Abstract
Electroencephalography (EEG) remains a “gold standard” for defining seizures; hence identification of epileptogenic zone for surgical treatment of epilepsy requires precise electrographic localization of the seizures. Routine scalp EEG recording is not sufficient in many instances, such as extratemporal lobe epilepsy or non-lesional temporal lobe epilepsy. In these individuals EEG recording from proximity of the seizure focus is necessary, which can be achieved by placing electrodes on the surface or in the substance of the brain. As this process requires invasive procedures (usually necessitating surgical intervention) EEG obtained via these electrodes is defined as invasive electroencephalography (iEEG). As only limited areas of the brain can be covered by these electrodes in an individual, precise targeting of the presumed seizure onset location is crucial. The presurgical planning includes where to place electrodes, which type of the electrodes to choose and planned duration of the intracranial recording. Though there are general principles that guide such endeavor, each center does it slightly differently depending upon the various technologies available to them and expertise and preferences of the epilepsy surgery team. Here we describe our approach to iEEG recording. We briefly describe the background, types of iEEG recording and rationale for each, various electrode types, and scenarios where iEEG might be useful. We also describe planning of iEEG recording once the need has been established as well as our decision making process of deciding about location of electrode placement, type of electrodes to use, length of recording, choice of arrays, mapping of eloquent cortex and finally surgical planning and decisions.
Keywords: Depth electrodes, electrocorticography, epidural peg electrodes, epilepsy surgery, intracranial electroencephalography, invasive electroencephalography, subdural electrodes
Introduction
Invasive electroencephalography (iEEG) can be defined as electroencephalography (EEG) recording utilizing invasive methods or using invasive intracranial electrodes placed surgically. In general, insertion of subcutaneous deep electrodes, such as sphenoidal electrodes done at the bedside, is not considered iEEG. A semi-invasive method of placing an electrode in close proximity to the temporal lobe under fluoroscopy in the vicinity of the foramen ovale is utilized by some practitioners.[1] The next more invasive method is to record EEG from the epidural space by creating a twist drill or small burr hole in the skull and placing an electrode with its tip touching the dura, commonly known as epidural peg electrode.[2] The most invasive method is to record EEG by placing electrodes intracranially. Intracranial EEG recording can be performed either using electrodes placed directly on the exposed surface of the brain (subdural grid and strip electrodes) or by electrodes inserted into the brain parenchyma or within a lesion (depth electrodes). Recording from the cortical surface using subdural electrodes is referred to as electrocorticography (ECoG), whereas EEG recording using multiple depth electrodes is referred to as stereoencephalography (SEEG). When recording is obtained for a short period of time during surgery, it is referred to as intraoperative ECoG and if recorded over hours to days outside the operative room it is known as extraoperative ECoG. For the purpose of this article, we will restrict the term iEEG to intracranial EEG and epidural peg electrode recording.
Why is iEEG Needed?
A randomized trial of surgery for temporal lobe epilepsy showed that surgery is superior to prolonged medical therapy.[3] Resection of the epileptogenic zone mapped with intracranial EEG is associated with an excellent seizure outcome.[4] The main purpose of iEEG is to determine the precise location and boundary of the epileptogenic zone. Some electrodes are also used to identify important functional areas of the cortex (eloquent cortex). This can be achieved either by recording evoked responses from these intracranial electrodes when specific sensory stimuli of a given modality are delivered peripherally, or by directly stimulating the electrodes with brief electrical current and observing its effects.
Can Intraoperative ECoG be an Alternate to iEEG Recording?
In cases of lesional epilepsy, it is generally believed that seizures arise from the vicinity of the lesion. However, the precise location is not certain and cannot be determined on the basis of imaging abnormalities. Over the years, many have tried to use EEG recording acutely during surgical intervention. A brief recording of cortical surface EEG using subdural grid/strip electrodes is attempted prior to removal of the lesion. Alternatively, a depth electrode may be inserted within a lesion to evaluate intrinsic epileptogenicity. This EEG recording is known as ECoG. If epileptiform activity is encountered on ECoG, a tailored resection can be performed to include the area of electrophysiological abnormality. This is shown to be helpful in lesional epilepsy cases[5] or temporal lobe epilepsy.[6] However, the success of this approach is limited. The main reason is that the interictal abnormality recorded during brief ECoG recording may not be indicative of the actual seizure onset zone.[7,8,9]
A presurgical evaluation using a variety of noninvasive tests (e.g., magnetic resonance imaging [MRI], positron emission tomography [PET], neuropsychology testing, magnetoencephalography, single photon emission computed tomography, etc.) may establish seizure onset from a temporal lobe, perhaps the medial temporal lobe in cases of hippocampal sclerosis identified on MRI. It can also identify other potential epileptogenic lesions such as cortical developmental malformation or brain tumors. In cases of medial or anterior temporal lobe seizures, one stage “standard” resection of the anteromedial temporal lobe or more selective amygdalohippocampectomy can provide lasting seizure freedom.[3,10,11,12,13,14] However, at times the presurgical evaluation may fail to provide definitive information regarding margins of the epileptogenic zone. In such instances, iEEG is often employed to help determine the epileptogenic zone (areas of seizure onset and early spread). Though general principles are well established, what triggers implantation of electrodes and iEEG recording may differ among different epilepsy centers and depend upon individual experience and availability of advanced technologies locally. The choice of electrodes is also dependent upon the individual's experience and expertise.
iEEG Electrodes
iEEGs are usually recorded with the help of subdural grid electrodes and/or depth electrodes. Subdural electrodes are small metal discs embedded in teflon or silastic sheath material and arranged in different configurations [Figure 1]. These electrodes are connected to a thin metal wire and they are individually insulated and ultimately bundled and covered with plastic material (tail). When the configuration of electrodes is in a single column, it is commonly referred as a subdural strip electrode. When it contains rows and columns, it is known as subdural grid electrode and is available in many different sizes and configurations. These grids or strips are implanted subdurally over the surface of the brain and their tails exit the meninges, skull and ultimately through the scalp to protrude on the scalp [Figure 2]. There they are connected to an amplifier through cables to record EEG signals from each individual electrode from the grid/strip. When the electrodes are placed on a hollow plastic tube that can be inserted in the brain tissue itself, the electrode is known as a depth electrode. The electrode usually comes with a stylet to provide necessary stiffness to insert in the brain. Depth electrodes can be placed in the deep structures (such as hippocampus, amygdala and insula) of the brain or within lesions and are commonly placed under neuronavigation guidance to optimize implantation into the desired location [Figure 3]. Epidural peg electrodes are another type of iEEG recording electrodes that are placed by creating a trephination of the skull with a small opening on the inner table and inserting the electrode in the trephine with the tip touching the dura. These electrodes stay extradural in the epidural space.
Figure 1.

Display of various size and configuration of subdural grid/strip electrodes produced by a manufacturer (PMT Corporation, Chanhassen, MN, USA). The electrodes are arranged in rows and are placed at equal distance from each other. Each electrode is a metal disc embedded in a sialastic material and connected to a thin insulated wire that traverses through a tail and connects it to an amplifier via a connector
Figure 2.
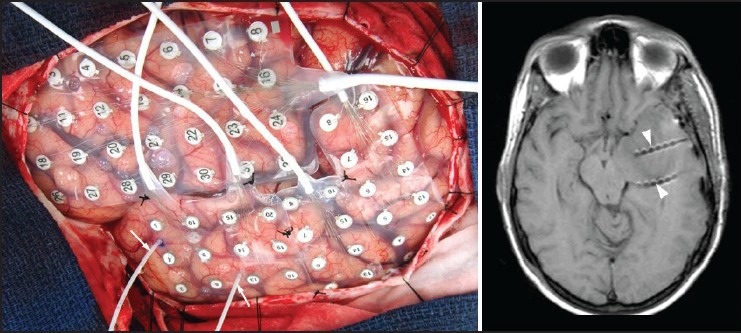
An intraoperative photograph (left panel) showing left cerebral hemisphere covered by various subdural grid electrode arrays. Two depth electrodes are also seen (arrows) entering the parenchyma, which were inserted under neuronavigation guidance via lateral approach. A T1W axial magnetic resonance imaging (right panel) obtained following electrode implantation shows depth electrodes traversing the temporal lobe with tips located in the mesial temporal structures (arrow heads)
Figure 3.
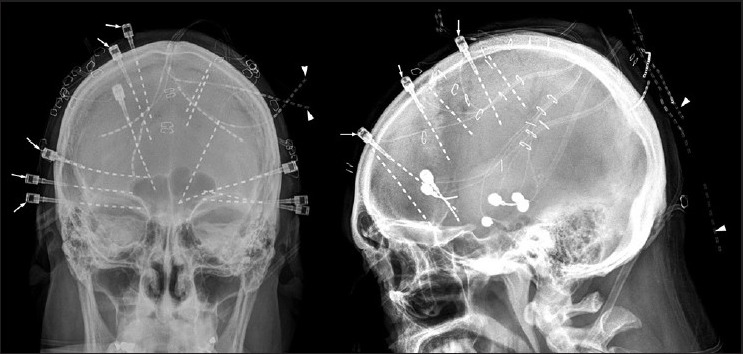
A post-implant skull X-ray showing multiple depth electrodes placed strategically under neuronavigation guidance. The targets are predetermined on the basis of presurgical evaluation. In this individual, the electrodes were placed in bilateral hippocampal formations, orbitofrontal gyri and cingulate gyri. Some of the depth electrodes were secured with an anchor to minimize post-implantation movements (arrows). The fainter electrodes ends represent the tails of the depth electrodes that connects to amplifier and are located over the scalp (arrowheads)
Electrodes are usually made of stainless steel or platinum. The platinum electrodes are more desirable as they are non-ferromagnetic making them more compatible with MRI, but they are more expensive.
Each of these electrode types provides certain advantages and is associated with its own challenges which are summarized in Table 1.
Table 1.
Comparison of various intracranial electrodes

This phase of iEEG recording is relatively short and consists of implantation of intracranial electrodes followed by extraoperative prolonged video-EEG recording using these electrodes to delineate the epileptogenic zone and also map the eloquent cortex. In a typical two-staged approach, a craniotomy is performed over the suspected epileptic hemisphere during the first stage of surgery to expose the cerebral cortex where subdural grids are to be implanted. Following implantation of the subdural grid, strip and/or depth electrodes, iEEG is recorded extraoperatively off antiseizure medications for an extended period of time (typically 3-14 days) to capture habitual seizures. Analysis of this data establishes the epileptogenic zone. During the extraoperative phase of video-EEG recording, intracranial electrodes can be stimulated with a square wave electrical pulse using an external electrical stimulator and clinical effects of such stimulation can be monitored and documented. Stimulation of various primary sensory cortices can lead to sensory experience of that modality, e.g., stimulation of visual cortex leads to visual experience of seeing phosphenes, colors, shapes, objects or even formed visual images depending upon the type of visual cortex being stimulated. If one stimulates the primary motor cortex of the precentral gyrus it can lead to time-locked motor movements of the corresponding body part on the contralateral side, e.g.; hand area stimulation resulting in twitching of hand/wrist. Similarly stimulation over the language area causes disruption of language tasks, such as dysnomia, interruption of spontaneous speech, etc. At the end of this phase of recording one expects to have a reasonably good idea of where the epileptogenic zone of habitual seizure is and where the eloquent cortex is located. At this point, the epilepsy team makes a decision regarding the resection margins and discusses that with the patient and his/her family members and caregivers. The risks of potential post-operative deficits can be discussed more precisely at this stage as one has a very good idea of where the eloquent cortex is located in relationship to the proposed resection margins for the epileptogenic zone. After this discussion one finalizes the resection margins on the basis of anatomical details and electrophysiological findings and resection ensues during the second stage of surgery.
Indications of iEEG
There are many circumstances where iEEG is useful, but the main indication of its use is when either seizures cannot be lateralized or localized with confidence with non-invasive methods or the epileptogenic zone is considered to be close to eloquent cortex and precise mapping of both epileptogenic zone and eloquent cortex is needed to provide the best possible outcome.
Complications of iEEG
As iEEG requires neurosurgical intervention the complications can be devastating. Fortunately the complication rates are low and most complications do not lead to permanent deficits. The most common adverse events reported include intracranial hemorrhage, superficial infection, elevated intracranial pressure and cerebral infections.[15,16,17,18]
Scenarios when iEEG is Recommended
There are no set standards to describe when iEEG is required. Each center follows their own guidelines depending upon their own preferences and experience. However, there are some general approaches and principles underlying the decision to employ iEEG.[19] Common scenarios where iEEG is used in our center and to a large extent at other comprehensive epilepsy centers include:
Scalp EEG pattern showing bilateral ictal discharges or ictal onset being obscured by movement and EMG artifacts consistently.
Interictal epileptiform activity contradicting ictal onset persistently.
Discordant results between various presurgical tests, such as neuroimaging (structural and functional), neuropsychological testing, EEG abnormalities.
Non-lesional extratemporal epilepsy.
Neocortical epilepsy (lesional or non-lesional) with suspected epileptogenic zone in close proximity to the eloquent cortex (motor or language).
Dual pathology.
Presence of bilateral independent temporal spikes or bilateral hippocampal sclerosis on MRI to lateralize the dominant epileptogenic temporal lobe.
Planning for iEEG
The techniques for craniotomy and placement of intracranial electrodes are straightforward. Planning for the iEEG session is the most critical aspect and requires collaboration and an excellent working relationship between the epilepsy neurosurgeon and the epileptologist. The following factors are important to consider:
Location of electrode placement
After making a decision to proceed with iEEG, this is probably the most critical aspect of iEEG planning. iEEG is limited mostly by where the electrodes can be placed. Even with the most ambitious plans, only parts of the cerebral cortex can be sampled with intracranial electrodes. Depth electrodes can be placed bilaterally, but they provide limited cortical surface coverage. The subdural grids/strips can provide excellent cortical surface coverage, but cannot access deeper structures and are usually used for unilateral placement. This is preferred because bilateral large craniotomies required to place subdural grid electrodes might lead to an unacceptable level of morbidity. Thus, limited coverage during iEEG recording may lead to sampling error as one does not know what is not recorded. If the seizure onset area is not sampled with iEEG electrodes, one may miss the onset entirely and fail to perform resection during second stage. Even worse would be when one electrode records the seizure spread area and assumes it to be the seizure initiation zone, leading to poor surgical outcome as the entire epileptogenic zone is not resected. Epidural peg electrodes might be an exception to this as it can be placed widely over the scalp, but again it lacks precise spatial information that can be obtained from the depth or subdural electrodes.
Type of electrodes to use
Though there are advantages and disadvantages of each type of electrodes used, ultimately the choice for the type of electrodes used primarily depends upon familiarity of the surgeon with a particular electrode type and the type of surgery planned. In cases where pre-surgical workup suggests temporal lobe seizures but cannot lateralize it conclusively, bilateral depth electrodes in temporal lobes can be used. Habitual seizures captured using these electrodes can help establish the side of seizure onset. Some centers prefer to use depth electrodes exclusively even in cases of extra-temporal epilepsy with strategic stereotactic implantation of depth electrodes in various brain regions where seizures are suspected to originate from on the basis of presurgical work-up. Other centers use subdural grids and strip electrodes exclusively. In cases where bilateral iEEG sampling is needed, there is a tendency to utilize subdural strip electrodes introduced via burr holes. This is certainly feasible, but introduction of subdural strips via burr holes can lead to misplaced leads as strips are guided blindly. Many other centers, including ours, use combinations of both depth electrodes and subdural grids/strips combination based on the individual case scenario. We commonly use depth electrodes to sample medial temporal structures along with subdural grid/strip coverage over the temporal or extratemporal neocortex as warranted by each case. In rare cases, where some information during presurgical work-up has raised a concern for a seizure focus on the other side, though the majority of data points to a single focus on one side, we use a combined approach with subdural grid ± depth electrodes placed following a craniotomy on one side and insert a few depth electrodes to sample a limited cortical area and medial temporal structures contralaterally.
Choice of arrays
The subdural grid and strip electrodes come in variety of sizes and shapes. On the other hand depth electrodes can vary in number of electrode contacts and inter-electrode distance. The choice of subdural grids/strips and/or depth electrodes to be used in an individual case depends upon local anatomy and area to be covered. If a large area is to be covered one may use strategically placed multiple smaller arrays, whereas cases of a lesion close to the eloquent cortex can best be addressed with a large array that covers the lesion and surrounding cortex.
Length of recording
As many of the iEEG recording sessions are performed in a two-staged epilepsy surgery fashion, both stages are usually planned before the first surgery. The duration of iEEG recording (short or longer duration recording) is usually determined by seizure frequency, number of seizures needed to make decision and time needed to plan mapping of the eloquent cortex. In children with intractable epilepsy a shorter duration might be adequate to gather all needed information, usually 3-5 days. In adults a slightly longer duration might be necessary as seizure frequency may not be as high (usually 5-7 days). However, one needs to be flexible and change the date of the second stage of surgery if adequate information is not gathered during planned duration of iEEG recording. Though there is no established standard, intracranial EEG recording is considered to be safe for up to 3 weeks. iEEG session duration longer than 2 weeks might increase chances of infection and longer duration also increases chances for lead malfunction making it more difficult to get satisfactory information.
Mapping of eloquent cortex
While planning for the iEEG session, one needs to take in consideration mapping of the eloquent cortex. Presurgical work-up in most instances should be able to provide information regarding language dominance. The other crucial functions are localized to certain brain regions with little variation between the subjects, e.g., the primary motor cortex is located in the precentral gyrus in most individuals, whereas primary visual cortex is located along the calcarine sulcus. This can be further evaluated with help of functional magnetic resonance imaging techniques to visualize the blood flow changes in association with activation of a certain brain area using the function of the area. Other presurgical work-up provides information regarding anatomical location of the potential epileptogenic zone. Structural information such as visualization of a lesion on MRI and functional information such as area of hypometabolism on fluorodeoxyglucose-PET scan, clinical semiology of seizure and ictal EEG recording all can help locate the epileptogenic zone. Once the information regarding the potential epileptogenic zone and eloquent cortex is available one can determine whether the eloquent cortex is likely to be in close proximity to the area of the possible surgical resection or not. In case they are in close proximity, planning should be made to place intracranial electrodes to cover both the potential epileptogenic zone as well as the nearby eloquent cortex. This provides ample opportunities to map both areas adequately and make conscious and informed decisions about resection margins and potential postoperative deficits.
Surgical planning and decisions
Finally, there is a need to determine type and size of craniotomy and whether or not to use neuronavigation during the surgical process of implanting intracranial electrodes. Again this depends upon several of the above-mentioned factors as well as the availability of the technology and expertise. The craniotomy needs to be fashioned in a way to provide adequate exposure of the cortical surface to place subdural grids in desired locations. One needs to keep in mind vascular anatomy while placing subdural electrodes, especially when they are to be placed in the interhemispheric fissure, subtemporally or over the insula. The craniotomy also needs to provide adequate exposure and access to the likely region(s) to be resected. Neuronavigation is very helpful for placing depth electrodes into the deeper or not easily accessible brain structures such as amygdala, hippocampus, insula, cingulate gyrus, etc.
Conclusion
Surgical intervention to treat intractable epilepsy is known to be effective. Currently, there is no class I data to prove that the iEEG recording is effective in management of intractable epilepsy and if it is effective what is the best way to approach it. However, iEEG remains a very useful tool in surgical management of epilepsy without which it cannot be performed adequately. Its role is well established in helping to delineate the epileptogenic zone precisely. The planning for iEEG recording requires expertise of many individuals and epitomizes the term “team approach”. Though it is guided by few basic principles, iEEG indication and planning varies significantly between centers depending upon local preferences, expertise and beliefs.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
References
- 1.Karakis I, Velez-Ruiz N, Pathmanathan JS, Sheth SA, Eskandar EN, Cole AJ. Foramen ovale electrodes in the evaluation of epilepsy surgery: Conventional and unconventional uses. Epilepsy Behav. 2011;22:247–54. doi: 10.1016/j.yebeh.2011.06.013. [DOI] [PubMed] [Google Scholar]
- 2.Beleza P, Rémi J, Feddersen B, Peraud A, Noachtar S. Epidural and foramen-ovale electrodes in the diagnostic evaluation of patients considered for epilepsy surgery. Epileptic Disord. 2010;12:48–53. doi: 10.1684/epd.2010.0297. [DOI] [PubMed] [Google Scholar]
- 3.Wiebe S, Blume WT, Girvin JP, Eliasziw M Effectiveness and Efficiency of Surgery for Temporal Lobe Epilepsy Study Group. A randomized, controlled trial of surgery for temporal-lobe epilepsy. N Engl J Med. 2001;345:311–8. doi: 10.1056/NEJM200108023450501. [DOI] [PubMed] [Google Scholar]
- 4.Jennum P, Dhuna A, Davies K, Fiol M, Maxwell R. Outcome of resective surgery for intractable partial epilepsy guided by subdural electrode arrays. Acta Neurol Scand. 1993;87:434–7. doi: 10.1111/j.1600-0404.1993.tb04131.x. [DOI] [PubMed] [Google Scholar]
- 5.Mikuni N, Ikeda A, Takahashi JA, Nozaki K, Miyamoto S, Taki W, et al. A step-by-step resection guided by electrocorticography for nonmalignant brain tumors associated with long-term intractable epilepsy. Epilepsy Behav. 2006;8:560–4. doi: 10.1016/j.yebeh.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 6.McKhann GM, 2nd, Schoenfeld-McNeill J, Born DE, Haglund MM, Ojemann GA. Intraoperative hippocampal electrocorticography to predict the extent of hippocampal resection in temporal lobe epilepsy surgery. J Neurosurg. 2000;93:44–52. doi: 10.3171/jns.2000.93.1.0044. [DOI] [PubMed] [Google Scholar]
- 7.McBride MC, Binnie CD, Janota I, Polkey CE. Predictive value of intraoperative electrocorticograms in resective epilepsy surgery. Ann Neurol. 1991;30:526–32. doi: 10.1002/ana.410300404. [DOI] [PubMed] [Google Scholar]
- 8.Fiol ME, Gates JR, Torres F, Maxwell RE. The prognostic value of residual spikes in the postexcision electrocorticogram after temporal lobectomy. Neurology. 1991;41:512–6. doi: 10.1212/wnl.41.4.512. [DOI] [PubMed] [Google Scholar]
- 9.Cascino GD, Trenerry MR, Jack CR, Jr, Dodick D, Sharbrough FW, So EL, et al. Electrocorticography and temporal lobe epilepsy: Relationship to quantitative MRI and operative outcome. Epilepsia. 1995;36:692–6. doi: 10.1111/j.1528-1157.1995.tb01048.x. [DOI] [PubMed] [Google Scholar]
- 10.Engel J, Jr, Driver MV, Falconer MA. Electrophysiological correlates of pathology and surgical results in temporal lobe epilepsy. Brain. 1975;98:129–56. doi: 10.1093/brain/98.1.129. [DOI] [PubMed] [Google Scholar]
- 11.Rao MB, Radhakrishnan K. Is epilepsy surgery possible in countries with limited resources? Epilepsia. 2000;41(Suppl 4):S31–4. doi: 10.1111/j.1528-1157.2000.tb01543.x. [DOI] [PubMed] [Google Scholar]
- 12.Bujarski KA, Hirashima F, Roberts DW, Jobst BC, Gilbert KL, Roth RM, et al. Long-term seizure, cognitive, and psychiatric outcome following trans-middle temporal gyrus amygdalohippocampectomy and standard temporal lobectomy. J Neurosurg. 2013;119:16–23. doi: 10.3171/2013.3.JNS12714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wendling AS, Hirsch E, Wisniewski I, Davanture C, Ofer I, Zentner J, et al. Selective amygdalohippocampectomy versus standard temporal lobectomy in patients with mesial temporal lobe epilepsy and unilateral hippocampal sclerosis. Epilepsy Res. 2013;104:94–104. doi: 10.1016/j.eplepsyres.2012.09.007. [DOI] [PubMed] [Google Scholar]
- 14.Josephson CB, Dykeman J, Fiest KM, Liu X, Sadler RM, Jette N, et al. Systematic review and meta-analysis of standard vs selective temporal lobe epilepsy surgery. Neurology. 2013;80:1669–76. doi: 10.1212/WNL.0b013e3182904f82. [DOI] [PubMed] [Google Scholar]
- 15.Arya R, Mangano FT, Horn PS, Holland KD, Rose DF, Glauser TA. Adverse events related to extraoperative invasive EEG monitoring with subdural grid electrodes: A systematic review and meta-analysis. Epilepsia. 2013;54:828–39. doi: 10.1111/epi.12073. [DOI] [PubMed] [Google Scholar]
- 16.Tanriverdi T, Ajlan A, Poulin N, Olivier A. Morbidity in epilepsy surgery: An experience based on 2449 epilepsy surgery procedures from a single institution. J Neurosurg. 2009;110:1111–23. doi: 10.3171/2009.8.JNS08338. [DOI] [PubMed] [Google Scholar]
- 17.Koubeissi MZ, Puwanant A, Jehi L, Alshekhlee A. In-hospital complications of epilepsy surgery: A six-year nationwide experience. Br J Neurosurg. 2009;23:524–9. doi: 10.1080/02688690903019589. [DOI] [PubMed] [Google Scholar]
- 18.Shah AK, Fuerst D, Sood S, Asano E, Ahn-Ewing J, Pawlak C, et al. Seizures lead to elevation of intracranial pressure in children undergoing invasive EEG monitoring. Epilepsia. 2007;48:1097–103. doi: 10.1111/j.1528-1167.2006.00975.x. [DOI] [PubMed] [Google Scholar]
- 19.Voorhies JM, Cohen-Gadol A. Techniques for placement of grid and strip electrodes for intracranial epilepsy surgery monitoring: Pearls and pitfalls. Surg Neurol Int. 2013;4:98. doi: 10.4103/2152-7806.115707. [DOI] [PMC free article] [PubMed] [Google Scholar]


