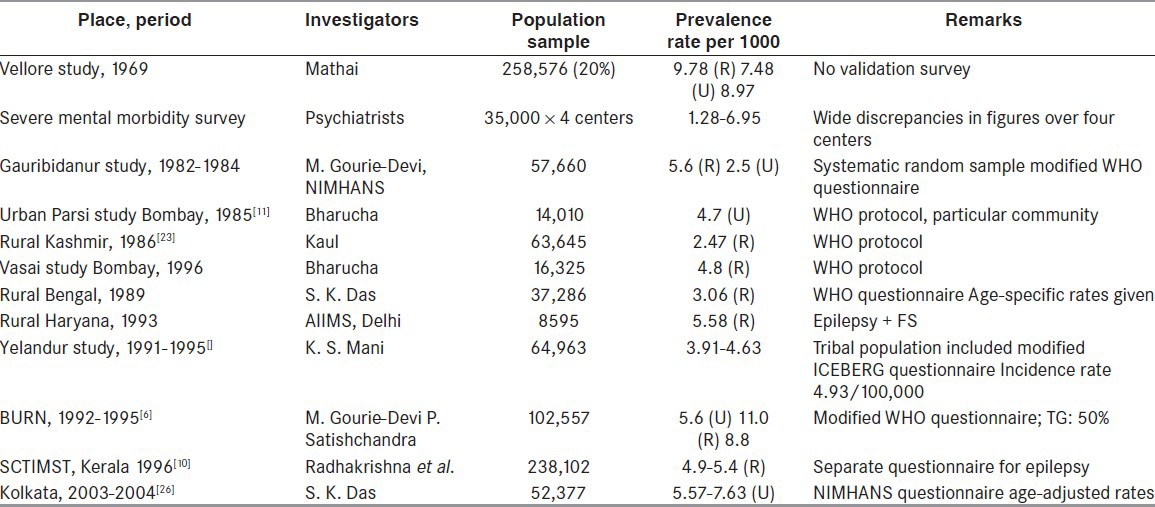
It is estimated in various studies that the overall prevalence of epilepsy in India is 5.59-10 per 1000.[3,6,8,9] The age-adjusted prevalence ratio of active epilepsy in Kerala is 4.7 per 1000 population.[10] Methodological differences in prevalence estimation, such as variations in defining the epilepsy, failure to exclude pseudo-seizures, syncope, and other mimickers of epilepsy, and missed cases because of concealment may influence the exact prevalence. More number of males getting affected by epilepsy was reported in a study conducted in India by Bharucha et al.[11] in which prevalence in males (5.1 per 1000) was significantly higher than that in females (2.2 per 100) [Tables 1, 2].
There are very few incidence studies from India, and the most recent one suggests an age-standardized incidence rate of 27.3/100,000 per year.[12] The exact magnitude of medically intractable epilepsy in India is unknown. Hot water epilepsy (HWE), a kind of reflex epilepsy in which seizures are induced by pouring hot water rapidly over the head, has been reported from South India, the prevalence of which varies from 1.14 to 2.99 cases/1000 population.[13,14] HWE was reported to account for 3.6-3.9% of all epilepsy cases in rural South India.[15]
Treatment gap
In many developing countries, people with epilepsy do not receive appropriate treatment for their condition, a phenomenon called treatment gap (TG), which is defined as the number of people with active epilepsy not on treatment (diagnostic and therapeutic) or on inadequate treatment, expressed as a percentage of the total number with active epilepsy.[16] The TG has two components: Those not accessing or unable to access biomedical facilities for diagnosis and treatment and, if accessing biomedical treatment, those not adhering to the prescribed antiepileptic drugs (AEDs). The gap is reported to be influenced by various factors, including lack of access to or knowledge of AEDs, poverty, cultural beliefs, stigma, poor health delivery infrastructure, and shortage of trained professionals.[16,17] The superstitions and cultural beliefs influence PWE to seek treatment from traditional healers instead of allopathic practitioners.[18,19,20,21]
The magnitude of epilepsy treatment gap in India ranges from 22% among urban, middle-income people to 90% in villages.[22] In order to reduce this gap in the context of limited resources, it would be necessary to specify the important cause of gap for a particular community and the most cost-effective resource for a particular situation.[11,23,24,25]
Etiology and Types of Epilepsy
The 1993 ILAE recommendations for epidemiologic studies suggested to divide the symptomatic epilepsies into “remote” and “progressive” types, wherein remote symptomatic epilepsies encompass cases developing following insults resulting in static lesions [such as those attributable to conditions such as stroke, head injury, or central nervous system (CNS) infections] and progressive symptomatic epilepsy encompasses epilepsies associated with progressive illnesses (e.g. brain tumors or degenerative disease). Bharucha et al.[11] reported that symptomatic epilepsy constituted 23% (progressive- 2%, remote symptomatic- 21%) and idiopathic epilepsy about 77%. They also reported that 54% had partial and 46% had generalized seizures. In another study by Koul et al.,[23] the seizure types were found to be partial seizures in 12%, generalized seizures in 79%, and unclassified type in 9% in a population in rural Kashmir.
All the three population-based case-control studies in India found febrile seizures to be a significant risk factor for developing epilepsy.[11,25,27] The Chandigarh Study[30] found in addition that head injury, developmental delay, and family history of epilepsy were significant risk factors. Among the risk factors of epilepsy, Kannoth et al.[28] further reported along with the family history of epilepsy, antecedent history of febrile seizures, birth by complicated delivery, and neonatal seizures as strong independent predictors of epilepsy. There were more similarities than differences in the distribution of risk factors between generalized and localization-related epilepsy syndromes.[28]
Single computed tomography enhancing lesion (SCTEL) is defined as disc or ring CT measuring less than 20 mm in size and enhancing on contrast administration. Single enhancing CT lesions are the commonest radiological abnormality in Indian patients with new-onset partial seizures. Histological studies suggest that SCTEL represents dying cysticerci that will spontaneously resolve as a result of host response. Infections of CNS including SCTEL account for 77% of patients with acute symptomatic epilepsy. Small single cerebral calcific CT lesion (SSCCCTL) is defined as single calcific lesion measuring less than 20 mm. Seizures are the commonest presentation of neurocysticercosis (50-80%)[29] Among 991 patients of symptomatic localization-related epilepsy studied, infections of CNS including single CT enhancing lesion (SCTEL) accounted for 77% of patients with acute symptomatic epilepsy. Cerebrovascular diseases were the risk factors in 48% of patients with remote symptomatic epilepsy. Neurocysticercosis, SCTEL, and SSCCCTL together accounted for 40% of etiological factors and neurotuberculosis for 10% of the factors. Infections of the CNS and SCTEL together were the putative risk factors in 52% of patients aged ≤40 years. Cerebrovascular diseases were the etiological factor in 64% of patients aged >40 years in South India, as reported by Murthy et al.[30] Mani et al.[31] reported a diagnosis of NCC in 2% of unselected series of epilepsy. At a tertiary referral center in New Delhi, NCC constituted 2.5% of all intracranial space-occupying lesions.[32] In a community survey of 50,617 individuals from South India, the prevalence of active epilepsy was 3.83 per 1000 and NCC was detected in 28.4% of them by CT.[33] Single cyst infection (range 47.7-53.4%) is the most common in the Indian subcontinent.[34,35] Serial magnetic resonance imaging (MRI) was performed in a cohort of patients with solitary cerebral cysticerci manifesting with new-onset seizures from this center. It was reported that albendazole therapy may hasten resolution of inflammation around the lesion, but affects neither the morphology of the NCC nor the process of degeneration and subsequent healing.[36]
The CNS infections are a major cause for acute symptomatic status epilepticus (SE), with an prevalence varying from 17.5 to 39.8% in various studies.[37,38,39,40] In an effort to understand the underlying pathogenesis of epilepsy, Sinha et al. studied the serum cytokine levels in 100 patients with epilepsy during the immediate post-ictal phase. They found that the detectable serum cytokines in the patient group (n = 100) were interleukin (IL)-6 (42), tumor necrosis factor (TNF)-alpha (36), IL-2 (22), IL-4 (22), IFN-gamma (20), and IL-1 (11) compared to the controls.[41]
HIV and seizures
Seizures are not uncommon in patients with human immunodeficiency virus (HIV) infection, and the upsurge in HIV infection may be an important cause for acute symptomatic seizures. Neurological disorders are the presenting manifestation in 5-10% of patients with acquired immune deficiency syndrome (AIDS) and CNS involvement is noted in 90% cases at autopsy.[42] New-onset seizures were noted in 20% of HIV-seropositive individuals with neurological manifestations at our center.[43] Cryptococcal meningitis was diagnosed in 33% cases; of these, 25% manifested with seizures. Toxoplasmosis with granuloma or acute encephalitis appears to be the commonest cause for recent-onset seizures associated with HIV infection. Focal neurological deficits occurred in 69% and seizure in 29% of toxoplasmosis of CNS.[44] Earlier series reported seizures in 44% of 99 patients with HIV-associated CNS tuberculosis.[43] Of 26 patients with HIV-associated progressive multifocal leukoencephalopathy (PML), 19% manifested with seizures. Only two cases of primary CNS lymphoma were seen among our cohort of 1005 HIV-infected individuals with neurological manifestations.[45,46] Seizures occur frequently in advanced HIV disease, with opportunistic infections being the main cause. Chadha et al. identified causes like cerebral toxoplasmosis in 7 (30.43%), cryptococcal meningitis in 4 (17.39%), tuberculoma in 3 (13.04%), AIDS dementia complex in 1 (4.34%), and PML in 1 (4.34%) patient.[47]
Other infections and seizures
Acute seizures occur in about 50% of children and in 5% of adults with tubercular meningitis.[48] Seizures occur in about 10% of patients with rabies.[49] Among other infections, Japanese B encephalitis and malaria cause seizures.
The term co-morbidities refers to the co-occurrence of more than one condition in the same person and in people living with epilepsy (PWE).[50] In a hospital-based case-control study conducted at National Institute of Mental Health and Neurosciences (NIMHANS), Bangalore, 60.8% PWE had at least one co-morbidity and 24.8% had at least two or more co-morbidities such as migraine (26%), sleep-related disorders like insomnia (6.9%), depression (5%), and anxiety (2.4%). The most common psychiatric conditions in adults with epilepsy were: Depression (5%), anxiety (2.4%), and psychoses (1.6%).[51]
Genetics and Epilepsy
About 12-27% of HWE epilepsy patients have positive family history. In an ongoing effort to understand the molecular basis of HWE, seven HWE families with several of their members affected with the disorder were examined and two loci for HWE at chromosomes 10q21.3-q22.3[52] and 4q24-q28[53] were identified. Exome sequencing suggested EATT3 or SLC1A1 gene, a glutamate transporter gene, as the causative gene. Absence of GABRA1 Ala 322Asp mutation has been noted in families of juvenile myoclonic epilepsy (JME) from South India.[54] Further, in another study, association of KCNQ3 gene was found in JME patients from Kerala.[55] There might be a protective role of CAG19 allele of hSKCa3 (calcium channel) gene in JME patients.[56] There is a susceptibility effect of CAG16 and CAG18 alleles of hSKCa3 gene in JME patients from Kerala.[56] A locus for JME has been mapped to 2q33-q36 and 5q12-q14 in South Indian population.[57] In another study from northern India, it is reported that polymorphisms in BRD2 and LGI4 might be risk factors for JME.[58]
Lafora body disease (LBD) is caused by mutation in the PME2 gene (EPM2A) on chromosome 6q and EPM2B gene.[59] The official name of this gene is “epilepsy, progressive myoclonus type 2A, Lafora disease (laforin).” EPM2A is the gene's official symbol. The EPM2A gene located at 6q24 provides instructions for making a protein called laforin.[60] Early brain developmental abnormalities involving neuronal migration and lamination are often implicated in childhood epilepsy. Reelin (RELN), a neuronal-signaling molecule, plays a crucial role in these migratory processes and is located on human chromosome 7q22. It is considered as a potential candidate gene for childhood epilepsy. In a study conducted at West Bengal in India, 63 patients with childhood-onset epilepsy and 103 healthy controls were recruited. Case-control analysis revealed significant over-representation of G/C and (G/C + C/C) genotypes and C allele of exon 22 G/C marker (rs362691) in cases as compared to controls.[61]
Autonomic Changes in Epilepsy
Activation of the autonomic nervous system is well known in patients with epilepsy during either electroconvulsive therapy (ECT) or spontaneous seizure.[62] Inter-ictal and ictal epileptic activity may involve many areas of central autonomic network (CAN) and induce autonomic symptoms during seizures, potentially affecting autonomic cardiac control inter-ictally and ictally.[63] Sathyaprabha et al. showed autonomic dysfunction in 56.3% of patients with chronic refractory epilepsy. Correlation of duration of illness with autonomic dysfunction was evident in this study, but AEDs did not show any significant role in autonomic functions.[64] In a retrospective analysis of eight patients with absence epilepsy, transient increase in heart rate during inter-ictal discharge (IED) phase was noted.[65] In another study at this center on drug-naïve new-onset 20 patients with epilepsy, subtle but definite cardiac autonomic dysfunction, especially in vagal tone, was identified.[66] Practice of yoga might have a role as an adjuvant therapy in the management of autonomic dysfunction in patients with refractory epilepsy.[67]
Progressive Myoclonic Epilepsy in India
Progressive myoclonic epilepsy (PME) is a disease complex characterized by the development of relentlessly progressive myoclonus, cognitive impairment, ataxia, and other neurological deficits. It encompasses different diagnostic entities, and the common causes include Lafora body disease [Figure 1a and b], neuronal ceroid lipofuscinosis (NCL), Unverricht-Lundborg disease (ULD), myoclonic epilepsy with ragged-red fiber (MERRF) syndrome, sialidoses, dentato-rubro-pallidal atrophy, storage diseases, and some of the inborn errors of metabolism, among others. A total of 147 patients with PME have been evaluated at NIMHANS, Bangalore, India till date, including cases with the following: LBD: 54; NCL: 65; ULD: 8; MERRF: 10; and Tay Sachs Disease (TSD): 10. Most of the patients presented with the classical triad: Myoclonus, cognitive decline, and neurological deficits.[68,69]
Figure 1.
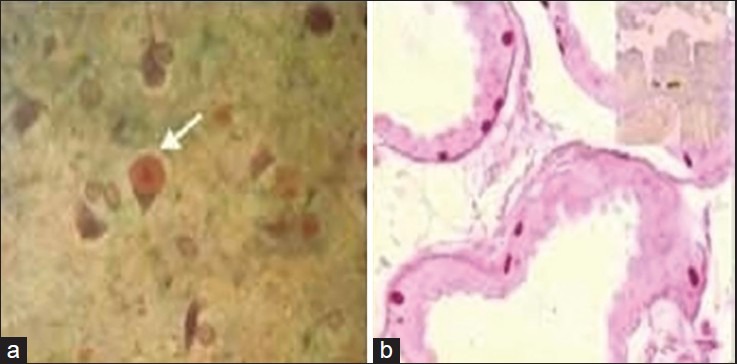
(a) Intraneuronal PAS-positive, diastase-resistant Lafora body in the cytoplasm of neurons in the cerebral cortex (×160); (b) PAS-positive diastase-resistant Lafora bodies along the base of apocrine sweat glands within the myoepithelial cells (×160)
Special Group of Epilepsies
Hot water epilepsy
Seizures precipitated by a specific sensory stimulus are described as reflex epilepsy. HWE is precipitated by the stimulus of bathing in hot water poured over the head, and is also variably known as water-immersion epilepsy or bathing epilepsy. It is from southern India that a large number of cases of HWE have been reported. The exact etiopathogenesis of this type of epilepsy is not clear, but several factors including genetic factors, environmental factors, consanguineous marriages, and habit of taking bath with high-temperature water has been postulated as probable reasons.[70] Reports from our center suggest that the cohort of patients with HWE is mainly from two adjoining districts, viz. Mandya-Mysore belt in Karnataka state of South India.[71] HWE was reported to account for 3.6-3.9% of all epilepsy cases in this part of India.[72] Triggering factors were mainly temperature of the water and contact of water on the scalp. Studies have shown a strong family history of epilepsy in these subjects from India (7-18%).[70]
Even though the exact mechanism of HWE is not clear, many theories have been proposed to explain the peculiar nature of seizures. Earlier, Satishchandra et al.[73] had postulated that patients with HWE probably have an aberrant thermoregulatory system and are probably sensitive to a rapid rise in temperature. The impaired sympathovagal balance is observed in HWE, characterized by increased sympathetic activity and reduced parasympathetic activity in patients with HWE. The hypothalamus is involved in both the pathogenesis of HWE and autonomic regulation. Intermittent therapy with clobazam, 1-1½ h prior to hot water head bath, is the method of treatment for this type of epilepsy. AEDs are required only if HWE is associated with non-reflex seizures.
Eating epilepsy
In 1984, 13 cases of eating epilepsy were reported from South India.[74] It was very frequently reported from Sri Lanka. Recently, six patients with eating epilepsy were studied in depth using MRI, single-photon emission computed tomography (SPECT) scan, and video encephalogram (EEG) from NIMHANS, and presence of lesions around perisylvian area has been identified. There might be an involvement of complex neuronal circuits around the Sylvian fissure (near area of face) responsible for eating epilepsy.[75]
Women with epilepsy
There are close to 1.5 million women with epilepsy (WWE) in reproductive age in India. About a sixth of WWE in the world are living in India. It is estimated that there are about 2.73 million WWE in India and 52% of them are in the reproductive (15-49 years) age group.[76] Sahota et al. reported that primary generalized seizures were seen in 53.6% women in their series. Sodium valproate was the most commonly used primary AED, alone or in combination with lamotrigine, followed by phenytoin and carbamazepine. Menstrual abnormalities, hirsutism, or both were seen in 19.4% women on these AEDs as untoward effects; of these, 60.2% received valproate, 25.3% received carbamazepine, 13.3% received phenytoin alone or in combination, and 1.2% received phenobarbitone alone. Polycystic ovaries were detected in 23.5% of valproate-treated women and 25% of carbamazepine-treated women.[77]
Exposure to AEDs in the first trimester of pregnancy has been associated with an increased risk of major congenital anomalies (MCAs) in offspring. MCM is defined as an abnormality that can interfere with the quality of life and warrants definite management. Nearly all studies on the adverse fetal effects of AEDs have methodological shortcomings. Studies done up to late 1990s suggest that the risk of MCAs in infants exposed to older-generation AEDs in the first trimester of pregnancy is about two to three times higher than in the general population (4-10% vs. 2-5%, respectively).[78,79,80] No single MCA has been associated most commonly with a specific AED, with the exception of spina bifida which is more common with exposure to valproic acid (1-5% of exposed offspring) and carbamazepine (0.5-1.0%) than with other AEDs. Valproic acid is also the only AED for which dose-dependency has been ascribed in several studies, with the increase in MCA risk, compared with other AEDs, being reportedly most evident at doses above 800-1000 mg/day.[81,82,83,84,85,86]
The data from Kerala Registry of Epilepsy and Pregnancy (KREP) indicate that anemia, ovarian cyst and fibroid uterus, and spontaneous abortions are more frequent in WWE.[87] In the study carried out by KREP, congenital malformations were detected in 12.5%. The odds ratio for the risk of malformation was much higher with sodium valproate (OR-6) than with carbamazepine (OR-1.2) or phenobarbitone (OR-0.8).[88]
Among the predictors of seizures during pregnancy in WWE, the main observations were that women with partial seizures on AED polytherapy or with at least one seizure in the pre-pregnancy month had higher risk of seizures during pregnancy.[89] In the study conducted by the same center regarding the recurrence of MCM, of 492 pregnancies in 246 women, 43 pregnancies had MCM (42 women). The MCM rates were 8.5% in index pregnancy and 8.9% in follow-up pregnancy. The data suggested that the malformation recurrence risk may be dose dependent, and at low dose, there may not be increased risk of recurrence.[90]
The AED usage may lead to folate deficiency which may predispose to neural tube defects.[91] Other mechanisms under consideration include alteration in the homeobox genes, retinoic acid signaling pathways, histone deacetylators, and polymorphisms involving AED transporters.[92,93] WWE who can potentially become pregnant need to be started on folic acid 5 mg daily, 1 or 2 months prior to anticipated pregnancy. All WWE who are using AEDs are advised to take vitamin K 10 mg intramuscular (IM) at 34 and 36 weeks of pregnancy.
Epilepsy and seizures may alter the hypothalamo-pituitary-ovarian axis and can lead to derangement of reproductive hormone levels. In a study conducted in Kerala on WWE and infertility, it was observed that the WWE-infertility group had different reproductive hormone profile than others; the luteinizing hormone (LH)/follicular stimulating hormone (FSH) ratio was significantly abnormal among the infertility group. This ratio was significantly abnormal in the follicular phase and luteal phase. The WWE-infertility group had 8.5 times higher risk of abnormal LH/FSH ratio.[94]
Status Epilepticus
The Commission on Classification and Terminology of the ILAE defines SE as a seizure that persists for a sufficient length of time or is repeated frequently enough that recovery between attacks does not occur (ILAE, 1981). Refractory status epilepticus (RSE) is a medical emergency with high morbidity and mortality, and the continuous or repetitive seizures are unresponsive to first- and/or second-line AED therapy. In a study involving 98 patients with RSE, Sinha et al. found that 34 had died and seizure control was achieved in two-third of patients.[95]
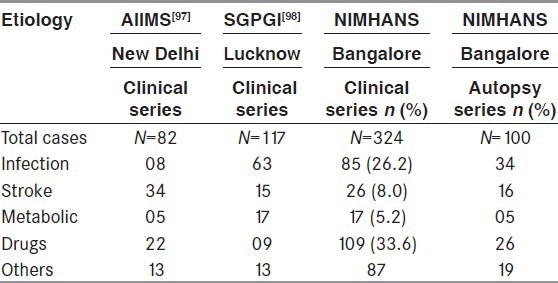
In the clinic-pathological study of 100 cases of SE conducted at NIMHANS, neuroinfection was found in 50% of cases of fatal SE followed by cerebrovascular accidents (21.2%). Patients with prior history of epilepsy had discontinued treatment in 71.4% of cases, resulting in SE. Majority of patients with SE had generalized convulsive seizures (75%).[96] The details of aetiology of SE in India is provided in Table 3.
Table 3.
Etiology of status epilepticus: Indian studies
Abnormalities close to the central sulcus may give rise to long-duration focal motor seizures. This condition is called epilepsia partialis continua (EPC). Analysis of 75 case records of patients with EPC with mean age at presentation of 30.2 ± 23.4 years and with duration of EPC of 47.02 ± 188.2 days was done. Majority of the patients had type I presentation (90.8%), while the remaining seven patients had Rasmusssen's encephalitis (RE) with type II EPC presentation. Patients of age >40 years had stroke, idiopathic, diabetic non-ketotic hyperosmolar coma, and metastasis as the common causes.[99]
In 68 patients with convulsive SE, seizures were aborted in 66% in the valproate group and 42% in the phenytoin group. As a second choice in refractory patients, sodium valproate may be preferred in convulsive SE because of its higher efficacy (79%) as compared to the phenytoin (PHT-25%)[100] Mishra et al. conducted a randomized, open-labeled pilot study comparing the efficacy and safety of levetiracetam and lorazepam in SE in 79 patients. Consecutive patients with convulsive or subtle convulsive SE were randomized to levetiracetam (LEV) 20 mg/kg intravenous (IV) over 15 min or lorazepam (LOR) 0.1 mg/kg over 2-4 min. Both were equally effective. In the first instance, the SE was controlled by LEV in 76.3% (29/38) and by LOR in 75.6% (31/41) of patients. The authors concluded that for the treatment of SE, levetiracetam is an alternative to lorazepam and may be preferred in patients with respiratory compromise and hypotension.[101]
Epilepsy in Elderly
Sinha et al. evaluated 64 elderly patients (32%) who presented with new-onset cluster attacks and/or SE.[102] Cluster seizures were observed in 26.4% and SE in 17% of the 201 elderly patients with seizures. The types of epilepsy syndrome included were: Acute symptomatic (37 patients; 57.8%), cryptogenic (23.4%), and remote symptomatic (18.8%). Inter-ictal EEG was abnormal in 80% of patients with critical presentation and two-third among 201 elderly patients with seizures.[102] Seizures were controlled with two AEDs in 70.6%. The SE and/or cluster seizures are common (32%) among elderly patients with epilepsy. Early and aggressive treatment is effective in a majority of them.[103] Another study from this center that evaluated the imaging (CT/MRI) observations in elderly patients manifesting with new-onset seizures showed that the CT scan (n = 201) revealed cerebral atrophy (139), focal lesions (98), infarcts (45), hemorrhages (18), granuloma (16), tumor (15), gliosis (4), hemispheric atrophy (1), white matter changes (75) and diffuse edema (21). The MRI (n = 43) showed variable degree of cerebral atrophy (31), white matter changes (20), focal cerebral lesions (24), infarct (7), intracranial hemorrhage (6), granuloma (5), tumor (6), gliosis (1), hemispheric atrophy (1), prominent Virchow-Robin spaces (7), and unidentified bright objects (UBOs-12). Patients with focal lesions on neuroimaging more often had partial seizures, symptomatic epilepsy, past stroke, focal deficit, absence of diffuse atrophy, focal EEG slowing, abnormal cerebrospinal fluid (CSF), and seizure recurrence at follow-up.[104]
Epilepsy in Children
A pediatric epilepsy study from Mumbai (using 1981/1989 classification) reported 55.3% partial, 27% generalized, 13.5% undetermined, and 4.1% specific epilepsy syndromes.[105] In a study of 123 children with “difficult-to-control” epilepsy, onset below 2 years of age, male sex, other neurological abnormalities, and certain seizure types emerged as risk factors for refractoriness. Perinatal insults seem to predominate the etiological spectrum.[106] They contributed to about 50% of symptomatic epilepsies with onset in the first 3 years of life in the study.[106] In later childhood and adolescence, symptomatic epilepsies due to an underlying structural cause tend to mainly constitute the refractory group. In children, NCC has been implicated in 0.4% of all neurological complaints.[107]
Refractory Epilepsy in India
Intractable epilepsy is defined as occurrence of two or more seizures per month for a period of more than 2 years despite using two or more AEDs in maximum tolerated (2 × 2 × 2) dose. According to the study in North India by Manjari T et al In the intractable group, 83% of patients had partial seizures, and 7% had generalized onset. The significant predictors of intractability were radiological evidence of structural cerebral abnormality, non-response to first AED, delayed milestones, high initial seizure frequency of more than one per month, partial seizure type, age of onset before 14 years, and febrile convulsions. The most common radiological features in the intractable group were known epileptogenic structural abnormalities such as mesial temporal sclerosis (MTS), dysembryoplastic neuroepithelial tumor (DNET), and perinatal hypoxic ischemic brain injuries.[108]
Surgical Management for Epilepsy
As many as 70-80% of persons with newly diagnosed epilepsy would eventually achieve remission, majority of them achieving it within 2 years of the onset of epilepsy.[109] About 20-30% of persons developing epilepsy continue to exhibit chronic recurrent seizures despite optimal treatment with AEDs.[110]
Epilepsy surgery in India
Nearly one-third of the patients with newly diagnosed epilepsy on long-term follow-up will have their seizures unsatisfactorily controlled by treatment with available AEDs.[111] Comprehensive epilepsy surgery centers with advanced imaging tools such as MRI, SPECT, positron emission tomography (PET), and magnetoencephalography (MEG), as well as a multidisciplinary team of neurologists, neurosurgeons, neuroradiologists, electrophysiologists, psychologists, psychiatrists, and medical social workers are needed in a country like ours. The first epilepsy surgery in India was performed on 25 August 1952 at Christian Medical College (CMC) in Vellore. The first epilepsy surgery at Sree Chitra Tirunal Institute for Medical Sciences and Technology (SCTMIST), Thiruvananthapuram was conducted in 1995. Almost simultaneously, epilepsy surgery programs were started at the All India Institute of Medical Sciences (AIIMS), New Delhi and NIMHANS, Bangalore. During the last one and half decades, these three centers together have undertaken over 2000 epilepsy surgeries.
India with over 1.2 billion people will have over 1 million people with medically refractory epilepsies, of which nearly one half are potential surgical candidates. The success of any epilepsy surgery program depends upon the early identification of potential surgical candidates. Eventually after temporal lobe resective epilepsy surgery, there is over 70% probability that the patient will be seizure-free and over 30% chance of being free of AEDs within 2 years after surgery.[112,113]
The decision making for epilepsy surgery needs a multidisciplinary approach in which different investigators involved with the program work in conjunction to create an integrated picture of epileptogenesis and its impact on the patient and care givers. Knowing when not to operate, because of the need for further investigations is as important as selecting which patient may benefit from surgery in a resource-limited setting.
IEA and Hindu Marriage Act
The Indian Epilepsy Association (IEA) was formed with nine members of the first governing council. These members registered the IEA in 1970. The IEA was registered in December 1971 as a Public Charity Trust with a mission to increase epilepsy awareness and also increase acceptance of PWE to provide relief and rehabilitation to patients and their families. It started with 16 chapters and has 25 chapters today all over India, with a total membership of 1597. The main functions of IEA are counseling of PWE and enhancing public awareness about epilepsy, reaching out to the poor and illiterate PWE for better quality of life. It creates awareness among doctors, paramedics, and lay public, and publishes newsletters. It has also fought legal battles to change the policies like Hindu Marriage Act of 1955. This along with the Special Marriage Act of 1954 stated that a marriage under these acts can be solemnized “if at the time of marriage, neither party suffers from recurrent attacks of insanity or epilepsy.” It took 12 years for Dr. K. S. Mani, often referred as “father of Indian epilepsy,” and the IEA to have the word “epilepsy” deleted from this law. This was achieved in December 1999.[114]
Conclusions
The burden of epilepsy could be reduced in India by alleviating poverty and by reducing the preventable causes, viz. perinatal insults, parasitic diseases, and head injuries. Empowering primary healthcare workers to diagnose and start treatment might significantly reduce the treatment gap and the disparities between rural and urban areas. In India with over 500,000 potential epilepsy surgery candidates, not more than 200 epilepsy surgeries per year are being undertaken today. Thus, only a miniscule of potential surgical candidates in our country ever gets a chance to undergo pre-surgical evaluation and surgery. This enormous surgical treatment gap can only be minimized by developing many more epilepsy surgery centers all over India.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
References
- 1.Hauser WA, Kurland LT. The epidemiology of epilepsy in Rochester, Minnesota, 1935 through 1967. Epilepsia. 1975;16:1–66. doi: 10.1111/j.1528-1157.1975.tb04721.x. [DOI] [PubMed] [Google Scholar]
- 2.Geneva: World Health Organization; 2006. WHO. Neurological Disorders: Public Health Challenges. [Google Scholar]
- 3.Sridharan R, Murthy BN. Prevalence and pattern of epilepsy in India. Epilepsia. 1999;40:631–6. doi: 10.1111/j.1528-1157.1999.tb05566.x. [DOI] [PubMed] [Google Scholar]
- 4.Leonardi M, Ustun TB. The global burden of epilepsy. Epilepsia. 2002;43(Suppl 6):21–5. doi: 10.1046/j.1528-1157.43.s.6.11.x. [DOI] [PubMed] [Google Scholar]
- 5.Pahl K, de Boer HM. Geneva: WHO; 2005. Epilepsy and rights. Atlas: Epilepsy Care in the World; pp. 72–3. [Google Scholar]
- 6.Gourie-Devi M, Gururaj G, Satishchandra P, Subbakrishna DK. Prevalence of neurological disorders in Bangalore, India: A community- based study with a comparison between urban and rural areas. Neuroepidemiology. 2004;23:261–8. doi: 10.1159/000080090. [DOI] [PubMed] [Google Scholar]
- 7.Jain S, Satishchandra P. Epilepsy: A Comprehensive Textbook. In: Engel J Jr, Pedley TA, editors. Vol. 2. New York: Cambridge University Press, Lippincott Williams and Wilkins; 2008. pp. 2885–9. [Google Scholar]
- 8.Sridharan R. Epidemiology of epilepsy. Curr Sci. 2002;82:664–70. [Google Scholar]
- 9.Goel D, Agarwal A, Dhanai JS, Semval VD, Mehrotra V, Saxena V, et al. Comprehensive rural epilepsy surveillance programme in Uttarakhand state of India. Neurol India. 2009;57:355–6. doi: 10.4103/0028-3886.53274. [DOI] [PubMed] [Google Scholar]
- 10.Radhakrishnan K, Pandian JD, Santhoshkumar T, Thomas SV, Deetha TD, Sarma PS, et al. Prevalence, knowledge, attitude, and practice of epilepsy in Kerala, South India. Epilepsia. 2000;41:1027–35. doi: 10.1111/j.1528-1157.2000.tb00289.x. [DOI] [PubMed] [Google Scholar]
- 11.Bharucha NE, Bharucha EP, Bharucha AE, Bhise AV, Schoenberg BS. Prevalence of epilepsy in the Parsi community of Bombay. Epilepsia. 1988;29:111–5. doi: 10.1111/j.1528-1157.1988.tb04405.x. [DOI] [PubMed] [Google Scholar]
- 12.Banerjee TK, Ray BK, Das SK, Hazra A, Ghosal MK, Chaudhuri A, et al. A longitudinal study of epilepsy in Kolkota, India. Epilepsia. 2010;51:2384–91. doi: 10.1111/j.1528-1167.2010.02740.x. [DOI] [PubMed] [Google Scholar]
- 13.Mani KS, Gopalkrishnan PN, Vyas JN, Pillai MS. “Hot-water epilepsy” — a peculiar type of reflex epilepsy. A preliminary report. Neurol India. 1968;16:107–10. [PubMed] [Google Scholar]
- 14.Satishchandra P, Shivaramakrishana A, Kaliaperumal VG, Schoenberg BS. Hot-water epilepsy: A variant of reflex epilepsy in Southern India. Epilepsia. 1988;29:52–6. doi: 10.1111/j.1528-1157.1988.tb05098.x. [DOI] [PubMed] [Google Scholar]
- 15.Gururaj G, Satishchandra P. Correlates of hot water epilepsy in rural south India: A descriptive study. Neuroepidemiology. 1992;11:173–9. doi: 10.1159/000110929. [DOI] [PubMed] [Google Scholar]
- 16.Meinardi H, Scott RA, Reis R, Sander JW ILAE Commission on the Developing World. The treatment gap in epilepsy: The current situation and ways forward. Epilepsia. 2001;42:136–49. doi: 10.1046/j.1528-1157.2001.32800.x. [DOI] [PubMed] [Google Scholar]
- 17.Scott RA, Lhatoo SD, Sander JW. The treatment of epilepsy in developing countries: Where do we go from here? Bull World Health Organ. 2001;79:344–51. [PMC free article] [PubMed] [Google Scholar]
- 18.Pal DK, Das T, Sengupta S. Case-control and qualitative study of attrition in a community epilepsy programme in rural India. Seizure. 2000;9:119–23. doi: 10.1053/seiz.1999.0357. [DOI] [PubMed] [Google Scholar]
- 19.Preux PM, Tiemagni F, Fodzo L, Kandem P, Ngouafong P, Ndonko F, et al. Antiepileptic therapies in the Mifi Province in Cameroon. Epilepsia. 2000;41:432–9. doi: 10.1111/j.1528-1157.2000.tb00185.x. [DOI] [PubMed] [Google Scholar]
- 20.Asawavichienjinda T, Sitthi-Amorn C, Tanyanont W. Compliance with treatment of adult epileptics in a rural district of Thailand. J Med Assoc Thai. 2003;86:46–51. [PubMed] [Google Scholar]
- 21.El Sharkawy G, Newton C, Hartley S. Attitudes and practices of families and health care personnel toward children with epilepsy in Kilifi, Kenya. Epilepsy Behav. 2006;8:201–12. doi: 10.1016/j.yebeh.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 22.Meyer AC, Dua T, Ma J, Saxena S, Birbeck G. Global disparities in the epilepsy treatment gap: A systematic review. Bull World Health Organ. 2010;88:260–6. doi: 10.2471/BLT.09.064147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koul R, Razdan S, Motta A. Prevalence and pattern of epilepsy (Lath/Mirgi/Laran) in rural Kashmir, India. Epilepsia. 1988;29:116–22. doi: 10.1111/j.1528-1157.1988.tb04406.x. [DOI] [PubMed] [Google Scholar]
- 24.Mani KS. Epidemiology of epilepsy in Karnataka, India. Neurosci Today. 1997;1:167–74. [Google Scholar]
- 25.Pal DK. Methodological issues in assessing risk factors for epilepsy in an epidemiologic study in India. Neurology. 1999;53:2058–63. doi: 10.1212/wnl.53.9.2058. [DOI] [PubMed] [Google Scholar]
- 26.Das SK, Biswas A, Roy T, Banerjee TK, Mukherjee CS, Raut DK, et al. A random sample survey for prevalence of major neurological disorders in Kolkata. Indian J Med Res. 2006;124:163–72. [PubMed] [Google Scholar]
- 27.Sawhney IM, Singh A, Kaur P, Suri G, Chopra JS. A case control study and one year follow-up of registered epilepsy cases in a resettlement colony of North India, a developing tropical country. J Neurol Sci. 1999;165:31–5. doi: 10.1016/s0022-510x(99)00069-6. [DOI] [PubMed] [Google Scholar]
- 28.Kannoth S, Unnikrishnan JP, Santhosh Kumar T, Sankara Sarma P, Radhakrishnan K. Risk factors for epilepsy: A population-based case-control study in Kerala, southern India. Epilepsy Behav. 2009;16:58–63. doi: 10.1016/j.yebeh.2009.07.019. [DOI] [PubMed] [Google Scholar]
- 29.Del Brutto OH, Santibañez R, Noboa CA, Aguirre R, Díaz E, Alarcón TA. Epilepsy due to neurocysticercosis: Analysis of 203 patients. Neurology. 1992;42:389–92. doi: 10.1212/wnl.42.2.389. [DOI] [PubMed] [Google Scholar]
- 30.Murthy JM, Yangala R. Etiological spectrum of symptomatic localization related epilepsies: A study from South India. J Neurol Sci. 1998;158:65–70. doi: 10.1016/s0022-510x(98)00093-8. [DOI] [PubMed] [Google Scholar]
- 31.Mani A, Ramesh CK, Ahuja GK. Cysticercosis presenting as epilepsy. Neurol India. 1974;22:30. [Google Scholar]
- 32.Wani MA, Banerji AK, Tandon PN, Bhargava S. Neurocysticercosis: Some uncommon presentations. Neurol India. 1981;29:58–63. [Google Scholar]
- 33.Rajshekhar V, Raghava MV, Prabhakaran V, Oommen A, Muliyil J. Active epilepsy as an index of burden of neurocysticercosis in Vellore district, India. Neurology. 2006;67:2135–9. doi: 10.1212/01.wnl.0000249113.11824.64. [DOI] [PubMed] [Google Scholar]
- 34.Prasad A, Gupta RK, Pradhan S, Tripathi M, Pandey CM, Prasad KN. What triggers seizures in neurocysticercosis? A MRI-based study in pig farming community from a district of North India. Parasitol Int. 2008;57:166–71. doi: 10.1016/j.parint.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 35.Prabhakaran V, Rajshekhar V, Murrell KD, Oommen A. Conformation-sensitive immunoassays improve the serodiagnosis of solitary cysticercus granuloma in Indian patients. Trans R Soc Trop Med Hyg. 2007;101:570–7. doi: 10.1016/j.trstmh.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 36.de Souza A, Nalini A, Kovoor JM, Yeshraj G, Siddalingaiah HS, Thennarasu K. Natural history of solitary cerebral cysticercosis on serial magnetic resonance imaging and the effect of albendazole therapy on its evolution. J Neurol Sci. 2010;288:135–41. doi: 10.1016/j.jns.2009.09.018. [DOI] [PubMed] [Google Scholar]
- 37.Misra UK, Kalita J, Nair PP. Status epilepticus in central nervous system infections: An experience from a developing country. Am J Med. 2008;121:618–23. doi: 10.1016/j.amjmed.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 38.Murthy JM, Yangala R. Acute symptomatic seizures — incidence and etiological spectrum: A hospital-based study from South India. Seizure. 1999;8:162–5. doi: 10.1053/seiz.1998.0251. [DOI] [PubMed] [Google Scholar]
- 39.Sinha S, Satishchandra P, Mahadevan A, Bhimani B, Kovur J, Shankar S. Fatal Status Epilepticus: A Clinico-pathological analysis among 100 patients: From a developing country perspective. Epilepsy Res. 2010;91:193–204. doi: 10.1016/j.eplepsyres.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 40.Swaminathan S, Sawhney IM, Jain S, Garg SK. Profile of status epilepticus. A prospective study. Neurol India. 1998;46:279–83. [PubMed] [Google Scholar]
- 41.Sinha S, Patil SA, Jayalekshmy V, Satishchandra P. Do cytokines have any role in epilepsy? Epilepsia Res. 2008;82:171–6. doi: 10.1016/j.eplepsyres.2008.07.018. [DOI] [PubMed] [Google Scholar]
- 42.Rachlis AR. Neurologic manifestations of HIV infection. Using imaging studies and antiviral therapy effectively. Postgrad Med. 1998;103:147. doi: 10.3810/pgm.1998.03.414. [DOI] [PubMed] [Google Scholar]
- 43.Sinha S, Satishchandra P, Nalini A, Ravi V, Subbakrishna DK, Jayakumar PN, et al. New-onset seizures among HIV infected drug naïve patients from south India. Neurol Asia. 2005;10:29–33. [Google Scholar]
- 44.Porter SB, Sande MA. Toxoplasmosis of the central nervous system in the acquired immunodeficiency syndrome. N Eng J Med. 1992;327:1643–8. doi: 10.1056/NEJM199212033272306. [DOI] [PubMed] [Google Scholar]
- 45.Satishchandra P, Nalini A, Gourie-Devi M, Khanna N, Santosh V, Ravi V, et al. Profile of neurologic disorders associated with HIV/AIDS from Bangalore, South India (1989-96) Indian J Med Res. 2000;111:14–23. [PubMed] [Google Scholar]
- 46.Satishchandra P, Sinha S. Seizures in HIV-seropositive individuals: NIMHANS experience and review. Epilepsia. 2008;49(Suppl 6):33–41. doi: 10.1111/j.1528-1167.2008.01754.x. [DOI] [PubMed] [Google Scholar]
- 47.Chadha DS, Handa A, Sharma SK, Varadarajulu P, Singh AP. Seizures in patients with human immunodeficiency virus infection. J Assoc Physicians India. 2000;48:573–6. [PubMed] [Google Scholar]
- 48.Udani PM, Parekh UC, Dastur DK. Neurological and related syndromes in CNS tuberculosis. Clinical features and pathogenesis. J Neurol Sci. 1971;14:341–57. doi: 10.1016/0022-510x(71)90222-x. [DOI] [PubMed] [Google Scholar]
- 49.Dupont JR, Earle KM. Human rabies encephalitis. A study of forty-nine fatal cases with a review of the literature. Neurology. 1965;15:1023–34. doi: 10.1212/wnl.15.11.1023. [DOI] [PubMed] [Google Scholar]
- 50.Feinstein AR. The pre-therapeutic classification of co-morbidity in chronic disease. J Chronic Dis. 1970;23:455–68. doi: 10.1016/0021-9681(70)90054-8. [DOI] [PubMed] [Google Scholar]
- 51.Babu CS, Satishchandra P, Sinha S, Subbakrishna DK. Co-morbidities in people living with epilepsy: Hospital based case-control study from a resource-poor setting. Epilepsy Res. 2009;86:146–52. doi: 10.1016/j.eplepsyres.2009.05.015. [DOI] [PubMed] [Google Scholar]
- 52.Ratnapriya R, Satishchandra P, Kumar SD, Gadre G, Reddy R, Anand A. A locus for autosomal dominant reflex epilepsy precipitated by hot water maps at chromosome 10q21.3-q22. Hum Genet. 2009;125:541–9. doi: 10.1007/s00439-009-0648-3. [DOI] [PubMed] [Google Scholar]
- 53.Ratnapriya R, Satishchandra P, Dilip S, Gadre G, Anand A. Familial autosomal dominant reflex epilepsy triggered by hot water maps to 4q24-q28. Hum Genet. 2009;126:677–83. doi: 10.1007/s00439-009-0718-6. [DOI] [PubMed] [Google Scholar]
- 54.Kapoor A, Vijai J, Ravishankar HM, Satishchandra P, Radhakrishnan K, Anand A. Absence of GABRA1 Ala322Asp mutation in juvenile myoclonic epilepsy families from India. J Genet. 2003;82:17–21. doi: 10.1007/BF02715876. [DOI] [PubMed] [Google Scholar]
- 55.Vijai J, Kapoor A, Ravishankar HM, Cherian PJ, Girija AS, Rajendran B, et al. Genetic association analysis of KCNQ3 and juvenile myoclonic epilepsy in a South Indian population. Hum Genet. 2003;113:461–3. doi: 10.1007/s00439-003-1003-8. [DOI] [PubMed] [Google Scholar]
- 56.Vijai J, Kapoor A, Ravishankar HM, Cherian PJ, Kuruttukulam G, Rajendran B, et al. Protective and susceptibility effects of hSKCa3 allelic variants on juvenile myoclonic epilepsy. J Med Genet. 2005;42:439–42. doi: 10.1136/jmg.2004.023812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ratnapriya R, Vijai J, Kadandale JS, Iyer RS, Radhakrishnan K, Anand A. A locus for juvenile myoclonic epilepsy maps to 2q33-q36. Hum Genet. 2010;128:123–30. doi: 10.1007/s00439-010-0831-6. [DOI] [PubMed] [Google Scholar]
- 58.Mehndiratta MM, Rao KB, Singh S, Ganesh S, Khwaja GA. Genetic study of juvenile myoclonic epilepsy (JME) patients and their family members in a University Hospital in North India. Neurol Asia. 2007;12(Suppl 1):105–6. [Google Scholar]
- 59.Acharya JN, Satishchandra P, Asha T, Shankar SK. Lafora's disease in South India: A clinical, electrophysiologic, and pathologic study. Epilepsia. 1993;34:476–87. doi: 10.1111/j.1528-1157.1993.tb02588.x. [DOI] [PubMed] [Google Scholar]
- 60.Minassian BA, Lee JR, Herbrick JA, Huizenga J, Soder S, Mungall AJ, et al. Mutations in a gene encoding a novel protein tyrosine phosphatase cause progressive myoclonus epilepsy. Nat Genet. 1998;20:171–4. doi: 10.1038/2470. [DOI] [PubMed] [Google Scholar]
- 61.Dutta S, Gangopadhyay PK, Sinha S, Chatterjee A, Ghosh S, Rajamma U. An association analysis of reelin gene (RELN) polymorphisms with childhood epilepsy in eastern Indian population from West Bengal. Cell Mol Neurobiol. 2011;31:45–56. doi: 10.1007/s10571-010-9551-7. [DOI] [PubMed] [Google Scholar]
- 62.Brown ML, Huston PE, Hines HM, Brown GW. Cardiovascular changes associated with electroconvulsive shock in monkeys; cerebral blood flow, blood pressure, and cardiac rate measurements before, during, and after electroconvulsive shock. AMA Arch Neurol Psychiatry. 1953;69:609–14. doi: 10.1001/archneurpsyc.1953.02320290061007. [DOI] [PubMed] [Google Scholar]
- 63.Lathers CM, Schraeder PL. Autonomic dysfunction in epilepsy: Characterization of autonomic cardiac neural discharge associated with pentylenetetrazol-induced epileptogenic activity. Epilepsia. 1982;23:633–47. doi: 10.1111/j.1528-1157.1982.tb05079.x. [DOI] [PubMed] [Google Scholar]
- 64.Sathyaprabha TN, Satishchandra P, Netravathi K, Sinha S, Thennarasu K, Raju TR. Cardiac autonomic dysfunctions in chronic refractory epilepsy. Epilepsy Res. 2006;72:49–56. doi: 10.1016/j.eplepsyres.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 65.Pradhan C, Sinha S, Thennarasu K, Jagadisha T. Quantitative analysis of heart rate variability in patients with absence epilepsy. Neurol India. 2011;59:25–9. doi: 10.4103/0028-3886.76852. [DOI] [PubMed] [Google Scholar]
- 66.Mativo P, Anjum J, Pradhan C, Sathyaprabha TN, Raju TR, Satishchandra P. Study of cardiac autonomic function in drug-naïve, newly diagnosed epilepsy patients. Epileptic Disord. 2010;12:212–6. doi: 10.1684/epd.2010.0325. [DOI] [PubMed] [Google Scholar]
- 67.Sathyaprabha TN, Satishchandra P, Pradhan C, Sinha S, Kaveri B, Thennarasu K, et al. Modulation of cardiac autonomic balance with adjuvant yoga therapy in patients with refractory epilepsy. Epilepsy Behav. 2008;12:245–52. doi: 10.1016/j.yebeh.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 68.Sinha S, Satishchandra P, Gayathri N, Yasha TC, Shankar SK. Progressive myoclonic epilepsy: A clinical, electrophysiological and pathological study from South India. J Neurol Sci. 2007;252:16–23. doi: 10.1016/j.jns.2006.09.021. [DOI] [PubMed] [Google Scholar]
- 69.Satishchandra P, Sinha S. Progressive myoclonic epilepsy. Neurol India. 2010;58:514–22. doi: 10.4103/0028-3886.68660. [DOI] [PubMed] [Google Scholar]
- 70.Satishchandra P. Hot-water epilepsy. Epilepsia. 2003;44(Suppl 1):29–32. doi: 10.1046/j.1528-1157.44.s.1.14.x. [DOI] [PubMed] [Google Scholar]
- 71.Meghana A, Sinha S, Sathyaprabha TN, Subbakrishna DK, Satishchandra P. Hot water epilepsy clinical profile and treatment – a prospective study. Epilepsy Res. 2012;102:160–6. doi: 10.1016/j.eplepsyres.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 72.Gururaj G, Satishchandra P. Correlates of hot water epilepsy in rural South India: A descriptive study. Neuroepidemiology. 1992;11:173–9. doi: 10.1159/000110929. [DOI] [PubMed] [Google Scholar]
- 73.Satishchandra P, Ullal GR, Shankar SK. Hot water epilepsy. Adv Neurol. 1998;75:283–93. [PubMed] [Google Scholar]
- 74.Nagaraja D, Chand RP. Eating epilepsy. Clin Neurol Neurosurg. 1984;86:95–9. doi: 10.1016/0303-8467(84)90072-6. [DOI] [PubMed] [Google Scholar]
- 75.Patel M, Satishchandra P, Saini J, Bharath RD, Sinha S. Eating epilepsy: Phenotype, MRI, SPECT and video-EEG observations. Epilepsy Res. 2013;107:115–20. doi: 10.1016/j.eplepsyres.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 76.Thomas SV. Managing epilepsy in pregnancy. Neurol India. 2011;59:59–65. doi: 10.4103/0028-3886.76860. [DOI] [PubMed] [Google Scholar]
- 77.Sahota P, Prabhakar S, Kharbanda PS, Bhansali A, Jain V, Das CP, et al. Seizure type, antiepileptic drugs, and reproductive dysfunction in Indian women with epilepsy: A cross-sectional study. Epilepsia. 2008;49:2069–77. doi: 10.1111/j.1528-1167.2008.01676.x. [DOI] [PubMed] [Google Scholar]
- 78.Samrén EB, van Duijn CM, Koch S, Hiilesmaa VK, Klepel H, Bardy AH, et al. Maternal use of antiepileptic drugs and the risk of major congenital malformations: A joint European prospective study of human teratogenesis associated with maternal epilepsy. Epilepsia. 1997;38:981–90. doi: 10.1111/j.1528-1157.1997.tb01480.x. [DOI] [PubMed] [Google Scholar]
- 79.Yerby MS. Contraception, pregnancy and lactation in women with epilepsy. Baillieres Clin Neurol. 1996;5:887–908. [PubMed] [Google Scholar]
- 80.Lindhout D, Omtzigt JG. Teratogenic effects of antiepileptic drugs: Implications for the management of epilepsy in women of childbearing age. Epilepsia. 1994;35(Suppl 4):19–28. doi: 10.1111/j.1528-1157.1994.tb05952.x. [DOI] [PubMed] [Google Scholar]
- 81.Kaneko S, Battino D, Andermann E, Wada K, Kan R, Takeda A, et al. Congenital malformations due to antiepileptic drugs. Epilepsy Res. 1999;33:145–58. doi: 10.1016/s0920-1211(98)00084-9. [DOI] [PubMed] [Google Scholar]
- 82.Vajda FJ, O’brien TJ, Hitchcock A, Graham J, Cook M, Lander C, et al. Critical relationship between sodium valproate dose and human teratogenicity: Results of the Australian register of anti-epileptic drugs in pregnancy. J Clin Neurosci. 2004;11:854–8. doi: 10.1016/j.jocn.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 83.Artama M, Auvinen A, Raudaskoski T, Isojärvi I, Isojärvi J. Antiepileptic drug use of women with epilepsy and congenital malformations in offspring. Neurology. 2005;64:1874–8. doi: 10.1212/01.WNL.0000163771.96962.1F. [DOI] [PubMed] [Google Scholar]
- 84.Morrow J, Russell A, Guthrie E, Parsons L, Robertson I, Waddell R, et al. Malformation risks of antiepileptic drugs in pregnancy: A prospective study from the UK Epilepsy and Pregnancy Register. J Neurol Neurosurg Psychiatry. 2006;77:193–8. doi: 10.1136/jnnp.2005.074203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Samrén EB, van Duijn CM, Koch S, Hiilesmaa VK, Klepel H, Bardy AH, et al. Maternal use of antiepileptic drugs and the risk of major congenital malformations: A joint European prospective study of human teratogenesis associated with maternal epilepsy. Epilepsia. 1997;38:981–90. doi: 10.1111/j.1528-1157.1997.tb01480.x. [DOI] [PubMed] [Google Scholar]
- 86.Mawer G, Clayton-Smith J, Coyle H, Kini U. Outcome of pregnancy in women attending an outpatient epilepsy clinic: Adverse features associated with higher doses of sodium valproate. Seizure. 2002;11:512–8. doi: 10.1016/s1059-1311(02)00135-8. [DOI] [PubMed] [Google Scholar]
- 87.Thomas SV, Sindhu K, Ajaykumar B, Sulekha Devi PB, Sujamol J. Maternal and obstetric outcome of women with epilepsy. Seizure. 2009;18:163–6. doi: 10.1016/j.seizure.2008.08.010. [DOI] [PubMed] [Google Scholar]
- 88.Thomas SV, Indrani L, Devi GC, Jacob S, Beegum J, Jacob PP, et al. Pregnancy in women with epilepsy: Preliminary results of Kerala registry of epilepsy and pregnancy. Neurol India. 2001;49:60–6. [PubMed] [Google Scholar]
- 89.Thomas SV, Syam U, Devi JS. Predictors of seizures during pregnancy in women with epilepsy. Epilepsia. 2012;53:e85–8. doi: 10.1111/j.1528-1167.2012.03439.x. [DOI] [PubMed] [Google Scholar]
- 90.Begum S, Sarma SP, Thomas SV. Malformation in index pregnancy in women with epilepsy is not followed by recurrence in subsequent pregnancy. Epilepsia. 2013;54:e163–7. doi: 10.1111/epi.12411. [DOI] [PubMed] [Google Scholar]
- 91.Dansky LV, Rosenblatt DS, Andermann E. Mechanisms of teratogenesis: Folic acid and antiepileptic therapy. Neurology. 1992;42(4 Suppl 5):32–42. [PubMed] [Google Scholar]
- 92.Wells PG, McCallum GP, Chen CS, Henderson JT, Lee CJ, Perstin J, et al. Oxidative stress in developmental origins of disease: Teratogenesis, neurodevelopmental deficits, and cancer. Toxicol Sci. 2009;108:4–18. doi: 10.1093/toxsci/kfn263. [DOI] [PubMed] [Google Scholar]
- 93.Jose M, Thomas SV. Role of multidrug transporters in neurotherapeutics. Ann Indian Acad Neurol. 2009;12:89–98. doi: 10.4103/0972-2327.53076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Thomas SV, Sarma PS, Nirmala C, Mathai A, Thomas SE, Thomas AC. Women with epilepsy and infertility have different reproductive hormone profile than others. Ann Indian Acad Neurol. 2013;16:544–8. doi: 10.4103/0972-2327.120460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sinha S, Prashantha DK, Thennarasu K, Umamaheshwara Rao GS, Satishchandra P. Refractory status epilepticus: A developing country perspective. J Neurol Sci. 2010;290:60–5. doi: 10.1016/j.jns.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 96.Sinha S, Satishchandra P, Mahadevan A, Bhimani BC, Kovur JM, Shankar SK. Fatal status epilepticus: A clinico-pathological analysis among 100 patients: From a developing country perspective. Epilepsy Res. 2010;91:193–204. doi: 10.1016/j.eplepsyres.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 97.Tripathi M, Vibha D, Choudhary N, Prasad K, Srivastava MV, Bhatia R, et al. Management of refractory status epilepticus at a tertiary care centre in a developing country. Seizure. 2010;19:109–11. doi: 10.1016/j.seizure.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 98.Kalita J, Nair PP, Misra UK. A clinical, radiological and outcome study of status epilepticus from India. J Neurol. 2010;257:224–9. doi: 10.1007/s00415-009-5298-9. [DOI] [PubMed] [Google Scholar]
- 99.Sinha S, Satishchandra P. Epilepsia Partialis Continua over last 14 years: Experience from a tertiary care center from south India. Epilepsy Res. 2007;74:55–9. doi: 10.1016/j.eplepsyres.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 100.Misra UK, Kalita J, Patel R. Sodium valproate vs phenytoin in status epilepticus: A pilot study. Neurology. 2006;67:340–2. doi: 10.1212/01.wnl.0000224880.35053.26. [DOI] [PubMed] [Google Scholar]
- 101.Misra UK, Kalita J, Maurya PK. Levetiracetam versus lorazepam in status epilepticus: A randomized, open labeled pilot study. J Neurol. 2012;259:645–8. doi: 10.1007/s00415-011-6227-2. [DOI] [PubMed] [Google Scholar]
- 102.Sinha S, Satishchandra P, Kalband BR, Thennarasu K. New-onset status epilepticus and cluster seizures in the elderly. J Clin Neurosci. 2013;20:423–8. doi: 10.1016/j.jocn.2012.02.050. [DOI] [PubMed] [Google Scholar]
- 103.Sinha S, Satishchandra P, Kalband BR, Thennarasu K. EEG observations in elderly with new onset seizures: From developing country perspective. J Clin Neurophysiol. 2011;28:388–93. doi: 10.1097/WNP.0b013e318227775c. [DOI] [PubMed] [Google Scholar]
- 104.Sinha S, Satishchandra P, Kalband BR, Bharath RD, Thennarasu K. Neuroimaging observations in a cohort of elderly manifesting with new onset seizures: Experience from a university hospital. Ann Indian Acad Neurol. 2012;15:273–80. doi: 10.4103/0972-2327.104335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Shah KN, Rajadhyaksha SB, Shah VS, Shah NS, Desai VG. Experience with the International league against epilepsy classifications of epileptic seizures (1981) and epilepsies and epileptic syndrome (1989) in epileptic children in a developing country. Epilepsia. 1992;33:1072–7. doi: 10.1111/j.1528-1157.1992.tb01761.x. [DOI] [PubMed] [Google Scholar]
- 106.Udani VP, Dharnidharka V, Nair A, Oka M. Difficult to control epilepsy in childhood — a long term study of 123 cases. Indian Pediatr. 1993;30:1199–206. [PubMed] [Google Scholar]
- 107.Udani V. Pediatric epilepsy — an Indian perspective. Indian J Pediatr. 2005;72:309–13. [PubMed] [Google Scholar]
- 108.Tripathi M, Padhy UP, Vibha D, Bhatia R, Padma Srivastava MV, Singh MB, et al. Predictors of refractory epilepsy in north India: a case-control study. Seizure. 2011;20:779–83. doi: 10.1016/j.seizure.2011.07.010. [DOI] [PubMed] [Google Scholar]
- 109.Sander JW. Some aspects of prognosis in the epilepsies: A review. Epilepsia. 1993;34:1007–16. doi: 10.1111/j.1528-1157.1993.tb02126.x. [DOI] [PubMed] [Google Scholar]
- 110.Engel J., Jr Etiology as a risk factor for medically refractory epilepsy: A case for early surgical intervention. Neurology. 1998;51:1243–4. doi: 10.1212/wnl.51.5.1243. [DOI] [PubMed] [Google Scholar]
- 111.Kwan P, Brodie MJ. Early identification of refractory epilepsy. N Engl J Med. 2000;342:314–9. doi: 10.1056/NEJM200002033420503. [DOI] [PubMed] [Google Scholar]
- 112.Rao MB, Radhakrishnan K. Is epilepsy surgery possible in countries with limited resources? Epilepsia. 2000;41(Suppl 4):S31–4. doi: 10.1111/j.1528-1157.2000.tb01543.x. [DOI] [PubMed] [Google Scholar]
- 113.Radhakrishnan K. Epilepsy surgery in India. Neurol India. 2009;57:4–6. doi: 10.4103/0028-3886.48791. [DOI] [PubMed] [Google Scholar]
- 114.D’Souza C. Epilepsy and discrimination in India. Neurol Asia. 2004;9(Suppl 1):53–4. [Google Scholar]


