Abstract
A multidisciplinary approach is required to understand the complex intricacies of drug-resistant epilepsy (DRE). A challenge that neurosurgeons across the world face is accurate localization of epileptogenic zone. A significant number of patients who have undergone resective brain surgery for epilepsy still continue to have seizures. The reason behind this therapy resistance still eludes us. Thus to develop a cure for the difficult to treat epilepsy, we need to comprehensively study epileptogenesis. Till date, most of the studies on DRE is focused on undermining the abnormal functioning of receptors involved in synaptic transmission and reduced levels of antiepileptic drugs around there targets. But recent advances in imaging and electrophysiological techniques have suggested the role epileptogenic networks in the process of epileptogenesis. According to this hypothesis, the local neurons recruit distant neurons through complex oscillatory circuits, which further recruit more distant neurons, thereby generating a hypersynchronus neuronal activity. The epileptogenic networks may be confined to the lesion or could propagate to distant focus. The success of surgery depends on the precision by which the epileptogenic network is determined while planning a surgical intervention. Here, we summarize various modalities of electrophysiological and imaging techniques to determine the functionally active epileptogenic networks. We also review evidence pertaining to the proposed role of epileptogenic network in abnormal synaptic transmission which is one of the major causes of epileptiform activity. Elucidation of current concepts in regulation of synaptic transmission by networks will help develop therapies for epilepsy cases that cannot be managed pharmacologically.
Keywords: Epilepsy surgery, epileptogenesis, epileptogenic networks, pharmaco-resistant epilepsy
Introduction
Epilepsy that cannot be controlled with known pharmacological management, also referred as drug-resistant epilepsy (DRE), patients are recommended for epilepsy surgery.[1] The genesis of DRE still eludes us, but abnormal synaptic transmission is one of the key features of this disorder. Epilepsy can be caused by multiple mechanisms and often they appear so diverse that one would suspect that no common hypothesis applies. However, one common principle that is applied to the process of epileptogenesis is disruption of mechanisms that normally create a balance between excitation and inhibition. Dysfunction of mechanisms that inhibit excitatory synaptic transmission or promoting the mechanisms that facilitate excitation can lead to epileptogenesis.[2,3] Although the concept of a balance provides a useful model for mechanisms that can initiate epileptiform activity, it is daunting to consider the array of potential causes. There are various controls that keep the balance between excitatory transmission and inhibitory transmission in normal neuronal network.
In addition to the two prevailing theories of DRE, target hypothesis and transporter hypothesis,[4] another recently proposed hypothesis is neural network hypothesis.[5] According to this hypothesis, seizure-induced alterations of brain plasticity including axonal sprouting, synaptic reorganization, neurogenesis, and gliosis could contribute to the formation of abnormal neural network. This in turn leads to loss of the inhibitory effect of endogenous antiepileptic system and also prevented the traditional antiepileptic drugs from entering their targets, eventually leading to DRE. It is important to understand the processes through which the neuronal activities are synchronized which in turn lead to generation of seizures.[5] To this end, localization of epileptic networks will help in guiding epilepsy surgery, deploying antiepileptic measures, and elucidating mechanisms underlying the process of epileptogenesis.
Hypersynchronus Neuronal Activity
Action potentials or electrical spikes are known to be the language of neuronal communication.[6] These action potentials are the result of inward and outward currents that pass through the ion channels, namely Na+ and K+ channels respectively, coupled with the capacitance in the cell membrane, over a time scale. When a stimulus is input in a neuron it accumulates the electrical charge till it crosses the threshold value[7,8,9] beyond which an output signal or action potential or spike is generated and transmitted to the adjacent neuron. The entire process takes place in a collective manner in a large number of neurons. As expected, strong inputs are capable of evoking spikes by overcoming the threshold, whereas weak signals are not.[10,11] Interestingly, weak signals can also evoke spikes when coupled with noise.[11,12,13] The case of single neuron although reveals lot of interesting features related to brain dynamics, it is an isolated and rather artificial situation. In fact, in the functioning of the brain, the most important factor is the interactions and communications within an ensemble of neurons. Synchronization of neurons is a correlated appearance in time of two or more events associated with various aspects of neuronal activity. It is being argued that during a number of physiological processes, the spiking trains of neurons get synchronized.[14,15] Zhou and Kurths[16] have demonstrated (numerically) that Gaussian noise enhances synchronization of weakly coupled neurons and coherence of the spike trains. Synchronization could be associated with chemical and electrical synaptic as well as ephaptic and nonspecific interactions. The local synchronization contributes to the generation of local field potentials. In case of electroencephalography (EEG) synchronization, chemical synaptic interactions as detected by distantly located electrodes.[5] Excessive neuronal synchronization is a hallmark of epileptic discharges. In epilepsy, synchronization occurs between cells and within local networks at different time-scales. It has been hypothesized that during epileptogenesis, clusters of pathological neurons are interconnected leading to burst of hypersynchronus action potentials.[17] Ligand-gated ion channel-mediated neurotransmission synchronizes over milliseconds to tens of milliseconds. While electrical mechanisms, gap junctions, and ephaptic or field interactions, which can operate over fractions of a millisecond to a few milliseconds contributes to faster synchronization. In case of slower synchronization which is in the range of hundreds of milliseconds, it depends on G-protein coupled receptors, fluctuations in the extracellular concentration of ions (most notably K+), interactions between neurons and glia, and the dynamics of interaction between coupled hyperexcitable regions.[5]
Tools to Study Epileptogenic Network
Development and application of integrated dynamic imaging approaches examining neuronal circuit function has significantly helped in our understanding of the mechanisms underlying epileptogenesis, epilepsy, and seizure generation [see Figure 1]. Progress in neuroimaging has led not only to successful identification of epileptic foci for surgical resection, but also to an improved understanding of the functional and microstructural changes in long-standing epilepsy. Positron emission tomography (PET), functional magnetic resonance imaging (fMRI), and diffusion tensor imaging are all promising tools that can assist in elucidating the underlying pathophysiology in chronic epilepsy.[18] In addition to conventional MRI, functional neuroimaging using PET and single-photon emission computed tomography (SPECT) can provide complementary information to help localize the epileptic focus and often provides additional information that cannot be obtained from conventional MRI sequences. Magnetoencephalography (MEG) has recently emerged as a clinical tool for neurology and neurosurgery could also be helpful in dysfunctional neuronal circuit by localization of the irritative zone and through functional mapping of eloquent (sensory, motor, and language) cortex. Recent MEG studies have demonstrated dramatic plastic shifts in brain function due to lesions or developmental abnormalities but not necessarily predicted by the anatomical changes.[18]
Figure 1.
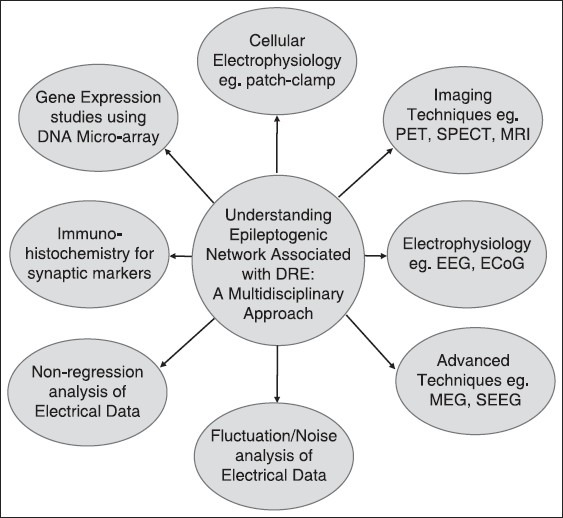
In order to understand the molecular basis of pharmacoresistant epilepsy, it is important to study the epileptogenic network using complementary and multidisciplinary approach. Creation of an infrastructure, which brings together a center with clinical facility for epilepsy surgery and a research institute, will help improve surgical therapies and increase the success rate of epilepsy surgeries. Center of excellence for epilepsy is one of its kind facility established under the aegis of Department of Biotechnology, Ministry of Science and Technology (Government of India) where a premier medical science institute, All India Institute of Medical Sciences, New Delhi is collaborating with National Brain Research Centre (NBRC), Manesar, a dedicated neuroscience research center
The major challenge that the epilepsy surgeons across the world face are those 30%-40% patients who still continue to have seizures despite undergoing an adequate epilepsy surgery.[19] The more straight forward pathologies have better outcomes, which are similar in most centers. The more complex pathologies, while requiring more extensive work up, does not translate into a better outcomes.[19,20,21,22] Localizing the dysfunctional network can explain the reasons for surgical failure and provide solutions to reduce the same. It is critical for the neurosurgeon to determine, how far the epileptogenic area is form the lesion. Growing evidences suggests that there is focal area where the onset of spike wave discharge (SWD) is located and these local SWDs propagate very quickly throughout the cortex and to thalamus at the millisecond scale.[23] It is important to determine with highest precision the extent of epileptogenic network while planning a surgical intervention. Quantitative methods of EEG signal analysis and epileptogenicity index are useful tools for functional analysis of neuronal networks associated with an epileptogenic lesion.[24] Posttemporal lobectomy, the presence of diffused pathology, is one of the reasons for recurrence after surgery. As suggested by positive neuroimaging in this case, maximal networks are close to the lesion.[25,26] Electrocorticography (ECoG) provides useful information for the prediction of surgical success in surgically remediable epilepsy. DRE and post resection ECoG correlation with its grade of severity and clinical outcome suggest network pattern of the lesions. Despite its widespread usage, there are still controversies regarding its utility especially keeping in mind its short period of application.[27,28,29,30] It also should be kept in mind that any electrode used potentially records from millions of neurons, for example, a circular electrode of the EcoG grid sits over 106 neurons, each having about 104 connections. Thus, this is equivalent to hanging a microphone among a population of 1 million people, each talking to 10,000 persons! Thus, the need of hour is to develop better amplifiers and also technologies which can analyze meaningfully such a huge amount of data.[31,32,33] The method of intraoperative coregistration of MRI, PET, and ECoG has shown to provide better objective localization of the epileptogenic foci.[29] Localizing eloquent cortex lesions, which present a surgical challenge, could be successfully achieved by combination of neuronavigation aided fMRI and diffusion tensor tractography along with cortical stimulation.[34] Source localization by the combination of MEG and EEG provided insight into the regional network associated with epileptogenic focus.[35] Stereoelctroencephalography- mediated electrophysiological recording helps in the computation of epileptogenecity index which helps determine if the epilieptogenic network is confined to the lesion or it involves other distant structures.[24] Recently, high frequency oscillations (HFOs) have been reportedly shown to arise from brain regions constituting epileptic networks and may be important to seizure generation.[36] HFOs are brief 50-500 Hz pathologic events measured in intracranial field and unit recordings in patients with refractory epilepsy. Basic research studies on HFOs indicate these local oscillatory field potentials correspond with an increase in rate and synchrony of neuronal discharges. Because HFOs can facilitate synaptic transmission through local networks, these events are implicated with information processing and consolidation of memory. Alterations to neuronal networks associated with epilepsy can also generate abnormal or pathologic HFOs that are believed to reflect fundamental neuronal disturbances associated with brain areas capable of generating spontaneous epileptic seizures.[37] There is compelling evidence supporting the view that normal hippocampal ripples and neocortical HFOs in the normal brain reflect inhibitory post-synaptic potentials (IPSPs) of interneurons that regulate the firing and timing of postsynaptic principal cells.
Mechanism of Neuronal Synchronization
Epileptiform synchronization could be generated due to loss of inhibition, reduction in after hyperpolarization, enhanced excitatory synaptic transmission, enhancement of inhibitory network activity, and depolarizing γ-amino butyric acid (GABA). The generation of interictal epileptiform discharges (IEDs) epilepsy is commonly ascribed to enhanced excitatory interactions within glutamatergic neuronal networks.[5] In terms of both pattern and underlying mechanisms, IEDs are heterogenous in nature. Evidence suggests that core region in which focal seizures are generated is surrounded by an area that generates hypersynchronous activity (denominated the “irritative region”) interposed between the seizure-onset area and the surrounding normal tissue.[38] IEDs are generated both in the epileptogenic zone and in the irritative region and can spread to (and thus be recorded from) adjacent “healthy” brain structures. Therefore, it is reasonable to conclude that interictal events are sustained by cellular and pharmacological mechanisms that vary according to the site of generation. It is possible that these differences may result in a different functional role with respect to seizure generation. Experiments on animal models with convulsants showed that the CA3 region of the hippocampus is involved in generating IEDs, which were soon related to abrupt “paroxysmal” depolarization shifts (PDSs) in pyramidal cells.[39,40,41] Hyperexcitation contributed to the PDS produced by most acute convulsants.[42,43] It has been found that the extensive axon collateral network of CA3 pyramidal cells, which connect with many other CA3 pyramidal cells in addition to their longer range projections to CA1, septum and contralateral CA3 is responsible to the aforesaid hyperexcitation. It has also been shown that integrity of CA3 region of the hippocampus is an important determinant of glutamatergic drive to the CA1 pyramidal neurons.[33] Ablation of CA3 field in rat brain slices lead to the reduction of glutamatergic synaptic transmission onto CA1 pyramidal neurons.[44] Altogether, these reports suggest that excessive excitatory drive generated from the CA3 region may contribute to the abnormal synchronous neuronal activity associated with epileptogenesis. These synaptic networks can play a role in the synchronization occurring during both seizures and IEDs. It is possibly strengthened by sprouting of new connections and other synaptic changes in chronic epileptic foci.
The duration of epilepsy is the most important predictor for long-term surgical outcome and active networks may attain permanent bi-stable stage with time.[45,46] It is also plausible that idling networks may return back to their active bi-stable stage after discontinuation of antiepileptic drugs (AEDs) in patients rendered seizure free by epilepsy surgery.[47] Altogether, the network concept provides better understanding of the epileptogenic lesion. Nonregression analysis of intracerebral EEG signals helps in the identification of epileptogenic networks.[48] In addition, fluctuation analysis has been an important aspect of research in neurophysiology.[49] Advanced cellular electrophysiological experiments has enabled us to record currents through a single or a group of channels from single neurons under voltage clamped conditions.[50] Recent development on quantification of noise at the ion channel level has thrown light on the phenomenon of transport of ions and metabolites across cell membrane and its mechanisms especially its relation to neuronal communications.[51,52,53,54,55,56,57,58,59,60,61] Synaptic noise, a kind of channel noise, plays an important role in this process.
Epiletogenic Zone Versus Network
Despite remarkable advances in epilepsy surgery, the principles of surgery have been only resective, disconnective, or neuromodulatory surgery. Since Wilkins et al.,'s[62] description of the famous case in 1886 of a patient with focal motor seizures with depressed fracture, who was cured of his epilepsy, when the lesion and also the surrounding area was resected led to the birth of epilepsy surgery. From here on the concept of “epileptic zone” took birth, where the lesion was assumed to be placed in the center surrounded by structurally normal but functionally deranged parenchyma. Luders further refined this concept by creating the concept of lesional zone, surrounded by ictal onset zone (as detected by video EEG) and further surrounded by a large irritative zone (as defined by inter ictal EEG). The epileptogenic zone was said to be placed between the ictal onset zone and the irritative zone.[63] Currently, there is no single investigation available to exactly delineate the epileptgenic zone. This is mostly because all the current investigations,[64,65] have different levels of spatial resolutions (ability to delineate the focus from the level of neuron to the brain) and temporal resolutions (ability to distinguish the onset of epilepsy at different time periods). Thus, PET has a lower temporal and spatial resolution as compared with SPECT. Thus, different modalities of investigations provide different snapshots of the epileptogenesis at different time periods and at variable magnifications. With the biomarkers of epilepsy limited to a few options (ictal EEG, HFO, and MEG), options turned on to devise more complex methods of data analysis to allow a greater sampling rate and larger sampling area. Linear mechanics to explain epiletogeneis were obviously doubtful to explain real-time situations. Thus, nonlinear mechanics like nonlinear regression analysis (Wendling et al.[48,66]) became more useful to develop computer models of epileptogenesis. This also led to use of more complex computational models like nonlinear dynamical systems and chaos theory which helped us understand epileptogenesis much better. These mostly included phase synchronization methods, generalized synchronized methods, and phase lag index.[67,68,69,70]
To understand epileptogenesis better, Aubert et al.,[24] developed the concept of epileptogenic index, which is essentially the propensity of the brain to generate high frequency oscillations and the time for this area to get involved in the seizure. He found that only in 1/3rd of cases with epilepsy there is a single epileptogenic zone, while in the rest cases; there was evidence of more than one area, which generated epilepsy. There is now cumulating evidence that the epileptogenic “zone” is essentially a simplified concept. In fact, the epileptic neurons are now recognized to have a bi-stable nature during epileptogenesis and during interictal period.[5,23,24,36,37,69,70,71] Thus, a small area of neurons, which epileptogenic stimulate more distant neurons, and through reentrant pathways forms a reverberating circuit (also called a “node”). Such circuits progressively recruit more distant circuits forming larger and larger networks over a period of time [Figure 2]. In fact, clinical studies (though indirectly) do also suggest existence of such networks. McIntosh et al.,[26] demonstrated best long-term outcome for temporal lobe epilepsy with presence of a demonstrable lesion, suggesting that the maximal networks are centered around the lesion. Another elegant study by Janszky et al.,[45] demonstrated that the seizure freedom was inversely proportional to the duration of epilepsy (being 90% Class I Engel for patients with duration of epilepsy between 1 and 10 years reducing to about 30% for patients with duration of epilepsy being >30 years at the time of surgery). While the findings of the study cannot be explained with the “zone” hypothesis, the “network” hypothesis can explain this by the fact that more neurons become recruited into the “epileptogenic network” over a period of time if epilepsy is not controlled. Similarly another landmark study by Schmidt et al.,[47] demonstrated that the seizure freedom falls down to 65% (Class I Engel) over 3 years after discontinuation of AEDs for temporal lobe epilepsy suggesting that “idling” networks may return back to the active bistable state. These studies suggest that the epileptogenic focus is not a “fixed” zone but a dynamic, constantly changing group of networks. The network hypothesis is further strengthened by Lin et al.,[72] who demonstrated diffuse thinning of neocortical grey matter in mesial temporal sclerosis suggesting clearly the complexity involved even in focal pathologies producing epilepsy. The network concept is important for the surgeon to understand that the epileptogenic “focus” may be far removed from the lesion and separated by normal parenchymal tissue. This has been very elegantly demonstrated in case study by Stefan et al.,[35] Here, the patient had a ventricular perinodular hetertopia (PNH). Placement of depths and a surface grid actually revealed ictal onset from the surface even though the lesion was deep within the ventricle. The patient underwent both resection of the PNH and surface neocortical temporal gyrus, following seizure freedom was achieved. We also had a similar case, where a distant focus was demonstrated [Figure 3].
Figure 2.
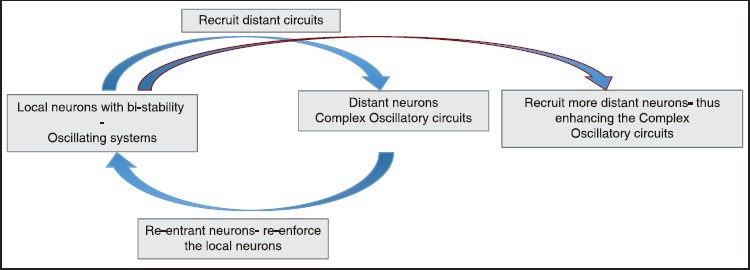
The following diagram shows the active, dynamic nature of epileptogenic networks. Epileptogenecity initially begins with a group of bistable local neurons which recruit more distant neurons and are joined back by reverberating circuits forming a “node.” When epilepsy remains untreated, more distant neurons are recruited within the network progressively increasing both its size and complexity. Thus, an epileptogenic network is constantly evolving as compared to the zone concept which was assumed to be static
Figure 3.
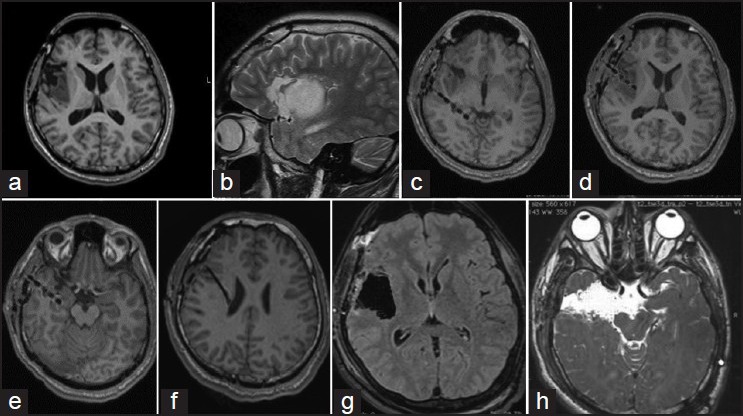
The following example demonstrates the concept of epilepsy network. This 19-year-old boy presented with symptoms of “choking” sensation, followed by generalization (about 5-6 episodes a day since 5 years of age). He underwent a partial excision for a right insular low grade glioma at another hospital [Figures 1a and b]. This semiology changed 1 year ago to choking sensation followed by an aura of epigastric “rising,” followed by deviation of eyes toward left side. Video electroencephalography suggested an ictal onset in F8 and T4. Ictal single-photon emission computed tomography showed activity from the mesial right temporal lobe. Four depths were placed, one within tail hippocampus [c], one within the tumor [d], one within the hippocampus [e], and the 4th superior to the tumor [f]. Ictal onset was recorded from the electrode from hippocampus. Surgery included a complete excision of the insular glioma [g] and amygdalohippocampectomy with anterior temporal lobectomy [f]. Histopathology of the hippocampus showed evidence of mesial temporal sclerosis. Following surgery, patient had an Engel I outcome. Thus, even though the primary lesion was the insular low grade glioma, the hippocampal sclerosis was probably induced by the virtue of insular connections to the temporal lobe
Multidisciplinary Approach to Study Epileptogenesis
In a developing country like India, the number of epilepsy patients is approximately five million, of which around one million epileptics suffer from the medically intractable epilepsy.[1] A significant number of patients who have undergone resective brain surgery for epilepsy still continue to have seizures. Understanding the intricacies of DRE still remains a challenge for neurosurgeons across the world. The investigation of abnormal neuronal network and it association with epileptogenic area may provide us answers regarding the cellular and molecular basis of the epileptogenesis. It is important to go through the growing phase and bridge the gap between clinical and basic research in the field of epilepsy through a complementary and multidisciplinary approach [Figure 1]. Even though animal models of epilepsy shed light on the process of epileptogenesis, the molecular mechanism underlying this phenomenon is unclear. The reason behind this road-block is that none of the animal models for epilepsy could replicate the etiopathological conditions in humans. The tissue removed during resective surgery of epilepsy patients could serve as an ideal model system to investigate the abnormalities at microscopic level.[73] The well-established epileptogenic zones in the resected brain sample serve as an ideal model to study the molecular mechanism of hypersynchronus epileptiform discharges. To this end, biochemical and cellular electrophysiological analysis of resected brain specimens obtained from epilepsy patients to investigate the molecular mechanism associated with epileptogenesis will provide useful insights.[47,48,49] The epileptogenic zone established using the above-mentioned imaging and electrical localization techniques provide a unique model system to study hyperexcitatory neuronal network. Patch-clamp technique provides an avenue to study the alteration in glutamatergic and GABAergic synaptic transmission on to specific neurons in the epileptogenic zone.[44,45,47,48,49,50,73,74,75] Moreover, the brain tissue specimens obtained in a graded manner from various areas of the above-defined epileptogenic zone could also be investigated for abnormal gene expression using deoxyribonucleic acid (DNA) microarray and to study changes in the excitatory and inhibitory neurotransmitter levels using immunohistochemistry. The correlations of above-mentioned molecular aspects of epileptogenic network with the clinical data will help enhance our understanding of epileptogenesis in general and DRE in specific [see figure 1].
Conclusion
Analysis of hypersynchronus neuronal network should be widely used for epilepsy surgery. Currently, utility and efficacy of determining epileptogenic network prior to surgery is controversial. But with high-resolution recording and imaging techniques, signal analysis methodologies and the possibility of studying dynamic brain states neuronal network analysis has gained strength. In India, the usage of noninvasive techniques like MEG and fMRI is now gaining popularity to identify network involvements both structurally and dynamically. Moreover, development of newer methods to analyze the dynamics of neuronal networks has gained momentum and has yielded a wide range of computer tools being tested in clinical and experimental environments. This has lead to development of newer concepts where the epileptogenic focus earlier thought to be a static zone centered around the lesion is now considered as an evolving active and a dynamic circuit of networks.
Footnotes
Source of Support: This work was supported by Centre of Excellence for Epilepsy Research (A NBRC-AIIMS Collaboration) grant from Department of Biotechnology, Ministry of Science & Technology, Government of India
Conflict of Interest: None declared
References
- 1.Chandra PS, Tripathi M. Epilepsy surgery: Recommendations for India. Ann Indian Acad Neurol. 2010;13:87–93. doi: 10.4103/0972-2327.64625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scharfman HE, Gray WP. Relevance of seizure-induced neurogenesis in animal models of epilepsy to the etiology of temporal lobe epilepsy. Epilepsia. 2007;48:33–41. doi: 10.1111/j.1528-1167.2007.01065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scharfman HE. Epileptogenesis in the parahippocampal region. Parallels with the dentate gyrus. Ann N Y Acad Sci. 2000;911:305–27. doi: 10.1111/j.1749-6632.2000.tb06734.x. [DOI] [PubMed] [Google Scholar]
- 4.Avoli M, Herbert H. Jasper and the basic mechanisms of the epilepsies. In: Noebels JL, Avoli M, Rogawski MA, Olsen RW, Delgado-Escueta AV, editors. Jasper's Basic Mechanisms of the Epilepsies. 4th ed. Bethesda (MD): 2012. [Google Scholar]
- 5.Fang M, Xi ZQ, Wu Y, Wang XF. A new hypothesis of drug refractory epilepsy: Neural network hypothesis. Med Hypotheses. 2011;76:871–6. doi: 10.1016/j.mehy.2011.02.039. [DOI] [PubMed] [Google Scholar]
- 6.Dumas JG, Rondepierre A. New York: Springer; 2003. Modelling the electrical activity of a neuron by a continuous and piecewise affine hybrid system: Computation and control. [Google Scholar]
- 7.Nelson P. New York: WH Freeman & Co; 2004. Biological physics; p. 508. [Google Scholar]
- 8.Silvia HC, Lucian CM, Reneto ME. Brazil: State University of Campinas; 2000. Brain and mind fundamentals, how brain cells work. [Google Scholar]
- 9.Yu Y, Wang W, Wang J, Liu F. Resonance-enhanced signal detection and transduction in the Hodgkin-Huxley neuronal systems. Phys Rev E Stat Nonlin Soft Matter Phys. 2001;63:021907. doi: 10.1103/PhysRevE.63.021907. [DOI] [PubMed] [Google Scholar]
- 10.Scott A. New York: Springer; 2002. Neuroscience: A mathematical primer. [Google Scholar]
- 11.Heneghan C, Chow CC, Collins JJ, Imhoff TT, Lowen SB, Teich MC. Information measures quantifying aperiodic stochastic resonance. Phys Rev E Stat Phys Plasmas Fluids Relat Interdiscip Topics. 1996;54:R2228–31. doi: 10.1103/physreve.54.r2228. [DOI] [PubMed] [Google Scholar]
- 12.Bratt GW, Rosenfeld MA, Williams DW. NIDCD/VA hearing aid clinical trial and follow-up: Background. J Am Acad Audiol. 2007;18:274–81. doi: 10.3766/jaaa.18.4.2. [DOI] [PubMed] [Google Scholar]
- 13.Douglass JK, Wilkens L, Pantazelou E, Moss F. Noise enhancement of information transfer in crayfish mechanoreceptors by stochastic resonance. Nature. 1993;365:337–40. doi: 10.1038/365337a0. [DOI] [PubMed] [Google Scholar]
- 14.Haken H. Berlin: Springer; 2002. Brain dynamics; pp. 54–73. [Google Scholar]
- 15.Glass L. Synchronization and rhythmic processes in physiology. Nature. 2001;410:277–84. doi: 10.1038/35065745. [DOI] [PubMed] [Google Scholar]
- 16.Zhou C, Kurths J. Noise-induced synchronization and coherence resonance of a Hodgkin-Huxley model of thermally sensitive neurons. Chaos. 2003;13:401–9. doi: 10.1063/1.1493096. [DOI] [PubMed] [Google Scholar]
- 17.Bragin A, Wilson CL, Engel J., Jr Chronic epileptogenesis requires development of a network of pathologically interconnected neuron clusters: A hypothesis. Epilepsia. 2000;41:S144–52. doi: 10.1111/j.1528-1157.2000.tb01573.x. [DOI] [PubMed] [Google Scholar]
- 18.Luat AF, Chugani HT. Molecular and diffusion tensor imaging of epileptic networks. Epilepsia. 2008;49:15–22. doi: 10.1111/j.1528-1167.2008.01506.x. [DOI] [PubMed] [Google Scholar]
- 19.Wiebe S, Jette N. Epilepsy surgery utilization: Who, when, where, and why? Curr Opin Neurol. 2012;25:187–93. doi: 10.1097/WCO.0b013e328350baa6. [DOI] [PubMed] [Google Scholar]
- 20.Jette N, Choi H, Wiebe S. Applying evidence to patient care: From population health to individual patient values. Epilepsy Behav. 2013;26:234–40. doi: 10.1016/j.yebeh.2012.08.017. [DOI] [PubMed] [Google Scholar]
- 21.Wiebe S, Jette N. Pharmacoresistance and the role of surgery in difficult to treat epilepsy. Nat Rev Neurol. 2012;8:669–77. doi: 10.1038/nrneurol.2012.181. [DOI] [PubMed] [Google Scholar]
- 22.Reid AY, Metcalfe A, Patten SB, Wiebe S, Macrodimitris S, Jette N. Epilepsy is associated with unmet health care needs compared to the general population despite higher health resource utilization - a Canadian population-based study. Epilepsia. 2012;53:291–300. doi: 10.1111/j.1528-1167.2011.03353.x. [DOI] [PubMed] [Google Scholar]
- 23.Berg AT, Scheffer IE. New concepts in classification of the epilepsies: Entering the 21st century. Epilepsia. 2011;52:1058–62. doi: 10.1111/j.1528-1167.2011.03101.x. [DOI] [PubMed] [Google Scholar]
- 24.Aubert S, Wendling F, Regis J, McGonigal A, Figarella-Branger D, Peragut JC, et al. Local and remote epileptogenicity in focal cortical dysplasias and neurodevelopmental tumours. Brain. 2009;132:3072–86. doi: 10.1093/brain/awp242. [DOI] [PubMed] [Google Scholar]
- 25.Vaugier L, Aubert S, McGonigal A, Trébuchon A, Guye M, Gavaret M, et al. Neural networks underlying hyperkinetic seizures of “temporal lobe” origin. Epilepsy Res. 2009;86:200–8. doi: 10.1016/j.eplepsyres.2009.06.007. [DOI] [PubMed] [Google Scholar]
- 26.McIntosh AM, Kalnins RM, Mitchell LA, Fabinyi GC, Briellmann RS, Berkovic SF. Temporal lobectomy: Long-term seizure outcome, late recurrence and risks for seizure recurrence. Brain. 2004;127:2018–30. doi: 10.1093/brain/awh221. [DOI] [PubMed] [Google Scholar]
- 27.Chandra SP, Bal CS, Jain S, Joshua SP, Gaikwad S, Garg A, et al. Intraoperative coregistration of magnetic resonance imaging, positron emission tomography, and electrocorticographic data for neocortical lesional epilepsies may improve the localization of the epileptogenic focus: A pilot study. World Neurosurg. 2013 doi: 10.1016/j.wneu.2013.02.057. In press. [DOI] [PubMed] [Google Scholar]
- 28.Bindra A, Chouhan RS, Prabhakar H, Dash HH, Chandra PS, Tripathi M. Comparison of the effects of different anesthetic techniques on electrocorticography in patients undergoing epilepsy surgery - a bispectral index guided study. Seizure. 2012;21:501–7. doi: 10.1016/j.seizure.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 29.Tripathi M, Garg A, Gaikwad S, Bal CS, Chitra S, Prasad K, et al. Intra-operative electrocorticography in lesional epilepsy. Epilepsy Res. 2010;89:133–41. doi: 10.1016/j.eplepsyres.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 30.Tripathi M, Singh MS, Padma MV, Gaikwad S, Bal CS, Tripathi M, et al. Surgical outcome of cortical dysplasias presenting with chronic intractable epilepsy: A 10-year experience. Neurol India. 2008;56:138–43. [PubMed] [Google Scholar]
- 31.Miller KJ, Honey CJ, Hermes D, Rao RP, Dennijs M, Ojemann JG. Broadband changes in the cortical surface potential track activation of functionally diverse neuronal populations. Neuroimage. 2014;85(Pt 2):711–20. doi: 10.1016/j.neuroimage.2013.08.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Miller KJ, denNijs M, Shenoy P, Miller JW, Rao RP, Ojemann JG. Real-time functional brain mapping using electrocorticography. Neuroimage. 2007;37:504–7. doi: 10.1016/j.neuroimage.2007.05.029. [DOI] [PubMed] [Google Scholar]
- 33.Leuthardt EC, Miller KJ, Schalk G, Rao RP, Ojemann JG. Electrocorticography-based brain computer interface — the Seattle experience. IEEE Trans Neural Syst Rehabil Eng. 2006;14:194–8. doi: 10.1109/TNSRE.2006.875536. [DOI] [PubMed] [Google Scholar]
- 34.Kumar A, Chandra PS, Sharma BS, Garg A, Rath GK, Bithal PK, et al. The role of neuronavigation-guided functional MRI and diffusion tensor tractography along with cortical stimulation in patients with eloquent cortex lesions. Br J Neurosurg. 2013 doi: 10.3109/02688697.2013.835370. [DOI] [PubMed] [Google Scholar]
- 35.Stefan H, Paulini-Ruf A, Hopfengartner R, Rampp S. Network characteristics of idiopathic generalized epilepsies in combined MEG/EEG. Epilepsy Res. 2009;85:187–98. doi: 10.1016/j.eplepsyres.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 36.Firpi H, Smart O, Worrell G, Marsh E, Dlugos D, Litt B. High-frequency oscillations detected in epileptic networks using swarmed neural-network features. Ann Biomed Eng. 2007;35:1573–84. doi: 10.1007/s10439-007-9333-7. [DOI] [PubMed] [Google Scholar]
- 37.Jacobs J, Staba R, Asano E, Otsubo H, Wu JY, Zijlmans M, et al. High-frequency oscillations (HFOs) in clinical epilepsy. Prog Neurobiol. 2012;98:302–15. doi: 10.1016/j.pneurobio.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Talairach J, Bancaud J. Lesion, “irritative” zone and epileptogenic focus. Confin Neurol. 1966;27:91–4. doi: 10.1159/000103937. [DOI] [PubMed] [Google Scholar]
- 39.Prince DA. Neurophysiology of epilepsy. Ann Rev Neurosci. 1978;1:395–415. doi: 10.1146/annurev.ne.01.030178.002143. [DOI] [PubMed] [Google Scholar]
- 40.Johnston D, Brown TH. Control theory applied to neural networks illuminates synaptic basis of interictal epileptiform activity. Adv Neurol. 1986;44:263–74. [PubMed] [Google Scholar]
- 41.Johnston D, Brown TH. Giant synaptic potential hypothesis for epileptiform activity. Science. 1981;211:294–7. doi: 10.1126/science.7444469. [DOI] [PubMed] [Google Scholar]
- 42.Traub RD, Miles R, Jefferys JG. Synaptic and intrinsic conductances shape picrotoxin-induced synchronized after-discharges in the guinea-pig hippocampal slice. J Physiol. 1993;461:525–47. doi: 10.1113/jphysiol.1993.sp019527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Traub RD, Wong RK. Cellular mechanism of neuronal synchronization in epilepsy. Science. 1982;216:745–7. doi: 10.1126/science.7079735. [DOI] [PubMed] [Google Scholar]
- 44.Banerjee J, Alkondon M, Albuquerque EX, Pereira EF. Contribution of CA3 and CA1 pyramidal neurons to the tonic alpha7 nAChR-dependent glutamatergic input to CA1 pyramidal neurons. Neurosci Lett. 2013;554:167–71. doi: 10.1016/j.neulet.2013.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Janszky J, Janszky I, Schulz R, Hoppe M, Behne F, Pannek HW, et al. Temporal lobe epilepsy with hippocampal sclerosis: Predictors for long-term surgical outcome. Brain. 2005;128:395–404. doi: 10.1093/brain/awh358. [DOI] [PubMed] [Google Scholar]
- 46.Janszky J, Jokeit H, Heinemann D, Schulz R, Woermann FG, Ebner A. Epileptic activity influences the speech organization in medial temporal lobe epilepsy. Brain. 2003;126:2043–51. doi: 10.1093/brain/awg193. [DOI] [PubMed] [Google Scholar]
- 47.Schmidt D, Baumgartner C, Loscher W. Seizure recurrence after planned discontinuation of antiepileptic drugs in seizure-free patients after epilepsy surgery: A review of current clinical experience. Epilepsia. 2004;45:179–86. doi: 10.1111/j.0013-9580.2004.37803.x. [DOI] [PubMed] [Google Scholar]
- 48.Wendling F, Bartolomei F, Senhadji L. Spatial analysis of intracerebral electroencephalographic signals in the time and frequency domain: Identification of epileptogenic networks in partial epilepsy. Philos Trans A Math Phys Eng Sci. 2009;367:297–316. doi: 10.1098/rsta.2008.0220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.White JA, Rubinstein JT, Kay AR. Channel noise in neurons. Trends Neurosci. 2000;23:131–7. doi: 10.1016/s0166-2236(99)01521-0. [DOI] [PubMed] [Google Scholar]
- 50.Benndorf K. Patch clamp analysis of Na channel gating in mammalian myocardium: Reconstruction of double pulse inactivation and voltage dependence of Na currents. Gen Physiol Biophys. 1988;7:353–77. [PubMed] [Google Scholar]
- 51.Akemann W, Lundby A, Mutoh H, Knopfel T. Effect of voltage sensitive fluorescent proteins on neuronal excitability. Biophys J. 2009;96:3959–76. doi: 10.1016/j.bpj.2009.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schmid G, Goychuk I, Hanggi P. Capacitance fluctuations causing channel noise reduction in stochastic Hodgkin-Huxley systems. Phys Biol. 2006;3:248–54. doi: 10.1088/1478-3975/3/4/002. [DOI] [PubMed] [Google Scholar]
- 53.Dorval AD, Jr, White JA. Channel noise is essential for perithreshold oscillations in entorhinal stellate neurons. J Neurosci. 2005;25:10025–8. doi: 10.1523/JNEUROSCI.3557-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rudolph M, Destexhe A. Characterization of subthreshold voltage fluctuations in neuronal membranes. Neural Comput. 2003;15:2577–618. doi: 10.1162/089976603322385081. [DOI] [PubMed] [Google Scholar]
- 55.Itami C, Samejima K, Nakamura S. Improved data processing for optical imaging of developing neuronal connectivity in the neonatal mouse barrel cortex. Brain Res Brain Res Protoc. 2001;7:103–14. doi: 10.1016/s1385-299x(01)00048-4. [DOI] [PubMed] [Google Scholar]
- 56.Redkozubov A. Elementary receptor currents elicited by a single pheromone molecule exhibit quantal composition. Pflugers Arch. 2000;440:896–901. doi: 10.1007/s004240000345. [DOI] [PubMed] [Google Scholar]
- 57.Rieke F, Baylor DA. Molecular origin of continuous dark noise in rod photoreceptors. Biophys J. 1996;71:2553–72. doi: 10.1016/S0006-3495(96)79448-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Konishi T, Salt AN. Electrochemical profile for potassium ions across the cochlear hair cell membranes of normal and noise-exposed guinea pigs. Hear Res. 1983;11:219–33. doi: 10.1016/0378-5955(83)90080-1. [DOI] [PubMed] [Google Scholar]
- 59.Werman R. The reversal potential as a diagnostic tool in transmitter identification. Adv Biochem Psychopharmacol. 1980;21:21–31. [PubMed] [Google Scholar]
- 60.Verveen AA, DeFelice LJ. Membrane noise. Prog Biophys Mol Biol. 1974;28:189–265. doi: 10.1016/0079-6107(74)90019-4. [DOI] [PubMed] [Google Scholar]
- 61.Lebovitz RM. A theoretical examination of ionic interactions between neural and non-neural membranes. Biophys J. 1970;10:423–44. doi: 10.1016/S0006-3495(70)86310-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wilkins RH, Horsley V, Cushing H. Neurosurgical Classics — XXI. J Neurosurg. 1964;21:713–23. doi: 10.3171/jns.1964.21.8.0713. [DOI] [PubMed] [Google Scholar]
- 63.Rosenow F, Luders H. Presurgical evaluation of epilepsy. Brain. 2001;124:1683–700. doi: 10.1093/brain/124.9.1683. [DOI] [PubMed] [Google Scholar]
- 64.Hazeltine E, Weinstein A, Ivry RB. Parallel response selection after callosotomy. J Cogn Neurosci. 2008;20:526–40. doi: 10.1162/jocn.2008.20030. [DOI] [PubMed] [Google Scholar]
- 65.Milner D. Cognitive neuroscience: The biology of the mind and findings and current opinion in cognitive neuroscience. Trends Cogn Sci. 1998;2:463. doi: 10.1016/s1364-6613(98)01226-1. [DOI] [PubMed] [Google Scholar]
- 66.Wendling F, Bellanger JJ, Bartolomei F, Chauvel P. Relevance of nonlinear lumped-parameter models in the analysis of depth-EEG epileptic signals. Biol Cybern. 2000;83:367–78. doi: 10.1007/s004220000160. [DOI] [PubMed] [Google Scholar]
- 67.Lehnertz K, Andrzejak RG, Arnhold J, Kreuz T, Mormann F, Rieke C, et al. Nonlinear EEG analysis in epilepsy: Its possible use for interictal focus localization, seizure anticipation, and prevention. J Clin Neurophysiol. 2001;18:209–22. doi: 10.1097/00004691-200105000-00002. [DOI] [PubMed] [Google Scholar]
- 68.Elger CE, Widman G, Andrzejak R, Dümpelmann M, Arnhold J, Grassberger P, et al. Value of nonlinear time series analysis of the EEG in neocortical epilepsies. Adv Neurol. 2000;84:317–30. [PubMed] [Google Scholar]
- 69.Elger CE, Widman G, Andrzejak R, Arnhold J, David P, Lehnertz K. Nonlinear EEG analysis and its potential role in epileptology. Epilepsia. 2000;41:S34–8. doi: 10.1111/j.1528-1157.2000.tb01532.x. [DOI] [PubMed] [Google Scholar]
- 70.Lehnertz K, Widman G, Andrzejak R, Arnhold J, Elger CE. Is it possible to anticipate seizure onset by non-linear analysis of intracerebral EEG in human partial epilepsies? Rev Neurol (Paris) 1999;155:454–6. [PubMed] [Google Scholar]
- 71.Faingold CL. Brainstem Networks: Reticulo-cortical synchronization in generalized convulsive seizures. In: Noebels JL, Avoli M, Rogawski MA, Olsen RW, Delgado-Escueta AV, editors. Jasper's Basic Mechanisms of the Epilepsies. 4th ed. Bethesda (MD): 2012. [PubMed] [Google Scholar]
- 72.Lin JJ, Salamon N, Lee AD, Dutton RA, Geaga JA, Hayashi KM, et al. Reduced neocortical thickness and complexity mapped in mesial temporal lobe epilepsy with hippocampal sclerosis. Cereb Cortex. 2007;17:2007–18. doi: 10.1093/cercor/bhl109. [DOI] [PubMed] [Google Scholar]
- 73.Banerjee J, Tripathi M, Chandra PS. Understanding complexities of synaptic transmission in medically intractable seizures: A paradigm of epilepsy research. Indian J Neurosurg. 2013:2. [Google Scholar]
- 74.Janszky J, Woermann FG, Barsi P, Schulz R, Halasz P, Ebner A. Right hippocampal sclerosis is more common than left after febrile seizures. Neurology. 2003;60:1209–10. doi: 10.1212/01.wnl.0000052823.29467.a0. [DOI] [PubMed] [Google Scholar]
- 75.Jepsen ML, Ewert SD, Dau T. A computational model of human auditory signal processing and perception. J Acoust Soc Am. 2008;124:422–38. doi: 10.1121/1.2924135. [DOI] [PubMed] [Google Scholar]


